Fabrication of Highly Porous and Pure Zinc Oxide Films Using Modified DC Magnetron Sputtering and Post-Oxidation
Abstract
:1. Introduction
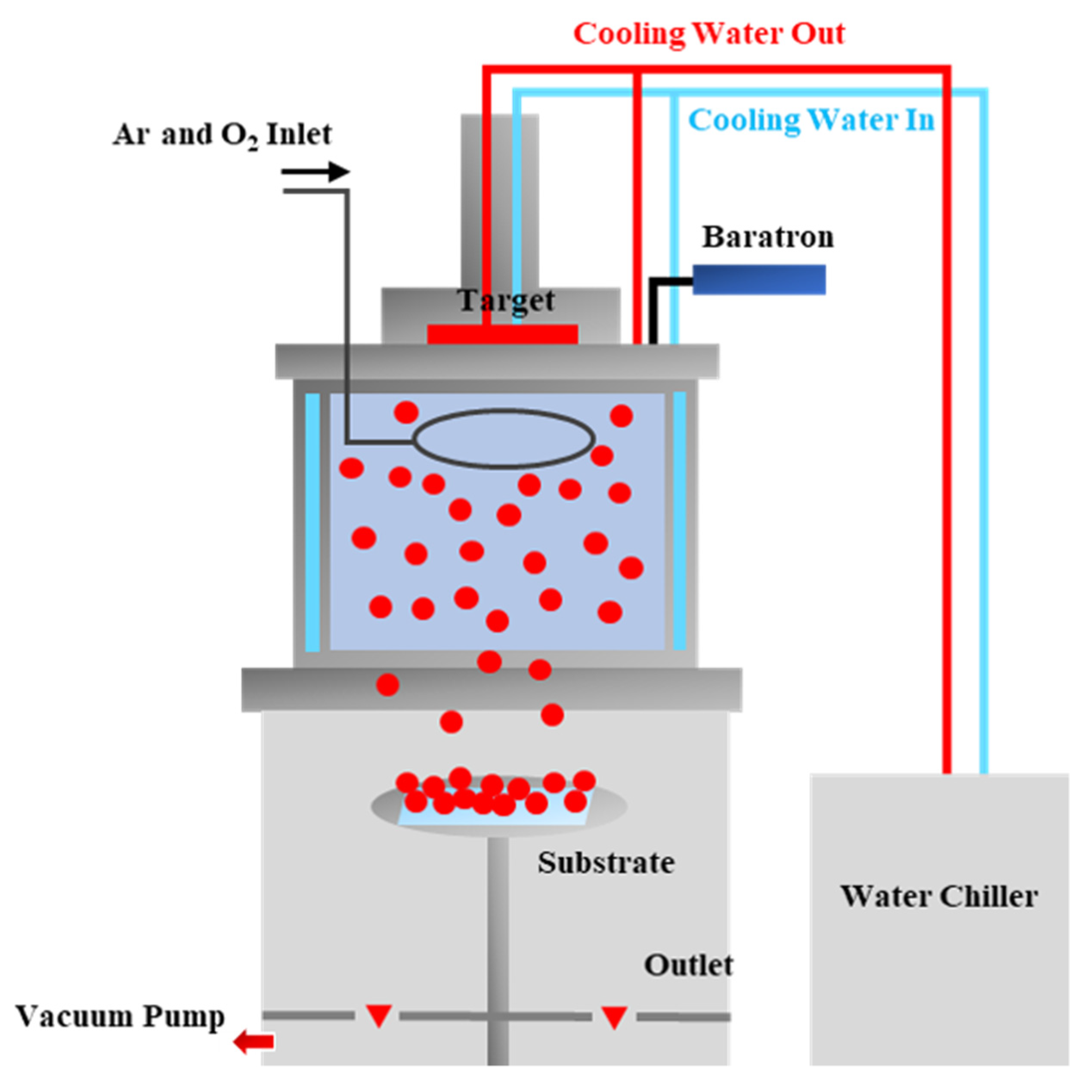
2. Materials and Methods
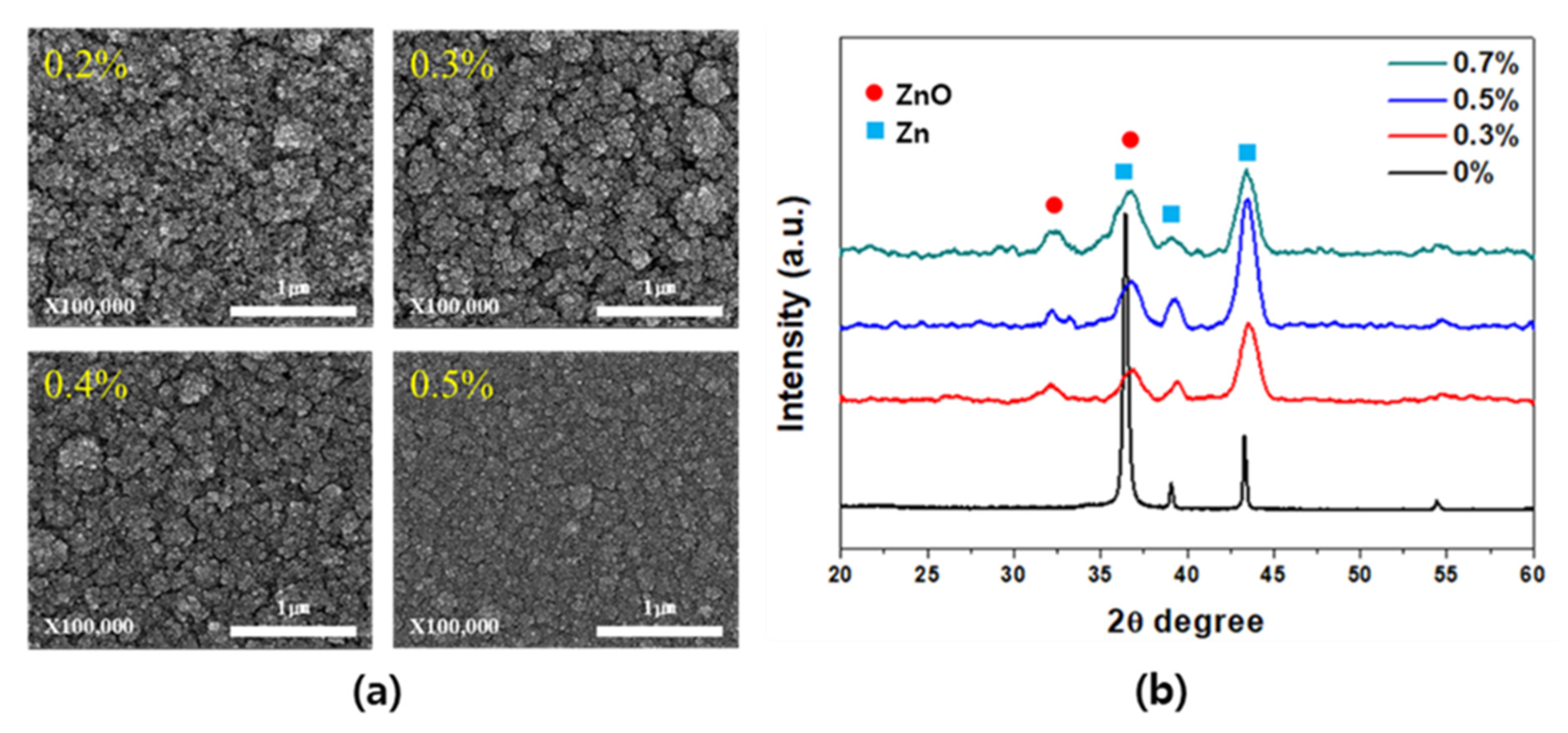
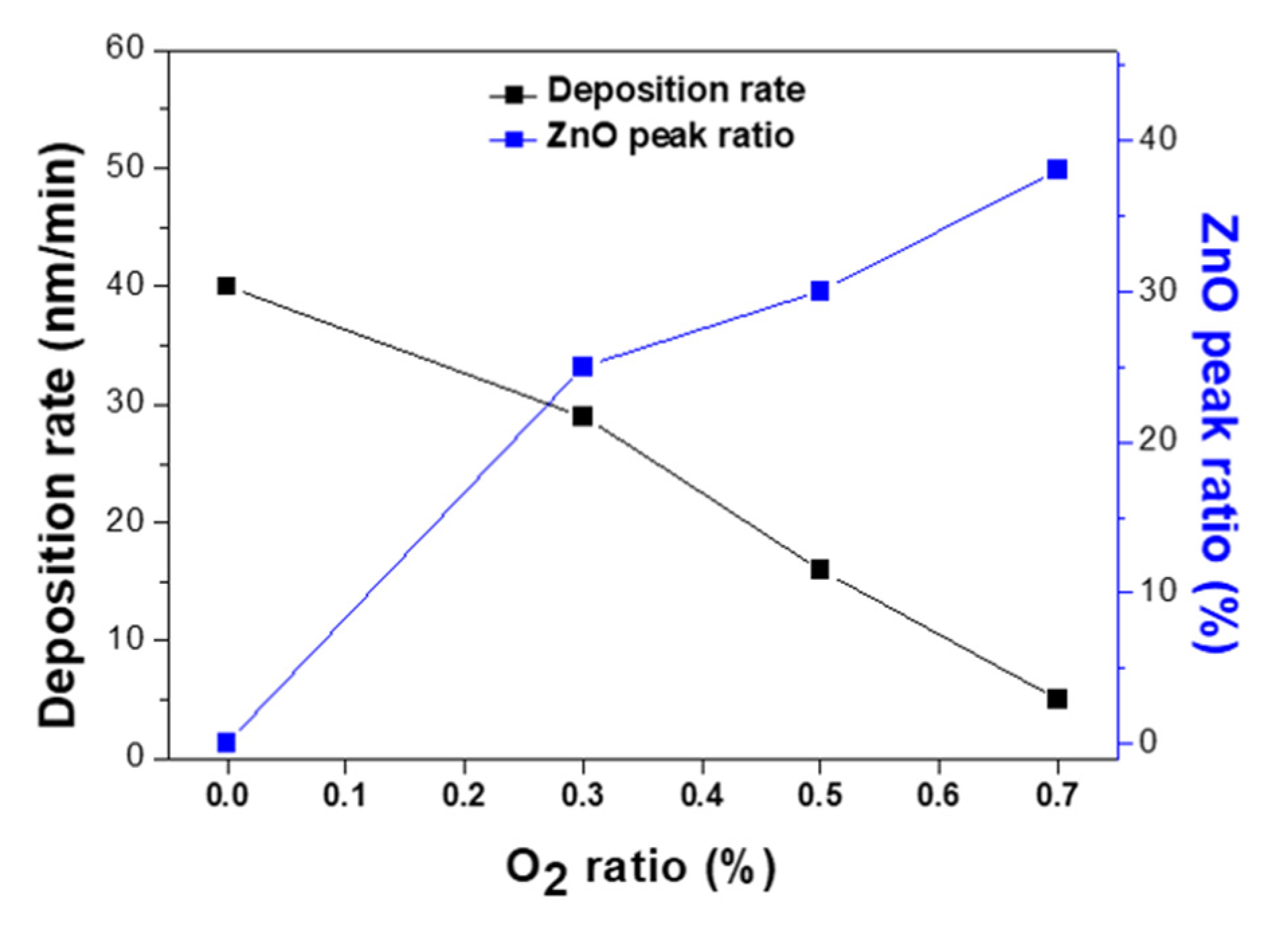
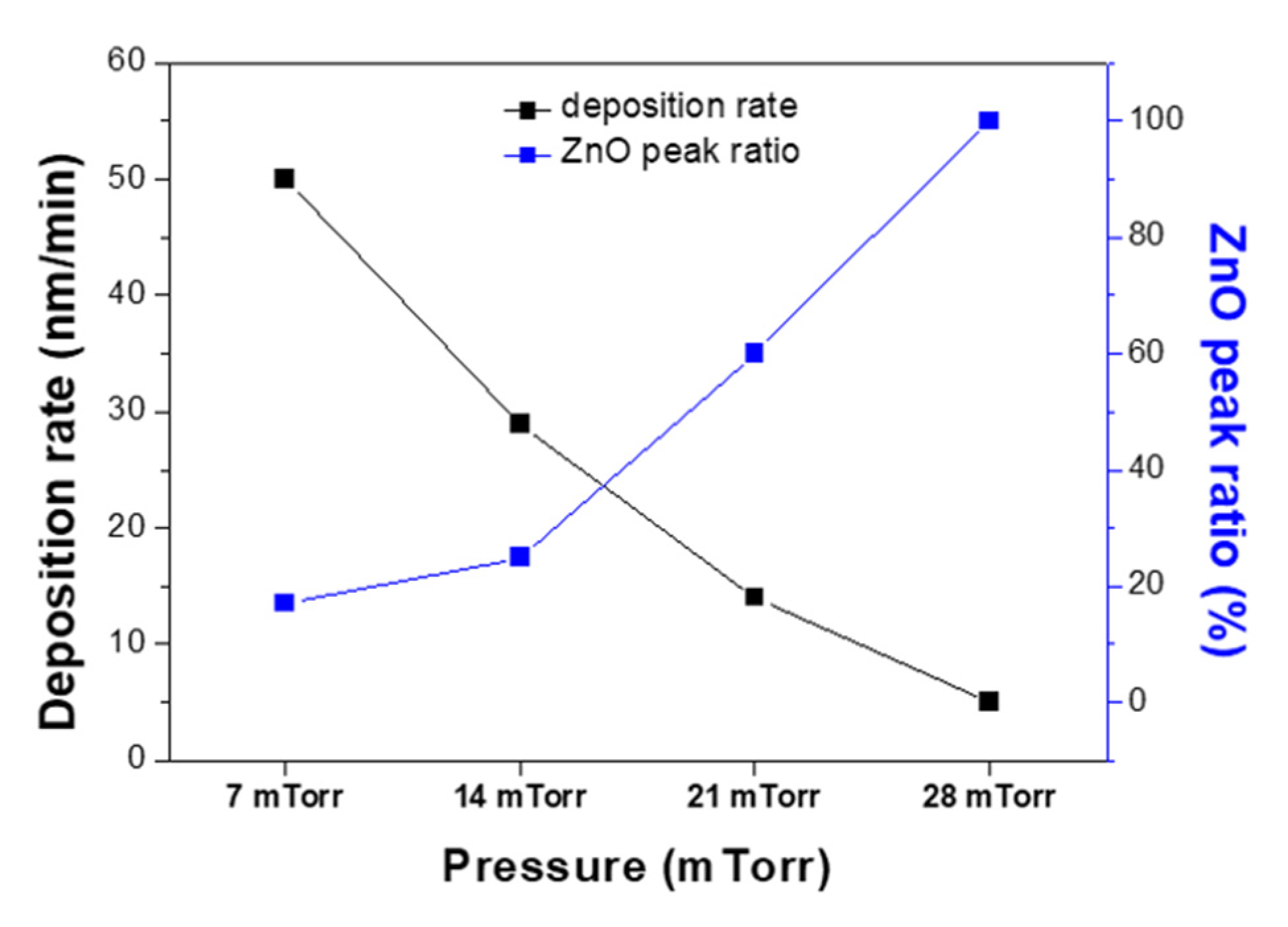
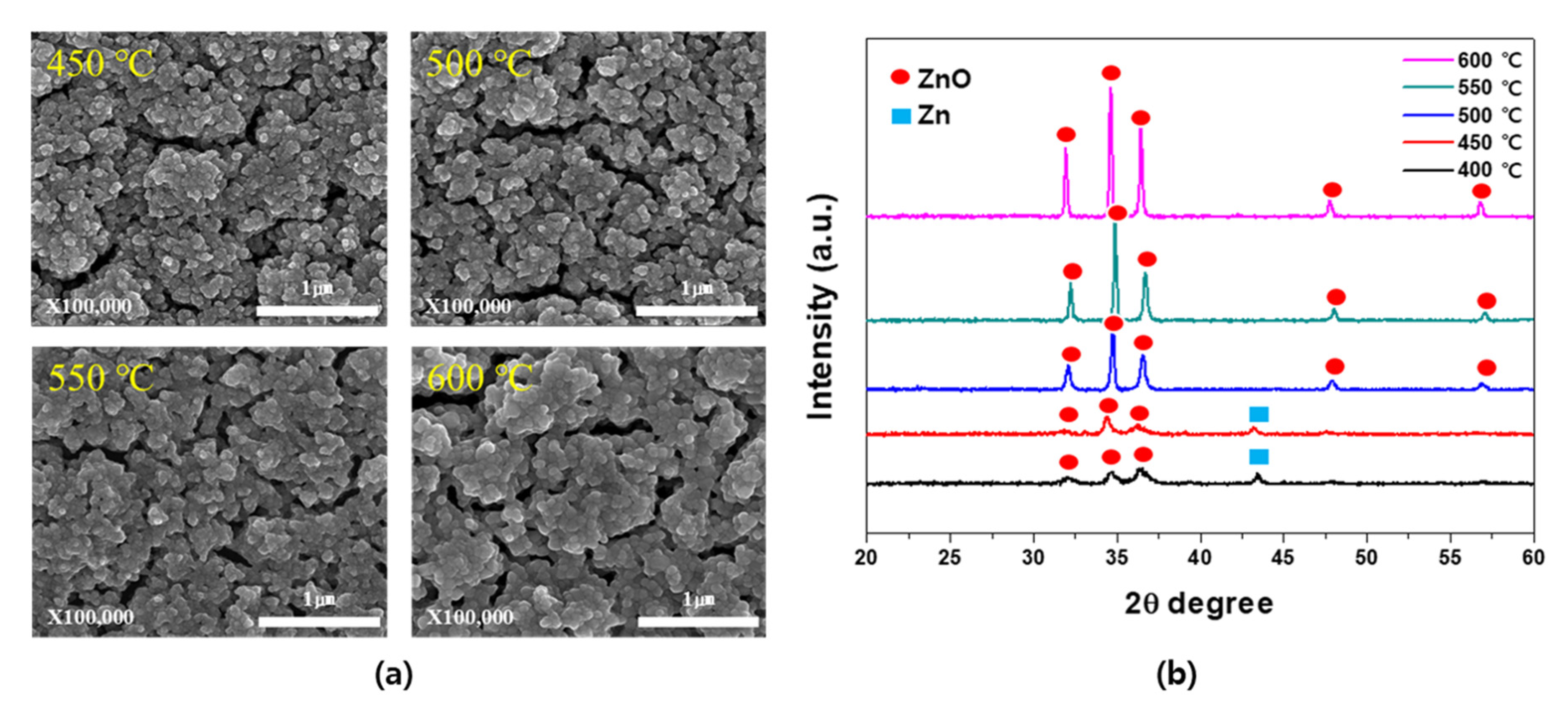
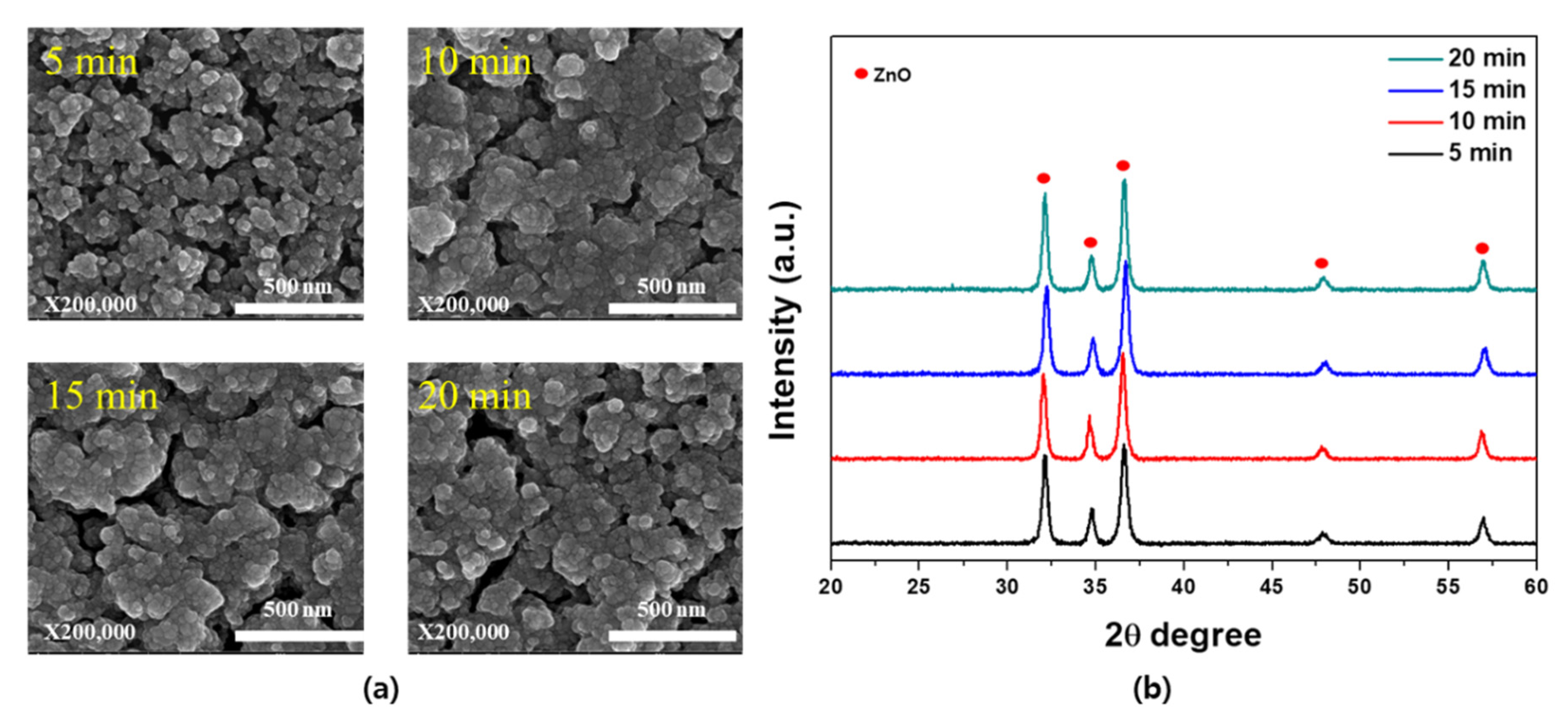
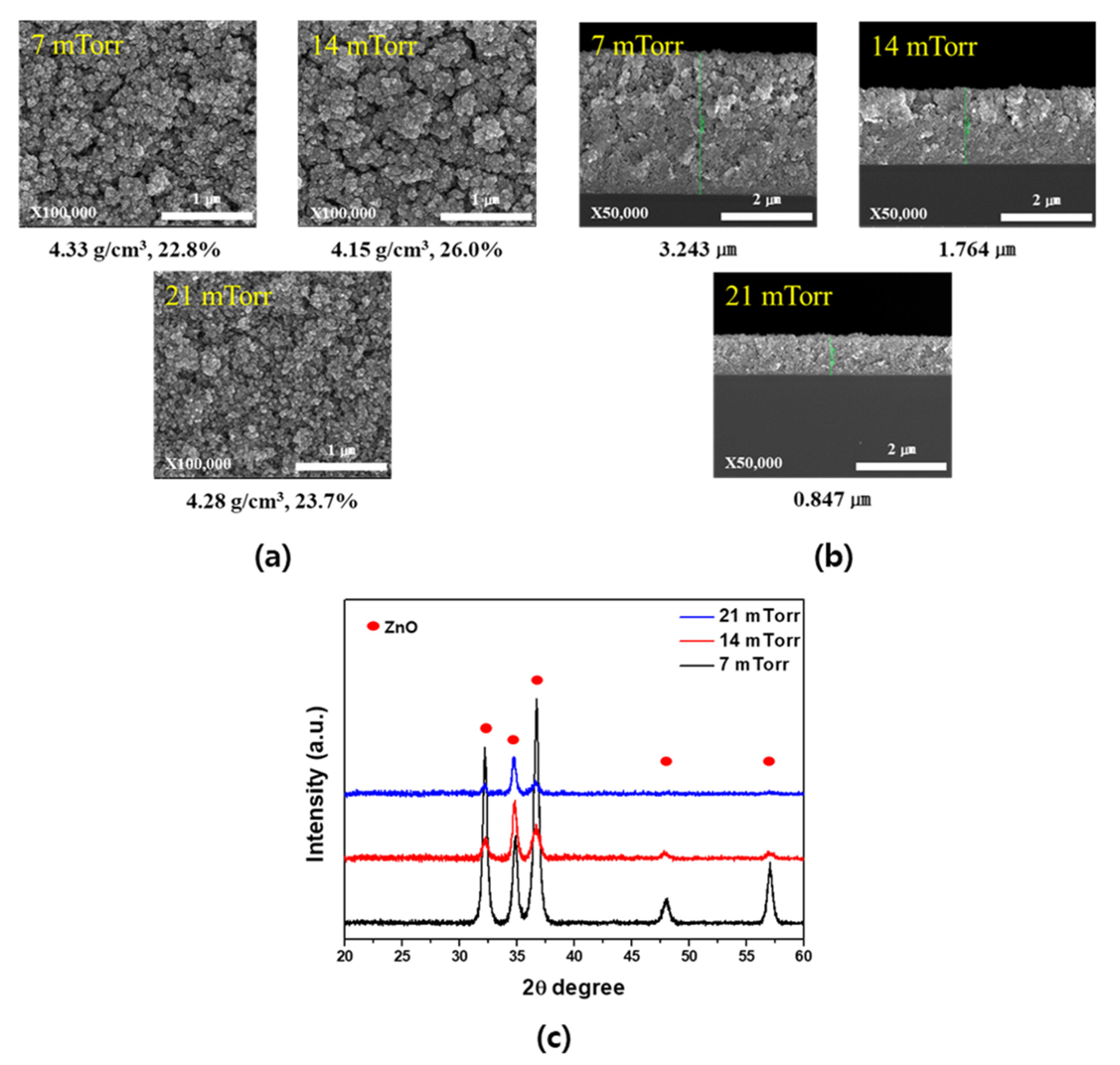
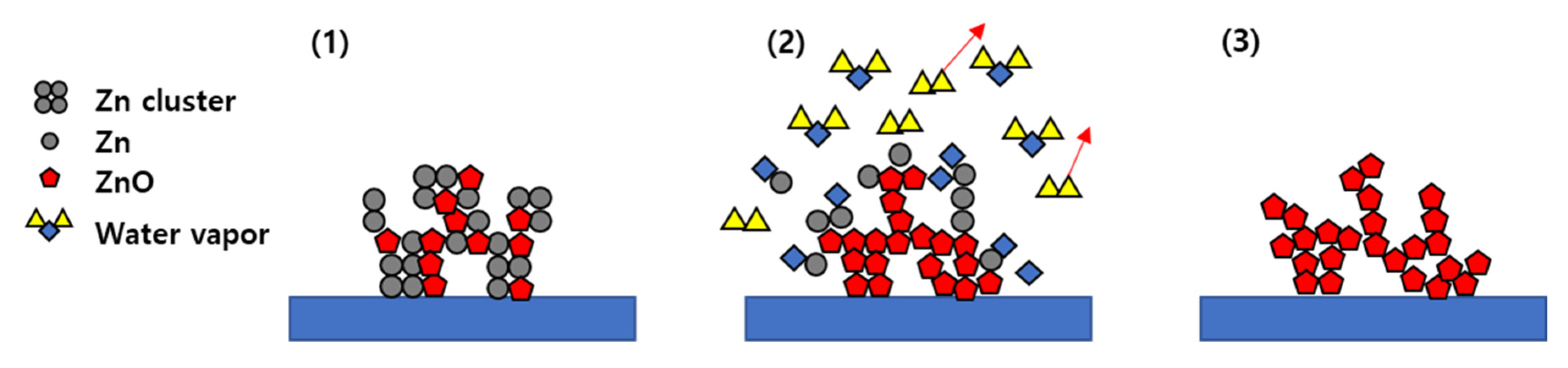
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q. (Max) Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wang, X. Pulsed laser deposition of the porous nickel oxide thin film at room temperature for high-rate pseudocapacitive energy storage. Electrochem. Commun. 2012, 18, 92–95. [Google Scholar] [CrossRef]
- Fei, H.; Yang, Y.; Fan, X.; Wang, G.; Ruan, G.; Tour, J.M. Tungsten-based porous thin-films for electrocatalytic hydrogen generation. J. Mater. Chem. A 2015, 3, 5798–5804. [Google Scholar] [CrossRef]
- Yang, Y.; Fei, H.; Ruan, G.; Tour, J.M. Porous cobalt-based thin film as a bifunctional catalyst for hydrogen generation and oxygen generation. Adv. Mater. 2015, 27, 3175–3180. [Google Scholar] [CrossRef]
- Dai, Z.; Xu, L.; Duan, G.; Li, T.; Zhang, H.; Li, Y.; Wang, Y.; Wang, Y.; Cai, W. Fast-response, sensitivitive and low-powered chemosensors by fusing nanostructured porous thin film and ides-microheater chip. Sci. Rep. 2013, 3, 1669. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kim, K.H.; Vellingiri, K.; Samaddar, P.; Kumar, P.; Deep, A.; Kumar, N. Hybrid porous thin films: Opportunities and challenges for sensing applications. Biosens. Bioelectron. 2018, 104, 120–137. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Oxygen vacancies enabled porous SnO2 thin films for highly sensitive detection of triethylamine at room temperature. ACS Appl. Mater. Interfaces 2020, 12, 20704–20713. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-K.; Kim, J.M.; Hwang, S.; Kim, J.-H.; Sung, C.H.; Moon, B.-M.; Chae, K.H.; Singh, J.P.; Kim, S.-H.; Jang, S.S.; et al. Porous strained Pt nanostructured thin-film electrocatalysts via dealloying for PEM fuel cells. Adv. Mater. Interfaces 2020, 7, 1901326. [Google Scholar] [CrossRef]
- Omri, K.; Bettaibi, A.; Khirouni, K.; El Mir, L. The optoelectronic properties and role of Cu concentration on the structural and electrical properties of Cu doped ZnO nanoparticles. Phys. B Condens. Matter 2018, 537, 167–175. [Google Scholar] [CrossRef]
- Omri, K.; Najeh, I.; El Mir, L. Influence of annealing temperature on the microstructure and dielectric properties of ZnO nanoparticles. Ceram. Int. 2016, 42, 8940–8948. [Google Scholar] [CrossRef]
- Omri, K.; Alyamani, A.; El Mir, L. Surface morphology, microstructure and electrical properties of Ca-doped ZnO thin films. J. Mater. Sci. Mater. Electron. 2019, 30, 16606–16612. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. Porous zinc oxide thin films: Synthesis approaches and applications. Coatings 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Simon, Q.; Barreca, D.; Gasparotto, A.; Maccato, C.; Montini, T.; Gombac, V.; Fornasiero, P.; Lebedev, O.I.; Turner, S.; Van Tendeloo, G. Vertically oriented CuO/ZnO nanorod arrays: From plasma-assisted synthesis to photocatalytic H2 production. J. Mater. Chem. 2012, 22, 11739–11747. [Google Scholar] [CrossRef]
- Shaikh, S.K.; Ganbavale, V.V.; Mohite, S.V.; Patil, U.M.; Rajpure, K.Y. ZnO nanorod based highly selective visible blind ultra-violet photodetector and highly sensitive NO2 gas sensor. Superlattices Microstruct. 2018, 120, 170–186. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Singh, S.G. Highly sensitive and ultra-fast responsive ammonia gas sensor based on 2D ZnO nanoflakes. Mater. Sci. Energy Technol. 2020, 3, 91–96. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Wu, P.; Kim, S.S. Design of supersensitive and selective ZnO-nanofiber-based sensors for H2 gas sensing by electron-beam irradiation. Sens. Actuators B Chem. 2019, 293, 210–223. [Google Scholar] [CrossRef]
- Ryu, H.W.; Park, B.S.; Akbar, S.A.; Lee, W.S.; Hong, K.J.; Seo, Y.J.; Shin, D.C.; Park, J.S.; Chio, G.P. ZnO sol–gel derived porous film for CO gas sensing. Sens. Actuators B Chem. 2003, 96, 717–722. [Google Scholar] [CrossRef]
- Pál, E.; Hornok, V.; Kun, R.; Oszkó, A.; Seemann, T.; Dékány, I.; Busse, M. Hydrothermal synthesis and humidity sensing property of ZnO nanostructures and ZnOIn(OH)3 nanocomposites. J. Colloid Interface Sci. 2012, 378, 100–109. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Soni, S.; Sharma, S.; Singh, R.C. Influence of different milling media on structural, morphological and optical properties of the ZnO nanoparticles synthesized by ball milling process. Mater. Sci. Semicond. Process. 2019, 98, 29–38. [Google Scholar] [CrossRef]
- Hjiri, M.; Dhahri, R.; El Mir, L.; Bonavita, A.; Donato, N.; Leonardi, S.G.; Neri, G. CO sensing properties of Ga-doped ZnO prepared by sol–gel route. J. Alloys Compd. 2015, 634, 187–192. [Google Scholar] [CrossRef]
- Quarta, A.; Novais, R.M.; Bettini, S.; Iafisco, M.; Pullar, R.C.; Piccirillo, C. A sustainable multi-function biomorphic material for pollution remediation or UV absorption: Aerosol assisted preparation of highly porous ZnO-based materials from cork templates. J. Environ. Chem. Eng. 2019, 7, 102936. [Google Scholar] [CrossRef]
- Lai, Y.F.; Huang, J.H.; Chen, Y.C.; Liu, C.P.; Yang, Y.W. Growth of large-area non-polar ZnO film without constraint to substrate using oblique-angle sputtering deposition. J. Eur. Ceram. Soc. 2013, 33, 1809–1814. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Late, D.J.; Bhise, A.B.; More, M.A.; Mulla, I.S.; Joag, D.S.; Vijayamohanan, K. ZnO multipods, submicron wires, and spherical structures and their unique field emission behavior. J. Phys. Chem. B 2006, 110, 18236–18242. [Google Scholar] [CrossRef]
- Jie, J.; Wang, G.; Chen, Y.; Han, X.; Wang, Q.; Xu, B.; Hou, J.G. Synthesis and optical properties of well-aligned ZnO nanorod array on an undoped ZnO film. Appl. Phys. Lett. 2005, 86, 031909. [Google Scholar] [CrossRef]
- Han, S.; Feng, X.; Lu, Z.H.; Johnson, D.; Wood, R. Erratum: “Transparent-cathode for top-emission organic light-emitting diodes”. Appl. Phys. Lett. 2003, 83, 2719. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Jeong, M.-C.; Myoung, J.-M. Fabrication and application potential of ZnO nanowires grown on GaAs(002) substrates bymetal–organic chemical vapour deposition. Nanotechnology 2003, 15, 254. [Google Scholar] [CrossRef]
- Greene, L.E.; Yuhas, B.D.; Law, M.; Zitoun, D.; Yang, P. Solution-grown zinc oxide nanowires. Inorg. Chem. 2006, 45, 7535–7543. [Google Scholar] [CrossRef]
- Vayssieres, L.; Keis, K.; Lindquist, S.-E.; Hagfeldt, A. Purpose-built anisotropic metal oxide material: 3D highly oriented microrod array of ZnO. J. Phys. Chem. B 2001, 105, 3350–3352. [Google Scholar] [CrossRef]
- Yan, X.; Li, Z.; Chen, R.; Gao, W. Template growth of ZnO nanorods and microrods with controllable densities. Cryst. Growth Des. 2008, 8, 2406–2410. [Google Scholar] [CrossRef]
- Chen, R.; Zou, C.; Yan, X.; Alyamani, A.; Gao, W. Growth mechanism of ZnO nanostructures in wet-oxidation process. Thin Solid Films 2011, 519, 1837–1844. [Google Scholar] [CrossRef]
- Li, Z.W.; Gao, W. Growth of zinc oxide thin films and nanostructures by wet oxidation. Thin Solid Films 2007, 515, 3323–3329. [Google Scholar] [CrossRef]
- Muñoz-Rojas, D.; Fraxedas, J.; Gómez-Romero, P.; Casañ-Pastor, N. Room temperature solid-state transformation from Ag2Cu2O3 to Ag2Cu2O4 by ozone oxidation. J. Solid State Chem. 2005, 178, 295–305. [Google Scholar] [CrossRef]
- Rho, S.-H.; Kim, H.-G.; Park, S.-Y.; Lee, H.-C. Study on the mechanism for the deposition of a porous zinc thin film by using a modified DC magnetron sputtering system. J. Korean Phys. Soc. 2021, 78, 679–687. [Google Scholar] [CrossRef]

| Deposition | Variable Parameters | Operating pressure (mTorr) | 7–28 |
| Gas ratio (O2, %) | 0–0.7 | ||
| Fixed Parameters | Chiller temperature (°C) | 20 | |
| Deposition time (min) | 60 | ||
| Power (W) | 150 | ||
| Argon flow rate (sccm) | 10 | ||
| Oxidation | Variable Parameters | Oxidation temperature (°C) | 400–600 |
| Oxidation time (min) | 5–30 | ||
| Fixed Parameters | Gas species | N2 + H2O | |
| Carrier gas flow rate (LPM) | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Rho, S.-H.; Lee, H.-S.; Kim, K.-M.; Lee, H.-C. Fabrication of Highly Porous and Pure Zinc Oxide Films Using Modified DC Magnetron Sputtering and Post-Oxidation. Materials 2021, 14, 6112. https://doi.org/10.3390/ma14206112
Park S-Y, Rho S-H, Lee H-S, Kim K-M, Lee H-C. Fabrication of Highly Porous and Pure Zinc Oxide Films Using Modified DC Magnetron Sputtering and Post-Oxidation. Materials. 2021; 14(20):6112. https://doi.org/10.3390/ma14206112
Chicago/Turabian StylePark, Se-Yong, Soon-Ho Rho, Hwan-Seok Lee, Kyoung-Min Kim, and Hee-Chul Lee. 2021. "Fabrication of Highly Porous and Pure Zinc Oxide Films Using Modified DC Magnetron Sputtering and Post-Oxidation" Materials 14, no. 20: 6112. https://doi.org/10.3390/ma14206112
APA StylePark, S.-Y., Rho, S.-H., Lee, H.-S., Kim, K.-M., & Lee, H.-C. (2021). Fabrication of Highly Porous and Pure Zinc Oxide Films Using Modified DC Magnetron Sputtering and Post-Oxidation. Materials, 14(20), 6112. https://doi.org/10.3390/ma14206112







