3D Printing of Macro Porous Sol-Gel Derived Bioactive Glass Scaffolds and Assessment of Biological Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioactive Glass Synthesis
2.2. Milling Procedures
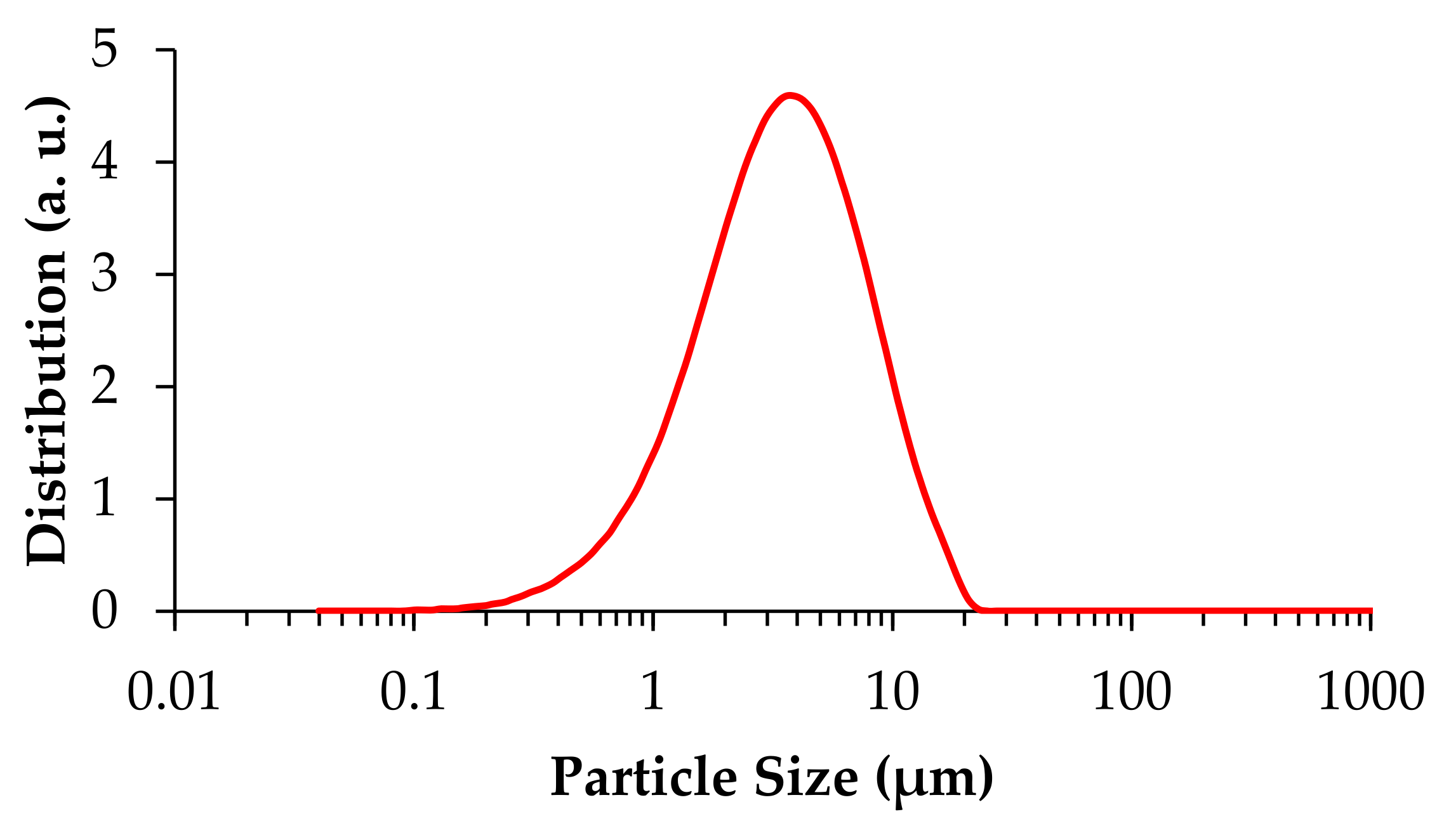
2.3. Particle Characterization
2.4. Paste Formulations
2.5. Rheological Characterization of the Inks
2.6. Printing Parameters
2.7. Debinding, Sintering, and Morphological Observation of the Scaffolds
2.8. Assessment of the Mechanical Properties through Compressive Strength Tests
2.9. Assessment of the Biological Properties through In Vitro Cytotoxicity Assays
3. Results and Discussion
3.1. Sol-Gel Synthesis
3.2. Milling Procedures
3.3. Paste Preparation
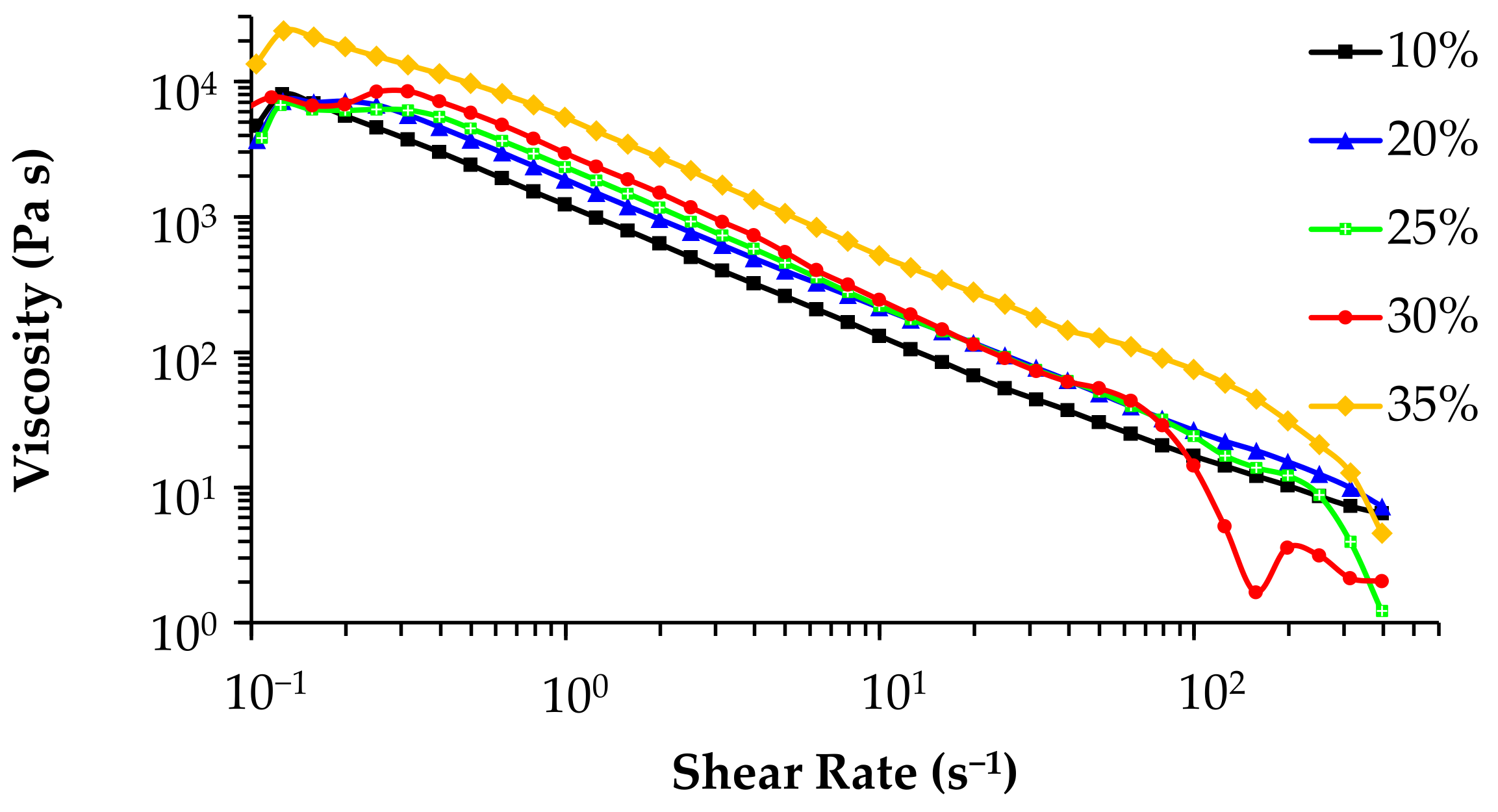
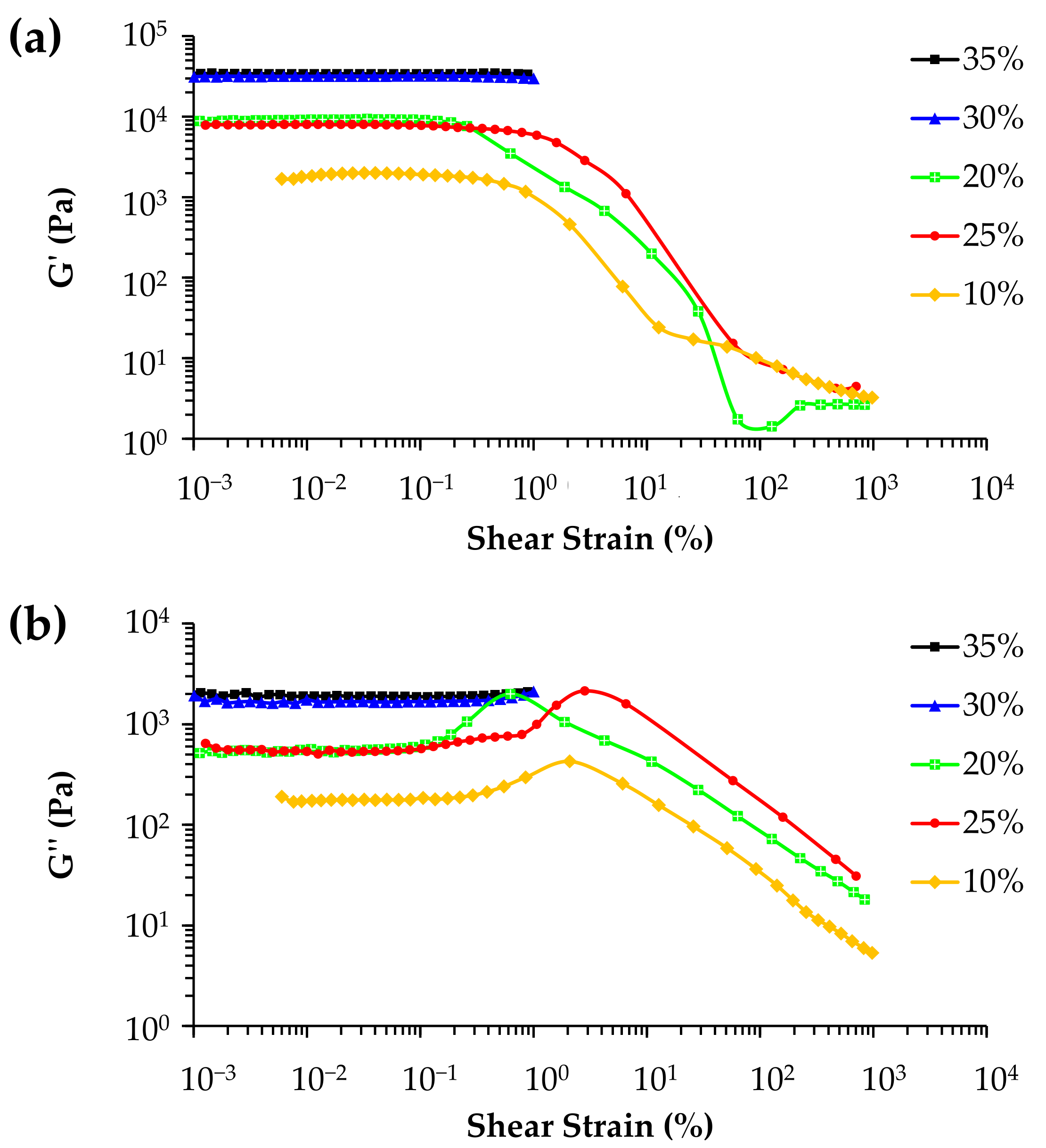
3.4. Rheological Properties of the Inks
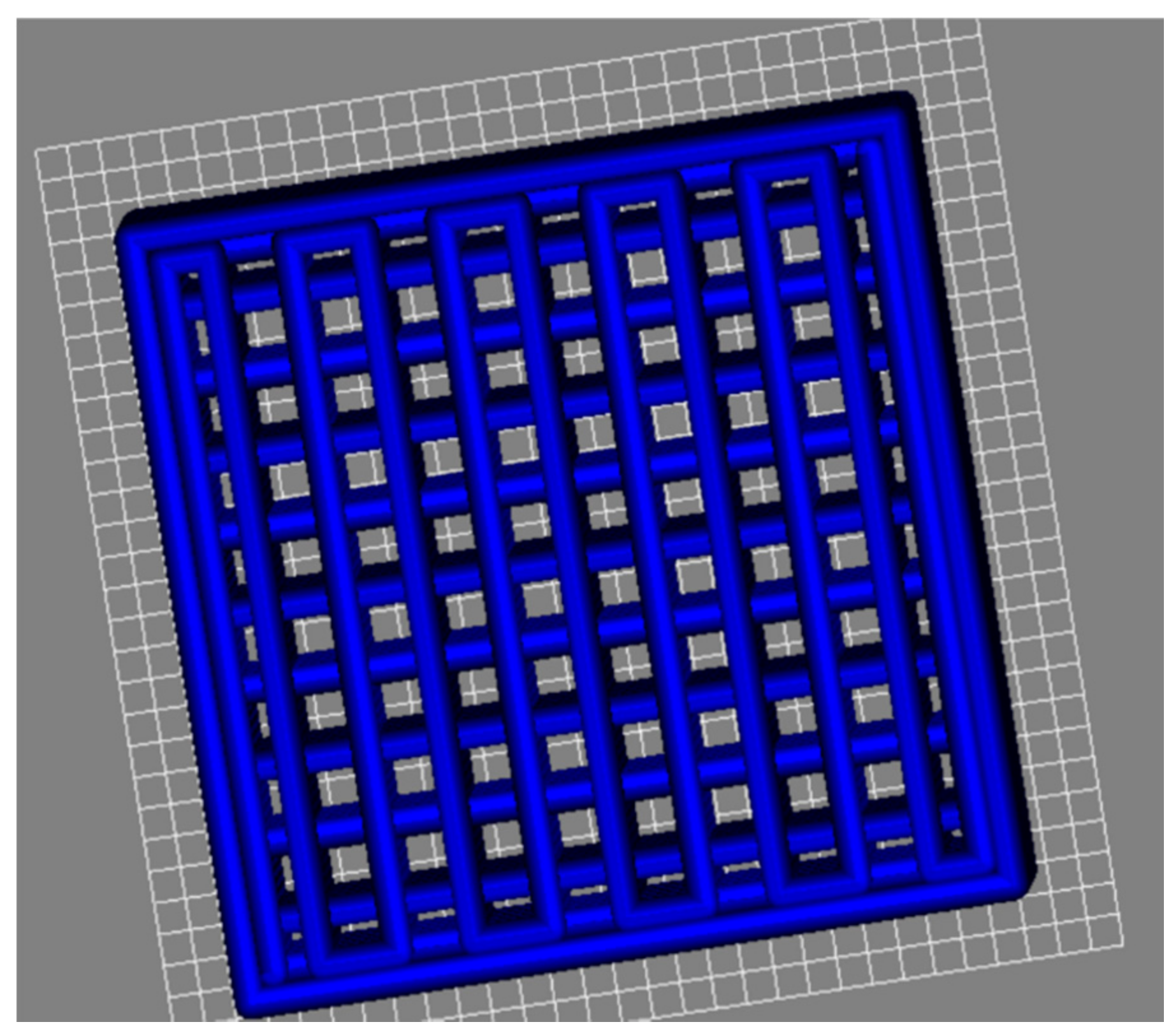
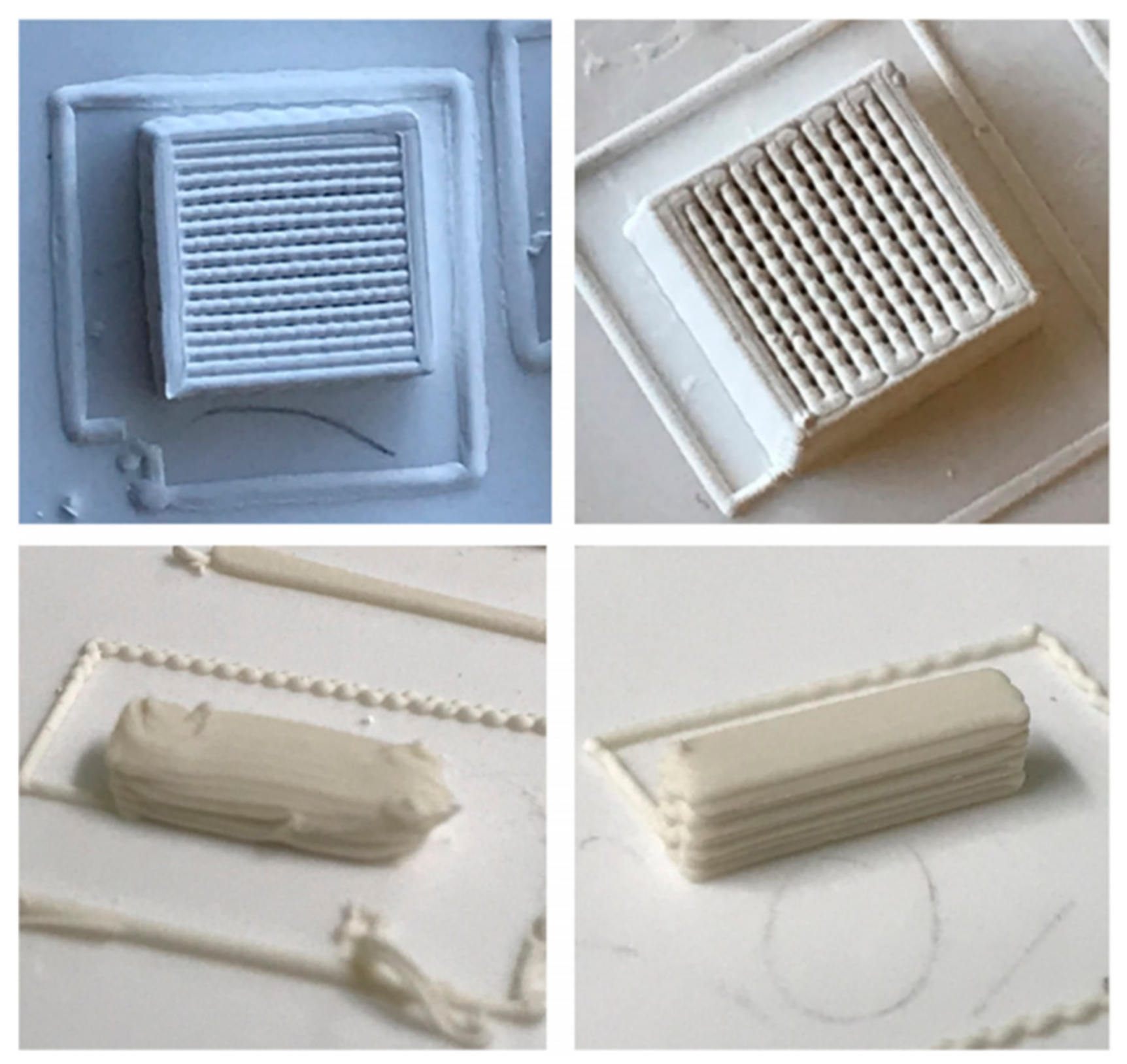
3.5. Printing
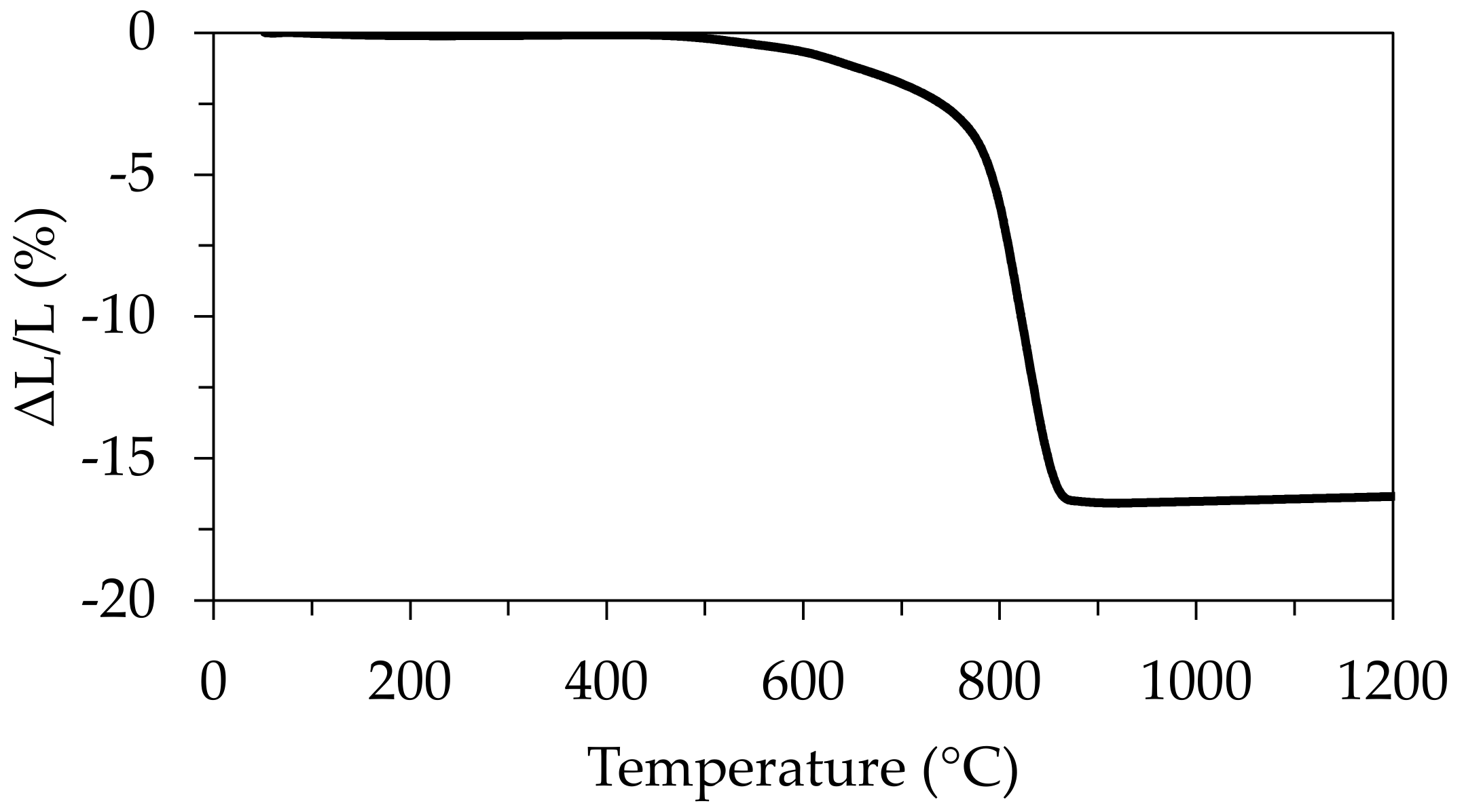
3.6. Thermal Treatments
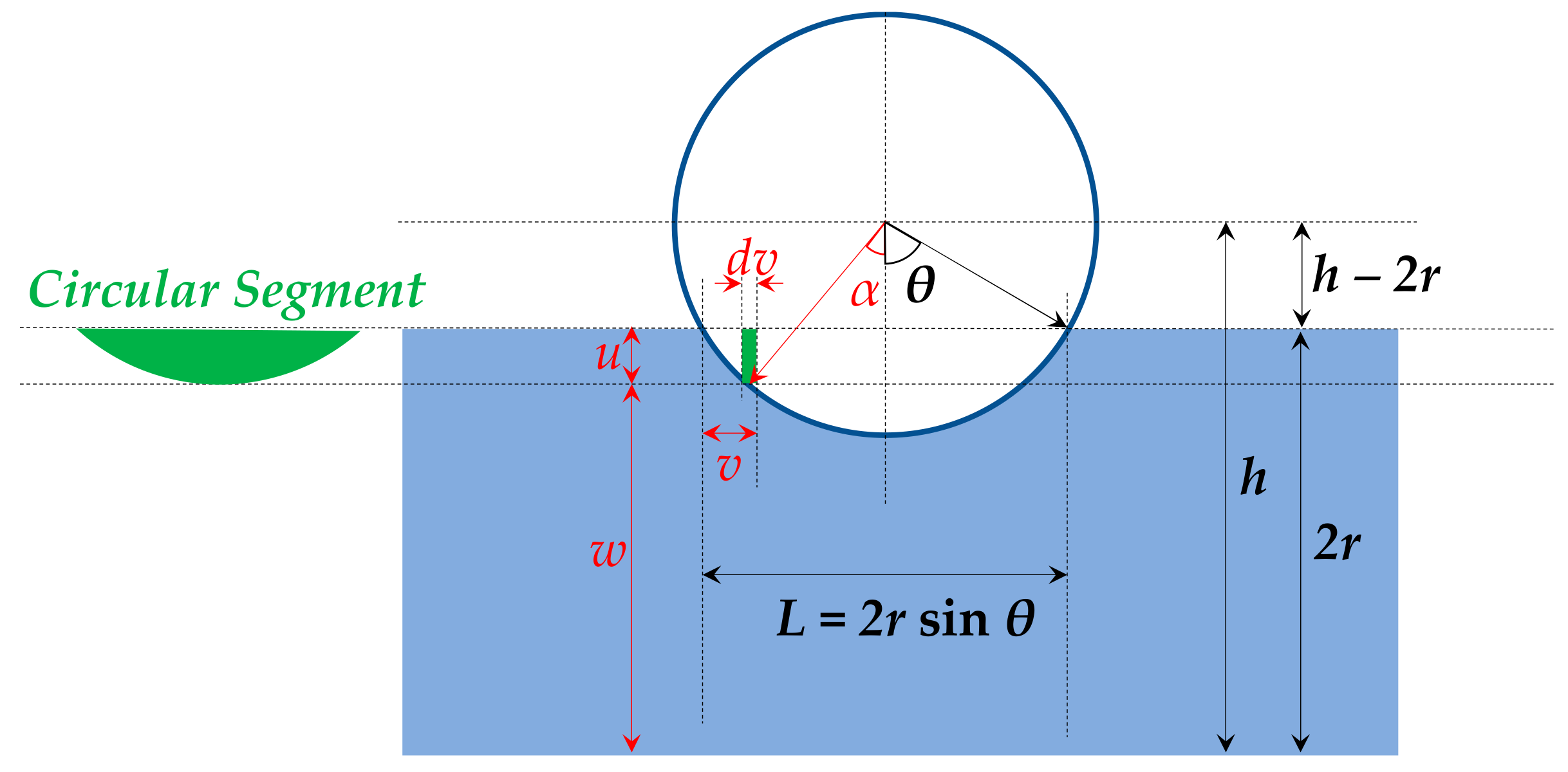
3.7. Preliminary Mechanical Properties Assessment through Compressive Strength Tests
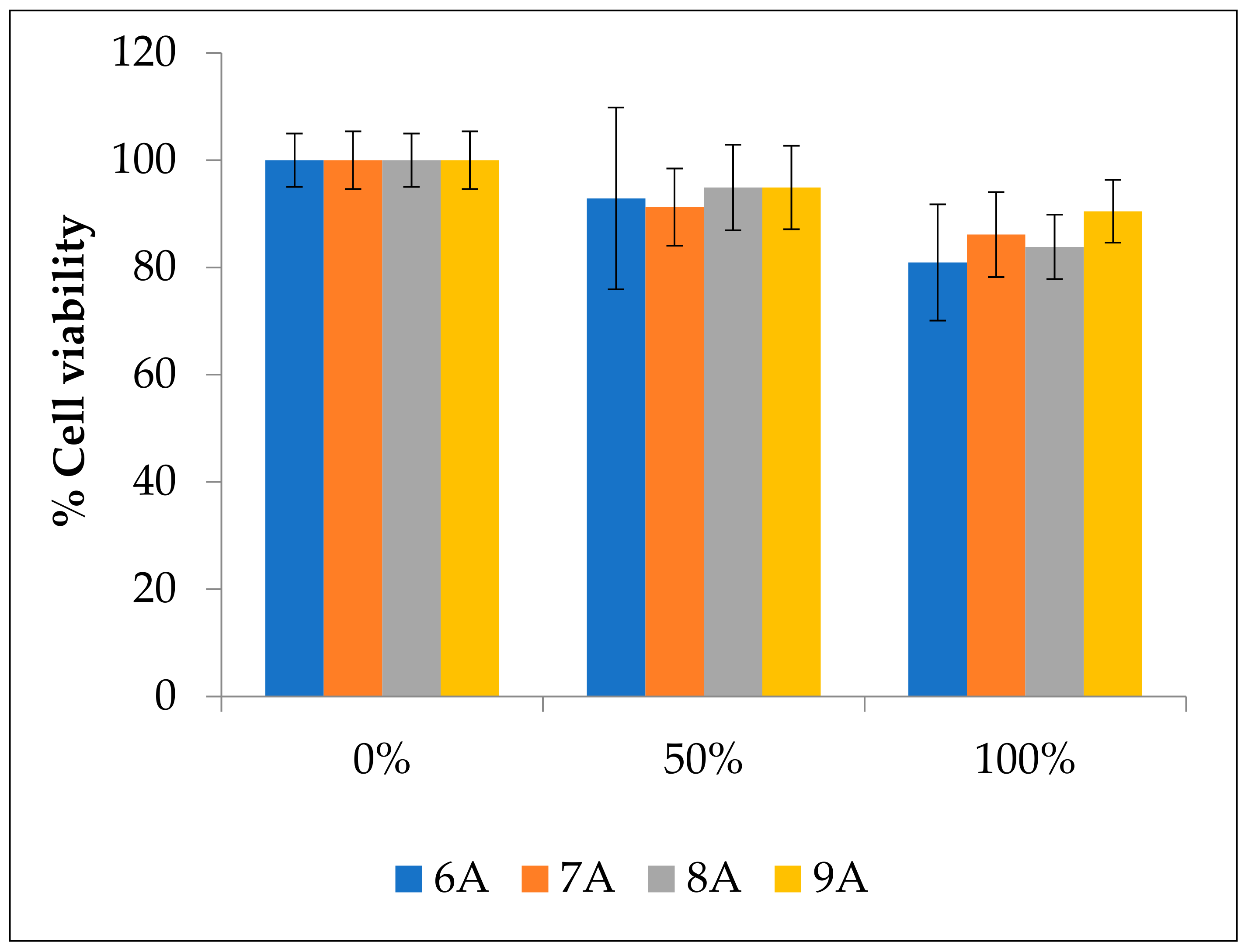
3.8. Biocompatibility Assays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Kunovjanek, M.; Knofius, N.; Reiner, G. Additive manufacturing and supply chains—A systematic review. Prod. Plan. Control 2020, 1–21. [Google Scholar] [CrossRef]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Watson, J.K.; Taminger, K.M.B. A decision-support model for selecting additive manufacturing versus subtractive manufacturing based on energy consumption. J. Clean. Prod. 2018, 176, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.T.; Zhu, Z.; Dhokia, V.; Shokrani, A. Process planning for additive and subtractive manufacturing technologies. CIRP Ann. 2015, 64, 467–470. [Google Scholar] [CrossRef]
- Karunakaran, K.P.; Suryakumar, S.; Pushpa, V.; Akula, S. Low cost integration of additive and subtractive processes for hybrid layered manufacturing. Robot. Comput. Integr. Manuf. 2010, 26, 490–499. [Google Scholar] [CrossRef]
- Singamneni, S.; Yifan, L.V.; Hewitt, A.; Chalk, R.; Thomas, W.; Jordison, D. Additive Manufacturing for the Aircraft Industry: A Review. J. Aeronaut. Aerosp. Eng. 2019, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- Manogharan, G.; Wysk, R.A.; Harrysson, O.L.A. Additive manufacturing–integrated hybrid manufacturing and subtractive processes: Economic model and analysis. Int. J. Comput. Integr. Manuf. 2016, 29, 473–488. [Google Scholar] [CrossRef]
- Cozmei, C.; Caloian, F. Additive Manufacturing Flickering at the Beginning of Existence. Procedia Econ. Financ. 2012, 3, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, C.; Delke, V.; Wasserman, M.E. Strategically Aligning Additive Manufacturing Supply Chains for Sustainability and Effectiveness. IFAC-PapersOnLine 2019, 52, 260–264. [Google Scholar] [CrossRef]
- Bonnin Roca, J.; Vaishnav, P.; Fuchs, E.R.H.; Morgan, M.G. Additive Manufacturing: Policy Needed. SSRN Electron. J. 2016. [Google Scholar] [CrossRef]
- Feilden, E.; Blanca, E.G.-T.; Giuliani, F.; Saiz, E.; Vandeperre, L. Robocasting of structural ceramic parts with hydrogel inks. J. Eur. Ceram. Soc. 2016, 36, 2525–2533. [Google Scholar] [CrossRef]
- Cesarano, J. A Review of Robocasting Technology. MRS Proc. 1998, 542, 133. [Google Scholar] [CrossRef] [Green Version]
- Cesarano, J., III; King, B.H.; Denham, H.B. Recent Developments in Robocasting of Ceramics and Multimaterial Deposition. In Proceedings of the International Solid Freeform Fabrication Symposium, Austin, TX, USA, 11–13 August 1998; pp. 697–703. [Google Scholar]
- Brazete, D.; Neto, A.S.; Ferreira, J.M.F. Optimization of zirconia inks to fabricate 3D porous scaffolds by robocasting. Clin. Technol. 2019, 49, 5–10. [Google Scholar]
- Smay, J.E.; Cesarano, J.; Lewis, J.A. Colloidal Inks for Directed Assembly of 3-D Periodic Structures. Langmuir 2002, 18, 5429–5437. [Google Scholar] [CrossRef]
- Tuttle, B.A.; Smay, J.E.; Cesarano, J.; Voigt, J.A.; Scofield, T.W.; Olson, W.R.; Lewis, J.A. Robocast Pb(Zr0.95Ti0.05)O3 Ceramic Monoliths and Composites. J. Am. Ceram. Soc. 2001, 84, 872–874. [Google Scholar] [CrossRef]
- Li, Q.; Lewis, J.A. Nanoparticle Inks for Directed Assembly of Three-Dimensional Periodic Structures. Adv. Mater. 2003, 15, 1639–1643. [Google Scholar] [CrossRef]
- Lewis, J.A.; Smay, J.E.; Stuecker, J.; Cesarano, J. Direct Ink Writing of Three-Dimensional Ceramic Structures. J. Am. Ceram. Soc. 2006, 89, 3599–3609. [Google Scholar] [CrossRef]
- Nan, B.; Galindo-Rosales, F.J.; Ferreira, J.M.F. 3D printing vertically: Direct ink writing free-standing pillar arrays. Mater. Today 2020, 35, 16–24. [Google Scholar] [CrossRef]
- Nan, B.; Gołębiewski, P.; Buczyński, R.; Galindo-Rosales, F.J.; Ferreira, J.M.F. Direct Ink Writing Glass: A Preliminary Step for Optical Application. Materials 2020, 13, 1636. [Google Scholar] [CrossRef] [Green Version]
- Munch, E.; Franco, J.; Deville, S.; Hunger, P.; Saiz, E.; Tomsia, A.P. Porous ceramic scaffolds with complex architectures. JOM 2008, 60, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Ben-Arfa, B.A.E.; Palamá, I.E.; Miranda Salvado, I.M.; Ferreira, J.M.F.; Pullar, R.C. Cytotoxicity and bioactivity assessments for Cu2+ and La3+ doped high-silica sol-gel derived bioglasses: The complex interplay between additive ions revealed. J. Biomed. Mater. Res. Part A 2019, 107, 2680–2693. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arfa, B.A.E.; Miranda Salvado, I.M.; Ferreira, J.M.F.; Pullar, R.C. A hundred times faster: Novel, rapid sol-gel synthesis of bio-glass nanopowders (Si-Na-Ca-P system, Ca:P = 1.67) without aging. Int. J. Appl. Glas. Sci. 2017, 8, 337–343. [Google Scholar] [CrossRef]
- Bento, R.; Gaddam, A.; Ferreira, J.M.F. Sol-gel synthesis and characterization of a quaternary bioglass for bone regeneration and tissue engineering. Materials 2021, 14, 4515. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Miranda Salvado, I.M.; Pullar, R.C.; Ferreira, J.M.F. The influence of processing parameters on morphology and granulometry of a wet-milled sol-gel glass powder. Ceram. Int. 2018, 44, 12754–12762. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Miranda Salvado, I.M.; Frade, J.R.; Pullar, R.C. Guidelines to adjust particle size distributions by wet comminution of a bioactive glass determined by Taguchi and multivariate analysis. Ceram. Int. 2019, 45, 3857–3863. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gaddam, A.; Brazete, D.S.; Neto, A.S.; Nan, B.; Ferreira, J.M.F. Three-dimensional printing of zirconia scaffolds for load bearing applications: Study of the optimal fabrication conditions. J. Am. Ceram. Soc. 2021, 104, 4368–4380. [Google Scholar] [CrossRef]
- Eqtesadi, S.; Motealleh, A.; Miranda, P.; Lemos, A.; Rebelo, A.; Ferreira, J.M.F. A simple recipe for direct writing complex 45S5 Bioglass® 3D scaffolds. Mater. Lett. 2013, 93, 68–71. [Google Scholar] [CrossRef]
- Eqtesadi, S.; Motealleh, A.; Miranda, P.; Pajares, A.; Lemos, A.; Ferreira, J.M.F. Robocasting of 45S5 bioactive glass scaffolds for bone tissue engineering. J. Eur. Ceram. Soc. 2014, 34, 107–118. [Google Scholar] [CrossRef]
- Nan, B.; Olhero, S.; Pinho, R.; Vilarinho, P.M.; Button, T.W.; Ferreira, J.M.F. Direct ink writing of macroporous lead-free piezoelectric Ba0.85Ca0.15Zr0.1Ti0.9O3. J. Am. Ceram. Soc. 2019, 102, 3191–3203. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Neto, A.S.; Miranda Salvado, I.M.; Pullar, R.C.; Ferreira, J.M.F. Robocasting: Prediction of ink printability in solgel bioactive glass. J. Am. Ceram. Soc. 2019, 102, 1608–1618. [Google Scholar] [CrossRef]
- Kao, H.-M.; Chen, C.-L.; Chiao, S.-W. Solid Polymer Electrolyte Based on Pluronic P123 Triblock Copolymer-Siloxane Organic-Inorganic Hybrid. J. Chin. Chem. Soc. 2005, 52, 693–699. [Google Scholar] [CrossRef]
- Park, J.; Yu, M.K.; Jeong, Y.Y.; Kim, J.W.; Lee, K.; Phan, V.N.; Jon, S. Antibiofouling amphiphilic polymer-coated superparamagnetic iron oxide nanoparticles: Synthesis, characterization, and use in cancer imaging in vivo. J. Mater. Chem. 2009, 19, 6412. [Google Scholar] [CrossRef]
- Dou, Q.; Abdul Karim, A.; Loh, X. Modification of Thermal and Mechanical Properties of PEG-PPG-PEG Copolymer (F127) with MA-POSS. Polymers 2016, 8, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, D.R.; Schwab, G.H.; Spengler, D.M. Tensile Fracture of Cancellous Bone. Acta Orthop. Scand. 1980, 51, 733–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Arfa, B.A.E.; Neto, S.; Miranda Salvado, I.M.; Pullar, R.C.; Ferreira, J.M.F. Robocasting of Cu2+ & La3+ doped sol–gel glass scaffolds with greatly enhanced mechanical properties: Compressive strength up to 14 MPa. Acta Biomater. 2019, 87, 265–272. [Google Scholar] [CrossRef] [PubMed]

| Bioactive Glass | Wet Milling Liquids | Milling Conditions | Recovered Powder | Sieve Mesh | Powder Fraction > 63 µm |
|---|---|---|---|---|---|
| 15 g | 15 g ethanol | 1 h 390 RPM | 12.88 g (86%) | 63 µm | 6.14 g (~47%) |
| 15 g acetone | 13.35 g (89%) | 12.88 g (~97%) |
| Weight (g) | Average Volume (cm3) | Density (g/cm3) |
|---|---|---|
| 1.59 | 0.595 | 2.672 ± 0.010 |
| Sample No. | Dimensions (mm) | Force (N) | Compressive Strength (MPa) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 1 | 9.64 | 804 | 3.43 | 804 | 8.48 |
| 2 | 9.66 | 944 | 3.41 | 944 | 9.96 |
| 3 | 9.60 | 657 | 3.48 | 657 | 7.14 |
| 4 | 9.78 | 846 | 3.34 | 846 | 8.76 |
| 5 | 9.64 | 716 | 3.42 | 716 | 7.62 |
| Average Compressive Strength (MPa): | 8.39 ± 0.20 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento, R.; Gaddam, A.; Oskoei, P.; Oliveira, H.; Ferreira, J.M.F. 3D Printing of Macro Porous Sol-Gel Derived Bioactive Glass Scaffolds and Assessment of Biological Response. Materials 2021, 14, 5946. https://doi.org/10.3390/ma14205946
Bento R, Gaddam A, Oskoei P, Oliveira H, Ferreira JMF. 3D Printing of Macro Porous Sol-Gel Derived Bioactive Glass Scaffolds and Assessment of Biological Response. Materials. 2021; 14(20):5946. https://doi.org/10.3390/ma14205946
Chicago/Turabian StyleBento, Ricardo, Anuraag Gaddam, Párástu Oskoei, Helena Oliveira, and José M. F. Ferreira. 2021. "3D Printing of Macro Porous Sol-Gel Derived Bioactive Glass Scaffolds and Assessment of Biological Response" Materials 14, no. 20: 5946. https://doi.org/10.3390/ma14205946
APA StyleBento, R., Gaddam, A., Oskoei, P., Oliveira, H., & Ferreira, J. M. F. (2021). 3D Printing of Macro Porous Sol-Gel Derived Bioactive Glass Scaffolds and Assessment of Biological Response. Materials, 14(20), 5946. https://doi.org/10.3390/ma14205946









