Synthesis, Self-Assembly and In Vitro Cellular Uptake Kinetics of Nanosized Drug Carriers Based on Aggregates of Amphiphilic Oligomers of N-Vinyl-2-pyrrolidone
Abstract
1. Introduction
2. Results and Discussion
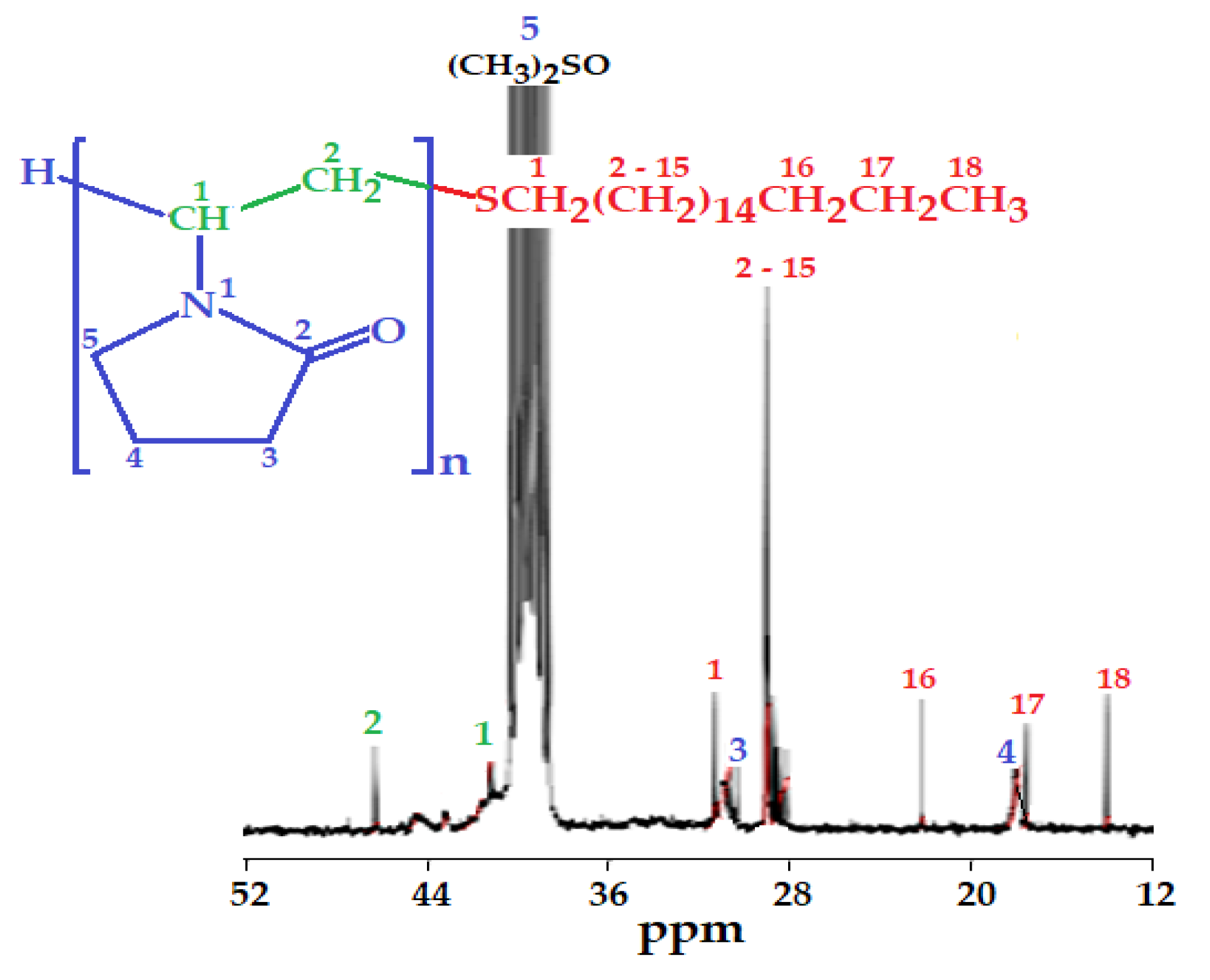
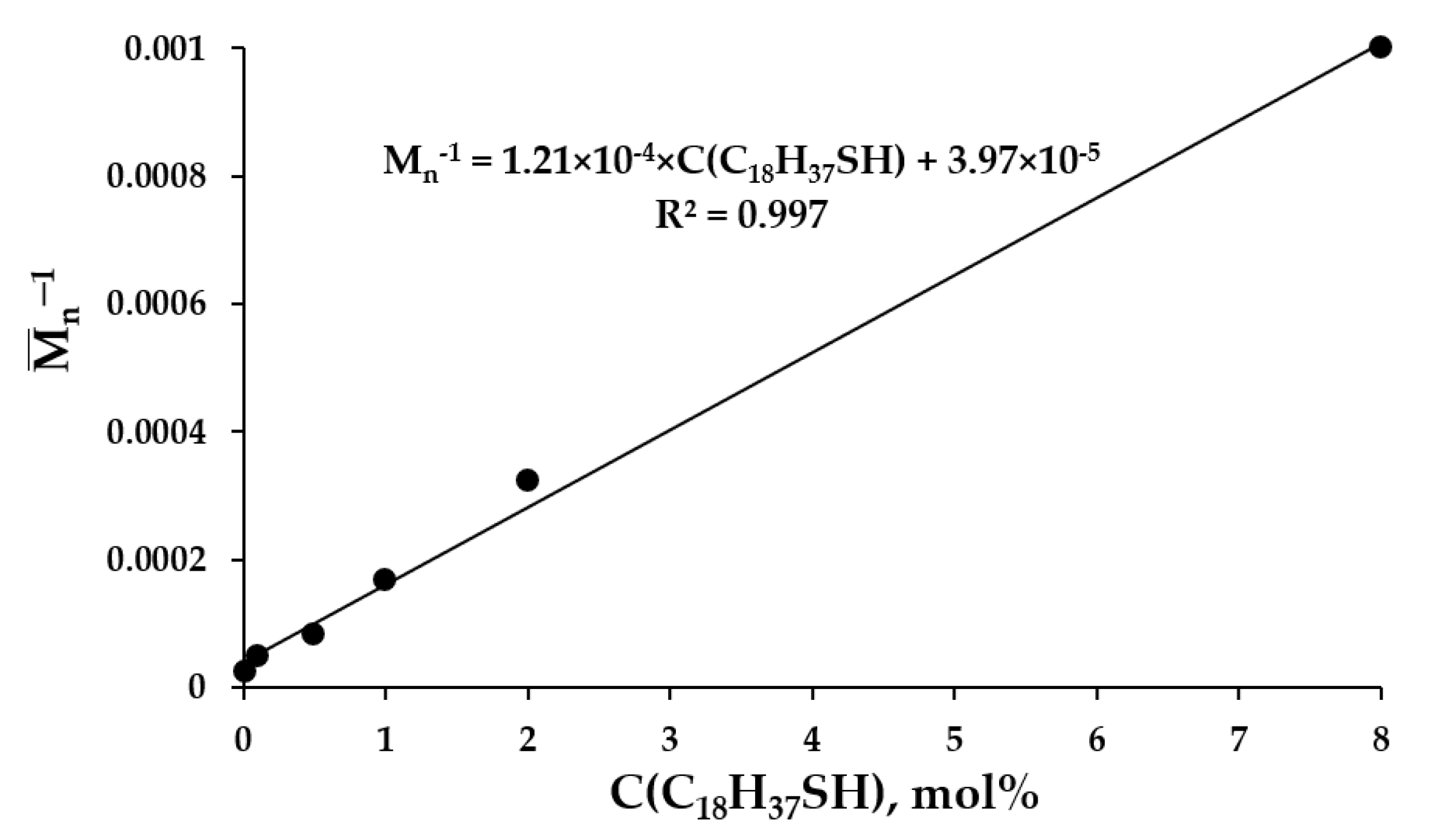
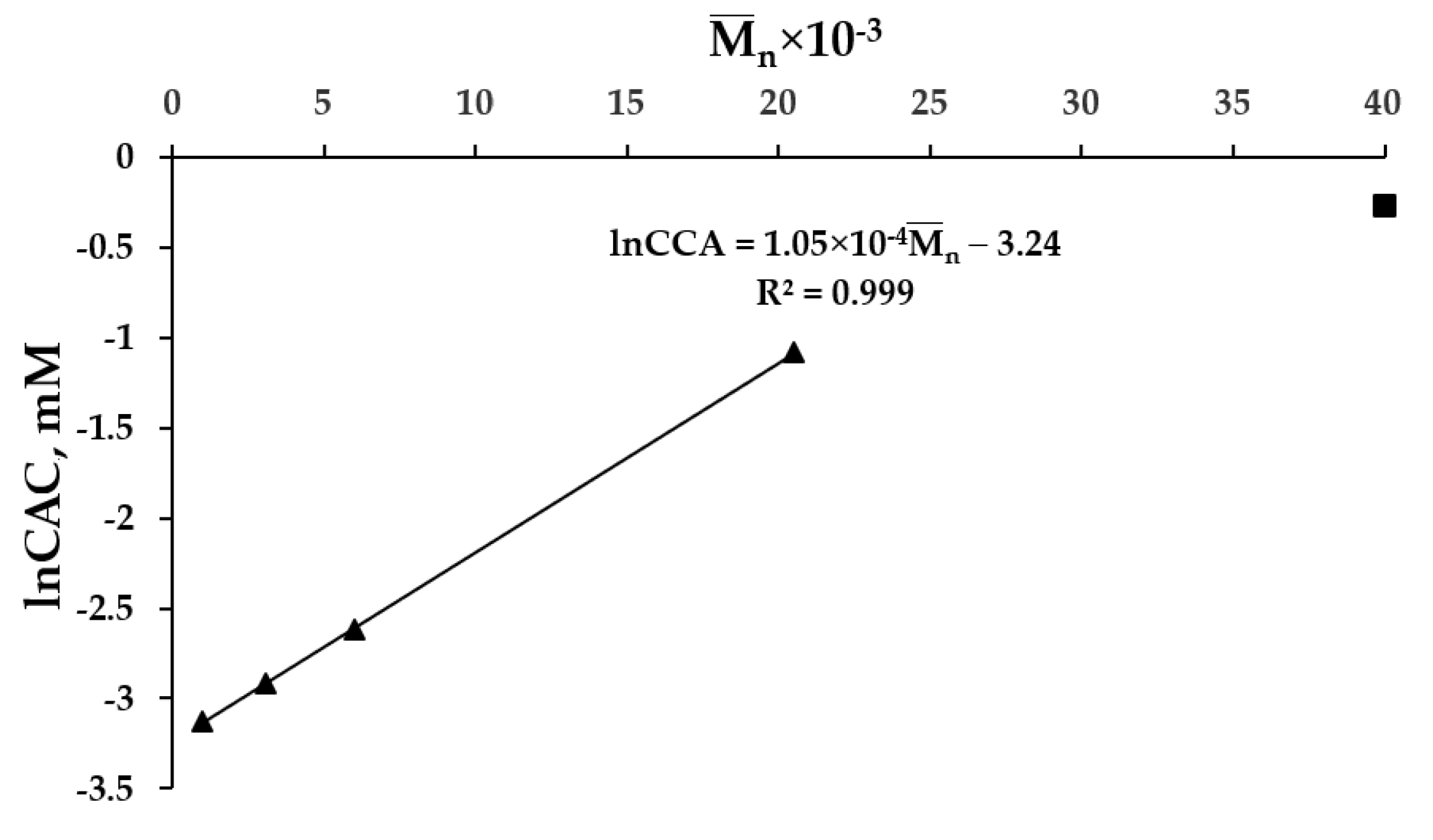
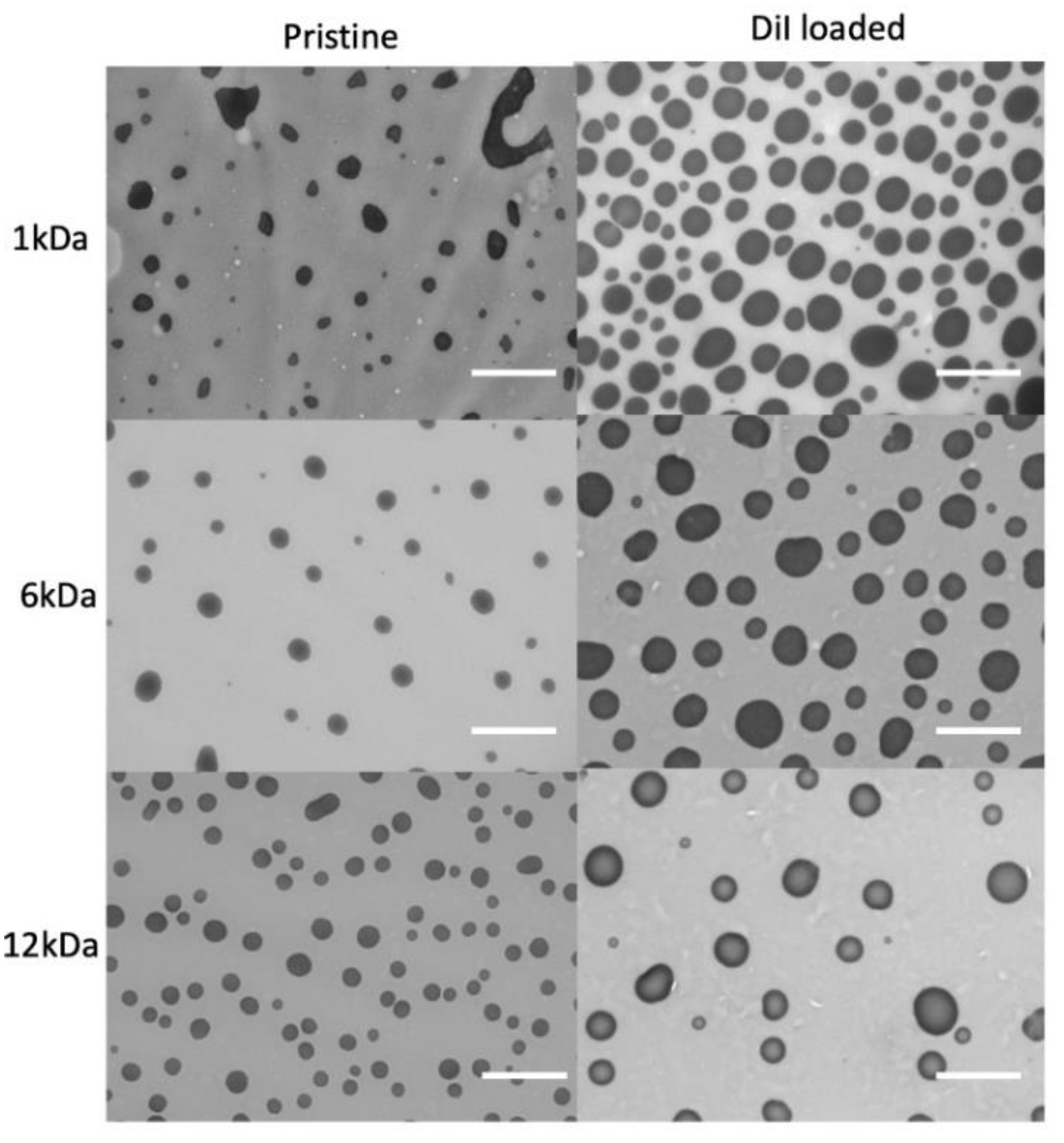
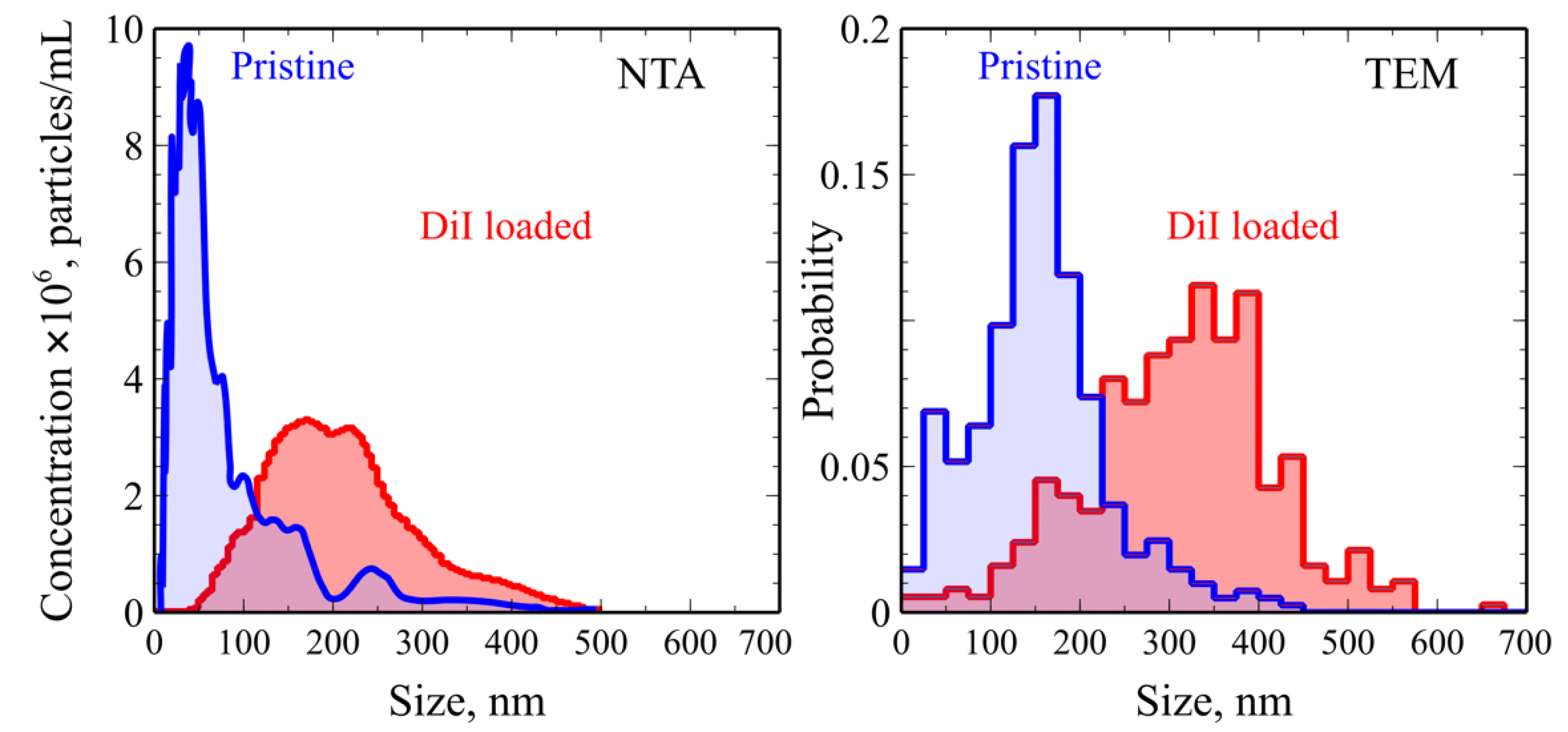
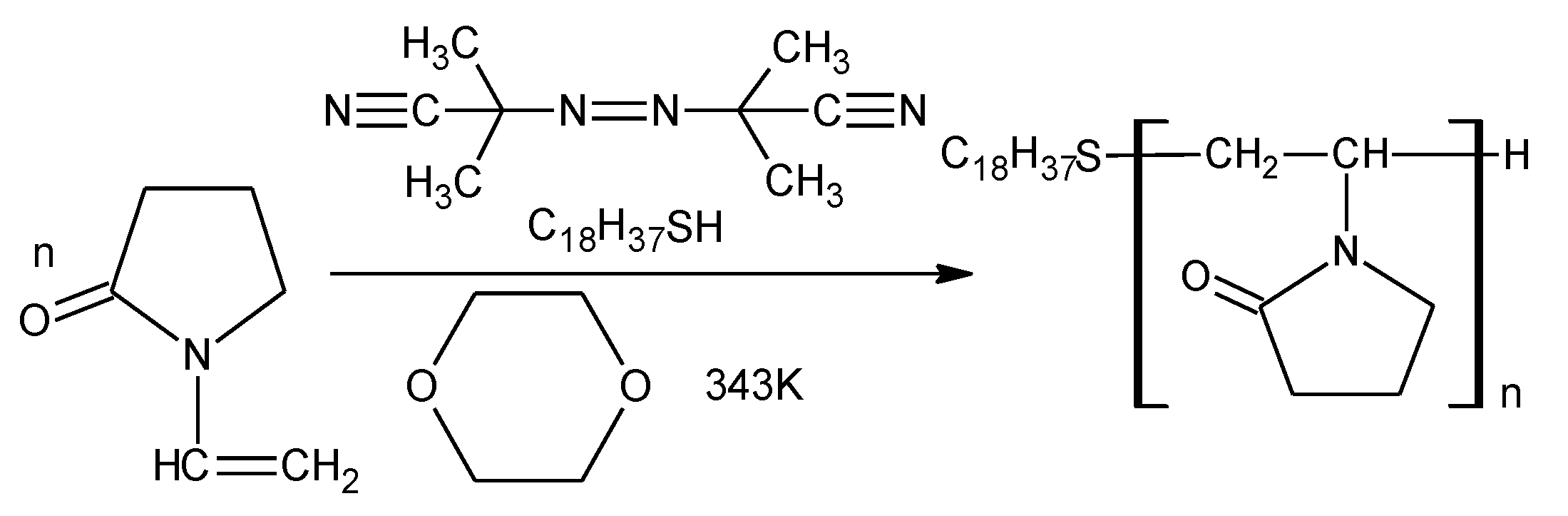
2.1. Synthesis and Characterization of Nanoaggregates Based on PVP-OD
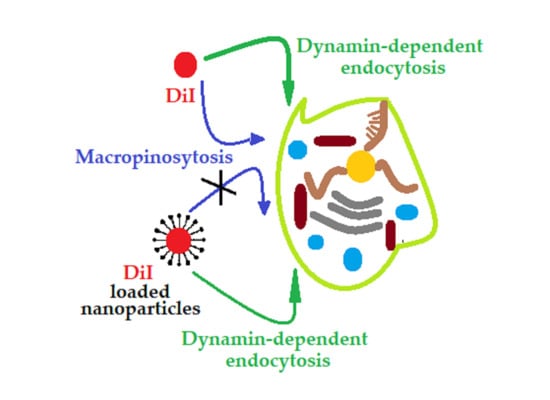
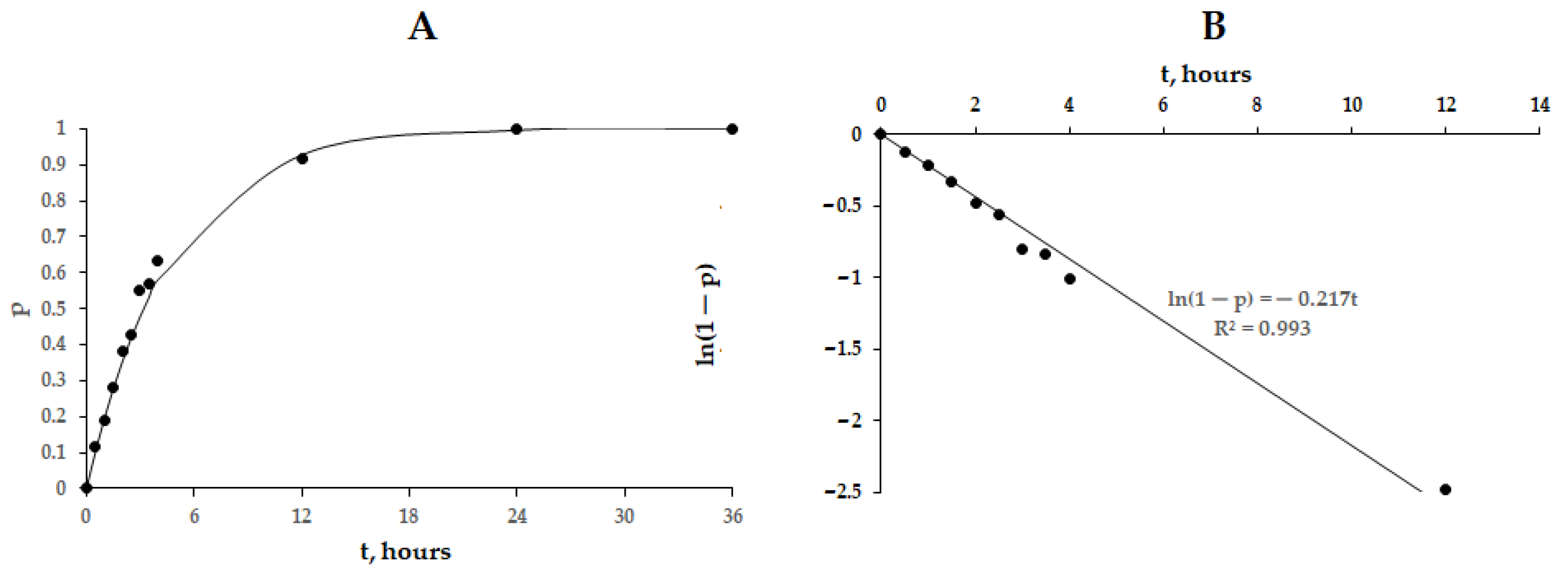
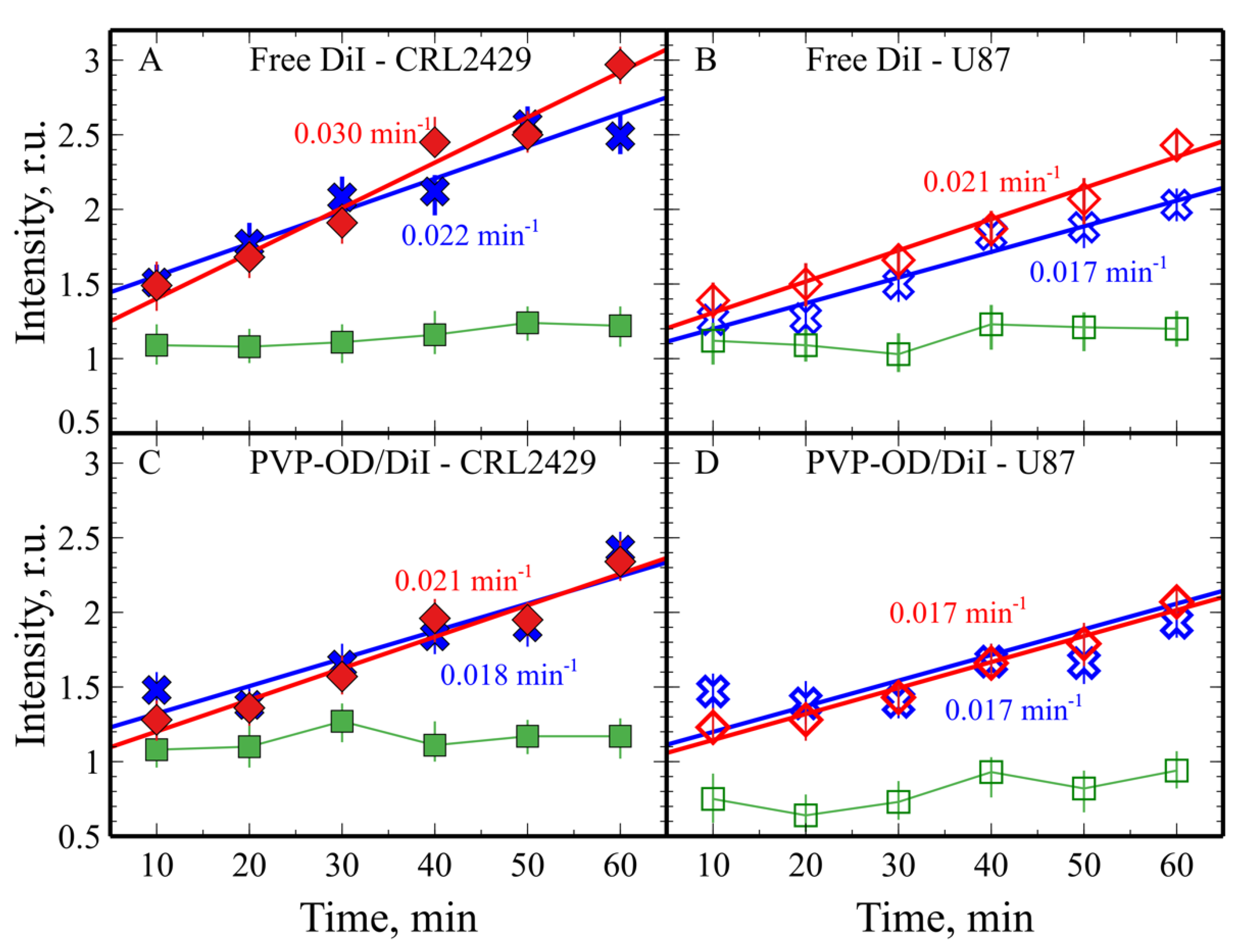
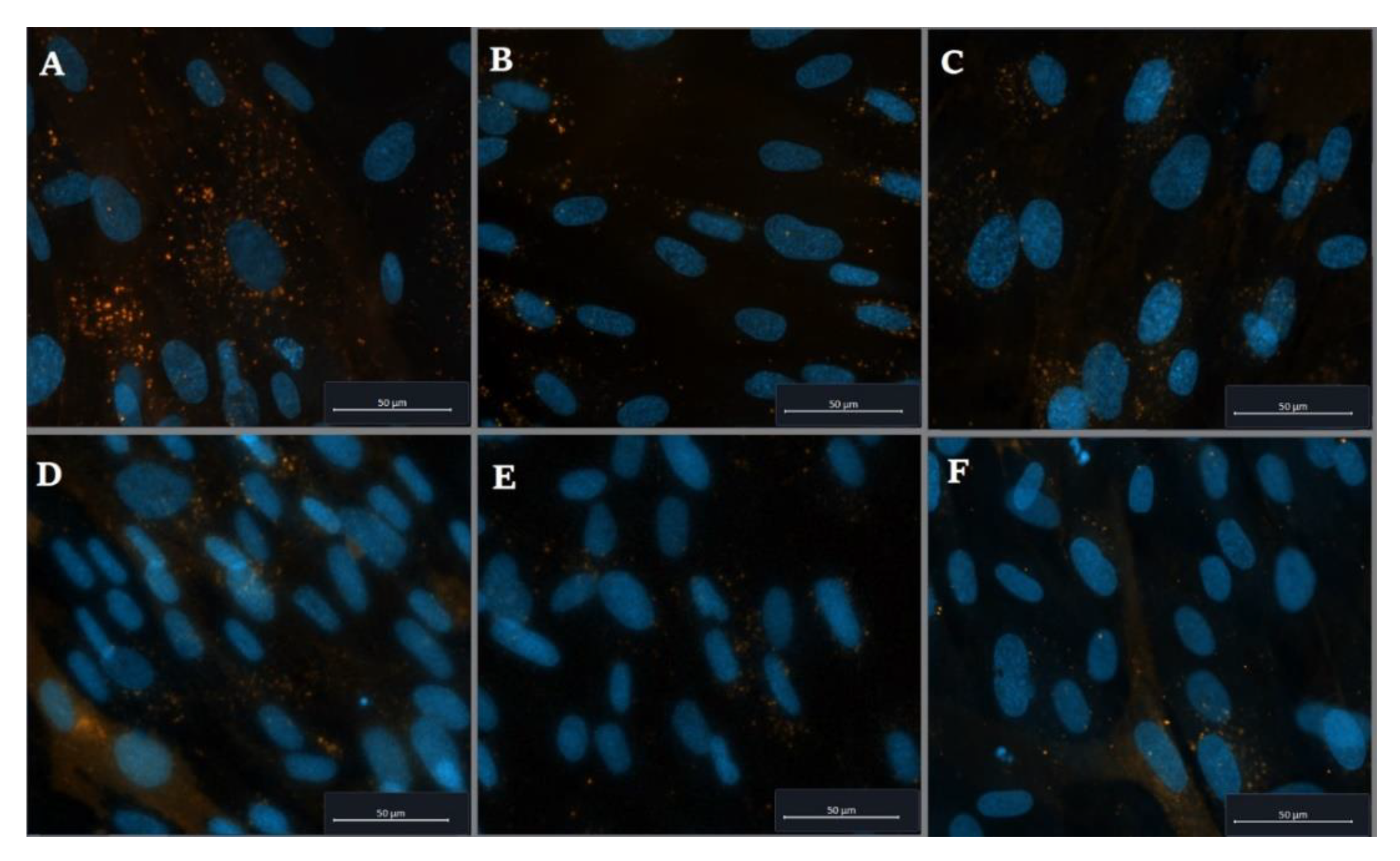
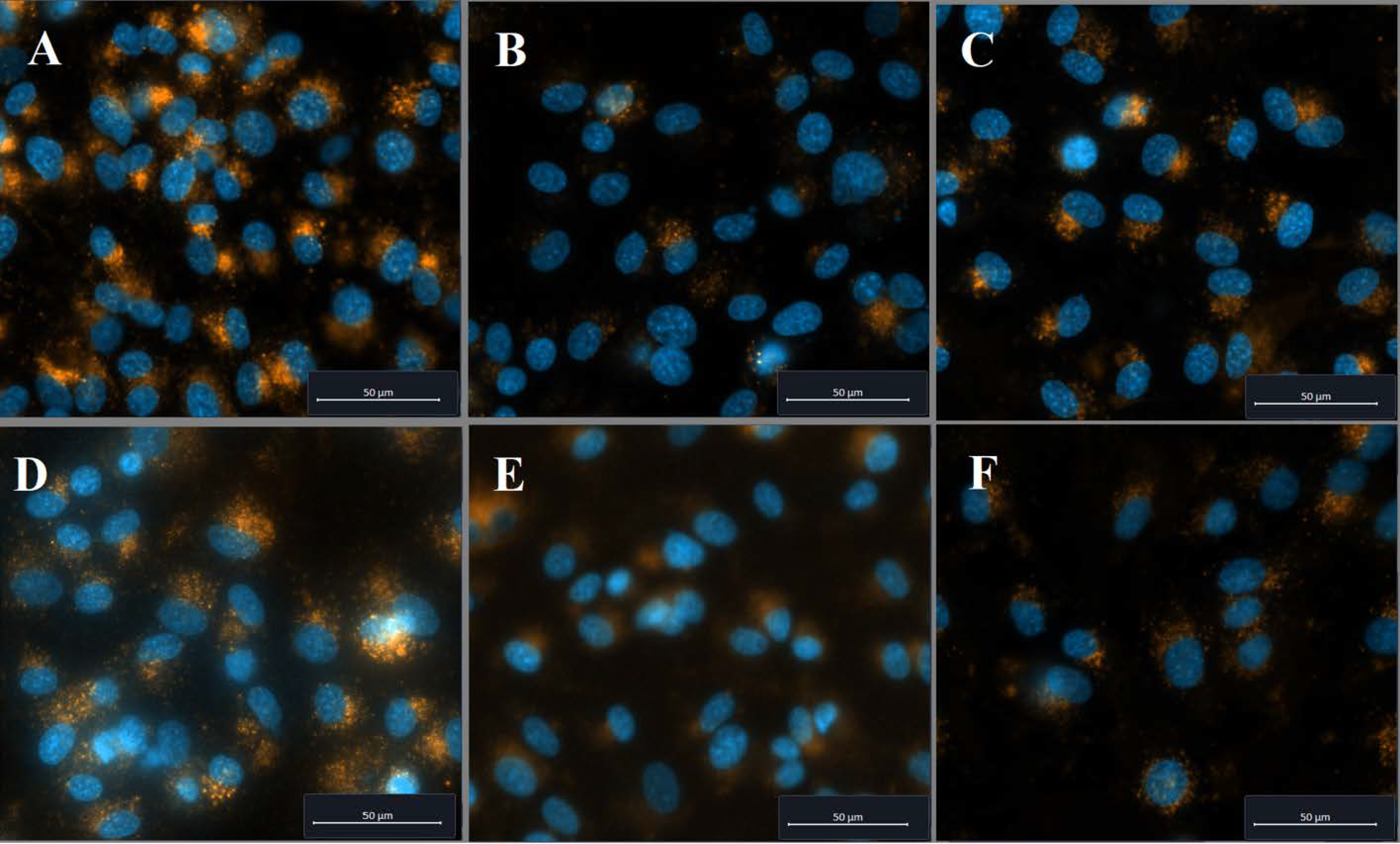
2.2. Cellular Uptake of PVP-OD Nanoaggregates
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiparissides, C.; Kammona, O. Nanoscale carriers for targeted delivery of drugs and therapeutic biomolecules. Can. J. Chem. Eng. 2013, 91, 638–651. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future pro-spects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.S.; Ma, L.L.; Natarajan, J.V.; Chattopadhyay, S. Polymer- and liposome-based nanoparticles in targeted drug delivery. Front. Biosci. (Schol Ed.) 2010, 2, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Aibani, N.; Khan, T.N.; Callan, B. Liposome mimicking polymersomes; A comparative study of the merits of polymersomes in terms of formulation and stability. Int. J. Pharm. X. 2020, 2, 100040. [Google Scholar] [CrossRef]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef]
- Handké, N.; Ficheux, D.; Rollet, M.; Delair, T.; Mabrouk, K.; Bertin, D.; Gigmes, D.; Verrier, B.; Trimaille, T. Lysine-tagged peptide coupling onto polylactide nanoparticles coated with activated ester-based amphiphilic copolymer: A route to highly pep-tide-functionalized biodegradable carriers. Colloids Surf. B Biointerfaces 2013, 103, 298–303. [Google Scholar] [CrossRef]
- Lu, A.; Petit, E.; Jelonek, K.; Orchel, A.; Kasperczyk, J.; Wang, Y.; Su, F.; Li, S. Self-assembled micelles prepared from bio-based hydroxypropyl methyl cellulose and polylactide amphiphilic block copolymers for anti-tumor drug release. Int. J. Biol. Macromol. 2020, 154, 39–47. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Li, S.; Deratani, A. Reverse micelles prepared from amphiphilic polylactide- b -poly(ethylene glycol) block copolymers for controlled release of hydrophilic drugs. Int. J. Pharm. 2015, 495, 154–161. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X. Microfluidics for producing poly (lactic-co-glycolic acid)-based pharmaceutical nanoparticles. Adv. Drug Deliv. Rev. 2018, 128, 101–114. [Google Scholar] [CrossRef]
- Taghizadehghalehjoughi, A.; Hacimuftuoglu, A.; Cetin, M.; Ugur, A.B.; Galateanu, B.; Mezhuev, Y.; Okkay, U.; Taspinar, N.; Taspinar, M.; Uyanik, A.; et al. Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: In vitro and in vivo studies. Nanomedicine 2018, 13, 1595–1606. [Google Scholar] [CrossRef]
- Kazempour, M.; Namazi, H.; Akbarzadeh, A.; Kabiri, R. Synthesis and characterization of PEG-functionalized graphene oxide as an effective pH-sensitive drug carrier. Artif. Cells Nanomed. Biotechnol. 2019, 47, 90–94. [Google Scholar] [CrossRef]
- Rabanel, J.-M.; Hildgen, P.; Banquy, X. Assessment of PEG on polymeric particles surface, a key step in drug carrier translation. J. Control. Release 2014, 185, 71–87. [Google Scholar] [CrossRef]
- Chu, B.; Qu, Y.; Huang, Y.; Zhang, L.; Chen, X.; Long, C.; He, Y.; Ou, C.; Qian, Z. PEG-derivatized octacosanol as micellar carrier for paclitaxel delivery. Int. J. Pharm. 2016, 500, 345–359. [Google Scholar] [CrossRef]
- Li, Y.-C.; Rissanen, S.; Stepniewski, M.; Cramariuc, O.; Róg, T.; Mirza, S.; Xhaard, H.; Wytrwal-Sarna, M.; Kepczynski, M.; Bunker, A. Study of Interaction Between PEG Carrier and Three Relevant Drug Molecules: Piroxicam, Paclitaxel, and Hematoporphyrin. J. Phys. Chem. B 2012, 116, 7334–7341. [Google Scholar] [CrossRef]
- Burnett, C.L. PVP (Polyvinylpyrrolidone). Int. J. Toxicol. 2017, 36, 50S–51S. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Stratidakis, A.; Goryachaya, A.; Tzatzarakis, M.; Stivaktakis, P.; Docea, A.; Berdiaki, A.; Nikitovic, D.; Velonia, K.; Shtilman, M.; et al. In vitro blood compatibility and in vitro cytotoxicity of amphiphilic poly-N-vinylpyrrolidone nanoparticles. Food Chem. Toxicol. 2019, 127, 42–52. [Google Scholar] [CrossRef]
- Torchilin, V.; Levchenko, T.; Whiteman, K.; Yaroslavov, A.; Tsatsakis, A.; Rizos, A.; Michailova, E.; Shtilman, M. Amphiphilic poly-N-vinylpyrrolidones:: Synthesis, properties and liposome surface modification. Biomater. 2001, 22, 3035–3044. [Google Scholar] [CrossRef]
- Benahmed, A.; Ranger, M.; Leroux, J. Novel polymeric micelles based on the amphiphilic diblock copolymer poly(N-vinyl-2-pyrrolidone)-block-poly(D,L-lactide). Pharm. Res. 2001, 18, 323–328. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, R.; Li, C.; Qiao, R. Self-assembled polypeptide-block-poly(vinylpyrrolidone) as prospective drug-delivery systems. Colloids Surfaces B Biointerfaces 2009, 74, 284–292. [Google Scholar] [CrossRef]
- Ali, M.S.; Ghosh, G.; Din, K.-U. Amphiphilic drug persuaded collapse of polyvinylpyrrolidone and poly(ethylene glycol) chains: A dynamic light scattering study. Colloids Surf. B Biointerfaces 2010, 75, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, P.P.; Kuskov, A.; Goryachaya, A.V.; Luss, A.; Shtil’Man, M.I. Amphiphilic poly-n-vinyl-2-pyrrolidone: Synthesis, properties, nanoparticles. Polym. Sci. Ser. D 2017, 10, 263–268. [Google Scholar] [CrossRef]
- Luss, A.L.; Kulikov, P.P.; Romme, S.B.; Andersen, C.L.; Pennisi, C.P.; O Docea, A.; Kuskov, A.N.; Velonia, K.; O Mezhuev, Y.; I Shtilman, M.; et al. Nanosized carriers based on amphiphilic poly-N-vinyl-2-pyrrolidone for intranuclear drug delivery. Nanomed. 2018, 13, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Nelemans, L.C.; Gurevich, L. Drug Delivery with Polymeric Nanocarriers—Cellular Uptake Mechanisms. Materials 2020, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Zhdanov, V.P. Kinetics of virus entry by endocytosis. Phys. Rev. E 2015, 91, 042715. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]
- Yi, X.; Gao, H. Kinetics of receptor-mediated endocytosis of elastic nanoparticles. Nanoscale 2017, 9, 454–463. [Google Scholar] [CrossRef]
- Li, Y.; Yue, T.; Yang, K.; Zhang, X. Molecular modeling of the relationship between nanoparticle shape anisotropy and endocytosis kinetics. Biomaterials 2012, 33, 4965–4973. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Yuan, H.; Gao, H.; Zhang, S. Role of Nanoparticle Geometry in Endocytosis: Laying Down to Stand Up. Nano Lett. 2013, 13, 4546–4550. [Google Scholar] [CrossRef]
- Aubry, L.; Martiel, J.-L.; Satre, M. Modelling of fluid-phase endocytosis kinetics in the amoebae of the cellular slime mouldDictyostelium discoideum. A multicompartmental approach. Acta Biotheor. 1995, 43, 319–333. [Google Scholar] [CrossRef]
- Lin, H.P.; Singla, B.; Ghoshal, P.; Faulkner, J.L.; Cherian-Shaw, M.; O’Connor, P.M.; She, J.X.; Belin de Chantemele, E.J.; Csányi, G. Identification of novel macropinocytosis inhibitors using a rational screen of Food and Drug Administration-approved drugs. Br. J. Pharmacol. 2018, 175, 3640–3655. [Google Scholar] [CrossRef]
- Jayashankar, V.; Edinger, A.L. Macropinocytosis confers resistance to therapies targeting cancer anabolism. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Kuskov, A.N.; Luss, A.L.; Gritskova, I.A.; Shtilman, M.I.; Motyakin, M.V.; Levina, I.I.; Nechaeva, A.M.; Sizova, O.Y.; Tsatsakis, A.M.; Mezhuev, Y.O. Kinetics and Mechanism of Synthesis of Carboxyl-Containing N-Vinyl-2-Pyrrolidone Telehelics for Pharma-cological Use. Polymers 2021, 13, 2569. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Montague, R.A. Controlled Synthesis of Polyphosphazenes with Chain-Capping Agents. Molecules 2021, 26, 322. [Google Scholar] [CrossRef]
- De Lisi, R.; Milioto, S.; Muratore, N. Thermodynamics of Surfactants, Block Copolymers and Their Mixtures in Water: The Role of the Isothermal Calorimetry. Int. J. Mol. Sci. 2009, 10, 2873–2895. [Google Scholar] [CrossRef]
- Wei, Z.; Ning, N.; Zhang, L.; Tian, M.; Mi, J. Density Functional Theory of Polymer Structure and Conformations. Polymers 2016, 8, 121. [Google Scholar] [CrossRef]
- Wipf, P.; Halter, R.J. Chemistry and biology of wortmannin. Org. Biomol. Chem. 2005, 3, 2053–2061. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Voskresenskaya, A.A.; Goryachaya, A.V.; Shtilman, M.I.; Spandidos, D.A.; Rizos, A.K.; Tsatsakis, A.M. Am-phiphilic poly-N-vinylpyrrolidone nanoparticles as carriers for non-steroidal anti-inflammatory drugs: Characterization and in vitro controlled release of indomethacin. Int. J. Mol. Med. 2010, 26, 85–94. [Google Scholar] [CrossRef][Green Version]
- Recouvreux, M.V.; Commisso, C. Macropinocytosis: A Metabolic Adaptation to Nutrient Stress in Cancer. Front. Endocrinol. 2017, 8, 261. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Ding, T.; Ji, N.; Zhao, H. The Dual Role of Macropinocytosis in Cancers: Promoting Growth and Inducing Methuosis to Participate in Anticancer Therapies as Targets. Front. Oncol. 2021, 10, 570108. [Google Scholar] [CrossRef]
- Arcaro, A.; Wymann, M. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: The role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 1993, 296, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Macia, E.; Ehrlich, M.; Massol, R.; Boucrot, E.; Brunner, C.; Kirchhausen, T. Dynasore, a Cell-Permeable Inhibitor of Dynamin. Dev. Cell 2006, 10, 839–850. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikov, P.P.; Luss, A.L.; Nelemans, L.C.; Shtilman, M.I.; Mezhuev, Y.O.; Kuznetsov, I.A.; Sizova, O.Y.; Christiansen, G.; Pennisi, C.P.; Gurevich, L. Synthesis, Self-Assembly and In Vitro Cellular Uptake Kinetics of Nanosized Drug Carriers Based on Aggregates of Amphiphilic Oligomers of N-Vinyl-2-pyrrolidone. Materials 2021, 14, 5977. https://doi.org/10.3390/ma14205977
Kulikov PP, Luss AL, Nelemans LC, Shtilman MI, Mezhuev YO, Kuznetsov IA, Sizova OY, Christiansen G, Pennisi CP, Gurevich L. Synthesis, Self-Assembly and In Vitro Cellular Uptake Kinetics of Nanosized Drug Carriers Based on Aggregates of Amphiphilic Oligomers of N-Vinyl-2-pyrrolidone. Materials. 2021; 14(20):5977. https://doi.org/10.3390/ma14205977
Chicago/Turabian StyleKulikov, Pavel P., Anna L. Luss, Levi C. Nelemans, Mikhail I. Shtilman, Yaroslav O. Mezhuev, Igor A. Kuznetsov, Oksana Yu. Sizova, Gunna Christiansen, Cristian P. Pennisi, and Leonid Gurevich. 2021. "Synthesis, Self-Assembly and In Vitro Cellular Uptake Kinetics of Nanosized Drug Carriers Based on Aggregates of Amphiphilic Oligomers of N-Vinyl-2-pyrrolidone" Materials 14, no. 20: 5977. https://doi.org/10.3390/ma14205977
APA StyleKulikov, P. P., Luss, A. L., Nelemans, L. C., Shtilman, M. I., Mezhuev, Y. O., Kuznetsov, I. A., Sizova, O. Y., Christiansen, G., Pennisi, C. P., & Gurevich, L. (2021). Synthesis, Self-Assembly and In Vitro Cellular Uptake Kinetics of Nanosized Drug Carriers Based on Aggregates of Amphiphilic Oligomers of N-Vinyl-2-pyrrolidone. Materials, 14(20), 5977. https://doi.org/10.3390/ma14205977