The Sono-Photocatalytic Performance of PAN/g-C3N4/CdS Nanofibers Heterojunction
Abstract
:1. Introduction
2. Methods/Experimental
2.1. Chemicals
2.2. Synthesis of PAN/C3N4/CdS Heterojunction
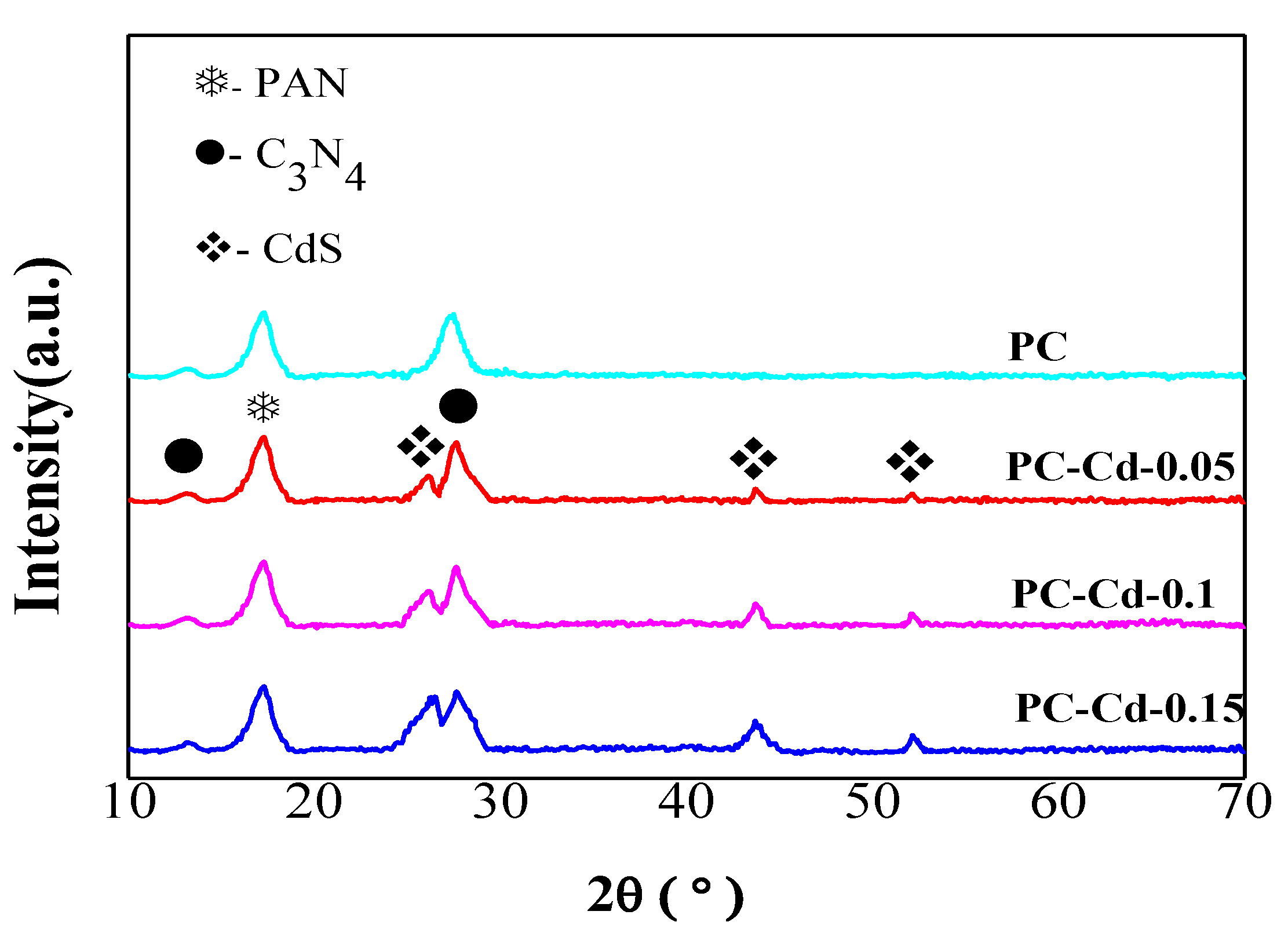
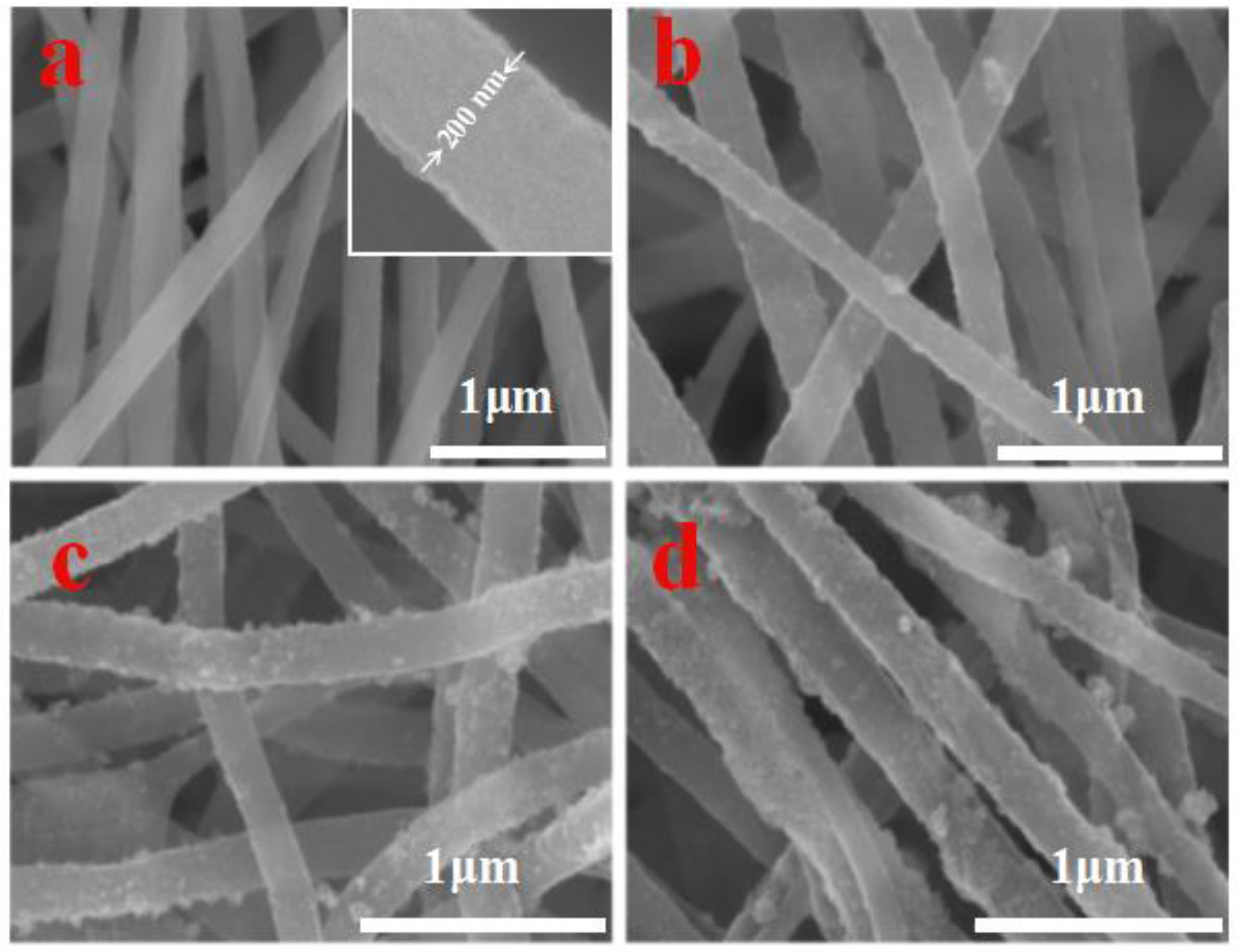
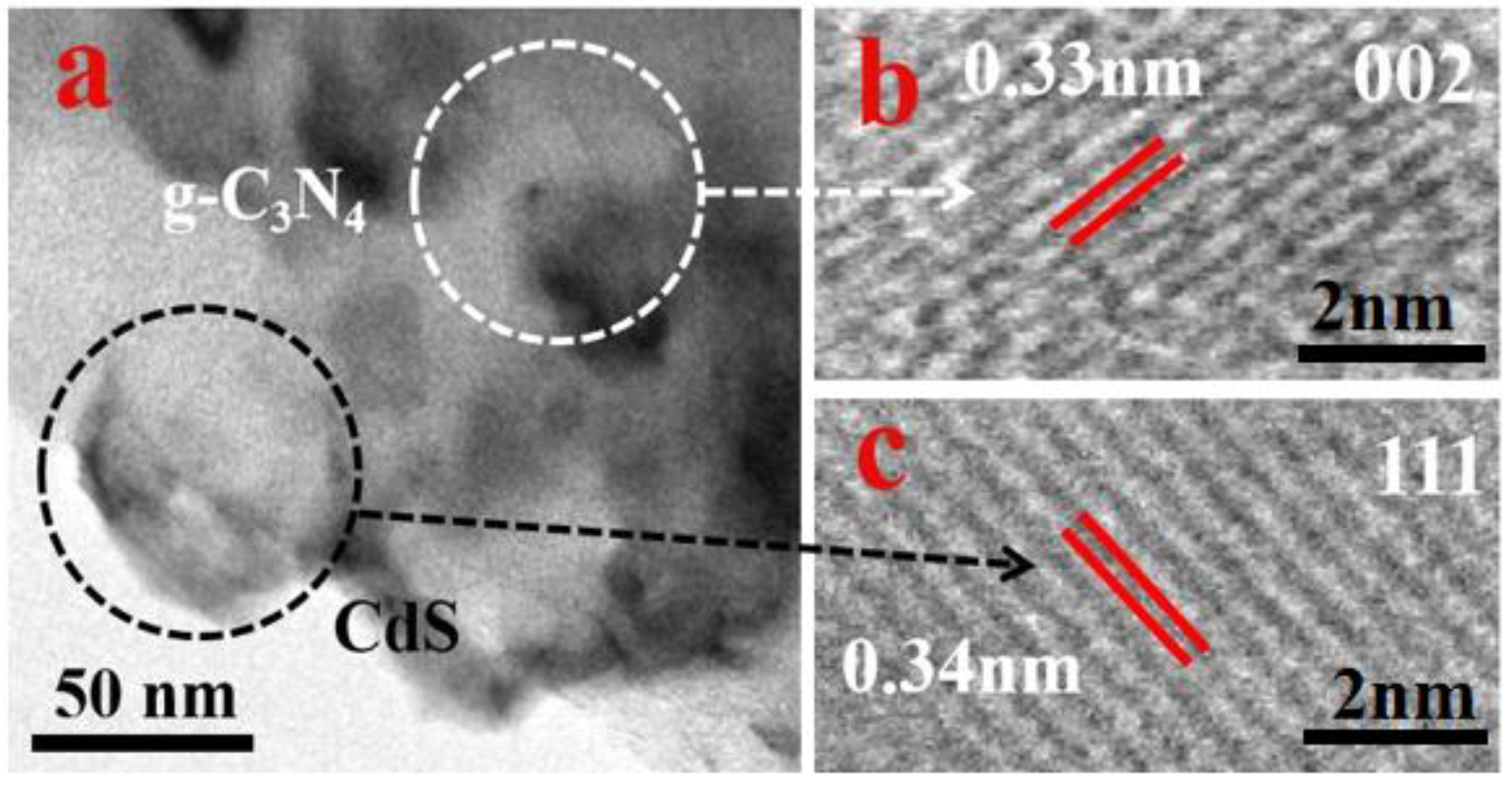
2.3. Characterization
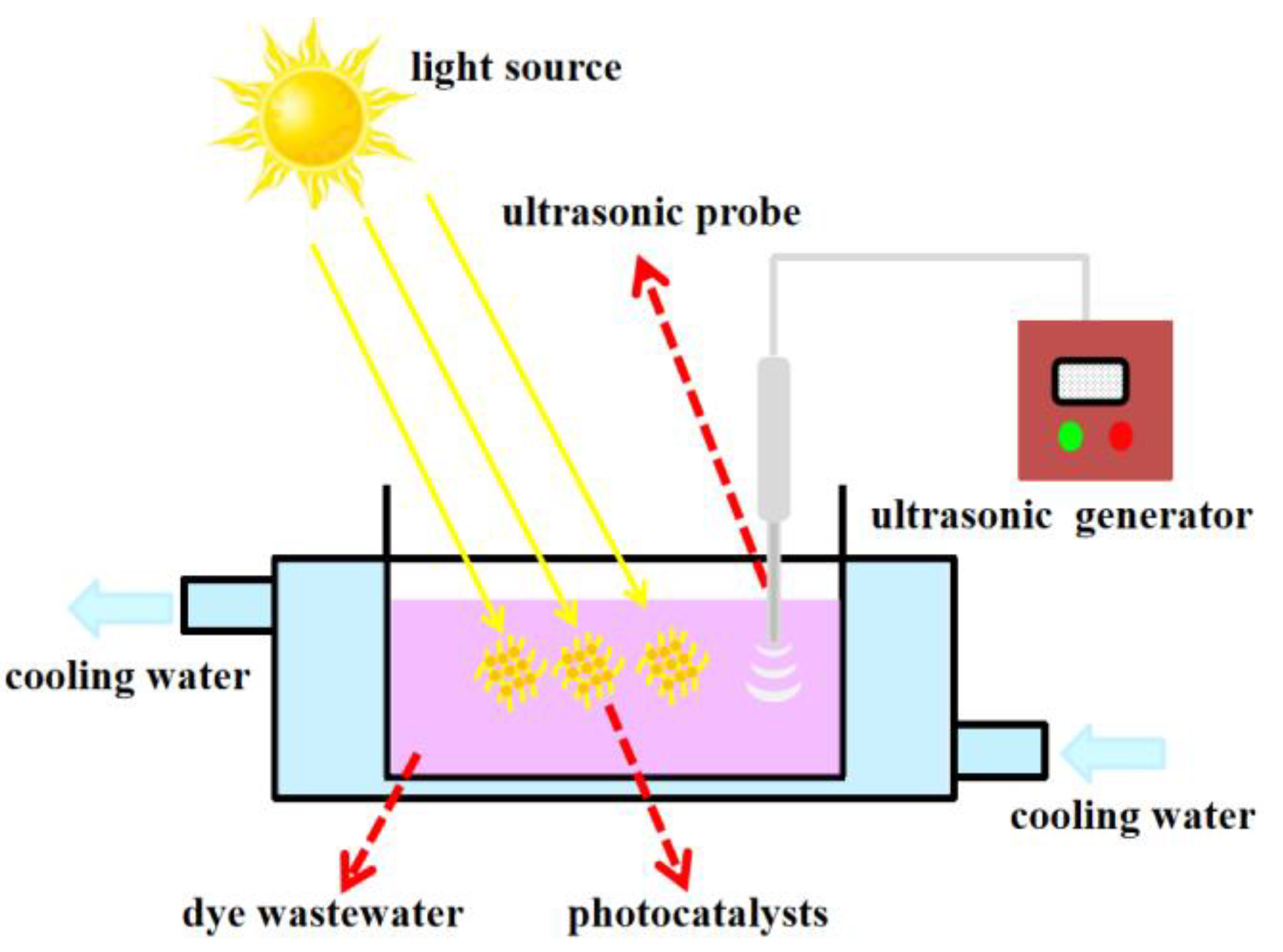
2.4. Sonophotocatalytic Activity Test
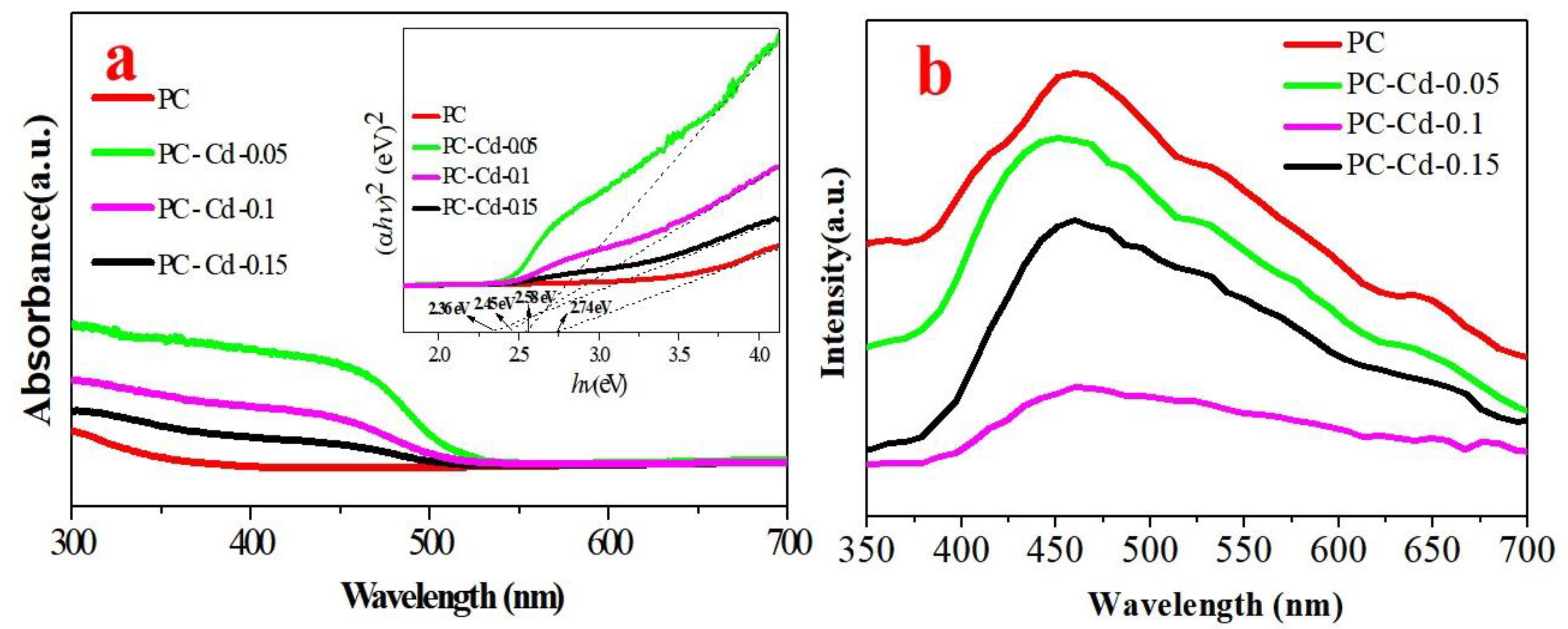
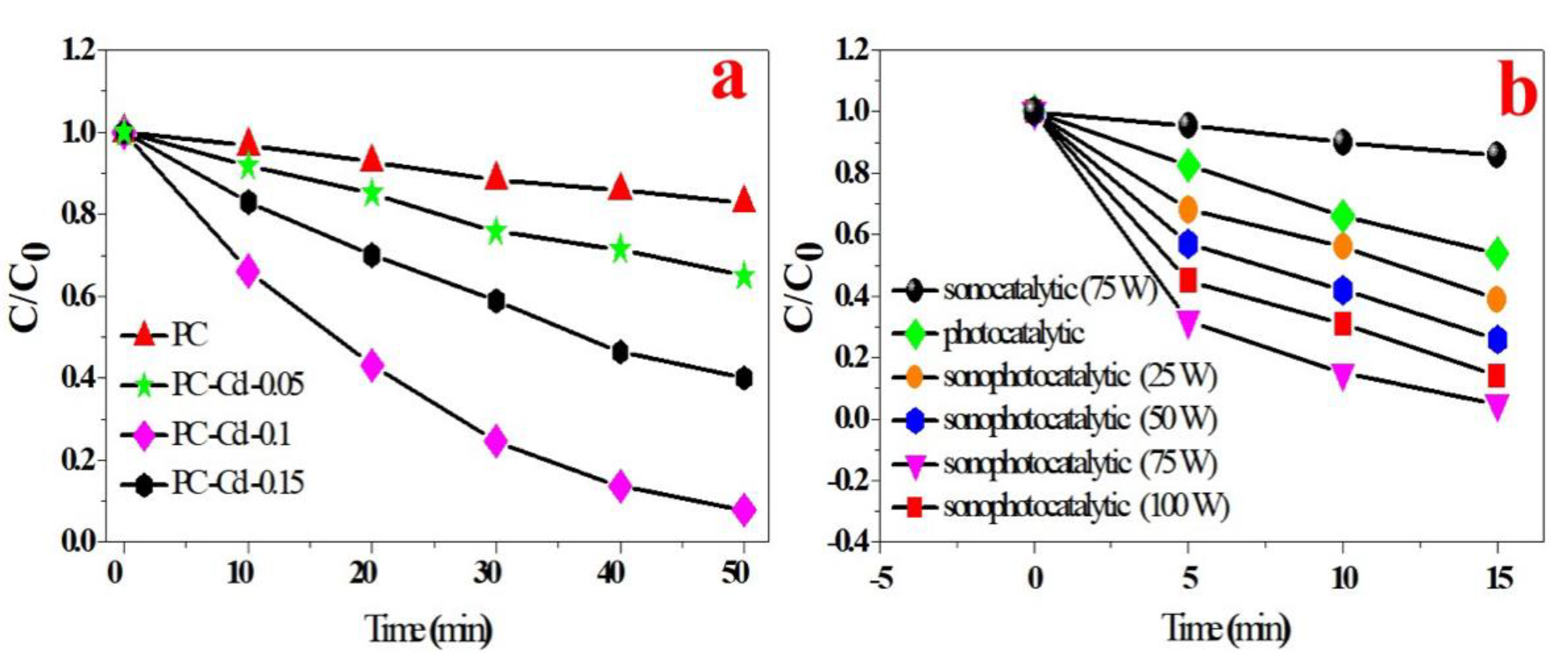
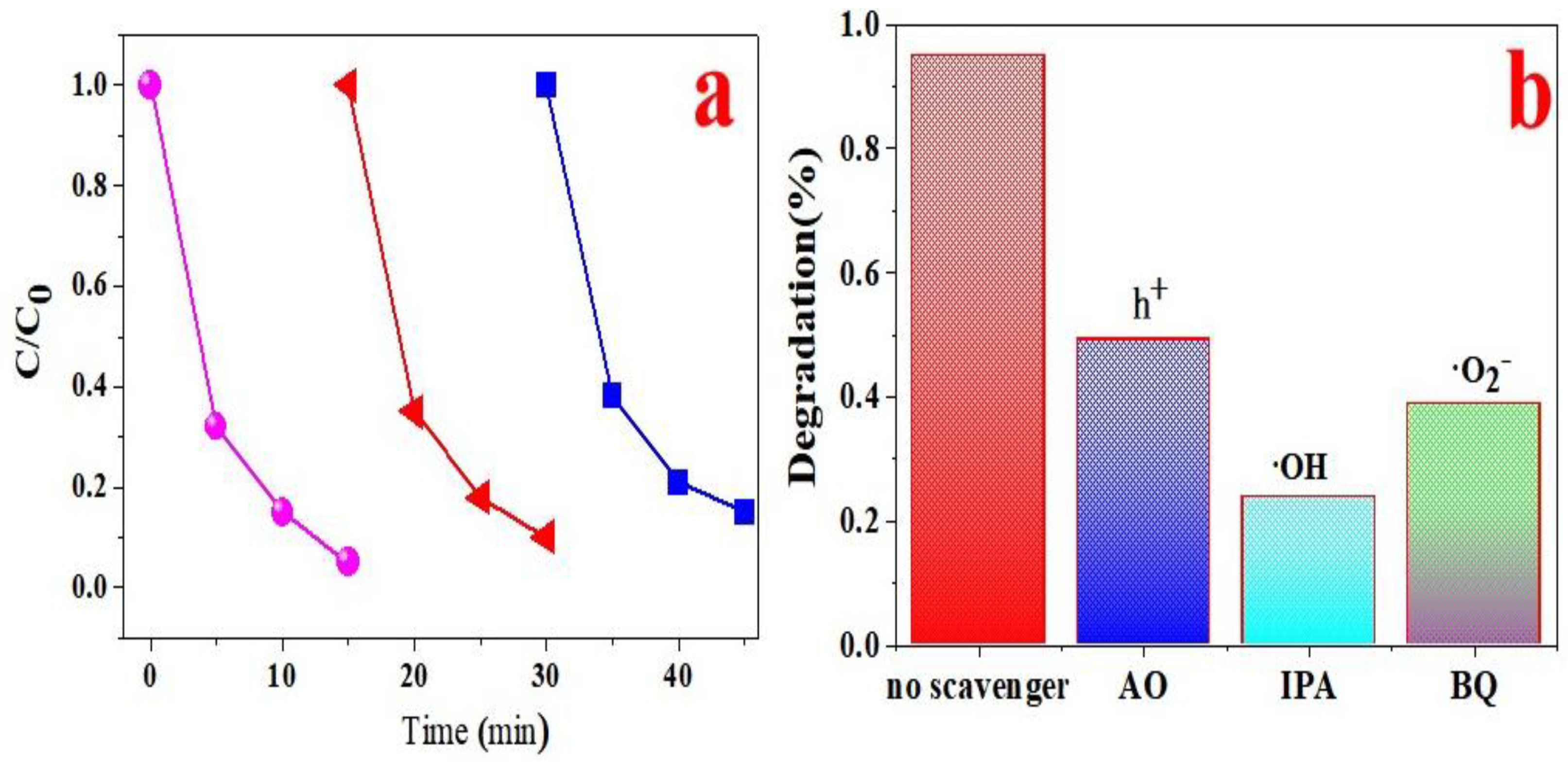
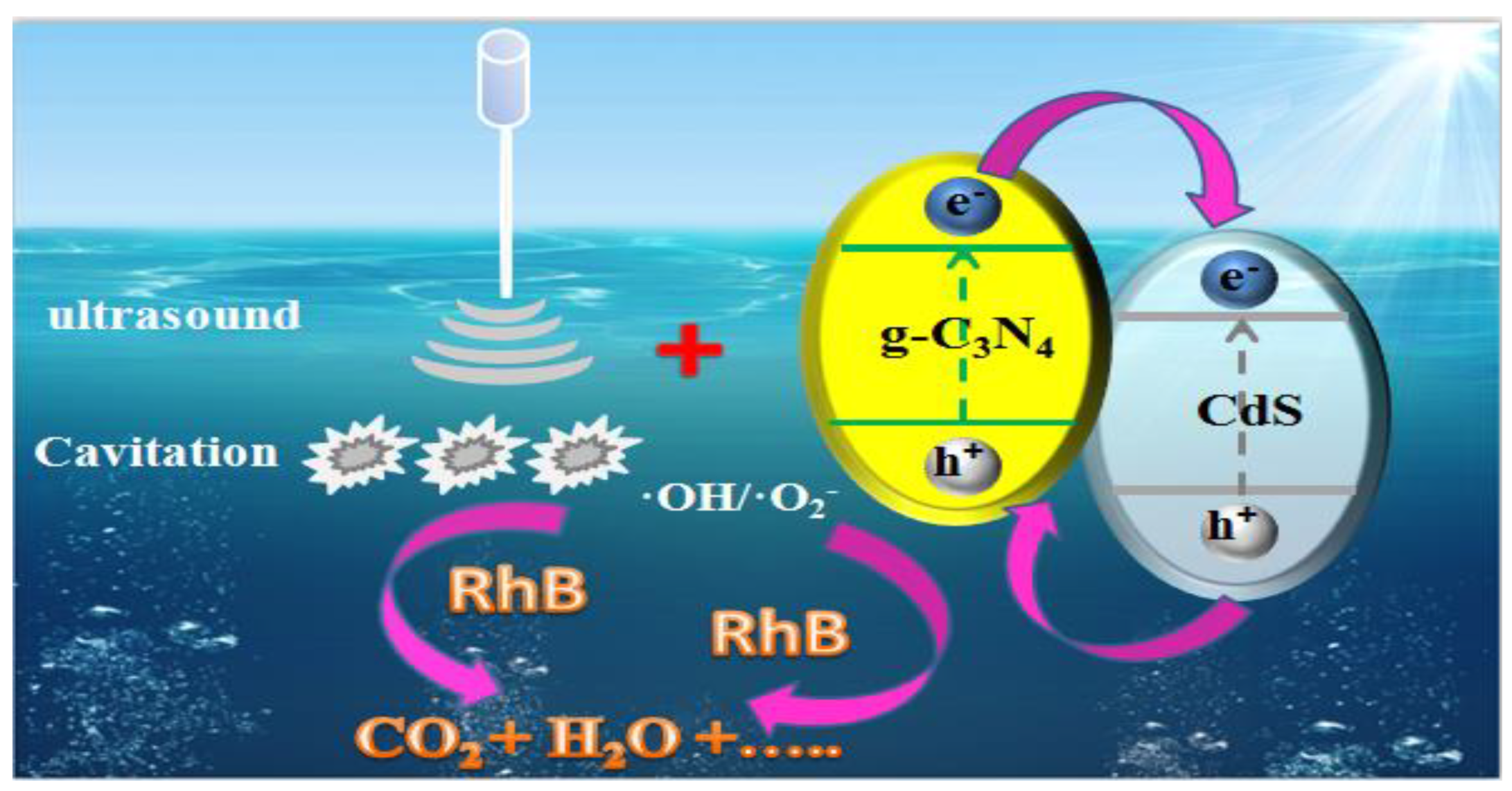
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.W.; Lu, Y.F.; Hai, G.T.; Dong, W.J.; Li, R.J.; Liu, J.H.; Ge, W. Bidentate Carboxylate linked TiO2 with NH2-MIL-101(Fe) photocatalyst: A conjugation effect platform for high photocatalytic activity under visible light irradiation. Sci. Bull. 2020, 65, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Amato, L.S.; Maddalena, P. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef]
- Luna, A.L.; Matter, F.; Schreck, M.; Wohlwend, J.; Tervoort, E.; ColbeauJustin, C.; Niederberger, M. Monolithic metal-containing TiO2 aerogels assembled from crystalline pre-formed nanoparticles as efficient photocatalysts for H2 generation. Appl. Catal. B Environ. 2020, 267, 118660. [Google Scholar] [CrossRef]
- Zhao, W.J.; Zhang, J.; Pan, J.Q.; Qiu, J.F.; Niu, J.T.; Li, C.R. One-step electrospinning route of SrTiO3-modified Rutile TiO2 nanofibers and its photocatalytic properties. Nanoscale Res. Lett. 2017, 12, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.X.; Jin, C.Q.; Jian, Z.Y.; Wei, Y.Y.; Nan, R.H.; Zhang, C.; Hu, L. Significantly enhanced photocatalytic performance of mesoporous C@ZnO hollow nanospheres via suppressing charge recombination. Chem. Phys. Lett. 2019, 716, 102–105. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Pirhashemi, M.; Ghosh, S. ZnO/ZnBi2O4 nanocomposites with p-n heterojunction as durable visible-light-activated photocatalysts for efficient removal of organic pollutants. J. Alloys Compd. 2020, 826, 154229. [Google Scholar] [CrossRef]
- Serrà, A.; Pip, P.; Gómez, E.; Philippe, L. Efficient magnetic hybrid ZnO-based photocatalysts for visible-light-driven removal of toxic cyanobacteria blooms and cyanotoxins. Appl. Catal. B Environ. 2020, 268, 118745. [Google Scholar] [CrossRef]
- Yuan, F.; Sun, Z.M.; Li, C.Q.; Tan, Y.; Zhang, X.W.; Zheng, S.L. Multi-component design and in-situ synthesis of visible-light-driven SnO2/g-C3N4/diatomite composite for high-efficient photoreduction of Cr(VI) with the aid of citric acid. J. Hazard. Mater. 2020, 396, 122694. [Google Scholar] [CrossRef]
- Sun, Y.K.; Zhu, Q.; Bai, B.; Li, Y.L.; He, C. Novel all-solid-state Z-scheme SnO2/Pt/In2O3 photocatalyst with boosted photocatalytic performance on water splitting and 2,4-dichlorophenol degradation under visible light. Chem. Eng. J. 2020, 390, 124518. [Google Scholar] [CrossRef]
- Huang, J.; Jing, H.X.; Li, N.; Li, L.X.; Jiao, W.Z. Fabrication of magnetically recyclable SnO2-TiO2/CoFe2O4 hollow core-shell photocatalyst: Improving photocatalytic efficiency under visible light irradiation. J. Solid State Chem. 2019, 271, 103–109. [Google Scholar] [CrossRef]
- Hong, X.D.; Wang, R.; Li, S.G.; Fu, J.W.; Chen, L.H.; Wang, X.L. Hydrophilic macroporous SnO2/rGO composite prepared by melamine template for high efficient photocatalyst. J. Alloys Compd. 2020, 816, 152550. [Google Scholar] [CrossRef]
- Kang, J.; Jin, C.Y.; Li, Z.L.; Wang, M.; Chen, Z.Q.; Wang, Y.Z. Dual Z-scheme MoS2/g-C3N4/Bi24O31Cl10 ternary heterojunction photocatalysts for enhanced visible-light photodegradation of antibiotic. J. Alloys Compd. 2020, 825, 153975. [Google Scholar] [CrossRef]
- Zhang, H.L.; Zhu, C.X.; Zhang, G.H.; Li, M.; Tang, Q.; Cao, J.L. Palladium modified ZnFe2O4/g-C3N4 nanocomposite as an efficiently magnetic recycling photocatalyst. J. Solid State Chem. 2020, 288, 121389. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.L.; Xiao, G.; Zhang, S. Steering charge kinetics in metal-free g-C3N4/melem hybrid photocatalysts for highly efficient visible-light-driven hydrogen evolution. Appl. Surf. Sci. 2020, 510, 145345. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.X.; Li, Y.X.; Peng, S.Q.; Liu, B. The nonmetal modulation of composition and morphology of g-C3N4-based photocatalysts. Appl. Catal. B Environ. 2020, 269, 118828. [Google Scholar] [CrossRef]
- Ma, L.T.; Fan, H.Q.; Fu, K.; Lei, S.H.; Hu, Q.Z.; Huang, H.T.; He, G.P. Protonation of graphitic carbon nitride (g-C3N4) for an electrostatically self-assembling carbon@g-C3N4 core-shell nanostructure toward high hydrogen evolution. ACS Sustain. Chem. Eng. 2017, 5, 7093–7103. [Google Scholar]
- Fu, J.; Chang, B.B.; Tian, Y.L.; Xi, F.N.; Dong, X.P. Novel C3N4-CdS composite photocatalysts with organic–inorganic heterojunctions: In situ synthesis, exceptional activity, high stability and photocatalytic mechanism. J. Mater. Chem. A 2013, 1, 3083–3090. [Google Scholar]
- Zhang, J.F.; Fu, J.W.; Wang, Z.L.; Cheng, B.; Dai, K.; Ho, W.K. Direct Z-scheme porous g-C3N4/BiOI heterojunction for enhanced visible-light photocatalytic activity. J. Alloys Compd. 2018, 766, 841–850. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, C.M. Rational design of a novel LaFeO3/g-C3N4/BiFeO3 double Z-scheme structure: Photocatalytic performance for antibiotic degradation and mechanistic insight. Chem. Eng. J. 2021, 423, 130076. [Google Scholar] [CrossRef]
- Raeisi-Kheirabadi, N.; Nezamzadeh-Ejhieh, A. A Z-scheme g-C3N4/Ag3PO4 nanocomposite: Its photocatalytic activity and capability for water splitting. Int. J. Hydrogen Energy 2020, 45, 33381–33395. [Google Scholar] [CrossRef]
- Shang, L.; Bian, T.; Yu, H.J.; Waterhouse, G.I.N.; Zhou, C.; Zhao, Y.F.; Tahir, M.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. CdS nanoparticle decorated Cd nanosheets for efficient visible light-driven photocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 201501241. [Google Scholar] [CrossRef]
- Gao, F.; Huang, X.Y.; Zhang, L.L.; Zhao, Y.; Feng, W.H.; Liu, P. Crafty design of chemical bonding to construct MoO2/CdS nanorod photocatalysts for boosting hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 24228–24236. [Google Scholar] [CrossRef]
- Li, N.; Fan, H.K.; Dai, Y.J.; Kong, J.; Ge, L. Insight into the solar utilization of a novel Z-scheme Cs0.33WO3/CdS heterostructure for UV–Vis-NIR driven photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 508, 145200. [Google Scholar] [CrossRef]
- Xu, J.J.; Li, X.P.; Niu, J.F.; Chen, M.D.; Yue, J.P. Synthesis of direct Z-Scheme Bi3TaO7/CdS composite photocatalysts with enhanced photocatalytic performance for ciprofloxacin degradation under visible light irradiation. J. Alloys Compd. 2020, 834, 155061. [Google Scholar] [CrossRef]
- Lei, D.S.; Xue, J.Q.; Bi, Q.; Tang, C.B.; Zhang, L. 3D/2D direct Z-scheme photocatalyst Zn2SnO4/CdS for simultaneous removal of Cr(VI) and organic pollutant. Appl. Surf. Sci. 2020, 517, 146030. [Google Scholar] [CrossRef]
- Su, L.; Luo, L.L.; Song, H.; Wu, Z.W.; Tu, W.X.; Wang, Z.J.; Ye, J.H. Hemispher-ical shell-thin lamellar WS2 porous structures composited with CdS photocatalysts for enhanced H2 evolution. Chem. Eng. J. 2020, 388, 124346. [Google Scholar] [CrossRef]
- Ma, Y.W.; Hai, G.T.; Atinafu, D.G.; Dong, W.J.; Li, R.J.; Hou, C.M.; Wang, G. Carbon inserted defect-rich MoS2 nanosheets@CdS nanospheres for efficient photocatalytic hydrogen evolution under visible light irradiation. J. Colloid Interf. Sci. 2020, 569, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Zhang, X.; Liu, Y.J.; Zhao, Y.B.; Xie, C.; Song, Y.X.; Yang, P. Crystallinity and phase controlling of g-C3N4/CdS hetrostructures towards high efficient photocatalytic H2 generation. Int. J. Hydrogen Energy 2019, 44, 30151–30159. [Google Scholar] [CrossRef]
- Gao, H.H.; Zhang, S.W.; Xu, J.Z.; Dou, Y.; Zhou, J.T.; Zhou, R. Activating and optimizing activity of CdS@g-C3N4 heterojunction for photocatalytic hydrogen evolution through the synergistic effect of phosphorus doping and defects. J. Alloys Compd. 2020, 834, 155201. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.M.; Chen, B.L. In situ photochemical fabrication of CdS/g-C3N4 nanocomposites with high performance for hydrogen evolution under visible light. Appl. Catal. B Environ. 2019, 256, 117848. [Google Scholar] [CrossRef]
- Song, X.F.; Wang, W.; Yang, J.Y.; Li, T.T.; Liu, G.; Han, Y.; Li, Y.S.; Liu, Y. Novel in-situ radiation construction of thioglycollic acid capped CdS quantum dots functionalized g-C3N4 nanohybrids with superior photocatalytic activity under visible light. Radiat. Phys. Chem. 2019, 165, 108449. [Google Scholar] [CrossRef]
- Zhou, X.J.; Shao, C.L.; Yang, S.; Li, X.W.; Guo, X.H.; Wang, X.X.; Li, X.H.; Liu, Y.C. heterojunction of g-C3N4/BiOI Immobilized on Flexible Electrospun Polyacrylonitrile Nanofibers: Facile Preparation and Enhanced Visible Photocatalytic Activity for Floating Photocatalysis. ACS Sustain. Chem. Eng. 2018, 6, 2316–2323. [Google Scholar] [CrossRef]
- Tao, R.; Yang, S.; Shao, C.L.; Li, X.H.; Li, X.W.; Liu, S.; Zhang, J.; Liu, Y.C. Reusable and Flexible g-C3N4/Ag3PO4/Polyacrylonitrile heterojunction Nanofibers for Photocatalytic Dye Degradation and Oxygen Evolution. ACS Appl. Nano. Mater. 2019, 2, 3081–3090. [Google Scholar] [CrossRef]
- Zhan, Y.F.; Lan, J.W.; Shang, J.J.; Yang, L.; Guan, X.M.; Li, W.X.; Chen, S.Q.; Qi, Y.; Lin, S.J. Durable ZIF-8/Ag/AgCl/TiO2 decorated PAN nanofibers with high visible light photocatalytic and antibacterial activities for degradation of dyes. J. Alloys Compd. 2020, 822, 153579. [Google Scholar] [CrossRef]
- Yu, D.D.; Bai, J.; Liang, H.O.; Ma, T.F.; Li, C.P. AgI-modified TiO2 supported by PAN nanofibers:A heterostructured composite with enhanced visible-light catalytic activity in degrading MO. Dyes Pigment. 2016, 133, 51–59. [Google Scholar] [CrossRef]
- Talukdar, K.; Saravanakumar, K.; Kim, Y.; Fayyaz, A.; Kim, G.; Yoon, Y.; Park, C.M. Rational construction of CeO2-ZrO2@MoS2 hybrid nanoflowers for enhanced sonophotocatalytic degradation of naproxen: Mechanisms and degradation pathways. Compos. Part B Eng. 2021, 215, 108780. [Google Scholar] [CrossRef]
- Liang, L.Y.; Tursun, Y.; Nulahong, A.; Dilinuer, T.; Tunishaguli, A.; Gao, G. Preparation and sonophotocatalytic performance of hierarchical Bi2WO6 structures and effects of various factors on the rate of Rhodamine B degradation. Ultrason. Sonochem. 2017, 39, 93–100. [Google Scholar] [CrossRef]
- Shi, X.L.; Liu, J.B.; Hosseini, M.; Shemshadi, R.; Razavi, R.; Parsaee, Z. Ultrasound-aasisted photodegradation of Alprazolam in aqueous media using a novel high performance nanocomosite hybridation g-C3N4/MWCNT/ZnO. Catal. Today 2019, 335, 582–590. [Google Scholar] [CrossRef]
- Yan, Z.X.; Zhang, L.L.; Zhao, Z.; Qi, H.; Li, Y.; Cang, D.Q. Enhanced antimicrobial activity of ZnO nanofluids in sonophotocatalysis and its mechanism. Ultrason. Sonochem. 2018, 47, 133–140. [Google Scholar] [CrossRef]
- You, M.Z.; Pan, J.Q.; Chi, C.Y.; Wang, B.B.; Zhao, W.J.; Song, C.S.; Zheng, Y.Y.; Li, C.R. The visible light hydrogen production of the Z-Scheme Ag3PO4/Ag/g-C3N4 nanosheets composites. J. Mater. Sci. 2018, 53, 1978–1986. [Google Scholar] [CrossRef]
- Li, Y.H.; Lv, K.L.; Ho, W.K.; Dong, F.; Wu, X.F.; Xia, Y. Hybridization of rutile TiO2 (rTiO2) with g-C3N4 quantum dots (CN QDs): An efficient visible-light-driven Z-scheme hybridized photocatalyst. Appl. Catal. B Environ. 2017, 202, 611–619. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.Y.; Tang, G.G.; Tang, H.; Jiang, H.B. The novel g-C3N4/MoS2/ZnS ternary nanocomposite with enhanced lithium storage properties. Appl. Surf. Sci. 2019, 492, 37–44. [Google Scholar] [CrossRef]
- Khalil, A.; Aboamera, N.M.; Nasser, W.S.; Mahmoud, W.H.; Mohamed, G.G. Photodegradation of organic dyes by PAN/SiO2-TiO2-NH2 nanofiber membrane under visible light. Sep. Purif. Technol. 2019, 224, 509–514. [Google Scholar] [CrossRef]
- Fang, J.J.; Chen, Y.K.; Wang, W.; Fang, L.; Lu, C.H.; Zhu, C.; Kou, J.H.; Ni, Y.R.; Xu, Z.Z. Highly efficient photocatalytic hydrogen generation of g-C3N4-CdS sheets based on plasmon-enhanced triplet–triplet annihilation upconversion. Appl. Catal. B Environ. 2019, 258, 117762. [Google Scholar] [CrossRef]
- Li, W.B.; Feng, C.; Dai, S.Y.; Yue, J.G.; Hua, F.X.; Hou, H. Fabrication of sulfur-doped g-C3N4/Au/CdS Z-scheme photocatalyst to improve the photocatalytic performance under visible light. Appl. Catal. B Environ. 2015, 168–169, 465–471. [Google Scholar] [CrossRef]
- Mkhalid, I.A.; Mohamed, R.M.; Ismail, A.A.; Alhaddad, M. Z-scheme g-C3N4 nanosheetphotocatalyst decorated with mesoporous CdS for the photoreduction of carbon dioxide. Ceram. Int. 2021, 47, 17210–17219. [Google Scholar] [CrossRef]
- Liu, X.M.; Liu, Y.; Zhang, W.K.; Zhong, Q.Y.; Ma, X.Y. In situ self-assembly of 3D hierarchical 2D/2D CdS/g-C3N4 hereojunction with excellent photocatalytic performance. Mat. Sci. Semicon. Proc. 2020, 105, 104734. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhao, W.; Pan, J.; Tang, R. The Sono-Photocatalytic Performance of PAN/g-C3N4/CdS Nanofibers Heterojunction. Materials 2021, 14, 5959. https://doi.org/10.3390/ma14205959
Zhang J, Zhao W, Pan J, Tang R. The Sono-Photocatalytic Performance of PAN/g-C3N4/CdS Nanofibers Heterojunction. Materials. 2021; 14(20):5959. https://doi.org/10.3390/ma14205959
Chicago/Turabian StyleZhang, Jing, Weijie Zhao, Jiaqi Pan, and Ruimin Tang. 2021. "The Sono-Photocatalytic Performance of PAN/g-C3N4/CdS Nanofibers Heterojunction" Materials 14, no. 20: 5959. https://doi.org/10.3390/ma14205959
APA StyleZhang, J., Zhao, W., Pan, J., & Tang, R. (2021). The Sono-Photocatalytic Performance of PAN/g-C3N4/CdS Nanofibers Heterojunction. Materials, 14(20), 5959. https://doi.org/10.3390/ma14205959



