Experimental Carbonation Study for a Durability Assessment of Novel Cementitious Materials
Abstract
:1. Introduction
2. Materials and Methods
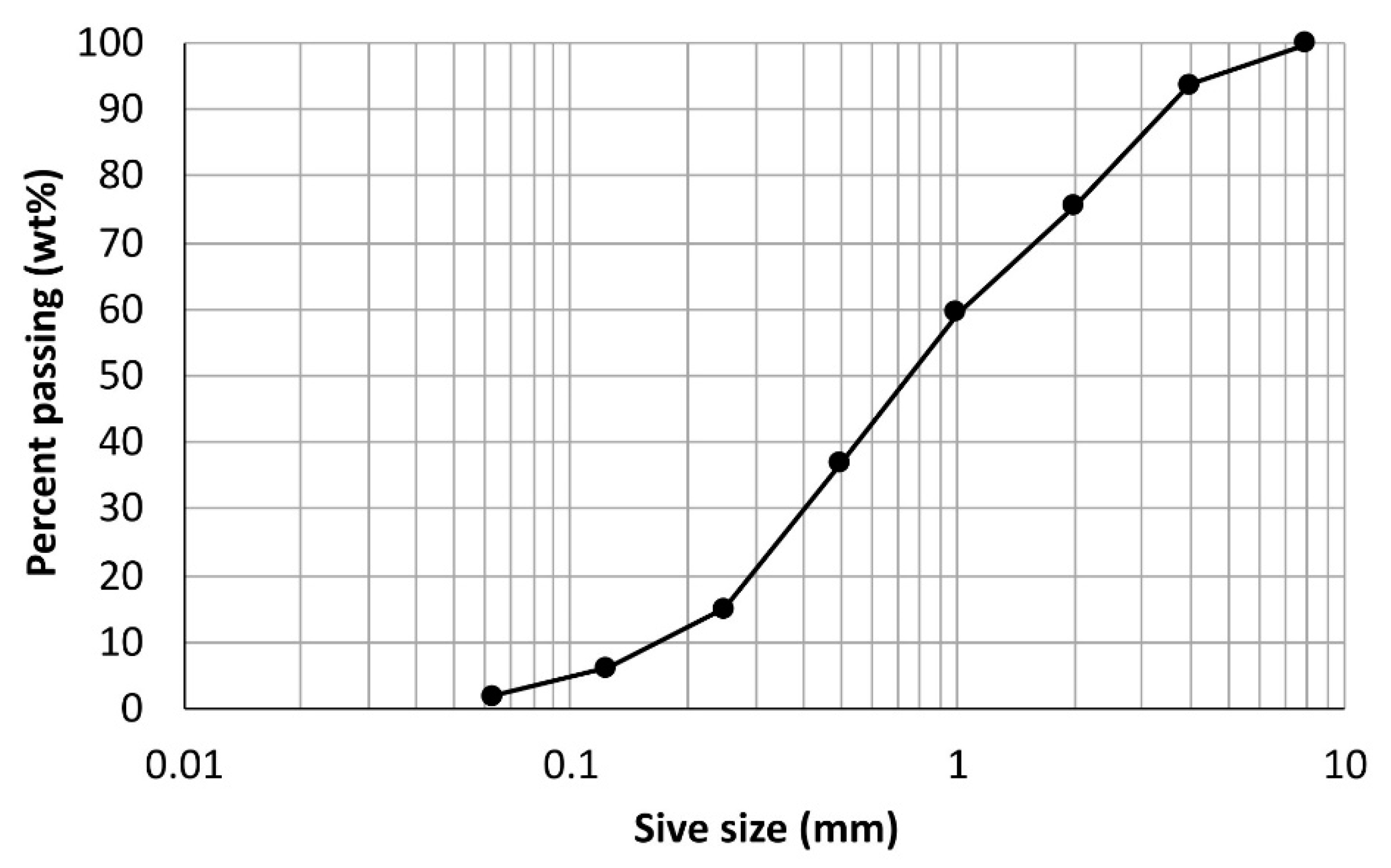
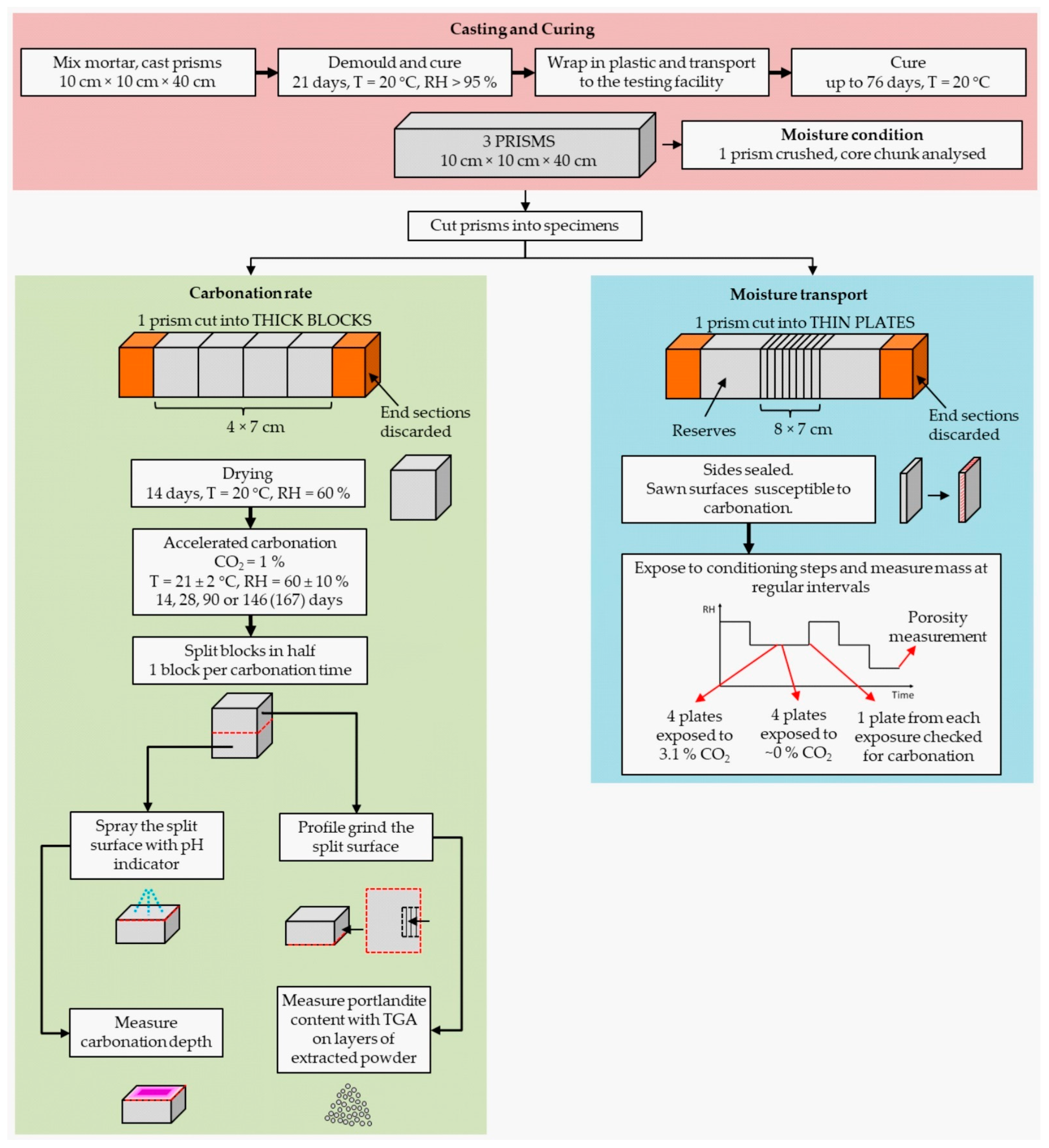
2.1. Specimen Preparation
2.2. Carbonation Rate
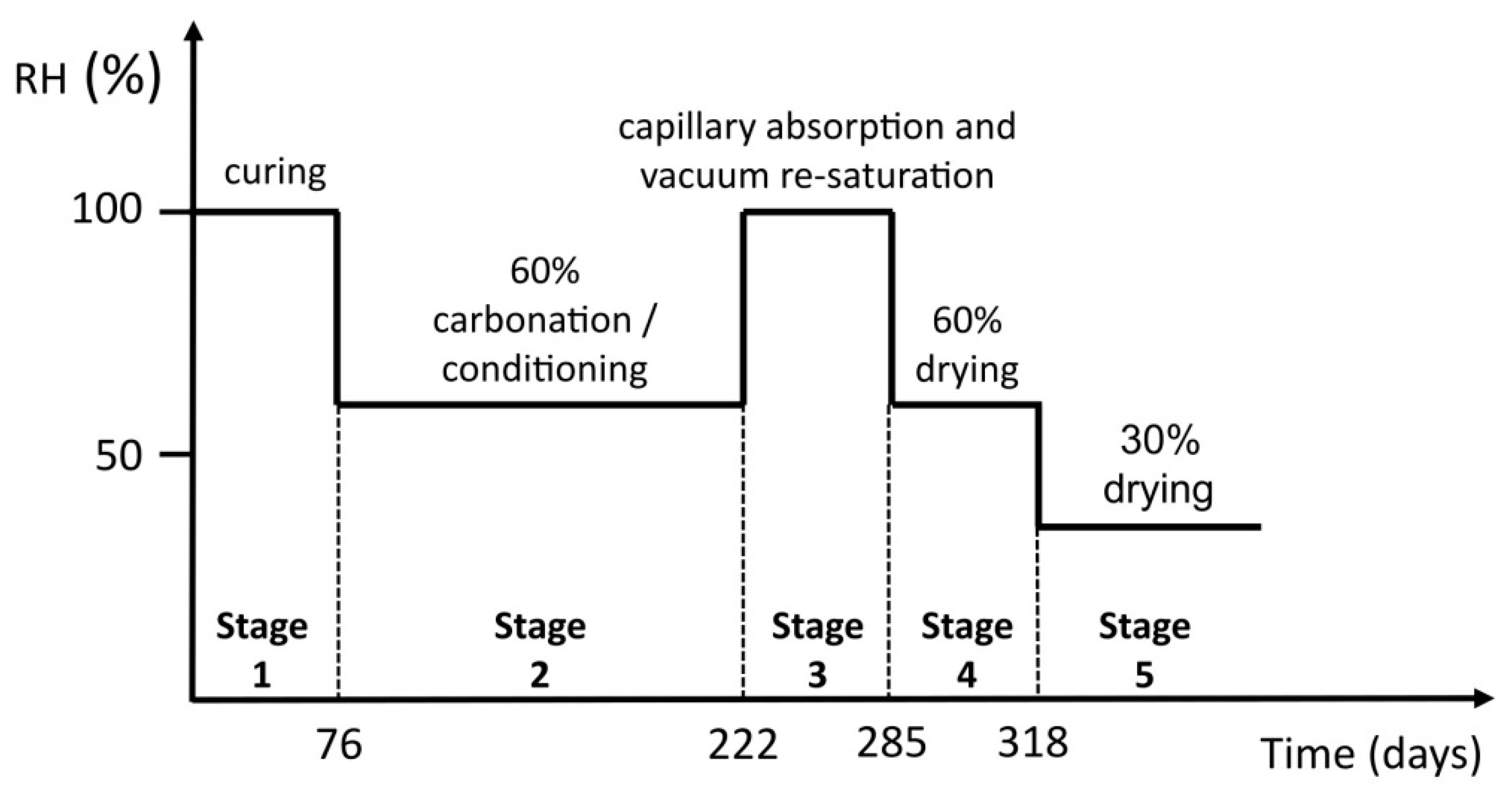
2.3. Moisture Transport
3. Results and Discussion
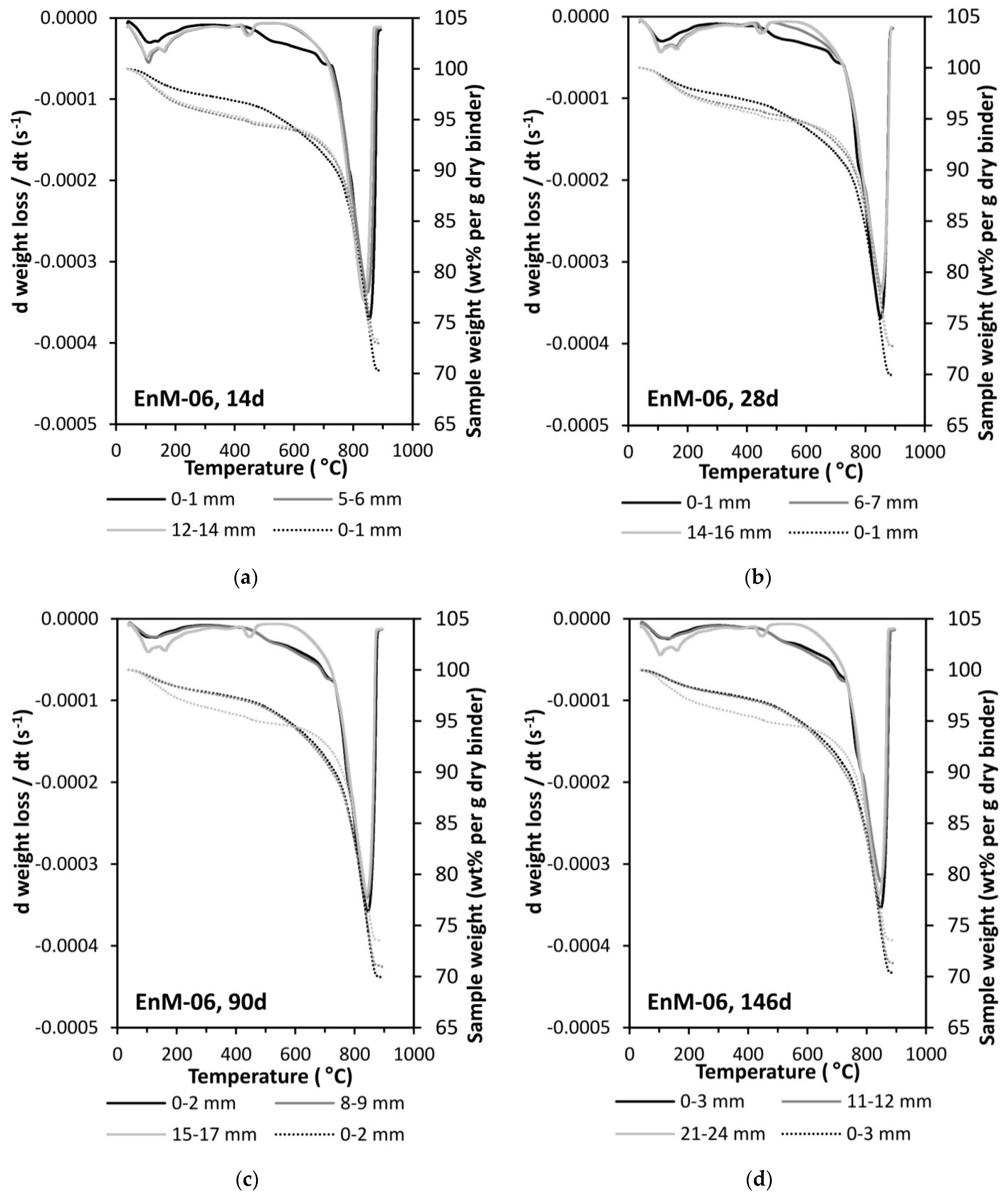
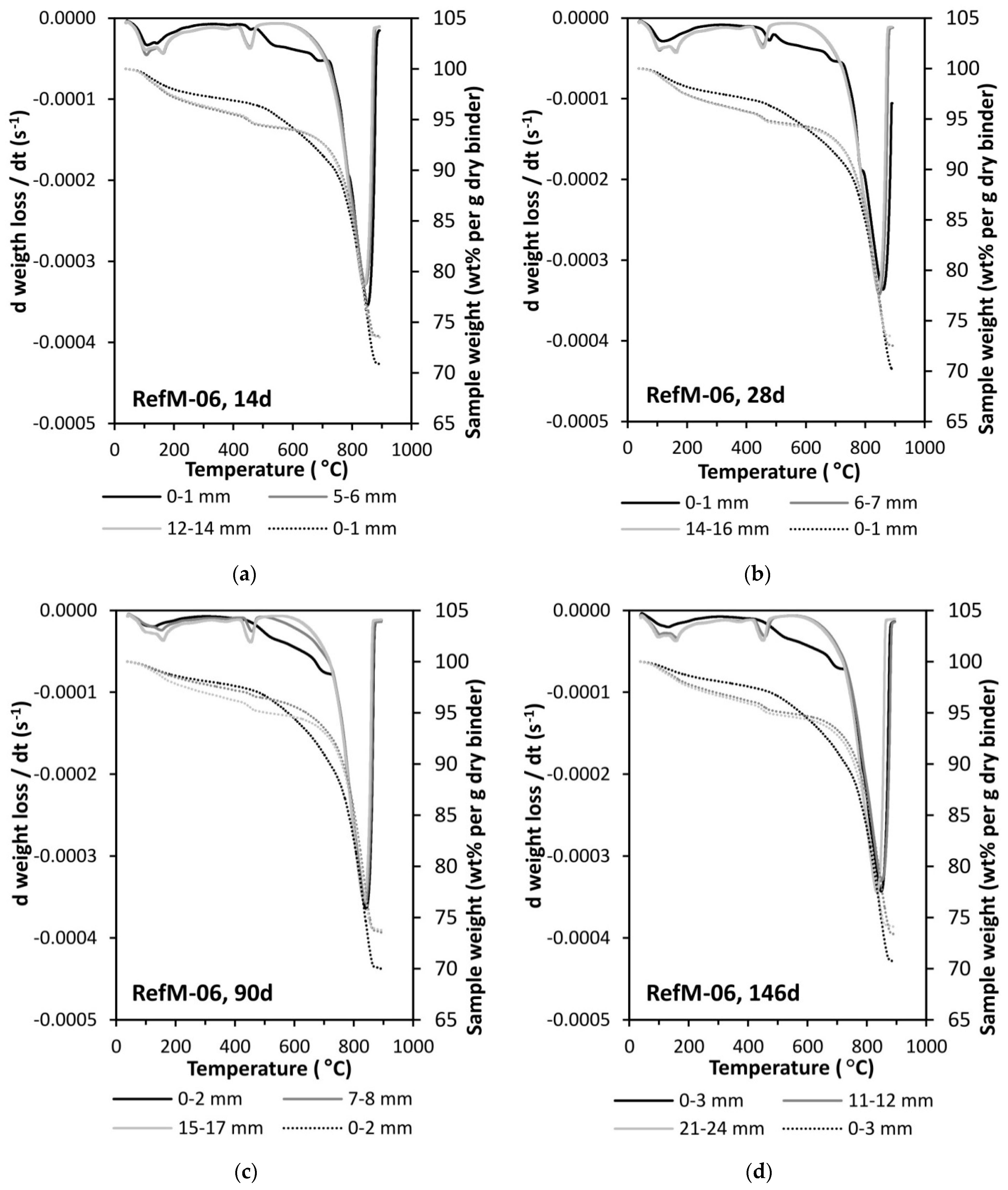
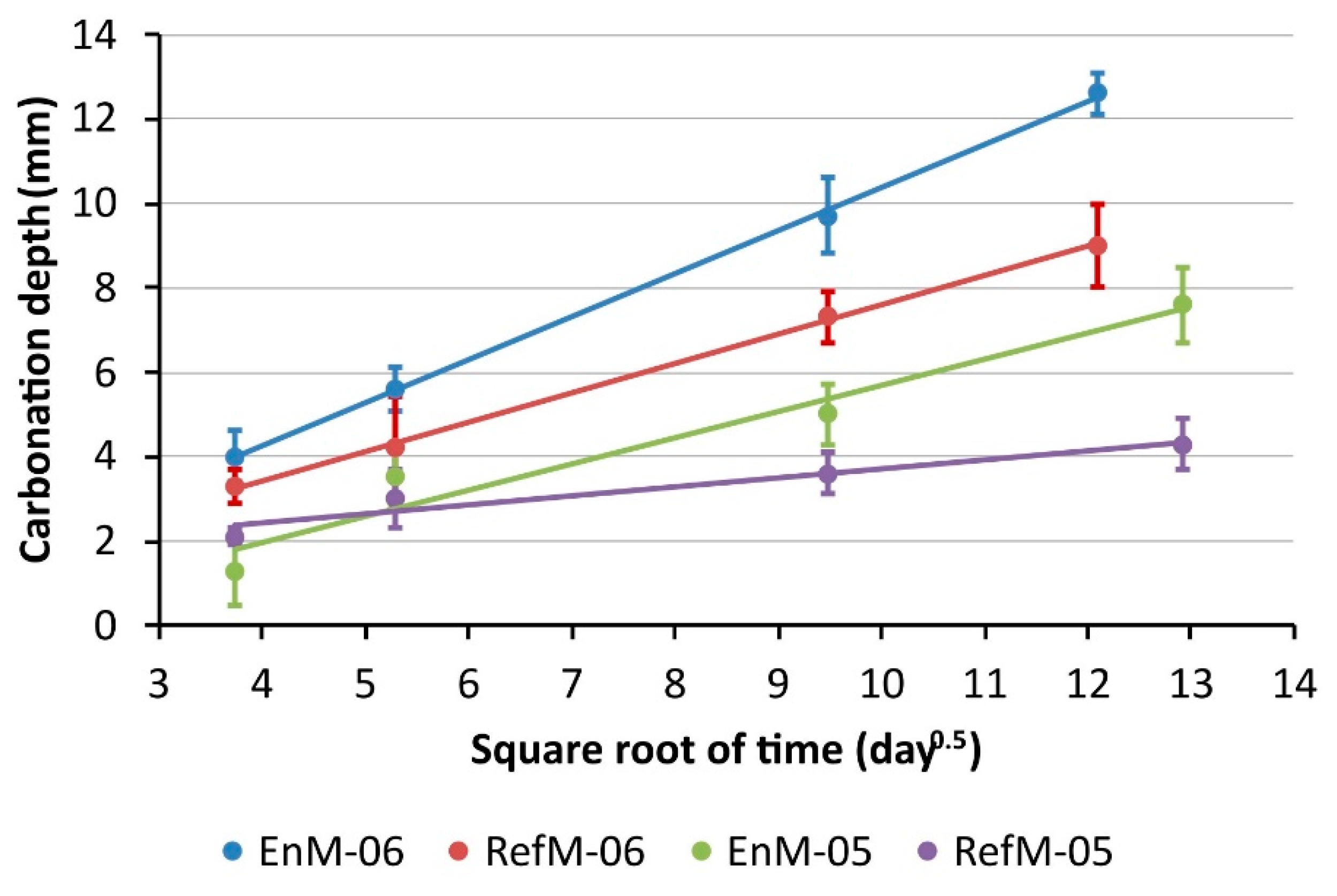
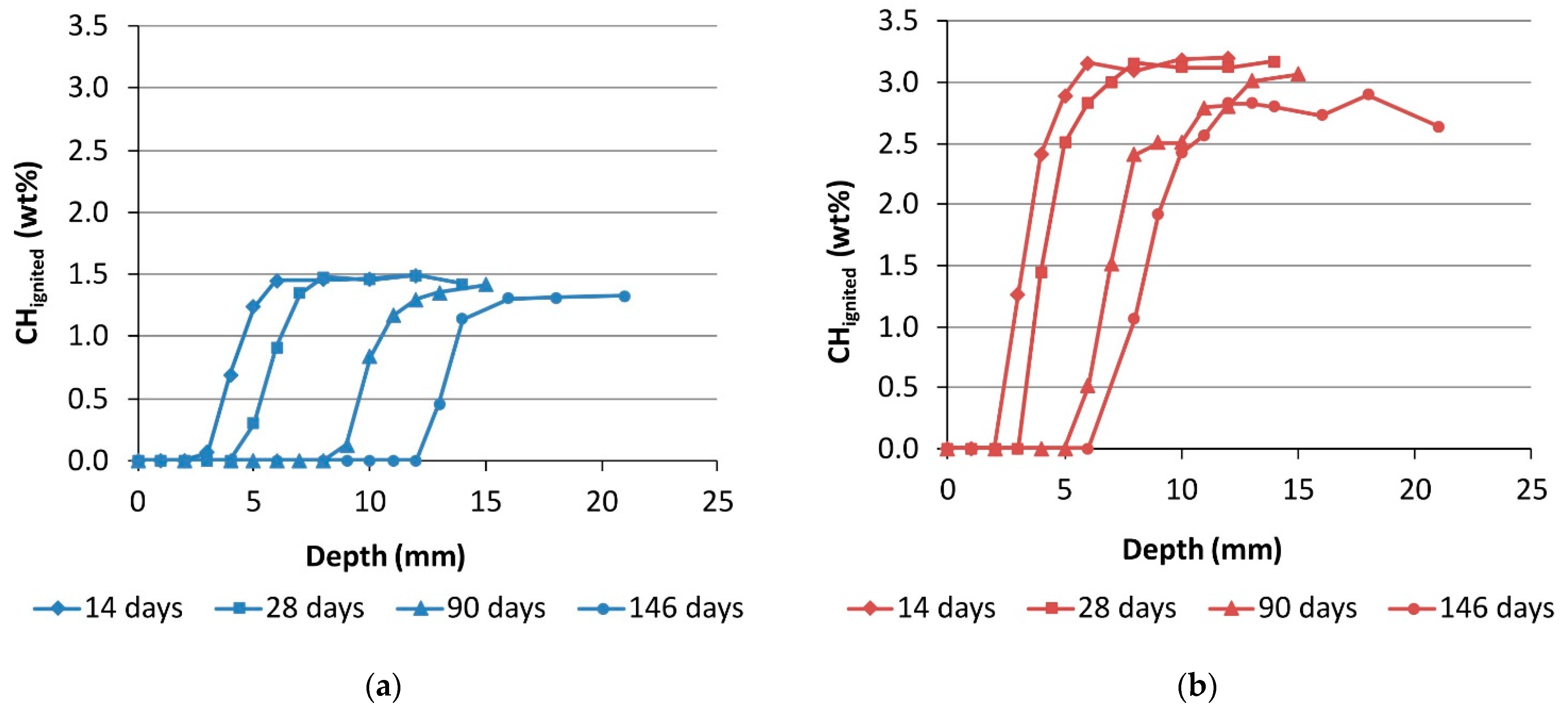
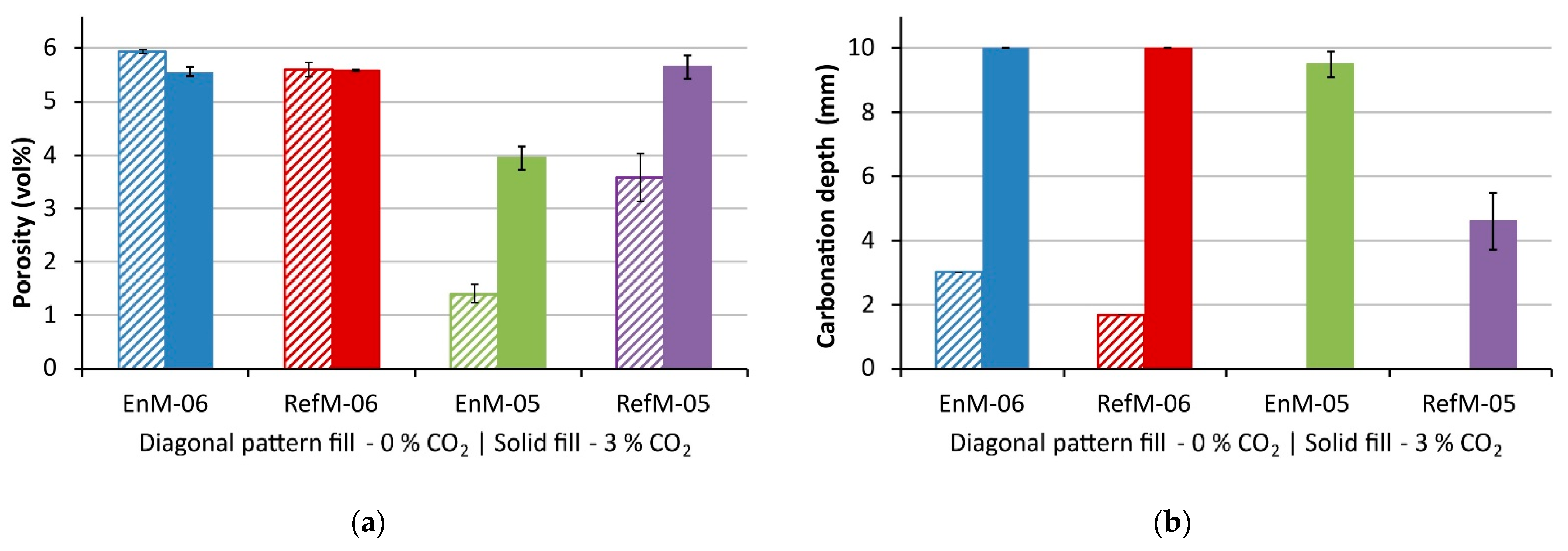
3.1. Carbonation Rate
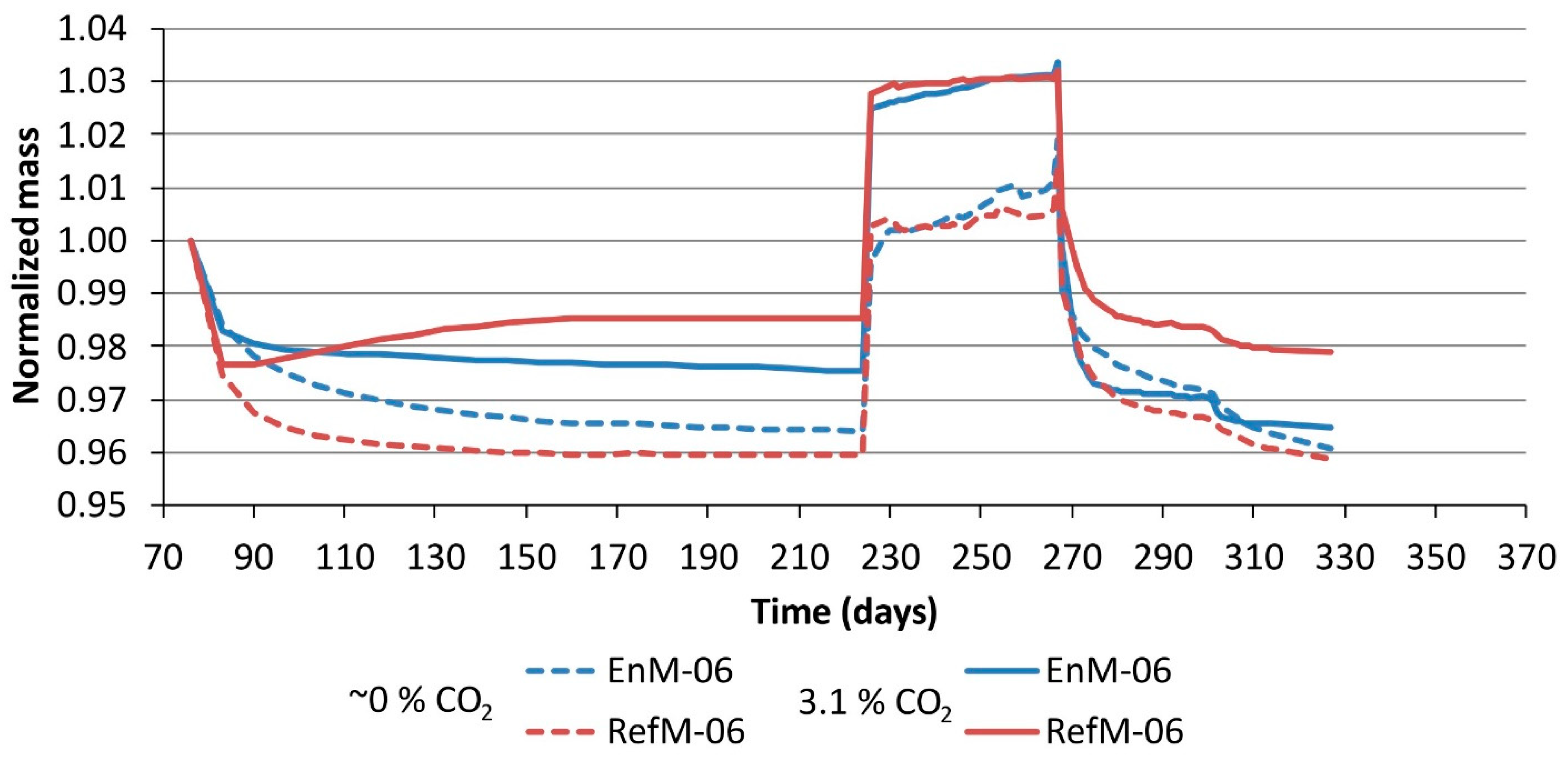
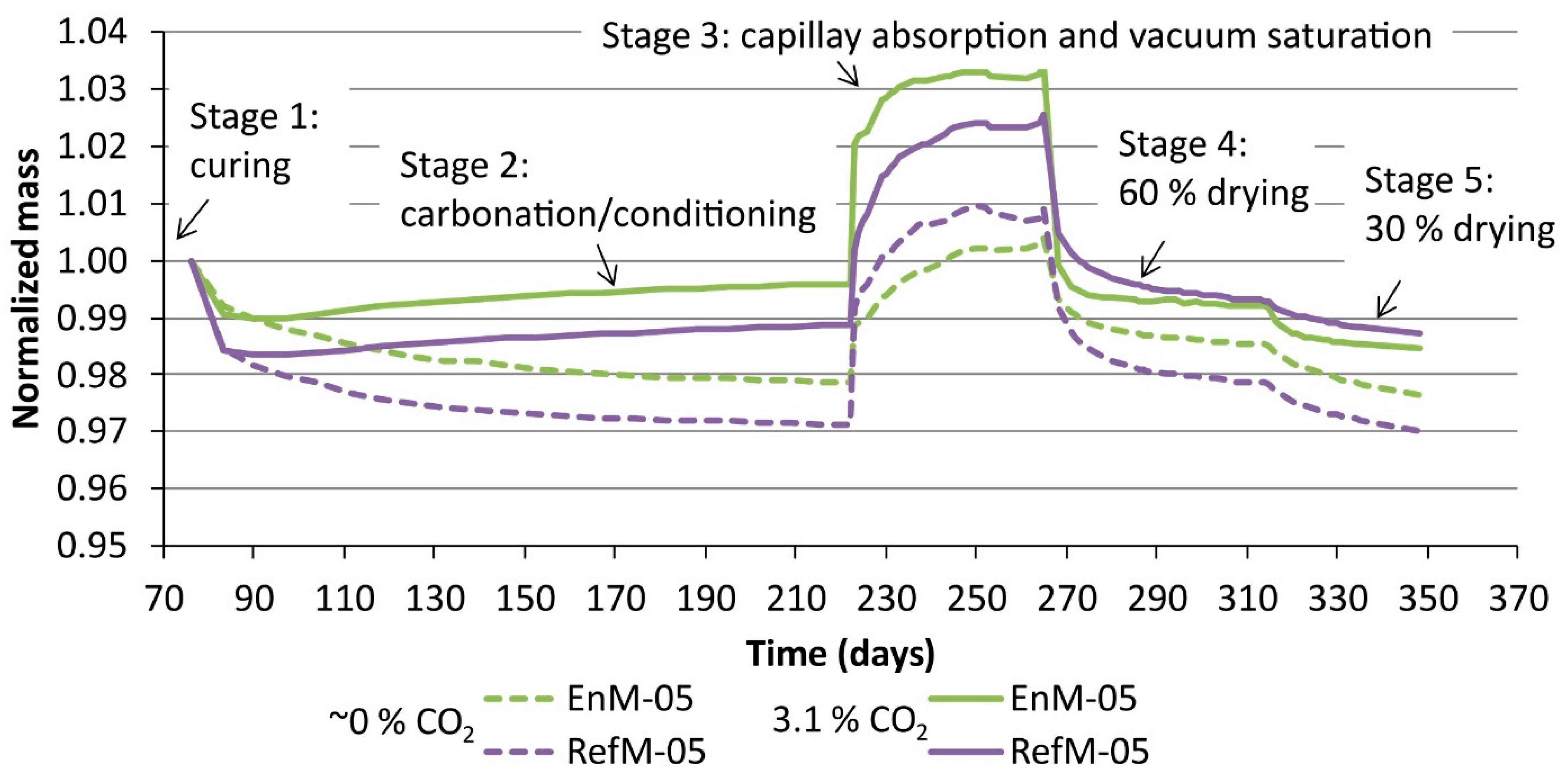
3.2. Moisture Transport
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Composition of Components (wt%) | Composition of Products (wt%) | ||||
|---|---|---|---|---|---|
| Title 1 | CEM I 52.5 R | S | LL | CEM II/C (S-LL) | CEM II/A-S |
| SiO2 | 20.35 | 35.21 | 0.85 | 24.34 | 21.50 |
| Al2O3 | 5.46 | 11.27 | 0.15 | 7.25 | 6.00 |
| TiO2 | 0.28 | 1.07 | 0.01 | 0.57 | 0.37 |
| MnO | 0.04 | 0.21 | 0.06 | 0.11 | 0.06 |
| Fe2O3 | 2.40 | 0.26 | 0.16 | 1.32 | 2.03 |
| CaO | 62.33 | 41.64 | 54.54 | 53.28 | 59.33 |
| MgO | 1.49 | 5.96 | 0.91 | 3.22 | 2.05 |
| K2O | 0.89 | 0.64 | 0.04 | 0.71 | 0.82 |
| Na2O | 0.20 | 0.22 | 0.01 | 0.19 | 0.20 |
| SO3 | 3.68 | 0.00 | 0.01 | 1.84 | 3.05 |
| P2O5 | 0.12 | 0.01 | 0.01 | 0.07 | 0.10 |
| LOI 1 | 1.87 | (+0.16) | 43.32 | / | / |
References
- EN 1992-1-1 Eurocode 2: Design of Concrete Structures. General Rules. Rules for Buildings; European Committee for Standardization (CEN): Brussels, Belgium, 2014.
- EN 1992-2 Eurocode 2: Design of Concrete Structures. Concrete Bridges. Design and Detailing Rules; European Committee for Standardization (CEN): Brussels, Belgium, 2008.
- International Federation for Structural Concrete. Fib Model Code for Concrete Structures 2010; Beverly, P., Ed.; Ernst & Sohn: Berlin, Germany, 2013. [Google Scholar]
- EnDurCrete. D4.3 Report on Modelling and Simulation of Materials at Mesoscale; The French Alternative Energies and Atomic Energy Commission (CEA): Paris, France, 2021.
- EnDurCrete|Home. Available online: http://www.endurcrete.eu/ (accessed on 30 September 2021).
- Bary, B.; Sellier, A. Coupled moisture—Carbon dioxide–Calcium transfer model for carbonation of concrete. Cem. Concr. Res. 2004, 34, 1859–1872. [Google Scholar] [CrossRef]
- Bary, B.; Mügler, C. Simplified modelling and numerical simulations of concrete carbonation in unsaturated conditions. Rev. Eur. Génie Civ. 2006, 10, 1049–1072. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Bary, B.; de Larrard, T. Coupled carbonation-rust formation-damage modeling and simulation of steel corrosion in 3D mesoscale reinforced concrete. Cem. Concr. Res. 2015, 74, 95–107. [Google Scholar] [CrossRef]
- Construction|Internal Market, Industry, Entrepreneurship and SMEs. Available online: https://ec.europa.eu/growth/sectors/construction_en (accessed on 5 February 2021).
- prEN 197-1 Cement. Composition, Specifications and Conformity Criteria for Common Cements; European Committee for Standardization (CEN): Brussels, Belgium, 2018.
- Bolte, G.; Zajac, M.; Skocek, J.; Ben Haha, M. Development of composite cements characterized by low environmental footprint. J. Clean. Prod. 2019, 226, 503–514. [Google Scholar] [CrossRef]
- Auroy, M.; Poyet, S.; Le Bescop, P.; Torrenti, J.-M.; Charpentier, T.; Moskura, M.; Bourbon, X. Impact of carbonation on unsaturated water transport properties of cement-based materials. Cem. Concr. Res. 2015, 74, 44–58. [Google Scholar] [CrossRef]
- EN 12390-2 Testing Hardened Concrete. Making and Curing Specimens for Strength Tests; European Committee for Standardization (CEN): Brussels, Belgium, 2019.
- Lindgård, J.; Sellevold, E.J.; Thomas, M.D.A.; Pedersen, B.; Justnes, H.; Rønning, T.F. Alkali–silica reaction (ASR)—Performance testing: Influence of specimen pre-treatment, exposure conditions and prism size on concrete porosity, moisture state and transport properties. Cem. Concr. Res. 2013, 53, 145–167. [Google Scholar] [CrossRef] [Green Version]
- Bjørndal, M.H. Carbonation Resistance of Concrete and Mortar Containing Novel Low Clinker Cement; Specialization project, Norwegian University of Science and Technology (NTNU): Trondheim, Norway, 2019. [Google Scholar]
- EN 13295 Products and Systems for the Protection and Repair of Concrete Structures—Test Methods. Determination of Resistance to Carbonation; European Committee for Standardization (CEN): Brussels, Belgium, 2004.
- prEN 12390-12 Testing Hardened Concrete. Determination of the Carbonation Resistance of Concrete—Accelerated Carbonation; European Committee for Standardization (CEN): Brussels, Belgium, 2017.
- Revert, A.B.; De Weerdt, K.; Hornbostel, K.; Geiker, M.R. Investigation of the effect of partial replacement of Portland cement by fly ash on carbonation using TGA and SEM-EDS. In Proceedings of the International RILEM Conference on Materials, Systems and Structures in Civil Engineering Conference, Lyngby, Denmark, 15–29 August 2016; pp. 413–422. [Google Scholar]
- Lothenbach, B.; Durdziński, P.T.; De Weerdt, K. Thermogravimetric Analysis. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K.L., Snellings, R., Lothenbach, B., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 177–211. [Google Scholar]
- Revert, A.B.; Geiker, M.R.; De Weerdt, K.; Jakobsen, U.H. SEM-EDS analysis of products formed under natural and accelerated carbonation of concrete with CEM I, CEM II/B-M and CEM II/B-V. In Proceedings of the XXIII Symposium on Nordic Concrete Research & Development, Aalborg, Denmark, 20–23 August 2017; pp. 85–88. [Google Scholar]
- Revert, A.B.; De Weerdt, K.; Jakobsen, U.H.; Geiker, M.R. Impact of Accelerated Carbonation on Microstructure and Phase Assemblage. Nord. Conc. Res. 2018, 59, 111–126. [Google Scholar] [CrossRef] [Green Version]
- EN 12390-11 Testing Hardened Concrete. Determination of the Chloride Resistance of Concrete, Unidirectional Diffusion; European Committee for Standardization (CEN): Brussels, Belgium, 2015.
- SIA 262-1 Concrete Structures. Complementary Specifications; Swiss Society for Engineers and Architects (SIA): Zurich, Switzerland, 2013.
- Boualleg, S.; Bencheikh, M.; Belagraa, L.; Daoudi, A.; Chikouche, M.A. The Combined Effect of the Initial Cure and the Type of Cement on the Natural Carbonation, the Portlandite Content, and Nonevaporable Water in Blended Cement. Adv. Mater. Sci. Eng. 2017, 2017, 5634713. [Google Scholar] [CrossRef] [Green Version]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental modeling and experimental investigation of concrete carbonation. ACI Mater. J. 1991, 88, 363–373. [Google Scholar]
- Shah, V.; Bishnoi, S. Carbonation resistance of cements containing supplementary cementitious materials and its relation to various parameters of concrete. Constr. Build. Mater. 2018, 178, 219–232. [Google Scholar] [CrossRef]
- Martins, R.O.G.; Alvarenga, R.d.C.S.S.; Pedroti, L.G.; de Oliveira, A.F.; Mendes, B.C.; de Azevedo, A.R.G. Assessment of the durability of grout submitted to accelerated carbonation test. Constr. Build. Mater. 2018, 159, 261–268. [Google Scholar] [CrossRef]
- Carneiro, A.F.B.; Daschevi, P.A.; Langaro, E.A.; Pieralisi, R.; Medeiros, M.H.F. Effectiveness of surface coatings in concrete: Chloride penetration and carbonation. J. Build. Pathol. Rehabil. 2021, 6, 3. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Costa, J.O.; Milestone, N.B.; Lynsdale, C.J.; Streatfield, R.E. Carbonation of CH and C–S–H in composite cement pastes containing high amounts of BFS. Cem. Concr. Res. 2010, 40, 284–292. [Google Scholar] [CrossRef]
- Shi, Z.; Lothenbach, B.; Geiker, M.R.; Kaufmann, J.; Leemann, A.; Ferreiro, S.; Skibsted, J. Experimental studies and thermodynamic modeling of the carbonation of Portland cement, metakaolin and limestone mortars. Cem. Concr. Res. 2016, 88, 60–72. [Google Scholar] [CrossRef]
- Lye, C.-Q.; Dhir, R.K.; Ghataora, G.S. Carbonation resistance of GGBS concrete. Mag. Concr. Res. 2016, 68, 936–969. [Google Scholar] [CrossRef] [Green Version]
- Sanjuán, M.A.; Andrade, C.; Cheyrezy, M. Concrete carbonation tests in natural and accelerated conditions. Adv. Cem. Res. 2003, 15, 171–180. [Google Scholar] [CrossRef]
- Leemann, A.; Nygaard, P.; Kaufmann, J.; Loser, R. Relation between carbonation resistance, mix design and exposure of mortar and concrete. Cem. Concr. Compos. 2015, 62, 33–43. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Justnes, H.; Skocek, J.; Østnor, T.A.; Engelsen, C.J.; Skjølsvold, O. Microstructural changes of hydrated cement blended with fly ash upon carbonation. Cem. Concr. Res. 2020, 137, 106192. [Google Scholar] [CrossRef]
- Wu, B.; Ye, G. Development of porosity of cement paste blended with supplementary cementitious materials after carbonation. Constr. Build. Mater. 2017, 145, 52–61. [Google Scholar] [CrossRef] [Green Version]
| Components | Mass of Component per 1 m3 of Mortar (kg) | ||||
|---|---|---|---|---|---|
| EnM-06 | RefM-06 | EnM-05 | RefM-05 | ||
| Cement | CEM II/C (S-LL) | 487 | / | 552 | / |
| CEM II/A-S | / | 487 | / | 552 | |
| Aggregate | Sand 0/4 | 1524 | 1524 | 1498 | 1496 |
| Admixture | Superplasticizer | 1.5 | 1.8 | 3.2 | 3.8 |
| Water | 292 | 292 | 264 | 263 | |
| Property | CEM II/C (S-LL) | CEM II/A-S | |
|---|---|---|---|
| Composition (wt%) | CEM I 52.5 R (K) | 50 | 83 |
| S | 40 | 13 | |
| LL | 10 | 4 | |
| Density (g cm−3) | 2.98 | 3.09 | |
| Specific surface area–Blaine (cm2 g−1) | 5210 | 3720 | |
| 28-day compressive strength (MPa) | 62.8 | 59.7 | |
| Property | Sand 0/4 |
|---|---|
| Oven-dry density (kg m−3) | 2730 |
| Saturated surface-dry density (kg m−3) | 2765 |
| Apparent density (kg m−3) | 2830 |
| Absorption (%) | 1.3 |
| Parameter | EnM-06 | RefM-06 | EnM-05 | RefM-05 |
|---|---|---|---|---|
| KAC (mm day−0.5) | 1.03 | 0.64 | 0.66 | 0.23 |
| a (mm) | 0.15 | 1.22 | −0.71 | 1.47 |
| R2 | 0.98 | 0.92 | 0.88 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanžič, L.; Robič, S.; Machner, A.; Bjørndal, M.H.; De Weerdt, K.; Gu, Y.; Bary, B.; Lample Carreras, R.M.; Šajna, A. Experimental Carbonation Study for a Durability Assessment of Novel Cementitious Materials. Materials 2021, 14, 6253. https://doi.org/10.3390/ma14216253
Hanžič L, Robič S, Machner A, Bjørndal MH, De Weerdt K, Gu Y, Bary B, Lample Carreras RM, Šajna A. Experimental Carbonation Study for a Durability Assessment of Novel Cementitious Materials. Materials. 2021; 14(21):6253. https://doi.org/10.3390/ma14216253
Chicago/Turabian StyleHanžič, Lucija, Sebastijan Robič, Alisa Machner, Marie Helene Bjørndal, Klaartje De Weerdt, Yushan Gu, Benoît Bary, Rosa Maria Lample Carreras, and Aljoša Šajna. 2021. "Experimental Carbonation Study for a Durability Assessment of Novel Cementitious Materials" Materials 14, no. 21: 6253. https://doi.org/10.3390/ma14216253
APA StyleHanžič, L., Robič, S., Machner, A., Bjørndal, M. H., De Weerdt, K., Gu, Y., Bary, B., Lample Carreras, R. M., & Šajna, A. (2021). Experimental Carbonation Study for a Durability Assessment of Novel Cementitious Materials. Materials, 14(21), 6253. https://doi.org/10.3390/ma14216253







