Low-Temperature Selective Catalytic Reduction DeNOX and Regeneration of Mn–Cu Catalyst Supported by Activated Coke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Activity Test
3. Results and Analysis
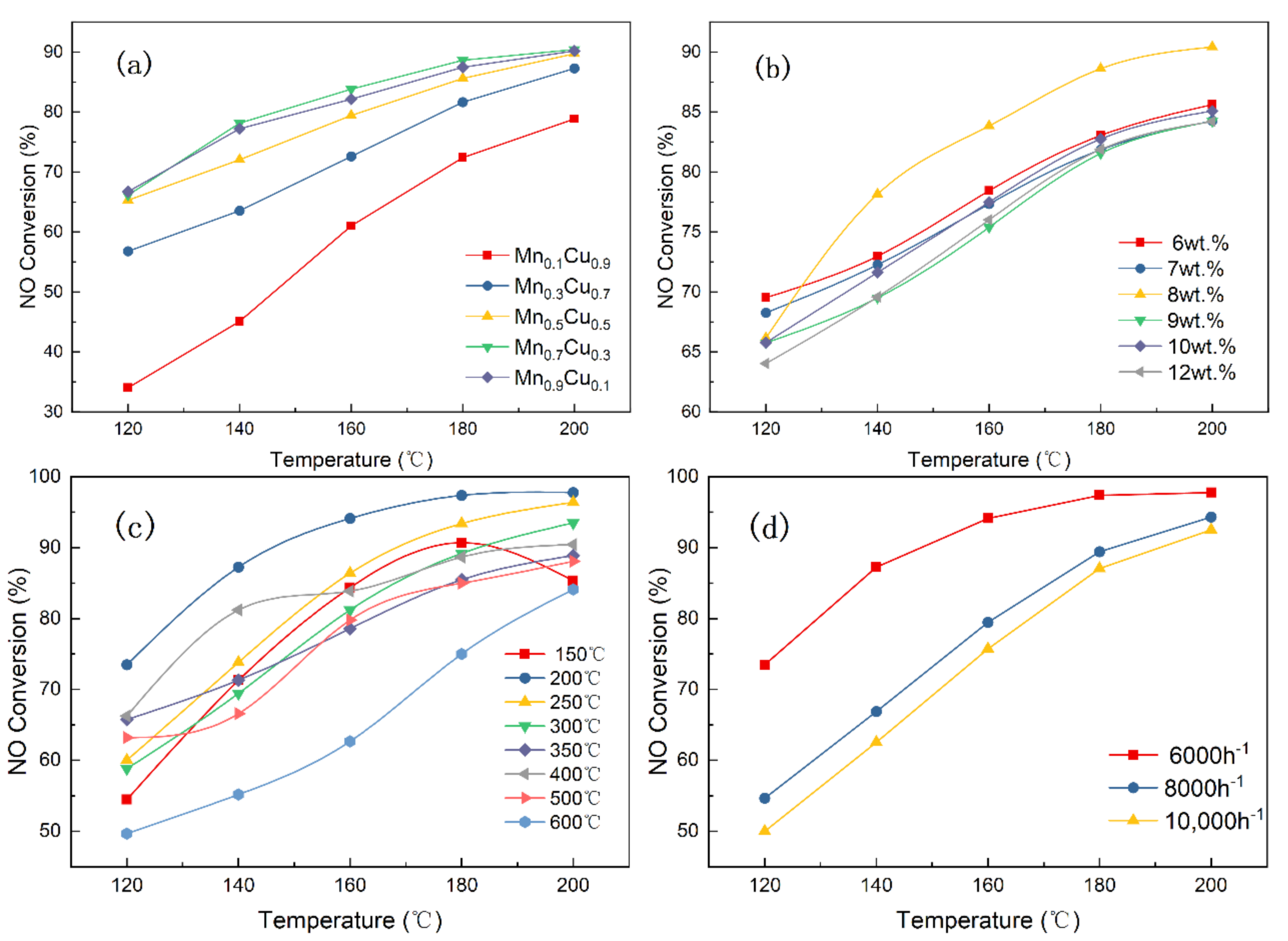
3.1. The Effects of Various Factors on Catalyst Activity of the Mn–Cu/AC Catalyst
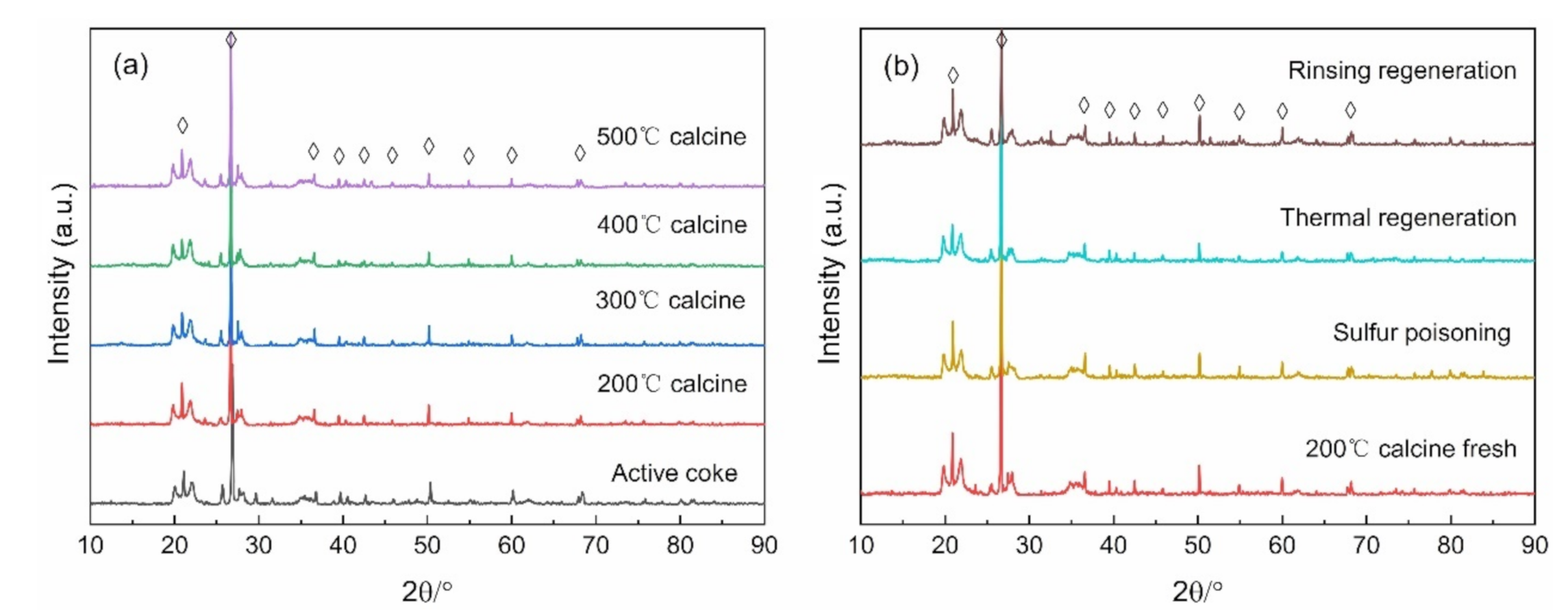
3.2. The Effects of the Regeneration Strategies and the Number of Times on the Catalytic Performance of Mn–Cu/AC Catalyst
3.3. The Physicochemical Properties of the Mn–Cu/AC Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Liu, J.; Liu, F.; Wang, Z.; Ding, J.; Huang, H. Reaction mechanism for NH3-SCR of NOx over CuMn2O4 catalyst. Chem. Eng. J. 2018, 361, 578–587. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z.; Liu, F. A skeletal reaction scheme for selective catalytic reduction of NOx with NH3 over CeO2/TiO2 catalyst. Fuel Process. Technol. 2018, 174, 17–25. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, N.; Lei, Z.; Chen, B. Selective Transformation of Various Nitrogen-Containing Exhaust Gases toward N2 over Zeolite Catalysts. Chem. Rev. 2016, 116, 3658–3721. [Google Scholar] [CrossRef]
- Liu, K.; Yu, Q.; Wang, B.; Qin, Q.; Wei, M.; Fu, Q. Low temperature selective catalytic reduction of nitric oxide with urea over activated carbon supported metal oxide catalysts. Environ. Technol. 2020, 41, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liebau, M.; Liu, B.; Li, L.; Zhang, S.; Gläser, R. Selective catalytic reduction of NOx with NH3 over Mn–Zr–Ti mixed oxide catalysts. J. Mater. Sci. 2019, 54, 6943–6960. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Liu, L.; Mi, L.; Wang, X. In situ DRIFTS studies on MnOx nanowires supported by activated semi-coke for low temperature selective catalytic reduction of NOx with NH3—ScienceDirect. Appl. Surf. Sci. 2016, 366, 139–147. [Google Scholar] [CrossRef]
- Raja, S.; Alphin, M.S. Systematic effects of Fe doping on the activity of V2O5/TiO2-carbon nanotube catalyst for NH3-SCR of NOx. J. Nanopart. Res. 2020, 22, 190. [Google Scholar] [CrossRef]
- Saramok, M.; Szymaszek, A.; Inger, M.; Antoniak-Jurak, K.; Samojeden, B.; Motak, M. Modified Zeolite Catalyst for a NOx Selective Catalytic Reduction Process in Nitric Acid Plants. Catalysts 2021, 11, 450. [Google Scholar] [CrossRef]
- Ahmad, N.; Ibrahim, N.; Ali, U.F.M.; Yusuf, S.Y.; Ridwan, F.M. Carbon-supported CuO Catalyst Prepared from Oil Palm Empty Fruit Bunch (EFB) for Low-temperature No Removal. Procedia Eng. 2016, 148, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, Y.; Zhu, T.; Su, H.; Zhu, J. Selective catalytic reduction of NOx by NH3 over Mn-Promoted V2O5/TiO2 Catalyst. Ind. Eng. Chem. Res. 2014, 53, 12964–12970. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.; Hu, H.; Han, J.; Shi, L.; Zhang, D. Highly dispersed V2O5/TiO2 modified with transition metals (Cu, Fe, Mn, Co) as efficient catalysts for the selective reduction of NO with NH3. Chin. J. Catal. 2015, 36, 1886–1899. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T.; Fuertes, A.B. Mechanism of low-temperature selective catalytic reduction of NO with NH3 over carbon-supported Mn3O4: Role of surface NH3 species: SCR mechanism. J. Catal. 2004, 226, 138–155. [Google Scholar] [CrossRef]
- You, F.-T.; Yu, G.-W.; Wang, Y.; Xing, Z.-J.; Liu, X.-J.; Li, J. Study of nitric oxide catalytic oxidation on manganese oxides-loaded activated carbon at low temperature. Appl. Surf. Sci. 2017, 413, 387–397. [Google Scholar] [CrossRef]
- Liu, T.; Qian, J.; Yao, Y.; Shi, Z.; Han, L.; Liang, C.; Li, B.; Dong, L.; Fan, M.; Zhang, L. Research on SCR of NO with CO over the Cu0.1La0.1Ce0.8O mixed-oxide catalysts: Effect of the grinding. J. Mol. Catal. A Chem. 2016, 430, 43–53. [Google Scholar]
- Cortés-Reyes, M.; Finocchio, E.; Herrera, C.; Larrubia, M.A.; Alemany, L.J.; Busca, G. A study of Cu-SAPO-34 catalysts for SCR of NOx by ammonia. Microporous Mesoporous Mater. 2017, 241, 258–265. [Google Scholar] [CrossRef]
- Li, W.; Tan, S.; Shi, Y.; Li, S. Utilization of sargassum based activated carbon as a potential waste derived catalyst for low temperature selective catalytic reduction of nitric oxides. Fuel 2015, 160, 35–42. [Google Scholar] [CrossRef]
- Liang, S.; Teng, F.; Bulgan, G.; Zong, R.; Zhu, Y. Effect of Phase Structure of MnO2 Nanorod Catalyst on the Activity for CO Oxidation. J. Phys. Chem. C 2010, 112, 5307–5315. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Ma, Q.; Yang, Y.; Whiddon, R.; Zhu, Y.; Cen, K. Catalytic deep oxidation of NO by ozone over MnOx loaded spherical alumina catalyst. Appl. Catal. B Environ. 2016, 198, 100–111. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Tang, W. Co-nanocasting synthesis of mesoporous Cu-Mn composite oxides and their promoted catalytic activities for gaseous benzene removal. Appl. Catal. B-Environ. 2015, 162, 110–121. [Google Scholar]
- Chusuei, C.C.; Brookshier, M.A.; Goodman, D.W. Correlation of relative X-ray photoelectron spectroscopy shake-up intensity with CuO particle size. Langmuir 1999, 15, 2806–2808. [Google Scholar] [CrossRef]
- Andonova, S.; Vovk, E.; Sjoblom, J.; Ozensoy, E.; Olsson, L. Chemical deactivation by phosphorous under lean hydrothermal conditions over Cu/BEA NH3-SCR catalysts. Appl. Catal. B Environ. 2014, 147, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Chen, L.; Yuan, F.; Li, R.; Zhang, T.; Bakhtiar, S.u.H.; Leng, X.; Niu, X.; Zhu, Y. Synergistic effect between copper and cerium on the performance of Cux-Ce0.5-x-Zr0.5 (x = 0.1–0.5) oxide catalysts for selective catalytic reduction of NO with ammonia. Appl. Catal. B Environ. 2017, 210, 223–234. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Ettireddy, P.R.; Kotrba, A.; Smirniotis, P.G. Influence of SiO2 on M/TiO2 (M = Cu, Mn, and Ce) Formulations for Low-Temperature Selective Catalytic Reduction of NOx with NH3: Surface Properties and Key Components in Relation to the Activity of NOx Reduction. Ind. Eng. Chem. Res. 2015, 54, 2261–2273. [Google Scholar] [CrossRef]
- Márquez, F.; Palomares, A.E.; Rey, F.; Corma, A. Characterisation of the active copper species for the NOx removal on Cu/Mg/Al mixed oxides derived from hydrotalcites: An in situ XPS/XAES study. J. Mater. Chem 2001, 11, 1675–1680. [Google Scholar] [CrossRef]
- Martin, L.; Martinez, H.; Poinot, D.; Pecquenard, B.; Le Cras, F. Comprehensive X-ray photoelectron spectroscopy study of the conversion reaction mechanism of CuO in lithiated thin film electrodes. J. Phys. Chem. C 2013, 117, 4421–4430. [Google Scholar] [CrossRef]
- Fang, J.; Bi, X.; Si, D.; Jiang, Z.; Huang, W. Spectroscopic studies of interfacial structures of CeO2–TiO2 mixed oxides. Appl. Surf. Sci. 2007, 253, 8952–8961. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Li, S.; Wang, Y.; Zhang, J.; Wang, T.; Zeng, G. Simultaneous removal of elemental mercury and NO in simulated flue gas over V2O5/ZrO2-CeO2 catalyst. Appl. Catal. B: Environ. 2016, 198, 420–430. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef]
- Darlatt, E.; Traulsen, C.H.H.; Poppenberg, J.; Richter, S.; Kühn, J.; Schalley, C.A.; Unger, W.E.S. Evidence of click and coordination reactions on a self-assembled monolayer by synchrotron radiation based XPS and NEXAFS. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 85–89. [Google Scholar] [CrossRef]
- Heister, K.; Zharnikov, M.; Grunze, M.; Johansson, L.S.O.; Ulman, A. Characterization of X-ray Induced Damage in Alkanethiolate Monolayers by High-Resolution Photoelectron Spectroscopy. Langmuir 2006, 17, 8–11. [Google Scholar] [CrossRef]
- Struis, R.P.W.J.; Schildhauer, T.J.; Czekaj, I.; Janousch, M.; Biollaz, S.M.A.; Ludwig, C. Sulphur poisoning of Ni catalysts in the SNG production from biomass: A TPO/XPS/XAS study. Appl. Catal. A Gen. 2009, 362, 121–128. [Google Scholar] [CrossRef]


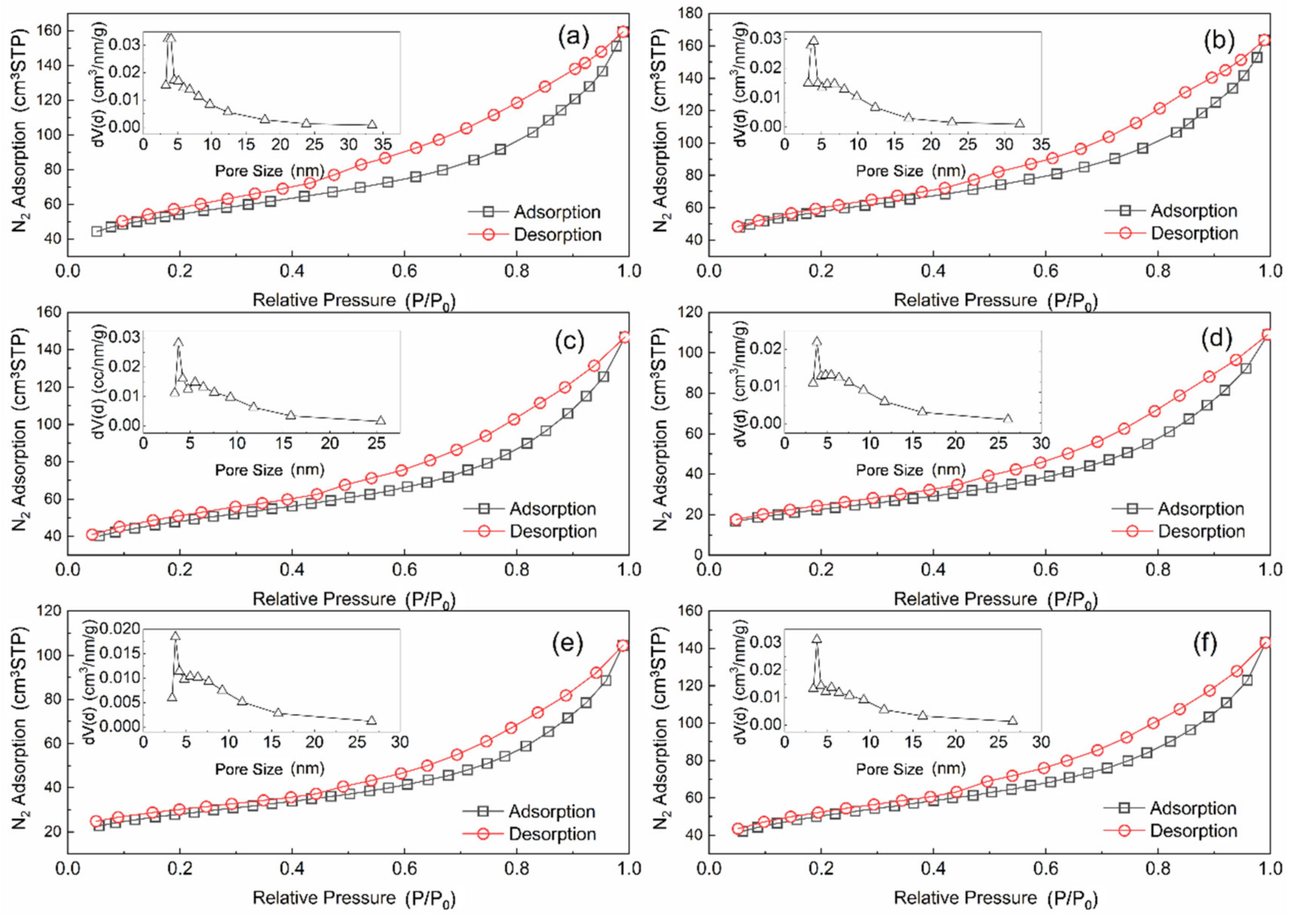
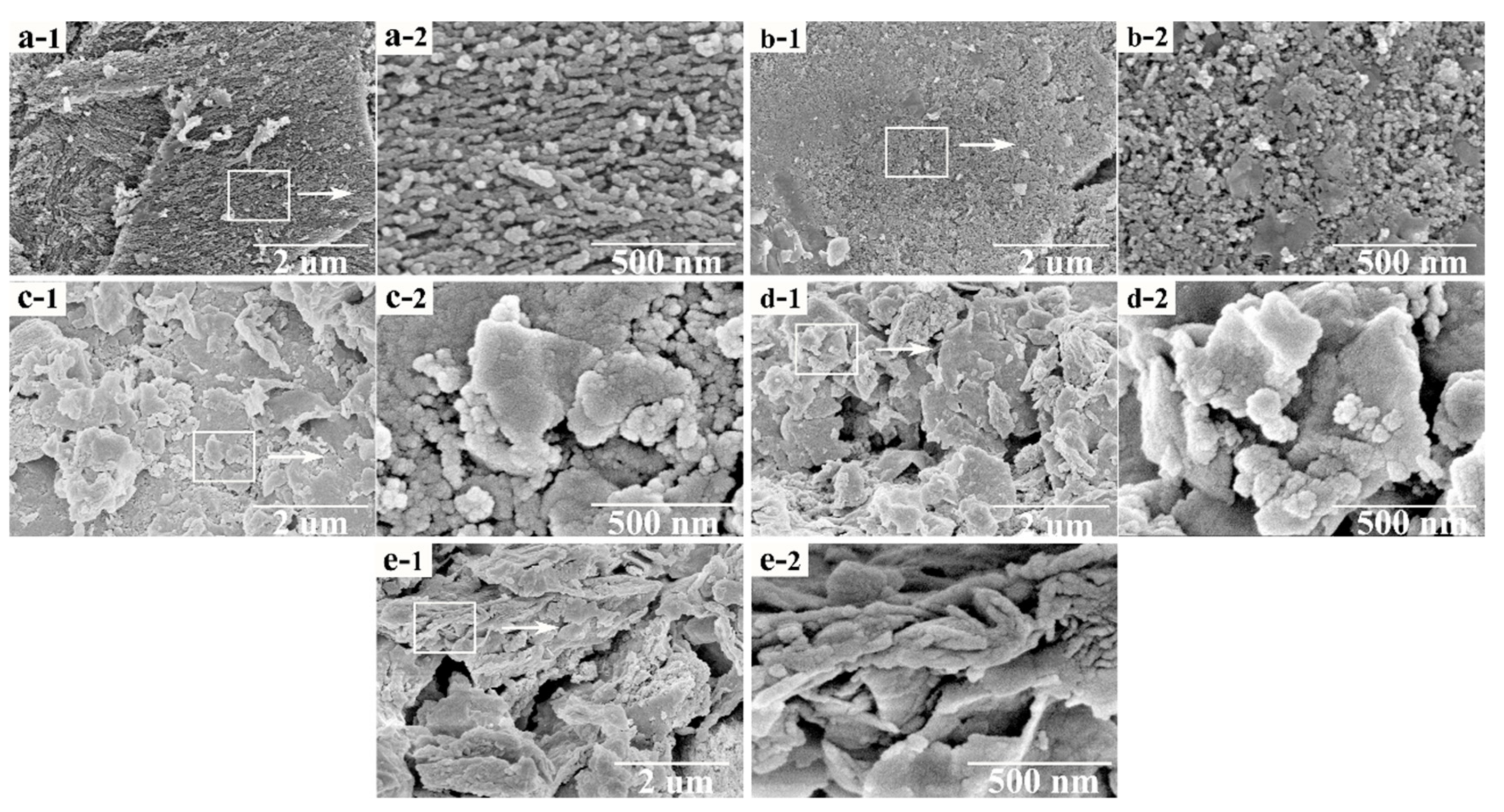
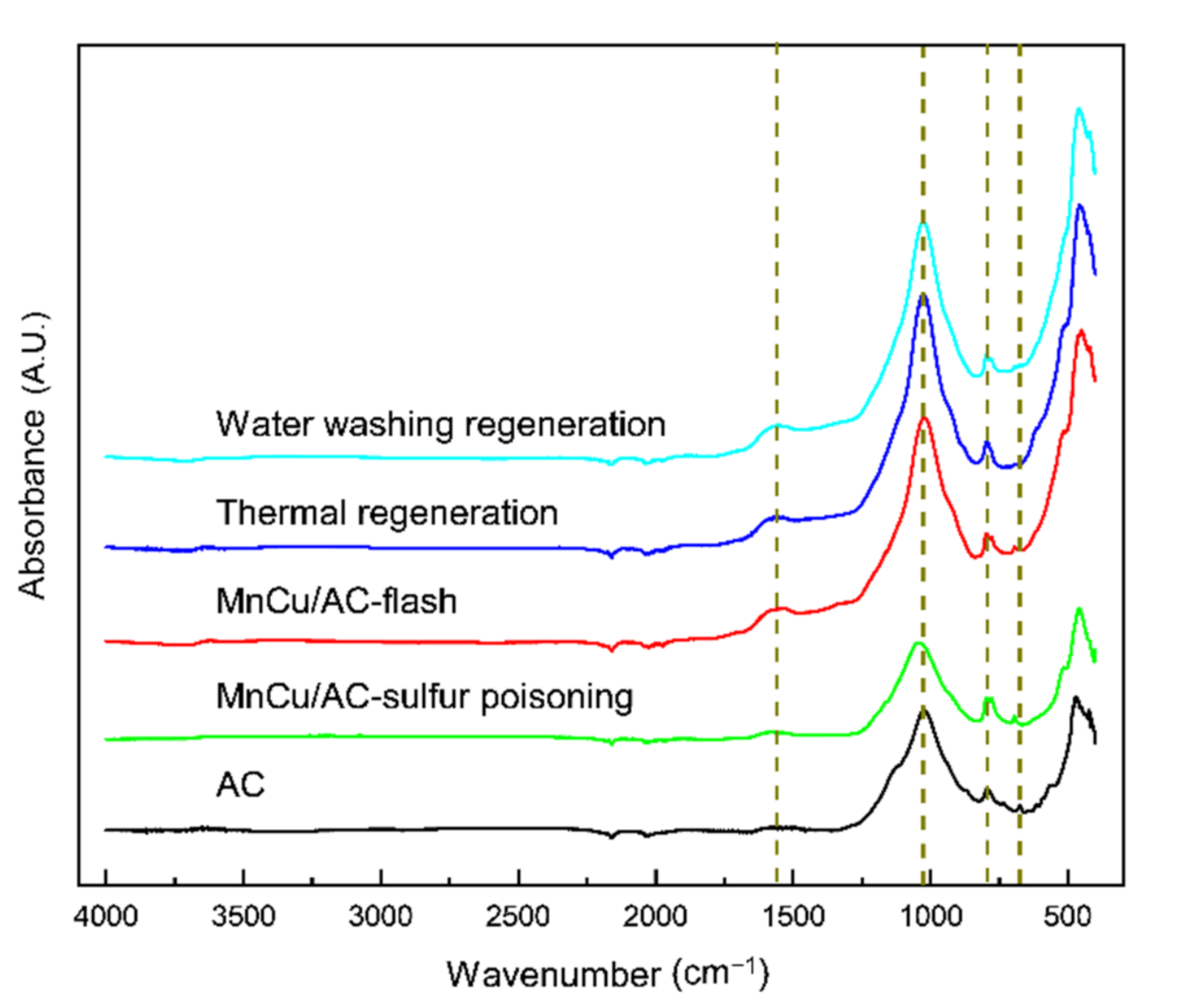
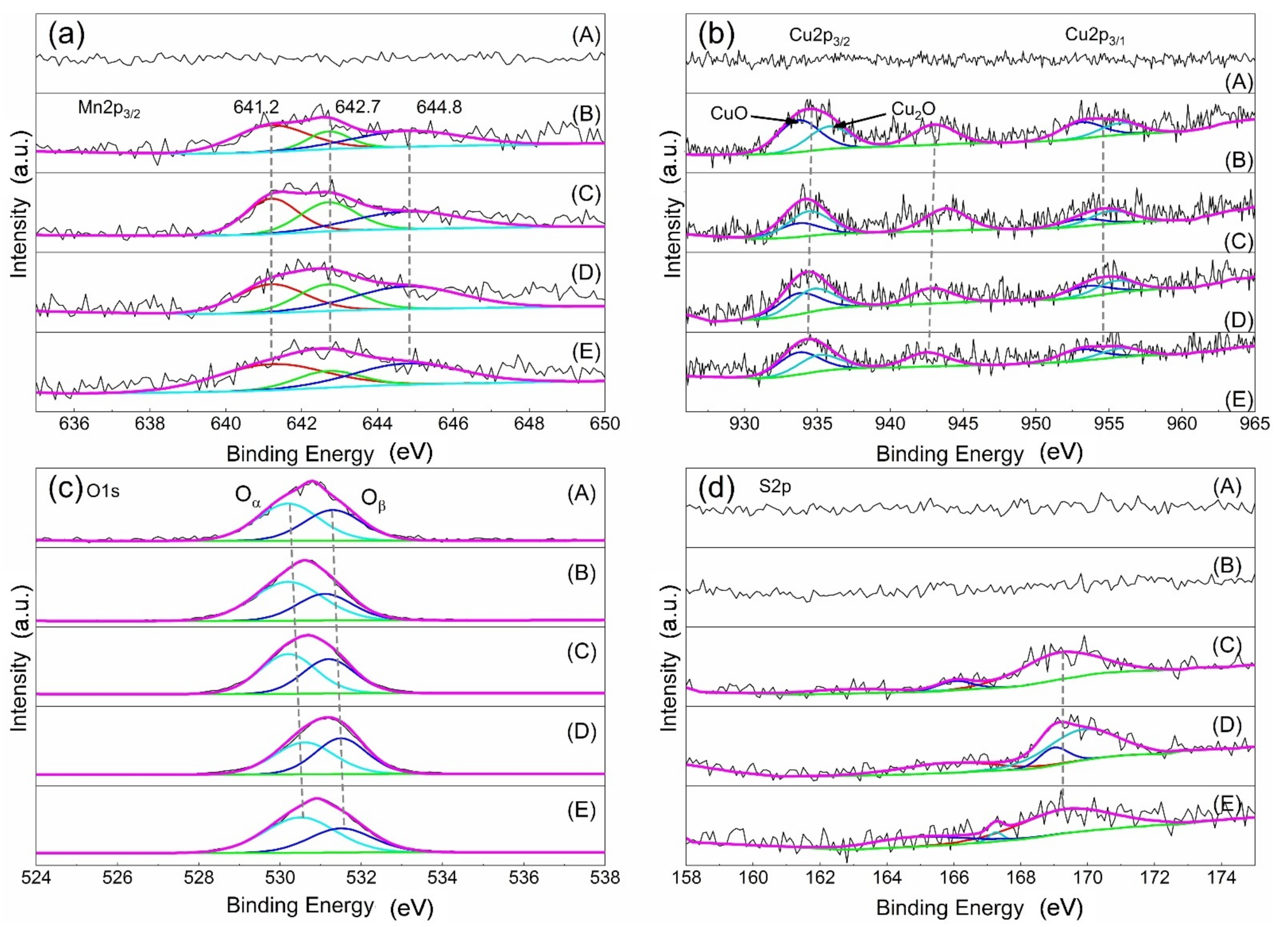
| Serial Number | Samples | Specific Area | Pore Volume | Pore Diameter |
|---|---|---|---|---|
| m2/g | cm3/g | nm | ||
| a | AC | 195.18 | 0.25 | 4.04 |
| b | Fresh catalyst | 207.36 | 0.26 | 3.99 |
| c | After deNOX | 172.04 | 0.23 | 3.77 |
| d | Sulfur poisoning | 81.45 | 0.17 | 3.78 |
| e | Thermal regeneration | 99.71 | 0.16 | 3.75 |
| f | Washing regeneration | 162.72 | 0.22 | 3.76 |
| Sample | Mn3+ | Mn4+ | Mn3+/Mn4+ | Cu+ | Cu2+ | Cu2+/Cu+ | Oα | Oβ | Oα/Oβ | SO32− | SO42− | SO32−/SO42− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | - | - | - | - | - | - | 530.2 | 531.3 | 1.3% | - | - | - |
| Fresh catalyst | 641.2 | 642.7 | 2.6% | 934.4 | 933.7 | 1.4% | 530.2 | 531.1 | 1.8% | - | - | - |
| Sulfur poisoning | 641.2 | 642.7 | 1.0% | 934.7 | 933.8 | 0.6% | 530.2 | 531.2 | 1.2% | 166.03 | 169.14 | 0.2% |
| Thermal regeneration | 641.3 | 642.7 | 1.2% | 935.1 | 933.7 | 0.9% | 530.6 | 531.5 | 1.0% | 165.84 | 169.84 | 0.4% |
| Rinsing regeneration | 641.2 | 642.7 | 2.6% | 935.8 | 933.7 | 1.4% | 530.5 | 531.5 | 1.6% | 167.25 | 169.35 | 0.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Ou, Z.; Wu, B. Low-Temperature Selective Catalytic Reduction DeNOX and Regeneration of Mn–Cu Catalyst Supported by Activated Coke. Materials 2021, 14, 5958. https://doi.org/10.3390/ma14205958
Ren X, Ou Z, Wu B. Low-Temperature Selective Catalytic Reduction DeNOX and Regeneration of Mn–Cu Catalyst Supported by Activated Coke. Materials. 2021; 14(20):5958. https://doi.org/10.3390/ma14205958
Chicago/Turabian StyleRen, Xusheng, Zhiliang Ou, and Bang Wu. 2021. "Low-Temperature Selective Catalytic Reduction DeNOX and Regeneration of Mn–Cu Catalyst Supported by Activated Coke" Materials 14, no. 20: 5958. https://doi.org/10.3390/ma14205958
APA StyleRen, X., Ou, Z., & Wu, B. (2021). Low-Temperature Selective Catalytic Reduction DeNOX and Regeneration of Mn–Cu Catalyst Supported by Activated Coke. Materials, 14(20), 5958. https://doi.org/10.3390/ma14205958






