Simple and Low-Cost Footstep Energy-Recover Barocaloric Heating and Cooling Device
Abstract
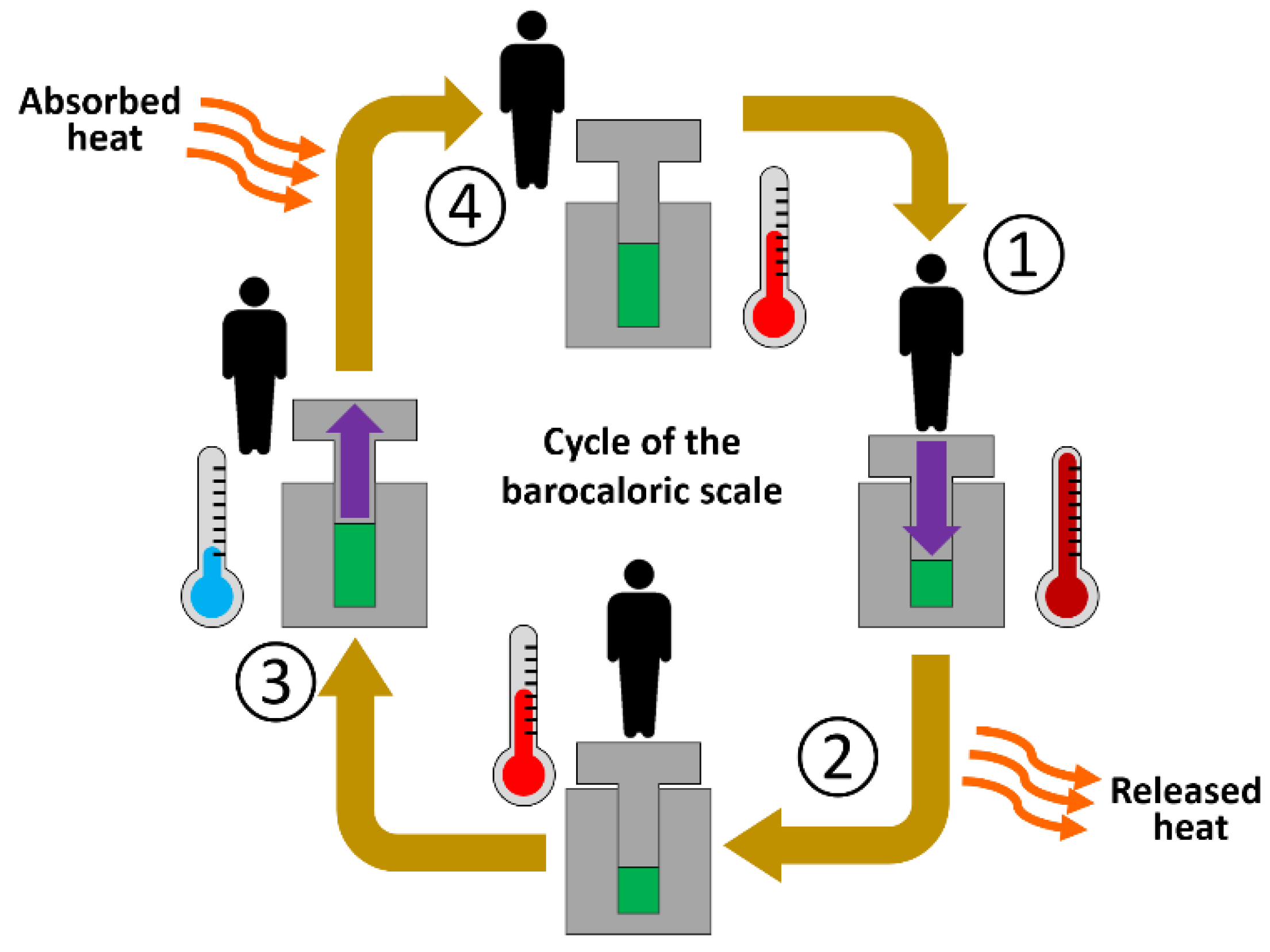
:1. Introduction
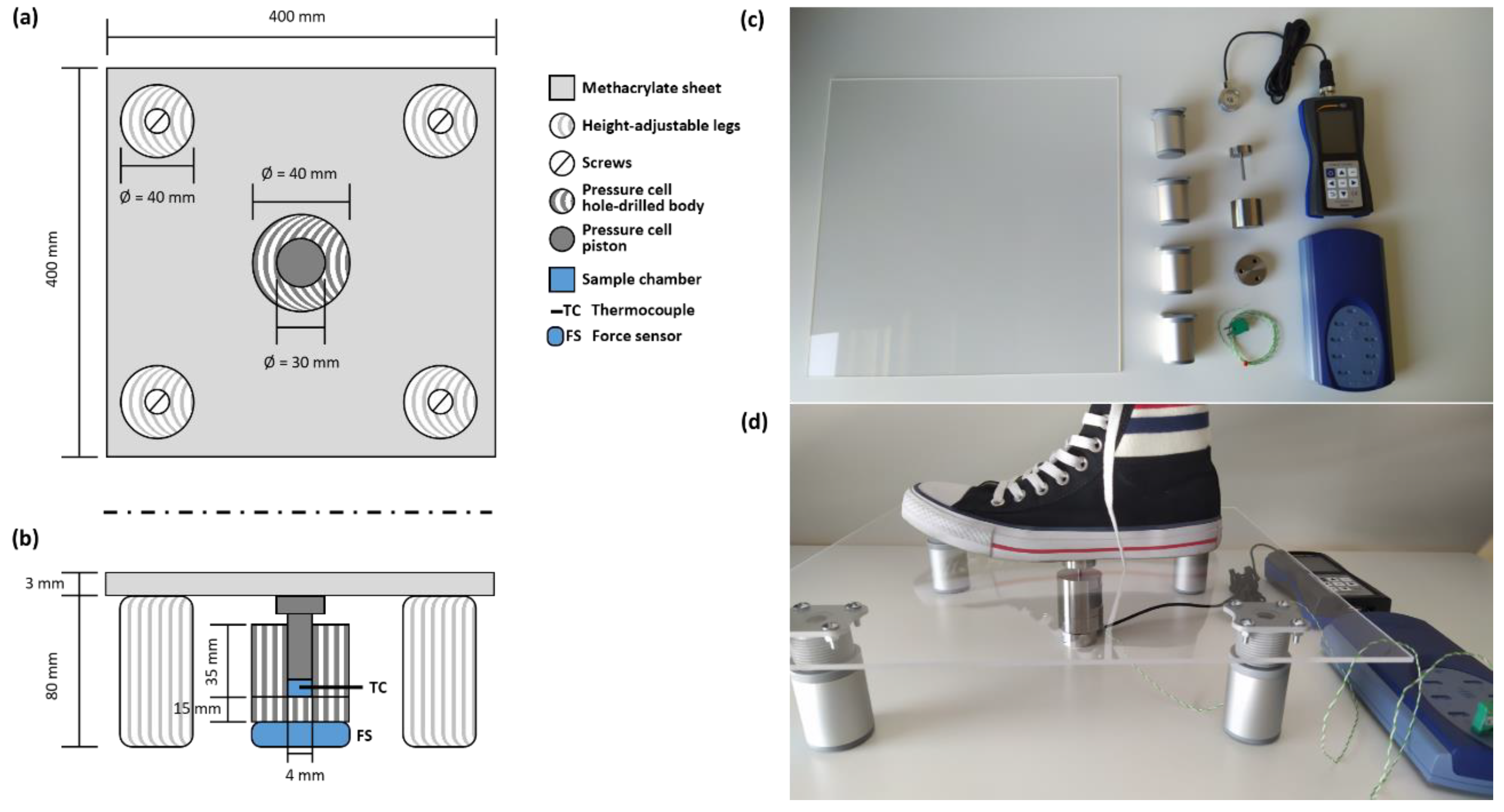
2. Design, Components, and Dimensions of the Barocaloric Scale
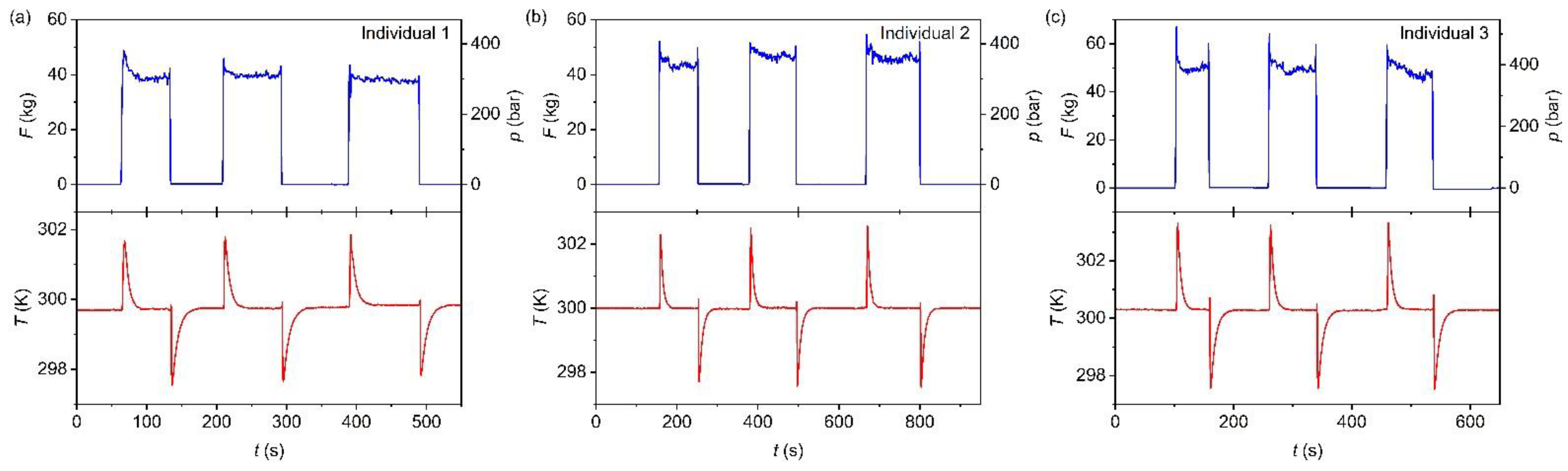
3. Measurement of the Temperature Changes Induced by Footstep in Trans-1,4-polyisoprene
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lloveras, P.; Tamarit, J.-L. Advances and obstacles in pressure-driven solid-state cooling: A review of barocaloric materials. MRS Energy Sustain. 2021, 8, 3–15. [Google Scholar] [CrossRef]
- Cazorla, C. Novel mechanocaloric materials for solid-state cooling applications. Appl. Phys. Rev. 2019, 6, 041316. [Google Scholar] [CrossRef] [Green Version]
- Boldrin, D. Fantastic barocalorics and where to find them. Appl. Phys. Lett. 2021, 118, 170502. [Google Scholar] [CrossRef]
- Moya, X.; Mathur, N.D. Caloric materials for cooling and heating. Science 2020, 370, 797–803. [Google Scholar] [CrossRef]
- Yuce, S.; Barrio, M.; Emre, B.; Stern-Taulats, E.; Planes, A.; Tamarit, J.L.; Mudryk, Y.; Gschneidner, K.A.; Pecharsky, V.K.; Mañosa, L. Barocaloric effect in the magnetocaloric prototype Gd5Si2Ge2. Appl. Phys. Lett. 2012, 101, 071906. [Google Scholar] [CrossRef]
- Mañosa, L.; González-Alonso, D.; Planes, A.; Bonnot, E.; Barrio, M.; Tamarit, J.-L.; Aksoy, S.; Acet, M. Giant solid-state barocaloric effect in the Ni-Mn-In magnetic shape-memory alloy. Nat. Mater. 2010, 9, 478–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern-Taulats, E.; Planes, A.; Lloveras, P.; Barrio, M.; Tamarit, J.L.; Pramanick, S.; Majumdar, S.; Frontera, C.; Mañosa, L. Barocaloric and magnetocaloric effects in Fe49Rh51. Phys. Rev. B-Condens. Matter Mater. Phys. 2014, 89, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matsunami, D.; Fujita, A.; Takenaka, K.; Kano, M. Giant barocaloric effect enhanced by the frustration of the antiferromagnetic phase in Mn3GaN. Nat. Mater. 2015, 14, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gorev, M.V.; Bogdanov, E.V.; Flerov, I.N.; Voronov, V.N.; Laptash, N.M. Barocaloric effect in oxyfluorides Rb2KTiOF5 and (NH4)2NbOF5. Ferroelectrics 2010, 397, 76–80. [Google Scholar] [CrossRef]
- Gorev, M.V.; Bogdanov, E.V.; Flerov, I.N.; Kocharova, A.G.; Laptash, N.M. Investigation of thermal expansion, phase diagrams, and barocaloric effect in the (NH4)2WO2F4 and (NH4)2MoO2F4 oxyfluorides. Phys. Solid State 2010, 52, 167–175. [Google Scholar] [CrossRef]
- Lloveras, P.; Stern-Taulats, E.; Barrio, M.; Tamarit, J.L.; Crossley, S.; Li, W.; Pomjakushin, V.; Planes, A.; Mañosa, L.; Mathur, N.D.; et al. Giant barocaloric effect at low pressure in ferrielectric ammonium sulphate. Nat Commun. 2015, 6, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Dunstan, D.; Lou, X.; Planes, A.; Mañosa, L.; Barrio, M.; Tamarit, J.-L.; Lloveras, P. Reversible barocaloric effects over a large temperature span in fullerite C60. J. Mater. Chem. A 2020, 8, 20354–20362. [Google Scholar] [CrossRef]
- Li, B.; Kawakita, Y.; Ohira-Kawamura, S.; Sugahara, T.; Wang, H.; Wang, J.; Chen, Y.; Kawaguchi, S.I.; Kawaguchi, S.; Ohara, K.; et al. Colossal barocaloric effects in plastic crystals. Nature 2019, 567, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Lloveras, P.; Aznar, A.; Barrio, M.; Negrier, P.; Popescu, C.; Planes, A.; Mañosa, L.; Stern-Taulats, E.; Avramenko, A.; Mathur, N.D.; et al. Colossal barocaloric effects near room temperature in plastic crystals of neopentylglycol. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Aznar, A.; Lloveras, P.; Barrio, M.; Negrier, P.; Planes, A.; Mañosa, L.; Mathur, N.; Moya, X.; Tamarit, J.L. Reversible and irreversible colossal barocaloric effects in plastic crystals. J. Mater. Chem. A 2020, 8, 639–647. [Google Scholar] [CrossRef]
- Sagotra, A.K.; Chu, D.; Cazorla, C. Room-temperature mechanocaloric effects in lithium-based superionic materials. Nat. Commun. 2018, 9, 3337. [Google Scholar] [CrossRef] [PubMed]
- Aznar, A.; Lloveras, P.; Romanini, M.; Barrio, M.; Tamarit, J.-L.; Cazorla, C.; Errandonea, D.; Mathur, N.D.; Planes, A.; Moya, X.; et al. Giant barocaloric effects over a wide temperature range in superionic conductor AgI. Nat. Commun. 2017, 8, 1851. [Google Scholar] [CrossRef]
- Vallone, S.P.; Tantillo, A.N.; Santos, A.M.; Molaison, J.J.; Kulmaczewski, R.; Chapoy, A.; Ahmadi, P.; Halcrow, M.A.; Sandeman, K.G. Giant Barocaloric Effect at the Spin Crossover Transition of a Molecular Crystal. Adv. Mater. 2019, 31, 1–7. [Google Scholar] [CrossRef]
- Von Ranke, P.J.; Alho, B.P.; Silva, P.H.S.; Ribas, R.M.; Nobrega, E.P.; De Sousa, V.S.R.; Colaço, M.V.; Marques, L.F.; Reis, M.S.; Scaldini, F.M.; et al. Large barocaloric effect in spin-crossover complex [CrI2(depe)2]. J. Appl. Phys. 2020, 127, 1–6. [Google Scholar] [CrossRef] [Green Version]
- von Ranke, P.J.; Alho, B.P.; Ribas, R.M.; Nobrega, E.P.; Caldas, A.; de Sousa, V.S.R.; Colaço, M.V.; Marques, L.F.; Rocco, D.L.; Ribeiro, P.O. Colossal refrigerant capacity in [Fe(hyptrz)3]A2·H2O around the freezing temperature of water. Phys. Rev. B 2018, 98, 1–5. [Google Scholar] [CrossRef]
- Bermúdez-García, J.M.; Sánchez-Andújar, M.; Castro-García, S.; López-Beceiro, J.; Artiaga, R.; Señarís-Rodríguez, M.A. Giant barocaloric effect in the ferroic organic-inorganic hybrid [TPrA][Mn(dca)3] perovskite under easily accessible pressures. Nat. Commun. 2017, 8, 15715. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-García, J.M.; Yáñez-Vilar, S.; García-Fernández, A.; Sánchez-Andújar, M.; Castro-García, S.; López-Beceiro, J.; Artiaga, R.; Dilshad, M.; Moya, X.; Señarís-Rodríguez, M.A. Giant barocaloric tunability in [(CH3CH2CH2)4N]Cd[N(CN)2]3 hybrid perovskite. J. Mater. Chem. C 2018, 6, 9867–9874. [Google Scholar] [CrossRef] [Green Version]
- Salgado-Beceiro, J.; Nonato, A.; Silva, R.X.; García-Fernández, A.; Sánchez-Andújar, M.; Castro-Garcia, S.; Stern-Taulats, E.; Señarís-Rodríguez, M.A.; Moya, X.; Bermúdez-García, J.M. Near-room-temperature reversible giant barocaloric effects in [(CH3)4N]Mn[N3]3 hybrid perovskite. Mater. Adv. 2020, 1, 3167–3170. [Google Scholar] [CrossRef]
- Szafrański, M.; Wei, W.J.; Wang, Z.M.; Li, W.; Katrusiak, A. Research Update: Tricritical point and large caloric effect in a hybrid organic-inorganic perovskite. APL Mater. 2018, 6, 100701. [Google Scholar] [CrossRef] [Green Version]
- Usuda, E.O.; Bom, N.M.; Carvalho, A.M.G. Large barocaloric effects at low pressures in natural rubber. Eur. Polym. J. 2017, 92, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.M.G.; Imamura, W.; Usuda, E.O.; Bom, N.M. Giant room-temperature barocaloric effects in PDMS rubber at low pressures. Eur. Polym. J. 2018, 99, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Usuda, E.O.; Imamura, W.; Bom, N.M.; Paixao, L.S.; Carvalho, A.M.G. Giant Reversible Barocaloric Effects in Nitrile Butadiene Rubber around Room Temperature. ACS Appl. Polym. Mater. 2019, 1, 1991–1997. [Google Scholar] [CrossRef] [Green Version]
- Imamura, W.; Usuda, É.O.; Paixao, L.S.; Bom, N.M.; Gomes, A.M.; Carvalho, A.M.G. Supergiant Barocaloric Effects in Acetoxy Silicone Rubber over a Wide Temperature Range: Great Potential for Solid-state Cooling. Chin. J. Polym. Sci. 2020, 38, 999–1005. [Google Scholar] [CrossRef]
- Bom, N.M.; Imamura, W.; Usuda, E.O.; Paixao, L.S.; Carvalho, A.M.G. Giant Barocaloric Effects in Natural Rubber: A Relevant Step toward Solid-State Cooling. ACS MacroLett. 2018, 7, 31–36, Correction in 2018, 7, 470–471. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Chauhan, A.; Vaish, R.; Thomas, P. Elastocaloric and barocaloric effects in polyvinylidene di-fluoride-based polymers. Appl. Phys. Lett. 2016, 108, 1–5. [Google Scholar] [CrossRef]
- Greco, A.; Aprea, C.; Maiorino, A.; Masselli, C. A review of the state of the art of solid-state caloric cooling processes at room-temperature before 2019. Int. J. Refrig. 2019, 106, 66–88. [Google Scholar] [CrossRef]
- Bom, N.M.; Usuda, E.O.; Guimarães, G.M.; Coelho, A.A.; Carvalho, A.M.G. Note: Experimental setup for measuring the barocaloric effect in polymers: Application to natural rubber. Rev. Sci. Instrum. 2017, 88, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Li, Y. Force Analysis and Energy Harvesting for Innovative Multi-functional Shoes. Front. Mater. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Turkmen, A.C.; Celik, C. Energy harvesting with the piezoelectric material integrated shoe. Energy 2018, 150, 556–564. [Google Scholar] [CrossRef]
- Qian, F.; Xu, T.; Zuo, L. Design, optimization, modeling and testing of a piezoelectric footwear energy harvester. Energy Convers. Manag. 2018, 171, 1352–1364. [Google Scholar] [CrossRef]
- Mañosa, L.; Planes, A.; Acet, M. Advanced materials for solid-state refrigeration. J. Mater. Chem. A 2013, 1, 4925–4936. [Google Scholar] [CrossRef] [Green Version]
- Kent, E.G.; Swinney, F.B. PROPERTIES AND APPLICATIONS OF trans-1,4- POLYISOPRENE. I&EC Prod. Res. Dev. 1966, 5, 134–138. [Google Scholar]
- Mañosa, L.; Planes, A. Materials with Giant Mechanocaloric Effects: Cooling by Strength. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Vasilev, A.; Lorenz, T.; Breitkopf, C. Thermal conductivity of polyisoprene and polybutadiene from molecular dynamics simulations and transient measurements. Polymers 2020, 12, 1081. [Google Scholar] [CrossRef]
- Miliante, C.M.; Christmann, A.M.; Usuda, E.O.; Imamura, W.; Paixao, L.S.; Carvalho, A.M.G.; Muniz, A.R. Unveiling the Origin of the Giant Barocaloric E ff ect in Natural Rubber. Macromolecules 2020, 53, 2606–2615. [Google Scholar] [CrossRef]
- Materials for the Energy Transition Roadmap: Caloric Energy Conversion Materials. 2020. Available online: https://www.royce.ac.uk/content/uploads/2020/10/M4ET-Caloric-Energy-Conversion-Materials-roadmap.pdf (accessed on 10 October 2021).
- John, C.; Alexis, S.; Peter, R.M.; Taylor, L.; Stevens, C.J.; Taylor, L. Endurance Performance is Influenced by Perceptions of Pain and Temperature: Theory, Applications and Safety Considerations. Sport. Med. 2018, 48, 525–537. [Google Scholar] [CrossRef]
- Department of Homeland Security (USA), System Assessment and Validation for Emergency Responders (SAVER), Personal Cooling Systems Market Survey Report, June 2016. Available online: https://www.dhs.gov/sites/default/files/publications/Personal-Cooling-Systems-MSR_0616-508.pdf (accessed on 10 October 2021).
- “Galicia Futura” museum exhibition in Gaiás Museum, Santiago de Compostela (Spain) from 14/07/2021 to 09/01/2022. Available online: https://www.cidadedacultura.gal/gl/system/files/downloads/2021/07/galiciafutura_english_texts.pdf (accessed on 10 October 2021).
- Orbita Laika Science Communication TV Program to Be Broadcasted in the National Spanish TVE Channel in October 2021. Available online: https://www.rtve.es/television/orbita-laika/ (accessed on 10 October 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Ben, J.; Delgado-Ferreiro, I.; Salgado-Beceiro, J.; Bermudez-Garcia, J.M. Simple and Low-Cost Footstep Energy-Recover Barocaloric Heating and Cooling Device. Materials 2021, 14, 5947. https://doi.org/10.3390/ma14205947
Garcia-Ben J, Delgado-Ferreiro I, Salgado-Beceiro J, Bermudez-Garcia JM. Simple and Low-Cost Footstep Energy-Recover Barocaloric Heating and Cooling Device. Materials. 2021; 14(20):5947. https://doi.org/10.3390/ma14205947
Chicago/Turabian StyleGarcia-Ben, Javier, Ignacio Delgado-Ferreiro, Jorge Salgado-Beceiro, and Juan Manuel Bermudez-Garcia. 2021. "Simple and Low-Cost Footstep Energy-Recover Barocaloric Heating and Cooling Device" Materials 14, no. 20: 5947. https://doi.org/10.3390/ma14205947
APA StyleGarcia-Ben, J., Delgado-Ferreiro, I., Salgado-Beceiro, J., & Bermudez-Garcia, J. M. (2021). Simple and Low-Cost Footstep Energy-Recover Barocaloric Heating and Cooling Device. Materials, 14(20), 5947. https://doi.org/10.3390/ma14205947






