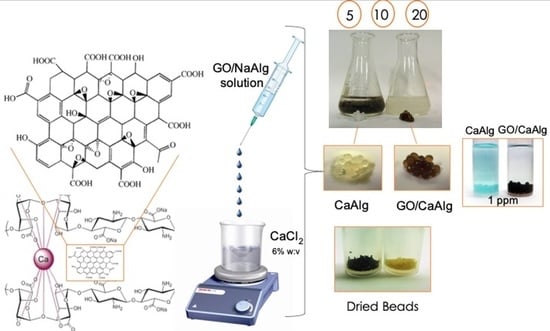
Activated Graphene Oxide-Calcium Alginate Beads for Adsorption of Methylene Blue and Pharmaceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Preparation of Graphene oxide (GO) Solution and Ca-Alg2/GO Beads
2.3. Acid Activation of the Beads Activation
2.4. Characterization
2.5. Adsorption Measurements
2.5.1. Initial Pollutant Concentration
2.5.2. Adsorbent Dose
2.5.3. pH
2.5.4. Temperature
2.5.5. Thermodynamics
2.5.6. Kinetics
2.5.7. Adsorption Isotherms
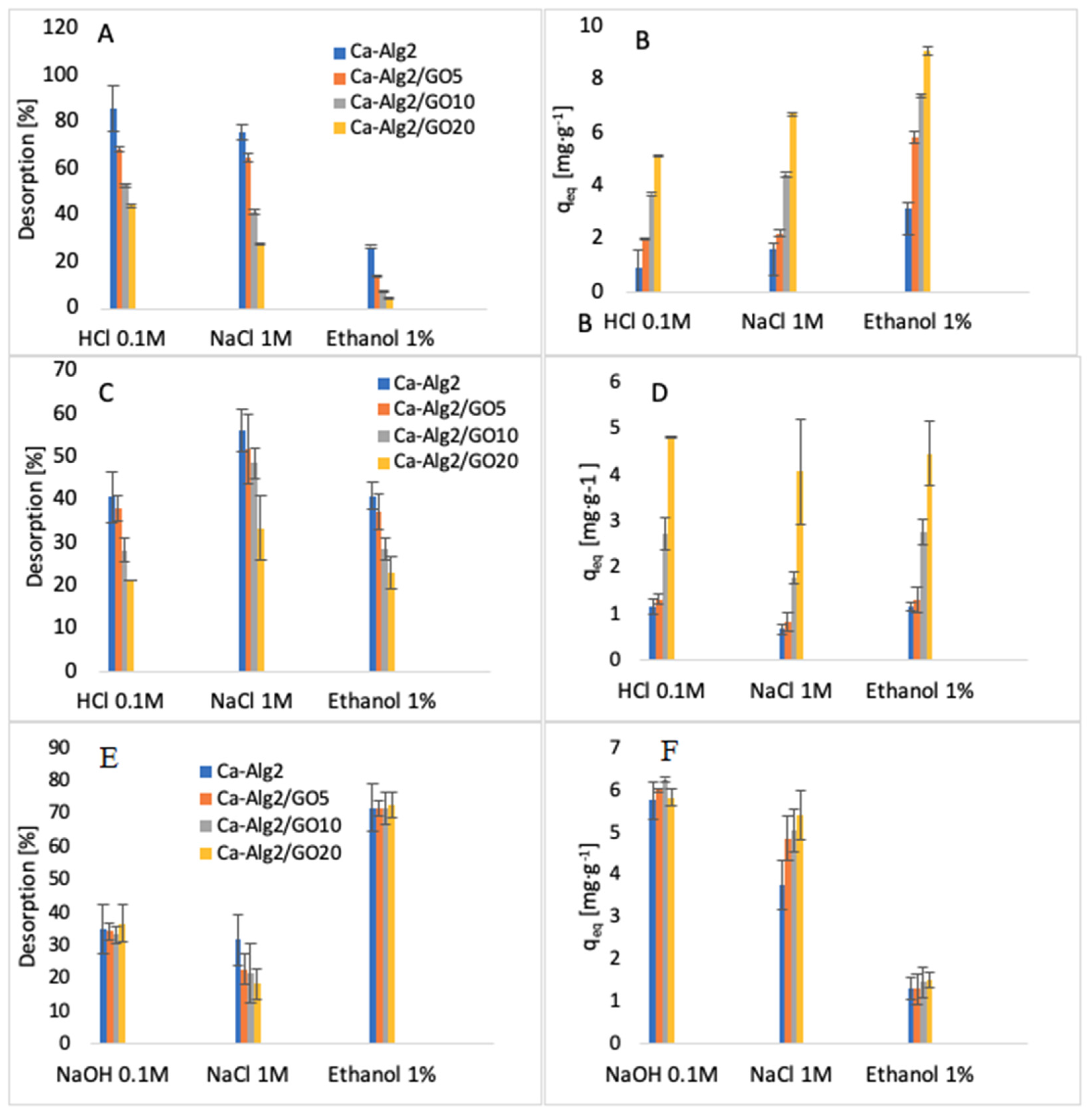
2.6. Desorption Studies
3. Results and Discussion
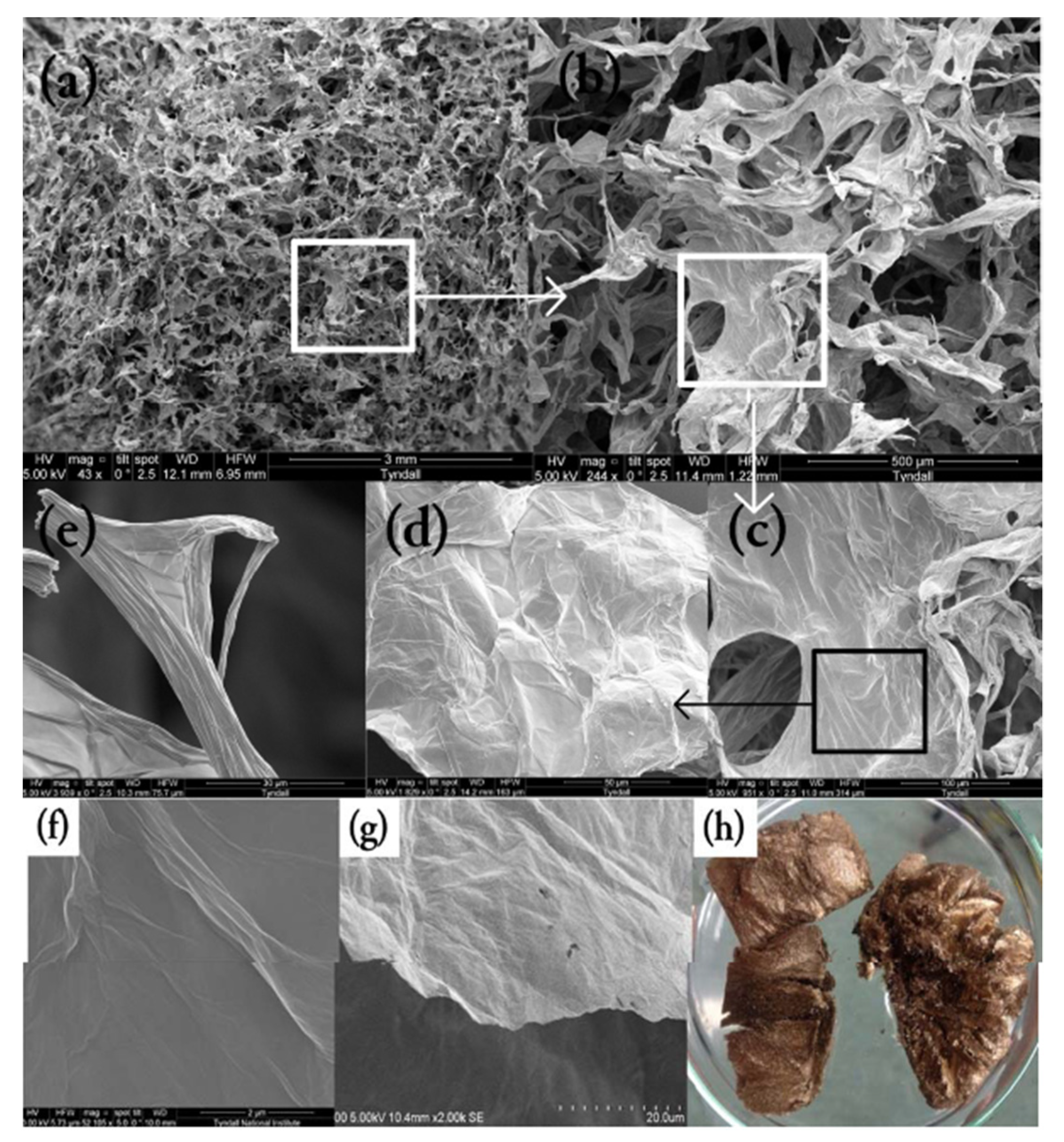
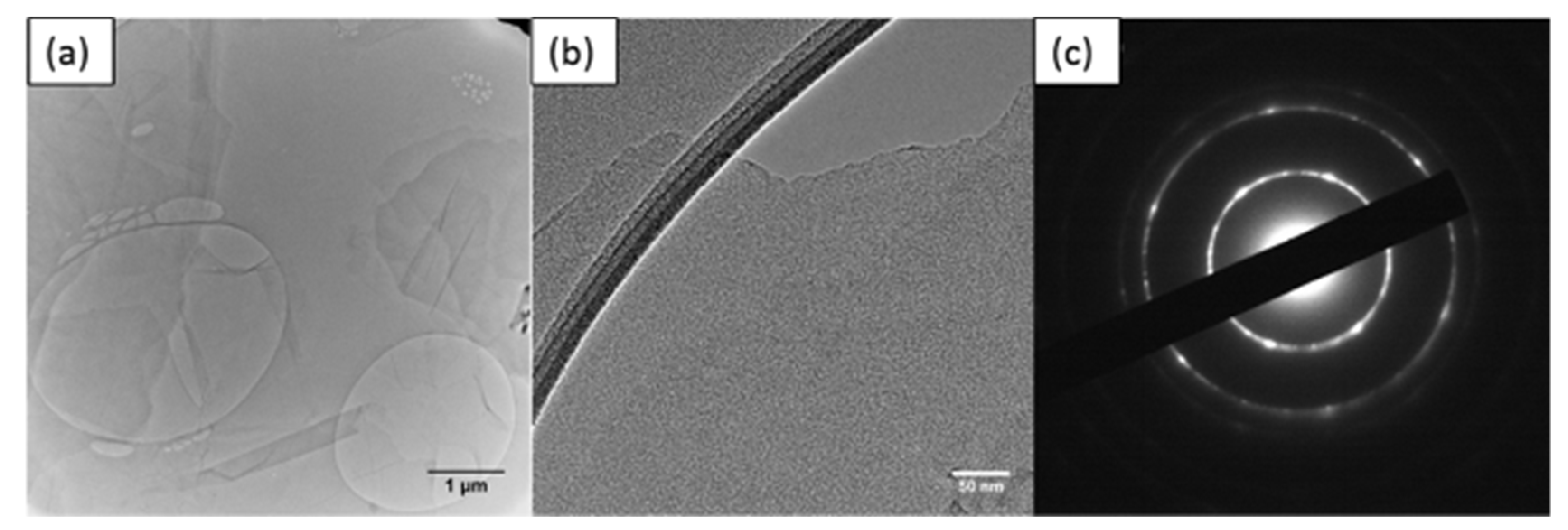
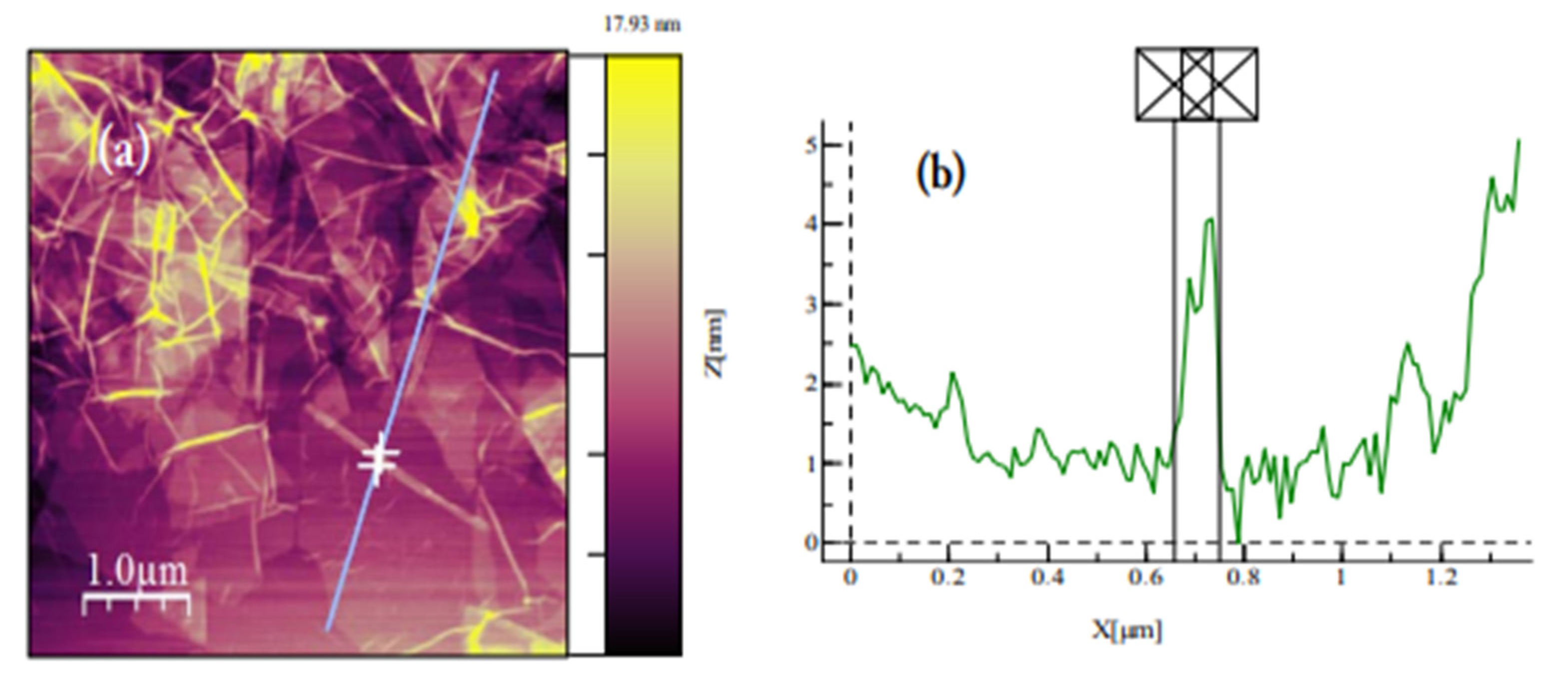
3.1. Characterisation of Beads
3.2. Effect of Operating Parameters on the Adsorption
3.2.1. Contact Time
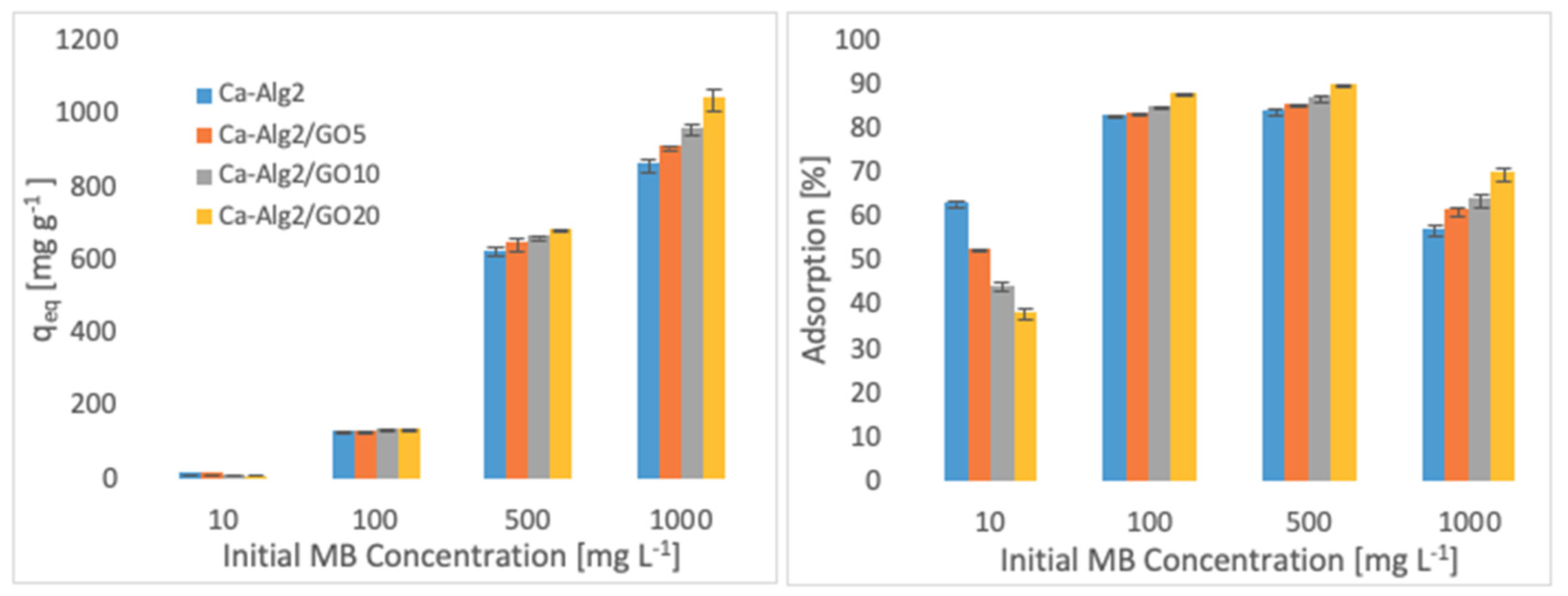
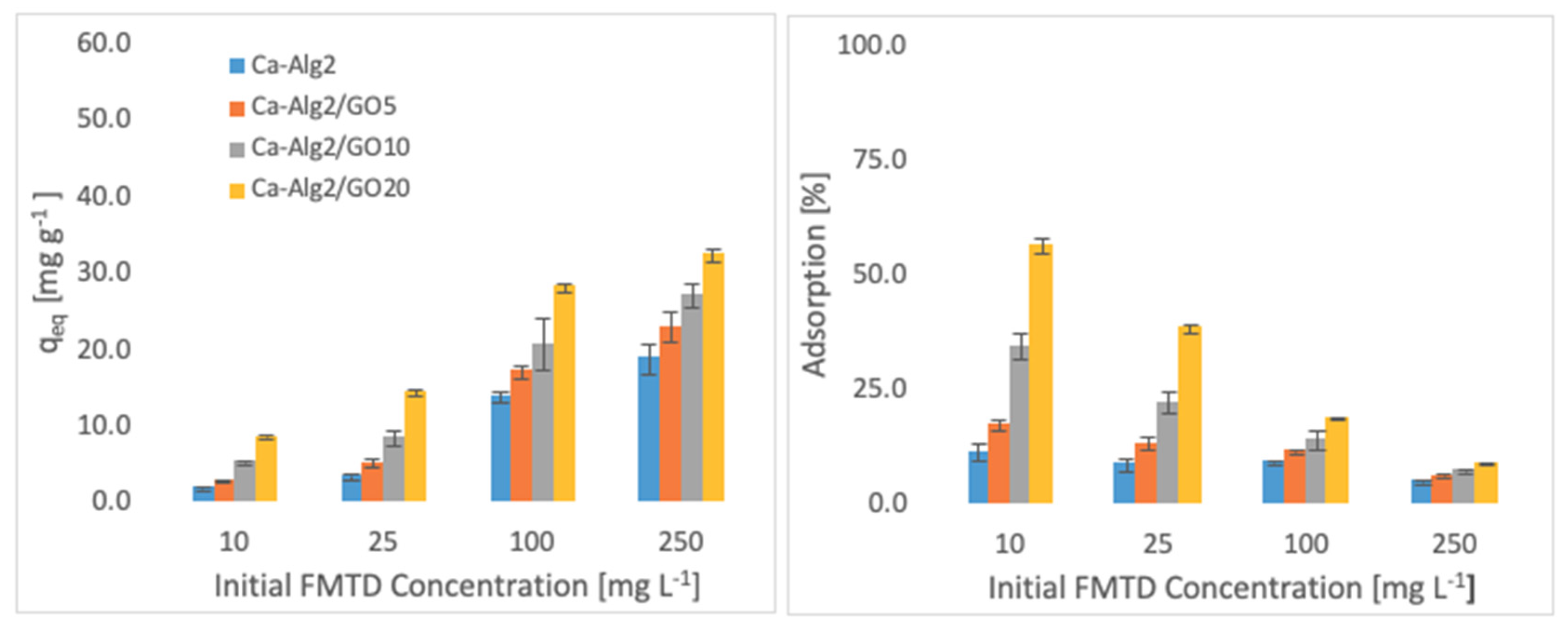
3.2.2. Pollutant Concentration
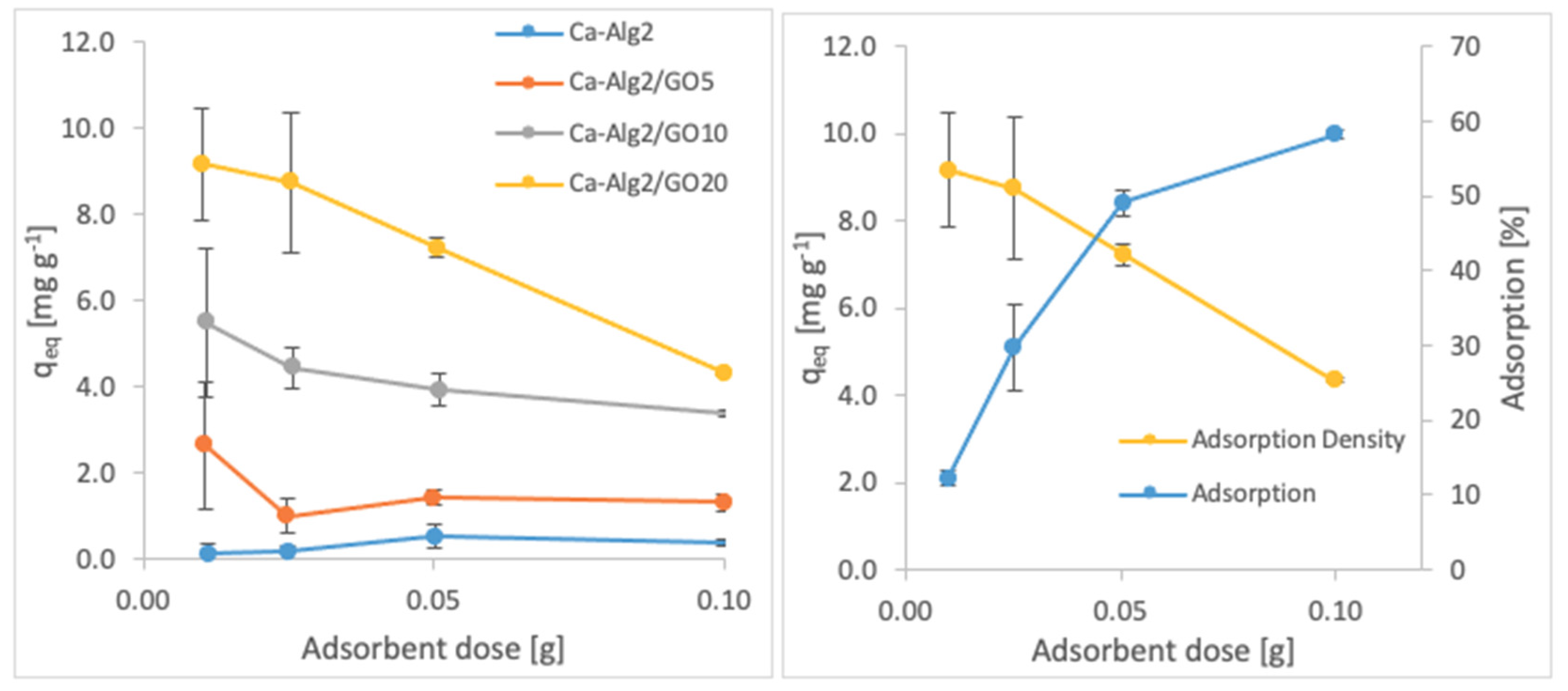
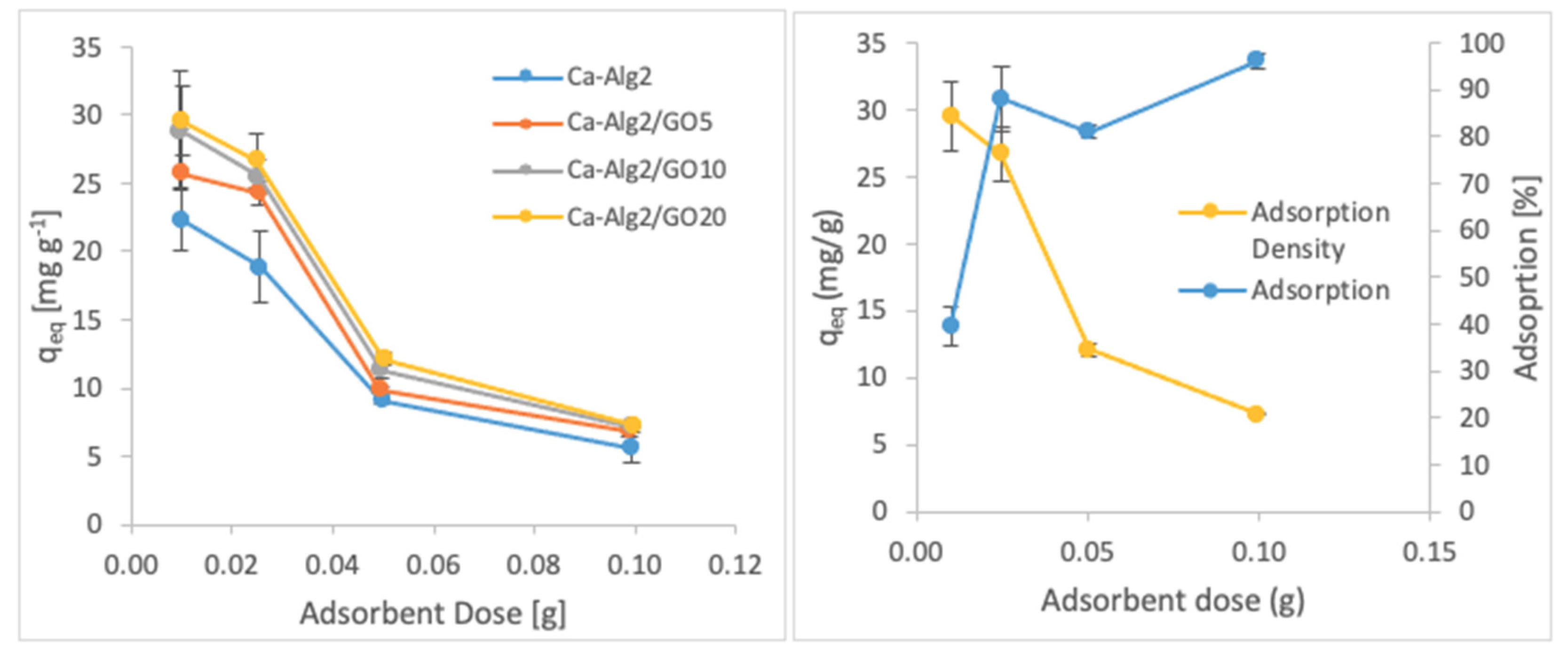
3.2.3. Adsorbent Dose
3.2.4. pH
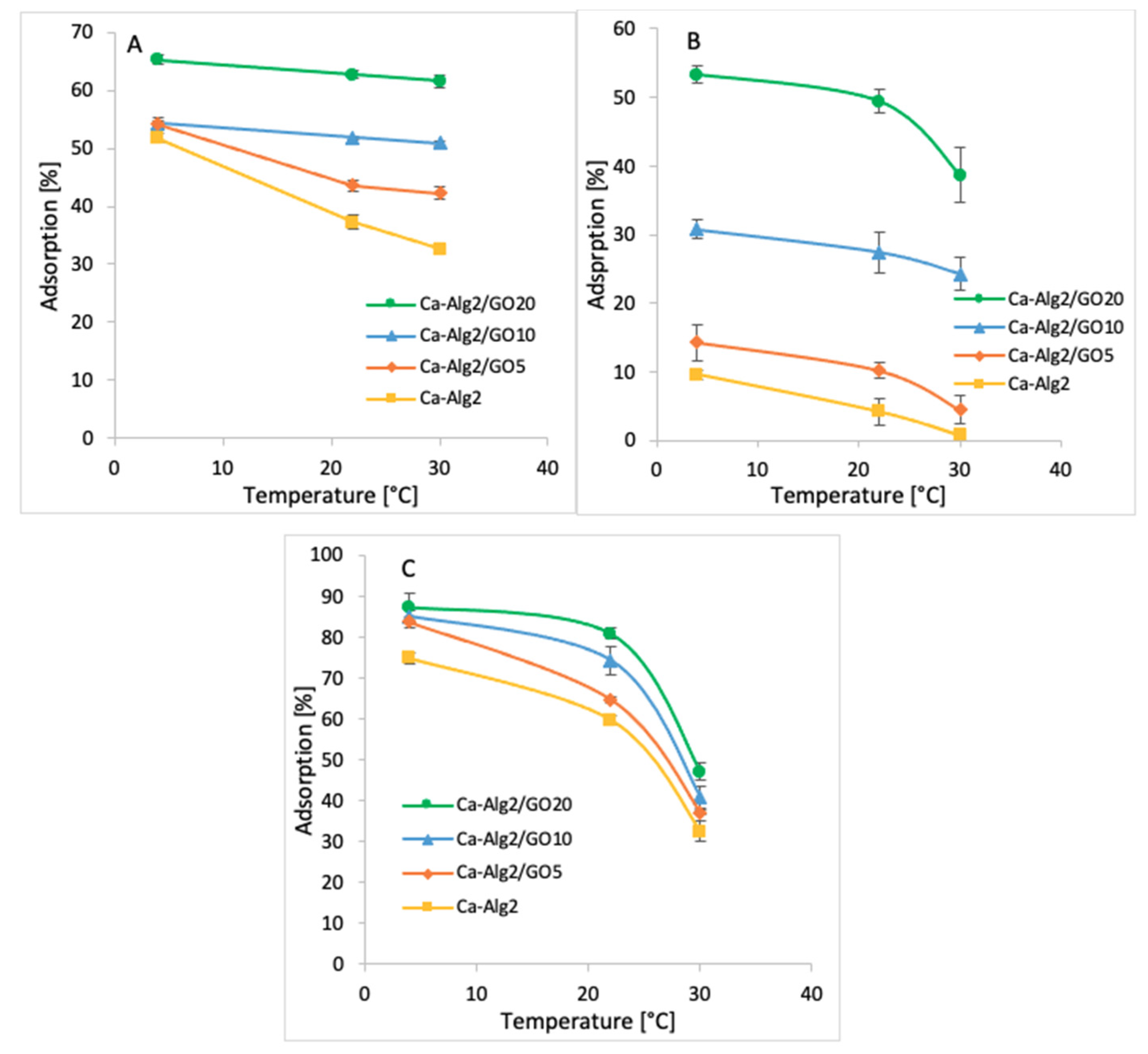
3.2.5. Temperature
3.3. Thermodynamics
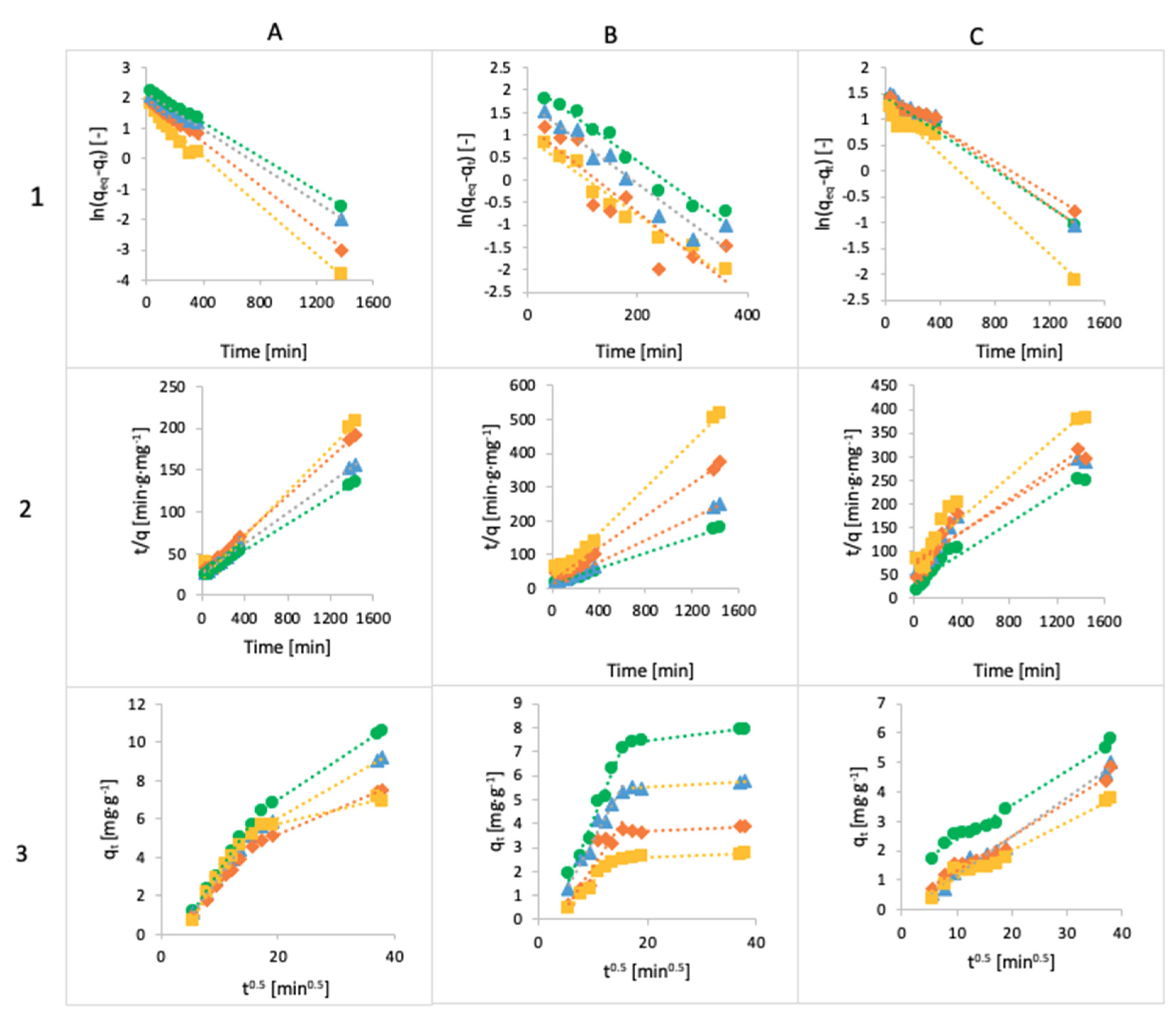
3.4. Kinetics
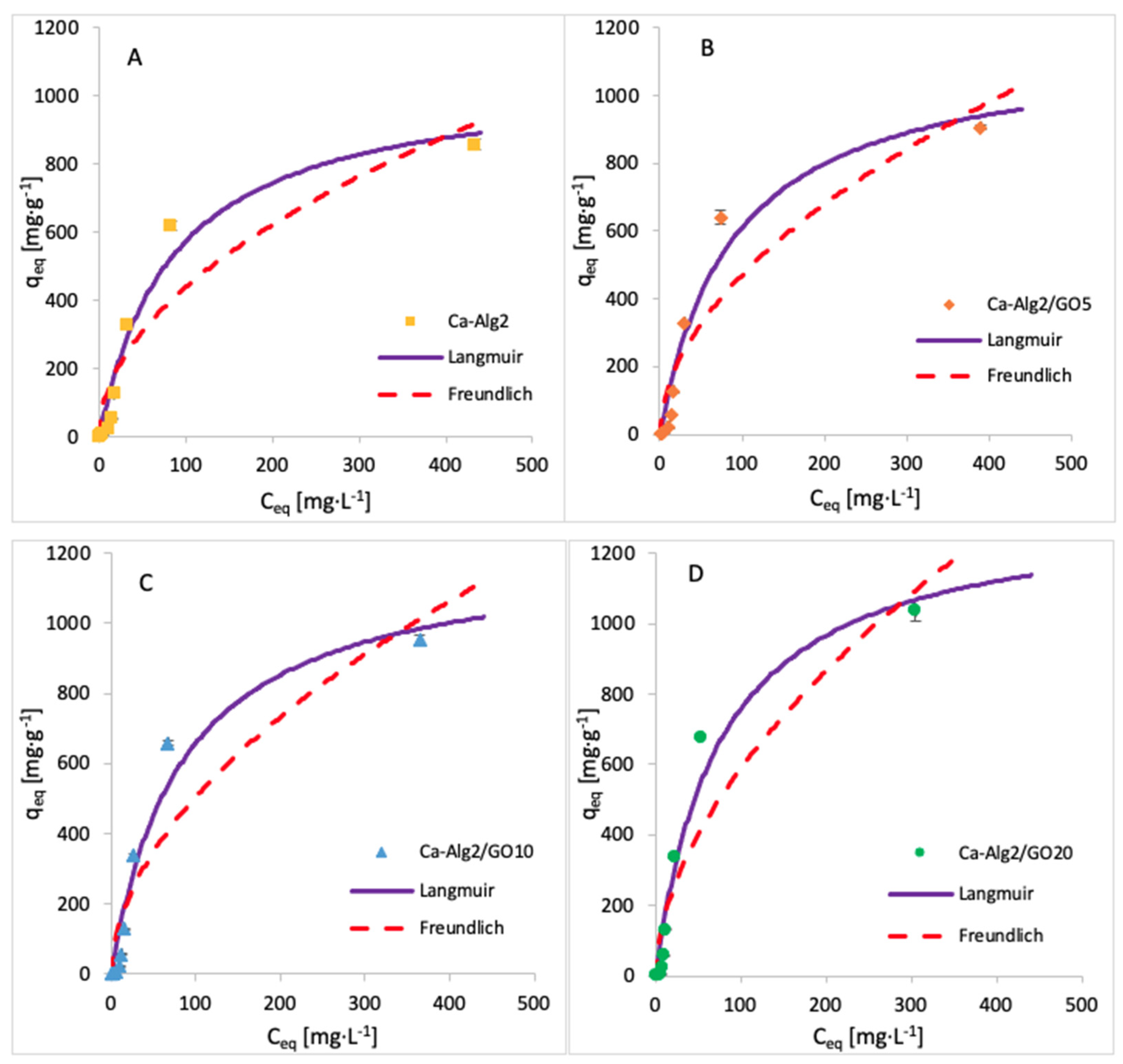
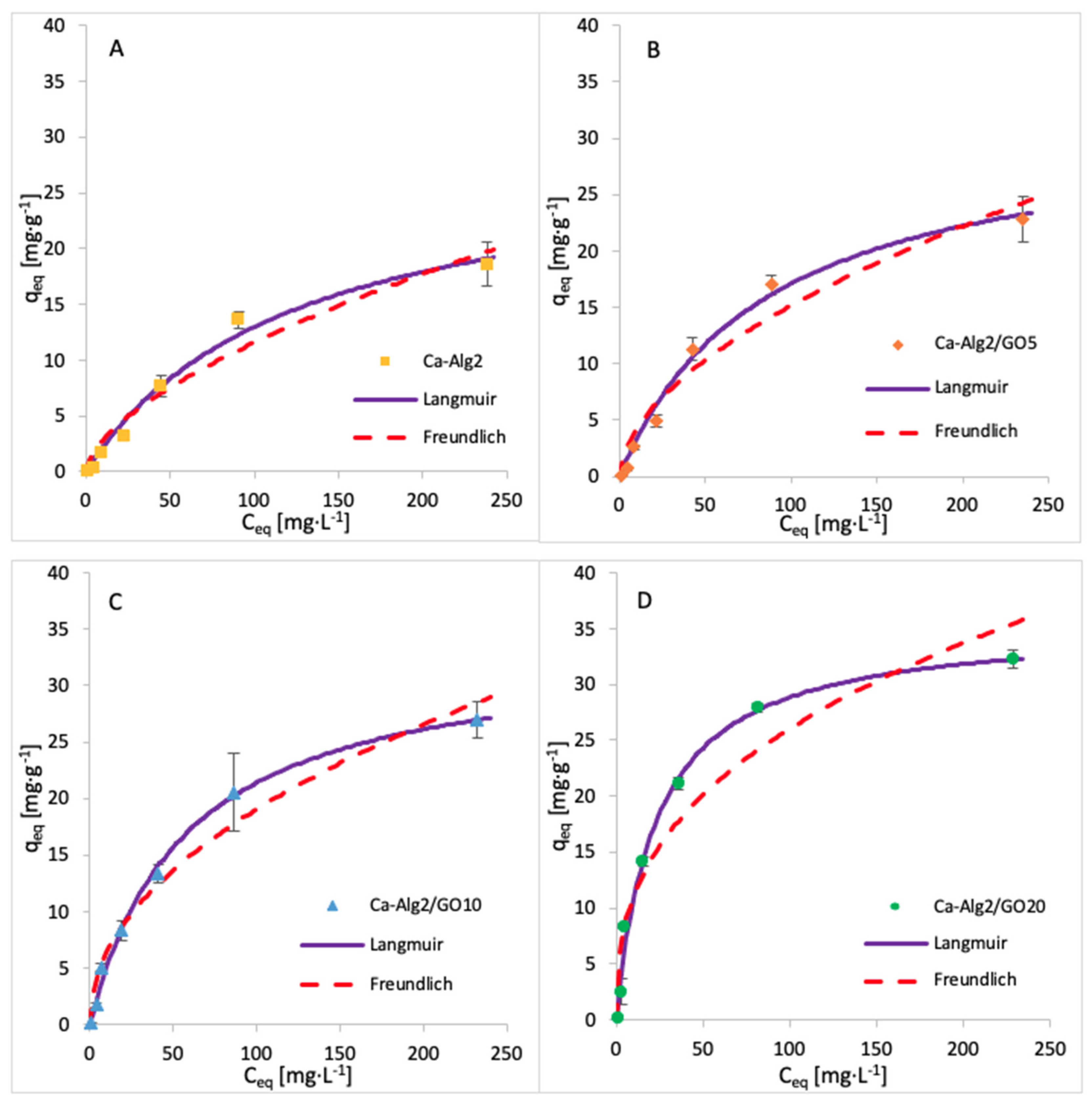
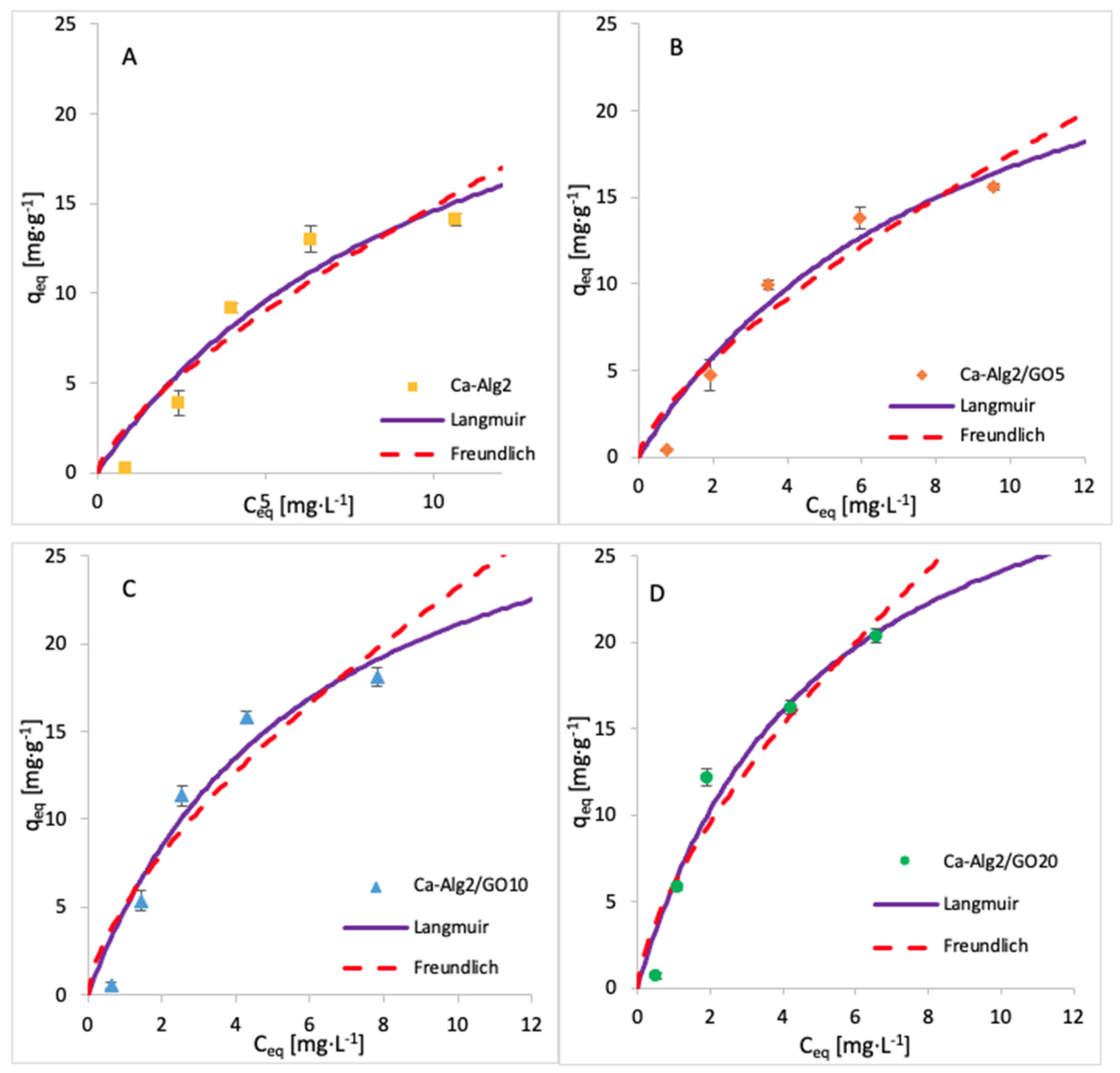
3.5. Adsorption Isotherms
3.6. Desorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Appendix A
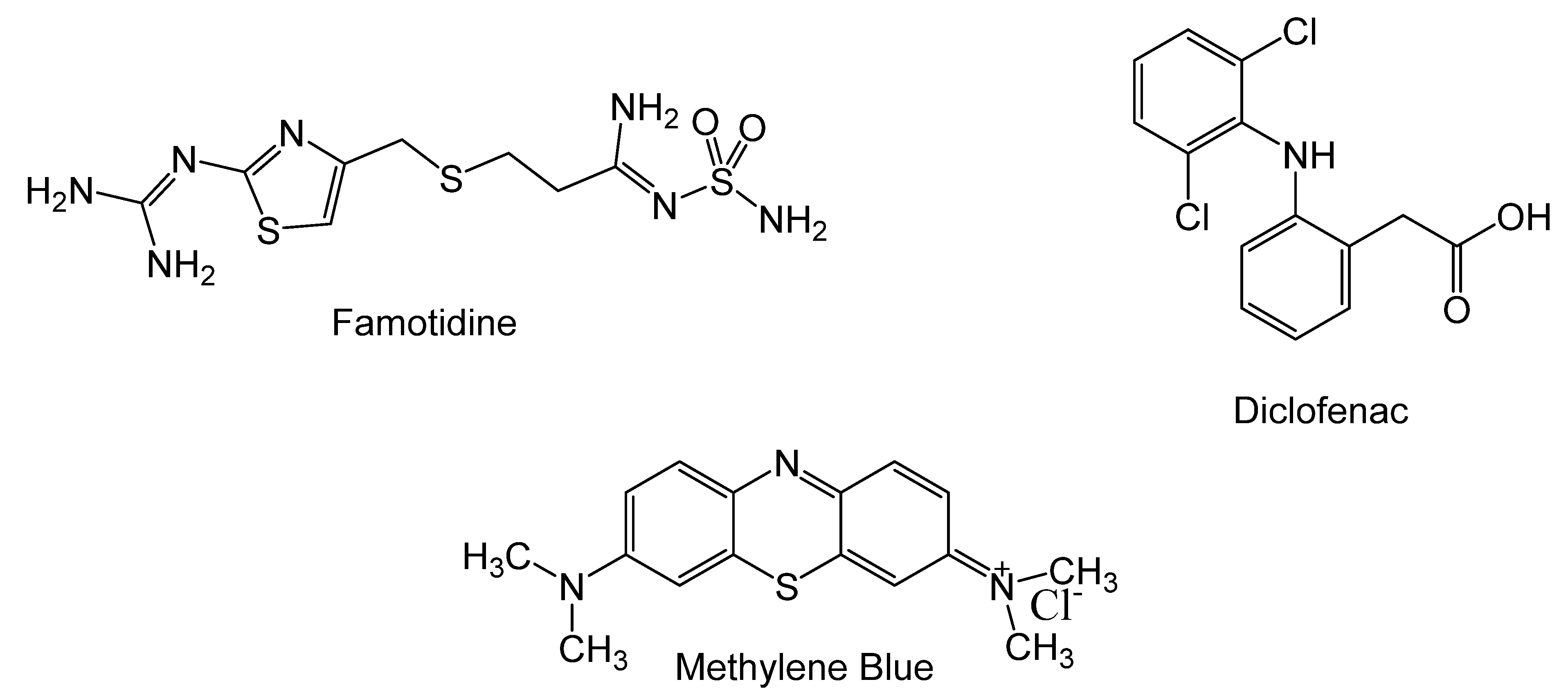
Appendix B
Appendix B.1. Preparation of GO
Appendix B.2. Preparation of Ca-Alg2/GO Beads


Appendix C


Appendix D
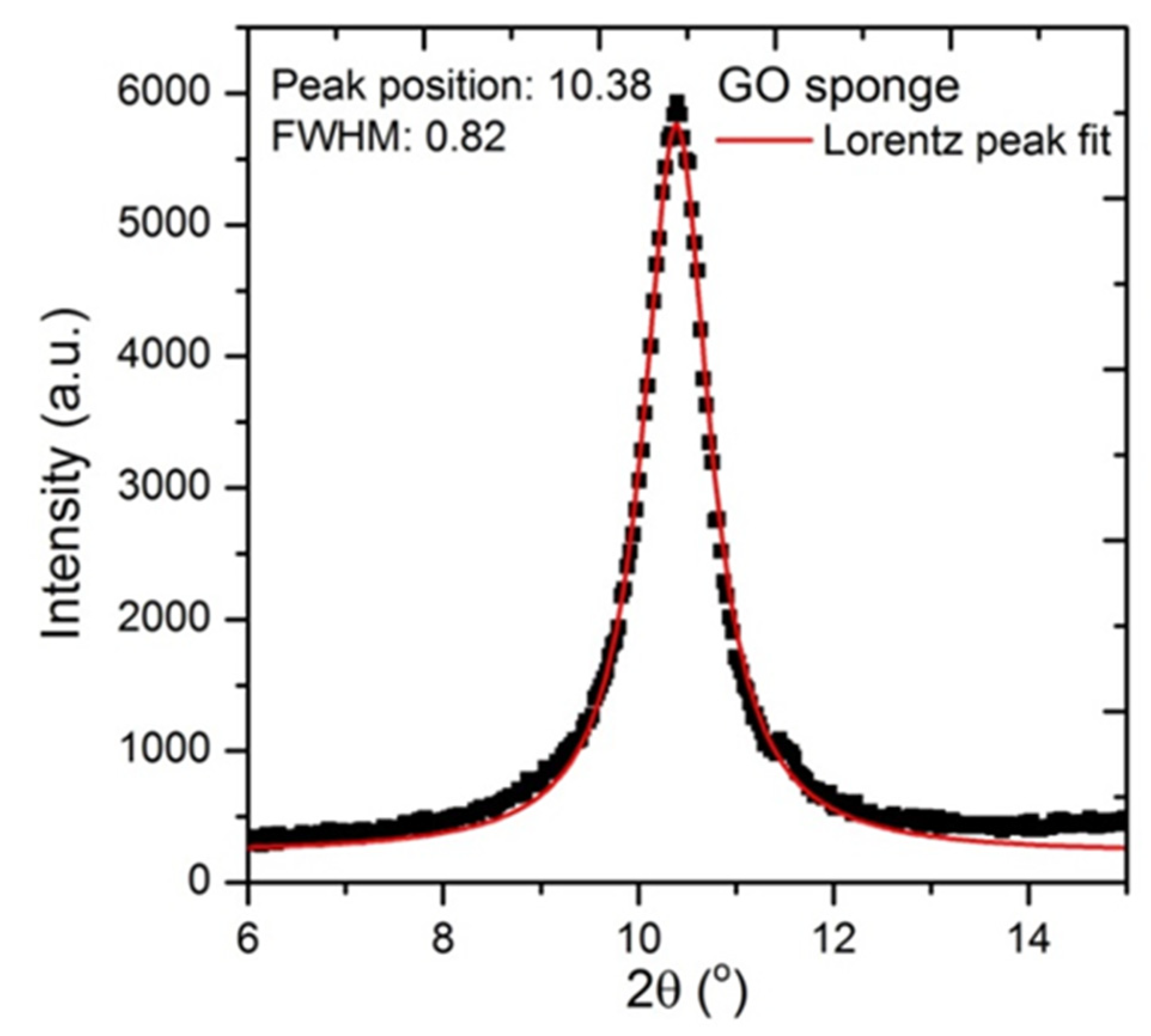
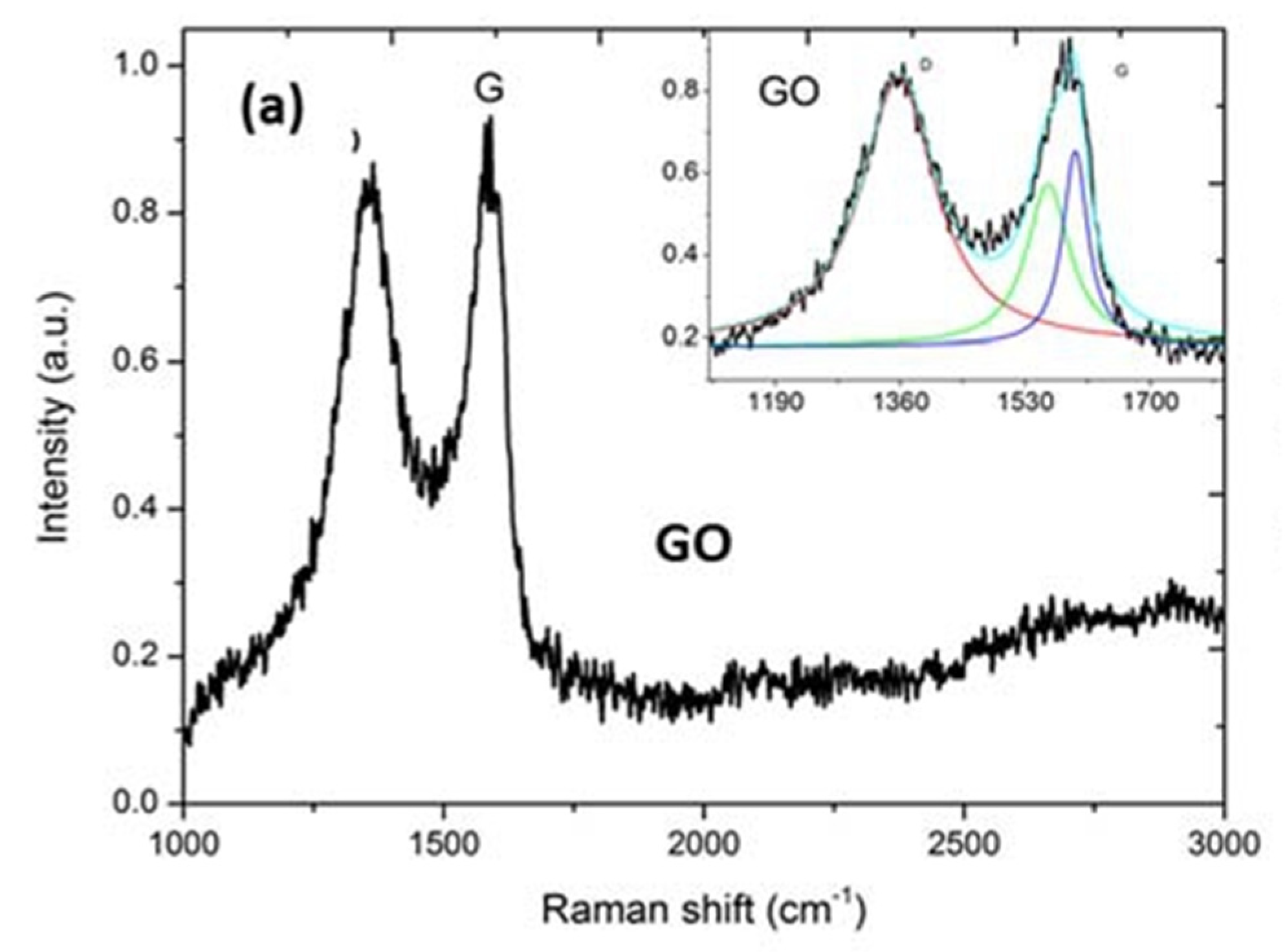
Appendix E
| Alginate | Graphene Oxide | Famotidine | Diclofenac | |||
|---|---|---|---|---|---|---|
| pKa = 3.38 | pKa = 3.65 | pKa = 4.3 | pKa = 6.6 | pKa = 9.8 | pKa=6.98 | pKa = 4.15 |
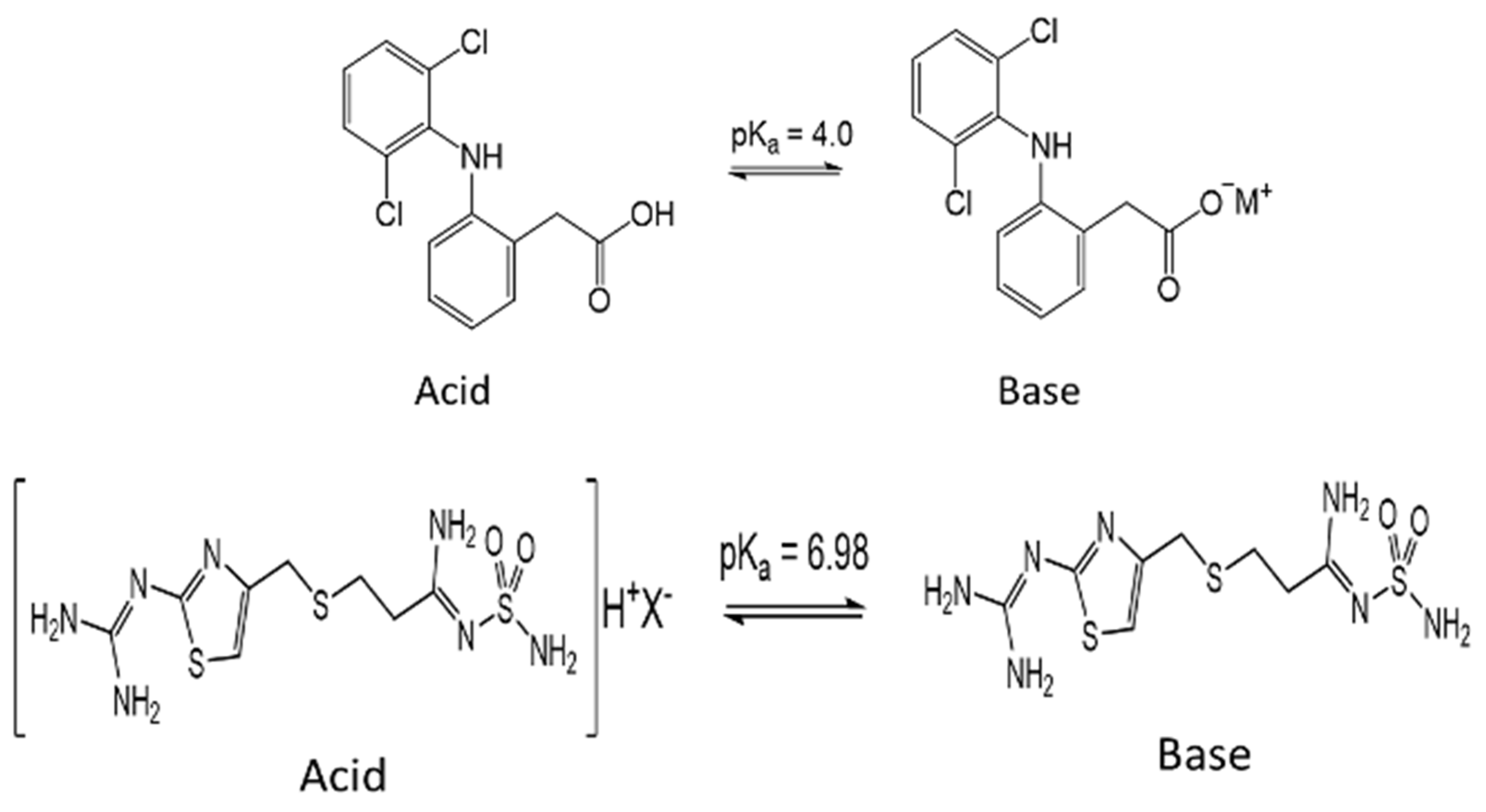
| pH of Solution | pH = 2 | pH = 3.5 | pH = 5 | pH = 7 |
|---|---|---|---|---|
| Acid/base | 100/1 | 3.16:1 | 0.1:1 | 0.001:1 |
Appendix F
References
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Guo, J.-Z.; Li, B.; Liu, L.; Lv, K. Removal of methylene blue from aqueous solutions by chemically modified bamboo. Chemosphere 2014, 111, 225–231. [Google Scholar] [CrossRef]
- Benstoem, F.; Becker, G.; Firk, J.; Kaless, M.; Wuest, D.; Pinnekamp, J.; Kruse, A. Elimination of micropollutants by activated carbon produced from fibers taken from wastewater screenings using hydrothermal carbonization. J. Environ. Manag. 2018, 211, 278–286. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The Challenge of Micropollutants in Aquatic Systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.H.; Bury, N.R.; Owen, S.; MacRae, J.I.; Barron, L.P. A review of the pharmaceutical exposome in aquatic fauna. Environ. Pollut. 2018, 239, 129–146. [Google Scholar] [CrossRef]
- Jeon, Y.S.; Lei, J.; Kim, J.-H. Dye adsorption characteristics of alginate/polyaspartate hydrogels. J. Ind. Eng. Chem. 2008, 14, 726–731. [Google Scholar] [CrossRef]
- Keskinkan, O.; Göksu, M.Z.L. Assessment of the dye removal capability of submersed aquatic plants in a laboratory-scale wetland system using anova. Braz. J. Chem. Eng. 2007, 24, 193–202. [Google Scholar] [CrossRef]
- Mittal, A.K. Stdie or Soretio of Dycr by Sulfomlcd Col dA Gamdema fucidutn. Indian J. Environ. Health 1989, 31, 105–111. [Google Scholar]
- El Qada, E.N.; Allen, S.J.; Walker, G. Adsorption of Methylene Blue onto activated carbon produced from steam activated bituminous coal: A study of equilibrium adsorption isotherm. Chem. Eng. J. 2006, 124, 103–110. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Hassan, A.; Abdel-Mohsen, A.-M.; Fouda, M.M. Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr. Polym. 2014, 102, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y. An Overview of Famotidine Polymorphs: Solid-State Characteristics, Thermodynamics, Polymorphic Transformation and Quality Control. Pharm. Res. 2014, 31, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Kristofco, L.A.; Brooks, B.W. Global scanning of antihistamines in the environment: Analysis of occurrence and hazards in aquatic systems. Sci. Total. Environ. 2017, 592, 477–487. [Google Scholar] [CrossRef]
- Murphy, S.; Saurel, C.; Morrissey, A.; Tobin, J.; Oelgemoeller, M.; Nolan, K. Photocatalytic activity of a porphyrin/TiO2 composite in the degradation of pharmaceuticals. Appl. Catal. B Environ. 2012, 119–120, 156–165. [Google Scholar] [CrossRef]
- Rad, T.S.; Khataee, A.; Kayan, B.; Kalderis, D.; Akay, S. Synthesis of pumice-TiO2 nanoflakes for sonocatalytic degradation of famotidine. J. Clean. Prod. 2018, 202, 853–862. [Google Scholar] [CrossRef]
- Bort, R.; Ponsoda, X.; Jover, R.; Gómez-Lechón, M.J.; Castell, J.V. Diclofenac toxicity to hepatocytes: A role for drug metabolism in cell toxicity. J. Pharmacol. Exp. Ther. 1999, 288, 65–72. [Google Scholar]
- McGettigan, P.; Henry, D. Cardiovascular Risk and Inhibition of Cyclooxygenase. JAMA 2006, 296, 1633–1644. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef]
- Triebskorn, R.; Casper, H.; Scheil, V.; Schwaiger, J. Ultrastructural effects of pharmaceuticals (carbamazepine, clofibric acid, metoprolol, diclofenac) in rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio). Anal. Bioanal. Chem. 2007, 387, 1405–1416. [Google Scholar] [CrossRef]
- Nambirajan, K.; Muralidharan, S.; Roy, A.A.; Manonmani, S. Residues of Diclofenac in Tissues of Vultures in India: A Post-ban Scenario. Arch. Environ. Contam. Toxicol. 2017, 74, 292–297. [Google Scholar] [CrossRef]
- Starukh, G. Photocatalytically Enhanced Cationic Dye Removal with Zn-Al Layered Double Hydroxides. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Huang, R.; He, L.; Zhang, T.; Li, D.; Tang, P.; Zhao, Y.; Feng, Y. Fabrication and Adsorption Behavior of Magnesium Silicate Hydrate Nanoparticles towards Methylene Blue. Nanomaterials 2018, 8, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basha, S.; Keane, D.; Nolan, K.; Oelgemoeller, M.; Lawler, J.; Tobin, J.M.; Morrissey, A. UV-induced photocatalytic degradation of aqueous acetaminophen: The role of adsorption and reaction kinetics. Environ. Sci. Pollut. Res. 2014, 22, 2219–2230. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, S.-T.; Feng, S.; Ma, Q.; Peng, X.; Wu, D. Preparation and Application of Carboxylated Graphene Oxide Sponge in Dye Removal. Int. J. Environ. Res. Public Health 2017, 14, 1301. [Google Scholar] [CrossRef] [Green Version]
- Cunha, D.L.; Kuznetsov, A.; Achete, C.A.; Machado, A.; Marques, M. Immobilized TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration process. PeerJ 2018, 6, e4464. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Odelius, K.; Rajarao, G.K.; Hakkarainen, M. Microwave carbonized cellulose for trace pharmaceutical adsorption. Chem. Eng. J. 2018, 346, 557–566. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Xu, P. Comparative study on pharmaceuticals adsorption in reclaimed water desalination concentrate using biochar: Impact of salts and organic matter. Sci. Total Environ. 2017, 601–602, 857–864. [Google Scholar] [CrossRef]
- Paunovic, O.; Pap, S.; Maletic, S.; Taggart, M.; Boskovic, N.; Sekulic, M.T. Ionisable emerging pharmaceutical adsorption onto microwave functionalised biochar derived from novel lignocellulosic waste biomass. J. Colloid Interface Sci. 2019, 547, 350–360. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Li, J.; Kong, F.; Jia, K.; He, S.; Wang, B. L-Lysine-grafted graphene oxide as an effective adsorbent for the removal of methylene blue and metal ions. Beilstein J. Nanotechnol. 2017, 8, 2680–2688. [Google Scholar] [CrossRef]
- Khan, M.A.; Hameed, B.H.; Lawler, J.; Kumar, M.; Jeon, B.-H. Developments in activated functionalized carbons and their applications in water decontamination: A review. DESALINATION Water Treat. 2014, 54, 422–449. [Google Scholar] [CrossRef]
- Wu, X.-L.; Shi, Y.; Zhong, S.; Lin, H.; Chen, J.-R. Facile synthesis of Fe3O4-graphene@mesoporous SiO2 nanocomposites for efficient removal of Methylene Blue. Appl. Surf. Sci. 2016, 378, 80–86. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, X.; Li, Y.; Du, Q.; Sun, J.; Wang, Y.; Wang, X.; Xia, Y.; Wang, Z.; Xia, L. Adsorption Properties of Doxorubicin Hydrochloride onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies. Materials 2013, 6, 2026–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Lü, L.; Li, H.; Luo, F. The adsorption properties of Pb(II) and Cd(II) on functionalized graphene prepared by electrolysis method. J. Hazard. Mater. 2010, 183, 923–930. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Sillanpää, M. Heavy metals adsorption by novel EDTA-modified chitosan–silica hybrid materials. J. Colloid Interface Sci. 2011, 358, 261–267. [Google Scholar] [CrossRef]
- Kadirvelu, K. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef]
- Algothmi, W.M.; Bandaru, N.M.; Yu, Y.; Shapter, J.; Ellis, A. Alginate–graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interface Sci. 2013, 397, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yu, J.; Wang, Q.; Li, L.; Zhang, W.; Ma, J.; Zhang, J.; Liu, P.; Li, D. Improved sodium storage properties of nickel sulfide nanoparticles decorated on reduced graphene oxide nanosheets as an advanced anode material. Nanotechnology 2021, 32, 195406. [Google Scholar] [CrossRef]
- Chandy, T.; Mooradian, D.L.; Rao, G.H. Evaluation of modified alginate-chitosan-polyethylene glycol microcapsules for cell encapsulation. Artif. Organs 1999, 23, 894–903. [Google Scholar] [CrossRef]
- Dutta, D.; Borah, B.J.; Saikia, L.; Pathak, M.G.; Sengupta, P.; Dutta, D.K. Synthesis and catalytic activity of Ni°-acid activated montmorillonite nanoparticles. Appl. Clay Sci. 2011, 53, 650–656. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Gazi, M. Enhanced removal of crystal violet by low cost alginate/acid activated bentonite composite beads: Optimization and modelling using non-linear regression technique. J. Water Process. Eng. 2014, 2, 43–52. [Google Scholar] [CrossRef]
- Tao, E.; Ma, D.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Compd. 2020, 832, 154833. [Google Scholar] [CrossRef]
- Kumar, M.; McGlade, D.; Ulbricht, M.; Lawler, J. Quaternized polysulfone and graphene oxide nanosheet derived low fouling novel positively charged hybrid ultrafiltration membranes for protein separation. RSC Adv. 2015, 5, 51208–51219. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Li, Q.; Zhong, S.; Chen, J.; Lin, H.; Wu, X.-L. Facile large scale fabrication of magnetic carbon nano-onions for efficient removal of bisphenol A. Mater. Chem. Phys. 2017, 198, 186–192. [Google Scholar] [CrossRef]
- Allen, S.; Mckay, G.; Porter, J. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Xia, B.; Du, Q.; Zhang, P.; Wang, D.; Wang, Z.; Xia, Y. Removal of copper from aqueous solution by carbon nanotube/calcium alginate composites. J. Hazard. Mater. 2010, 177, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Popuri, S.R.; Vijaya, Y.; Boddu, V.M.; Abburi, K. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour. Technol. 2009, 100, 194–199. [Google Scholar] [CrossRef]
- Hameed, B. Evaluation of papaya seeds as a novel non-conventional low-cost adsorbent for removal of methylene blue. J. Hazard. Mater. 2009, 162, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.M.; Narurkar, M.M. Solubility, Stability and Ionization Behaviour of Famotidine. J. Pharm. Sci. 1993, 45, 682–686. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef]
- Francis, N.L.; Hunger, P.M.; Donius, A.E.; Riblett, B.W.; Zavaliangos, A.; Wegst, U.G.K.; Wheatley, M.A. An ice-templated, linearly aligned chitosan-alginate scaffold for neural tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3493–3503. [Google Scholar] [CrossRef]
- Rocher, V.; Siaugue, J.-M.; Cabuil, V.; Bee, A. Removal of organic dyes by magnetic alginate beads. Water Res. 2008, 42, 1290–1298. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Pocostales, P.; Alvarez, P.; Oropesa, A.L. Diclofenac removal from water with ozone and activated carbon. J. Hazard. Mater. 2009, 163, 768–776. [Google Scholar] [CrossRef]
- Al-Degs, Y.; El-Barghouthi, M.; El-Sheikh, A.; Walker, G. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Kumar, M.; Tripathi, B.P.; Shahi, V.K. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. J. Hazard. Mater. 2009, 172, 1041–1048. [Google Scholar] [CrossRef]
- Weng, C.-H.; Lin, Y.-T.; Tzeng, T.-W. Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazard. Mater. 2009, 170, 417–424. [Google Scholar] [CrossRef]
- Han, S.S.; Lee, H.M. Adsorption properties of hydrogen on (10,0) single-walled carbon nanotube through density functional theory. Carbon 2004, 42, 2169–2177. [Google Scholar] [CrossRef]
- Wang, P.; Cao, M.; Wang, C.; Ao, Y.; Hou, J.; Qian, J. Kinetics and thermodynamics of adsorption of methylene blue by a magnetic graphene-carbon nanotube composite. Appl. Surf. Sci. 2014, 290, 116–124. [Google Scholar] [CrossRef]
- Doğan, M.; Özdemir, Y.; Alkan, M. Adsorption kinetics and mechanism of cationic methyl violet and methylene blue dyes onto sepiolite. Dye. Pigment. 2007, 75, 701–713. [Google Scholar] [CrossRef]
- Cheung, A.; Szeto, Y.S.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Yaagoob, I.Y.; Mazumder, M.A.; Al-Muallem, H.A. Fast removal of methylene blue and Hg(II) from aqueous solution using a novel super-adsorbent containing residues of glycine and maleic acid. J. Hazard. Mater. 2019, 369, 642–654. [Google Scholar] [CrossRef]
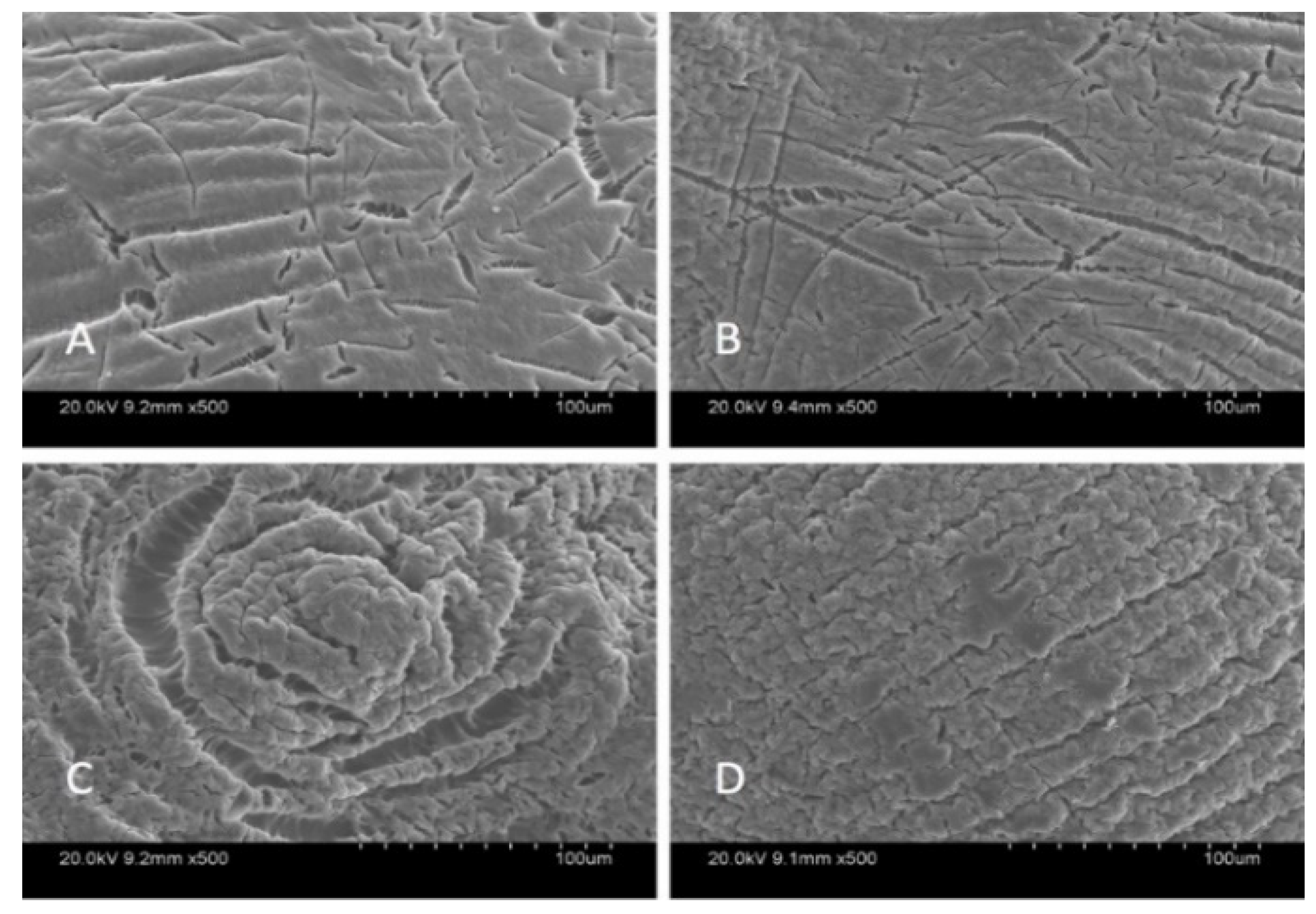
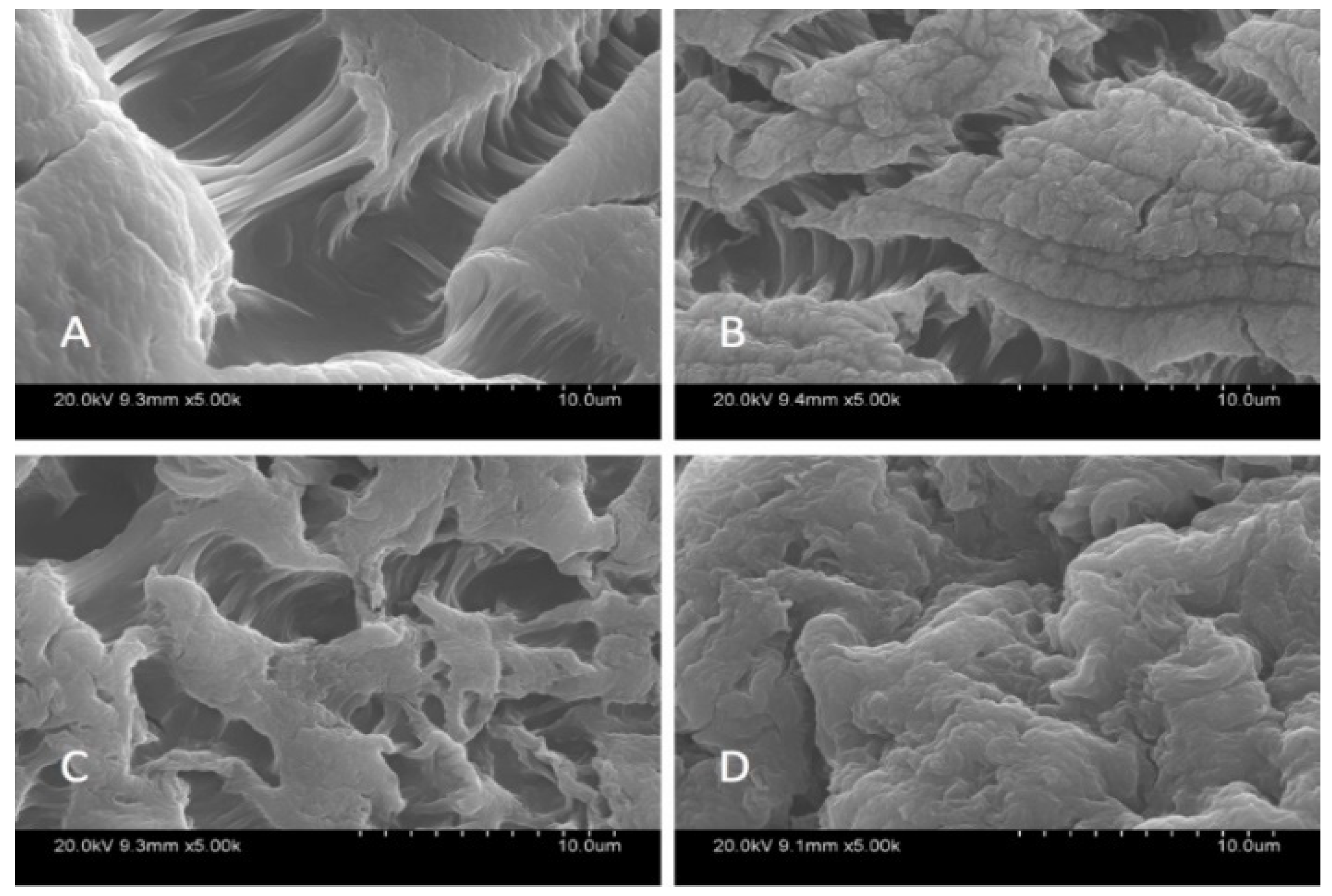




| Adsorbent | Kd | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J·K−1·mol−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 4 °C | 22 °C | 30°C | 4 °C | 22 °C | 30 °C | |||
| Ca-Alg2 | 1.07 | 0.59 | 0.48 | −0.144 | 1.240 | 1.855 | −21.44 | −76.9 |
| Ca-Alg2/GO5 | 1.18 | 0.77 | 0.73 | −0.341 | 0.504 | 0.879 | −13.34 | −46.9 |
| Ca-Alg2/GO10 | 1.19 | 1.08 | 1.04 | −0.401 | −0.186 | 0.091 | −3.712 | −12.0 |
| Ca-Alg2/GO20 | 1.88 | 1.68 | 1.60 | −1.459 | −1.272 | −1.189 | −4.338 | −10.4 |
| Adsorbent | Kd | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS°(J·K−1·mol−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 4 °C | 22 °C | 30 °C | 4 °C | 22 °C | 30 °C | |||
| Ca-Alg2 | 0.11 | 0.04 | 0.01 | 4.747 | 9.465 | 11.56 | −67.85 | −262 |
| Ca-Alg2/GO5 | 0.17 | 0.11 | 0.05 | 3.926 | 6.226 | 7.248 | −31.46 | −128 |
| Ca-Alg2/GO10 | 0.44 | 0.38 | 0.32 | 1.847 | 2.505 | 2.797 | −8.277 | −36.6 |
| Ca-Alg2/GO20 | 1.14 | 0.98 | 0.63 | −0.418 | 0.473 | 0.868 | −14.13 | −49.5 |
| Adsorbent | Kd | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J·K−1·mol−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 4 °C | 22 °C | 30 °C | 4 °C | 22 °C | 30 °C | |||
| Ca-Alg2 | 2.90 | 1.55 | 0.55 | −2.758 | −0.042 | 1.165 | −44.56 | −151 |
| Ca-Alg2/GO5 | 4.96 | 1.80 | 0.58 | −3.982 | −0.704 | 0.753 | −54.43 | −182 |
| Ca-Alg2/GO10 | 5.48 | 2.61 | 0.74 | −4.352 | −1.342 | −0.004 | −50.67 | −167 |
| Ca-Alg2/GO20 | 6.88 | 3.81 | 0.93 | −4.896 | −2.047 | −0.781 | −48.74 | −158 |
| Adsorbent | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qeq (mg g−1) | k1 (min−1) | R2 (-) | qeq (mg g−1) | k2 (g·mg−1·min−1) | R2 (-) | kid (mg g−1·min−0.5) | C (mg g−1) | R2 (-) | |
| Ca-Alg2 | 6.27 | 5.1 × 10−3 | 0.9657 | 7.84 | 8.3 × 10−4 | 0.9907 | 0.479 | −1.68 | 0.9580 |
| Ca-Alg2/GO5 | 7.22 | 3.6 × 10−3 | 0.9974 | 8.70 | 5.0 × 10−4 | 0.9995 | 0.358 | −0.957 | 0.9997 |
| Ca-Alg2/GO10 | 8.55 | 3.0 × 10−3 | 0.9979 | 10.81 | 3.5 × 10−4 | 0.9991 | 0.409 | −1.10 | 0.9996 |
| Ca-Alg2/GO20 | 9.79 | 2.8 × 10−3 | 0.9991 | 12.66 | 2.7 × 10−4 | 0.9993 | 0.457 | −1.28 | 0.9998 |
| Adsorbent | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qeq (mg g−1) | k1 (min−1) | R2 (-) | qeq (mg g−1) | k2 (g·mg−1·min−1) | R2 (-) | kid (mg g−1·min−0.5) | C (mg g−1) | R2 (-) | |
| Ca-Alg2 | 3.70 | 2.4 × 10−3 | 0.9756 | 4.64 | 5.5 × 10−4 | 0.9405 | 0.248 | −0.882 | 0.8809 |
| Ca-Alg2/GO5 | 4.28 | 1.6 × 10−3 | 0.9820 | 5.72 | 4.4 × 10−4 | 0.9147 | 0.340 | −1.23 | 0.9355 |
| Ca-Alg2/GO10 | 4.74 | 1.9 × 10−3 | 0.9865 | 6.31 | 3.3 × 10−4 | 0.9422 | 0.409 | −0.821 | 0.8817 |
| Ca-Alg2/GO20 | 4.26 | 1.8 × 10−3 | 0.9879 | 6.33 | 7.7 × 10−4 | 0.9712 | 0.556 | −1.44 | 0.9992 |
| Adsorbent | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qeq (mg g−1) | k1 (min−1) | R2 (-) | qeq (mg g−1) | k2 (g·mg−1·min−1) | R2 (-) | kid (mg g−1·min−0.5) | C (mg g−1) | R2 (-) | |
| Ca-Alg2 | 3.70 | 2.4 × 10−3 | 0.9756 | 4.64 | 5.5 × 10−4 | 0.9405 | 0.095 | 0.107 | 0.9684 |
| Ca-Alg2/GO5 | 4.28 | 1.6 × 10−3 | 0.9820 | 5.72 | 4.4 × 10−4 | 0.9147 | 0.117 | 0.110 | 0.9740 |
| Ca-Alg2/GO10 | 4.74 | 1.9 × 10−3 | 0.9865 | 6.31 | 3.3 × 10−4 | 0.9422 | 0.131 | −0.130 | 0.9858 |
| Ca-Alg2/GO20 | 4.26 | 1.8 × 10−3 | 0.9879 | 6.33 | 7.7 × 10−4 | 0.9712 | 0.117 | 1.190 | 0.9835 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax (mg·g−1) | KL (L·g−1) | R2 (-) | KF (L·g−1) | n (-) | R2 (-) | |
| Ca-Alg2 | 1064 | 85.56 | 0.9778 | 43.26 | 0.503 | 0.9270 |
| Ca-Alg2/GO5 | 1153 | 88.93 | 0.9716 | 41.02 | 0.530 | 0.9109 |
| Ca-Alg2/GO10 | 1212 | 84.21 | 0.9782 | 42.64 | 0.537 | 0.8941 |
| Ca-Alg2/GO20 | 1334 | 76.21 | 0.9894 | 45.10 | 0.558 | 0.8541 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax (mg·g−1) | KL (L·g−1) | R2 (-) | KF (L·g−1) | n (-) | R2 (-) | |
| Ca-Alg2 | 28.96 | 123.2 | 0.9809 | 0.680 | 0.615 | 0.9351 |
| Ca-Alg2/GO5 | 31.69 | 85.39 | 0.9733 | 1.190 | 0.552 | 0.9173 |
| Ca-Alg2/GO10 | 33.57 | 57.02 | 0.9611 | 2.099 | 0.479 | 0.8593 |
| Ca-Alg2/GO20 | 35.50 | 23.10 | 0.9214 | 4.647 | 0.374 | 0.7491 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax (mg·g−1) | KL (L·g−1) | R2 (-) | KF (L·g−1) | n (-) | R2 (-) | |
| Ca-Alg2 | 30.74 | 11.10 | 0.9457 | 2.795 | 0.725 | 0.8937 |
| Ca-Alg2/GO5 | 31.81 | 9.020 | 0.9175 | 3.441 | 0.705 | 0.8707 |
| Ca-Alg2/GO10 | 33.72 | 5.988 | 0.8886 | 5.055 | 0.662 | 0.8401 |
| Ca-Alg2/GO20 | 36.35 | 5.066 | 0.8872 | 5.992 | 0.672 | 0.8322 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunes, B.; Jaquet, Y.; Sánchez, L.; Pumarino, R.; McGlade, D.; Quilty, B.; Morrissey, A.; Gholamvand, Z.; Nolan, K.; Lawler, J. Activated Graphene Oxide-Calcium Alginate Beads for Adsorption of Methylene Blue and Pharmaceuticals. Materials 2021, 14, 6343. https://doi.org/10.3390/ma14216343
Gunes B, Jaquet Y, Sánchez L, Pumarino R, McGlade D, Quilty B, Morrissey A, Gholamvand Z, Nolan K, Lawler J. Activated Graphene Oxide-Calcium Alginate Beads for Adsorption of Methylene Blue and Pharmaceuticals. Materials. 2021; 14(21):6343. https://doi.org/10.3390/ma14216343
Chicago/Turabian StyleGunes, Burcu, Yannick Jaquet, Laura Sánchez, Rebecca Pumarino, Declan McGlade, Brid Quilty, Anne Morrissey, Zahra Gholamvand, Kieran Nolan, and Jenny Lawler. 2021. "Activated Graphene Oxide-Calcium Alginate Beads for Adsorption of Methylene Blue and Pharmaceuticals" Materials 14, no. 21: 6343. https://doi.org/10.3390/ma14216343
APA StyleGunes, B., Jaquet, Y., Sánchez, L., Pumarino, R., McGlade, D., Quilty, B., Morrissey, A., Gholamvand, Z., Nolan, K., & Lawler, J. (2021). Activated Graphene Oxide-Calcium Alginate Beads for Adsorption of Methylene Blue and Pharmaceuticals. Materials, 14(21), 6343. https://doi.org/10.3390/ma14216343