Dual Ion Releasing Nanoparticles for Modulating Osteogenic Cellular Microenvironment of Human Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Method of ZIOs
2.3. Characterization
2.4. Cell Culture
2.5. Neutral Red Assay and Live/Dead Assay
2.6. TUNEL Staining
2.7. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.8. F-Actin Staining
2.9. Alkaline Phosphatase (ALP) Staining
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Reactive Oxygen Species (ROS) Staining
2.12. Statistical Analysis
3. Results and Discussion
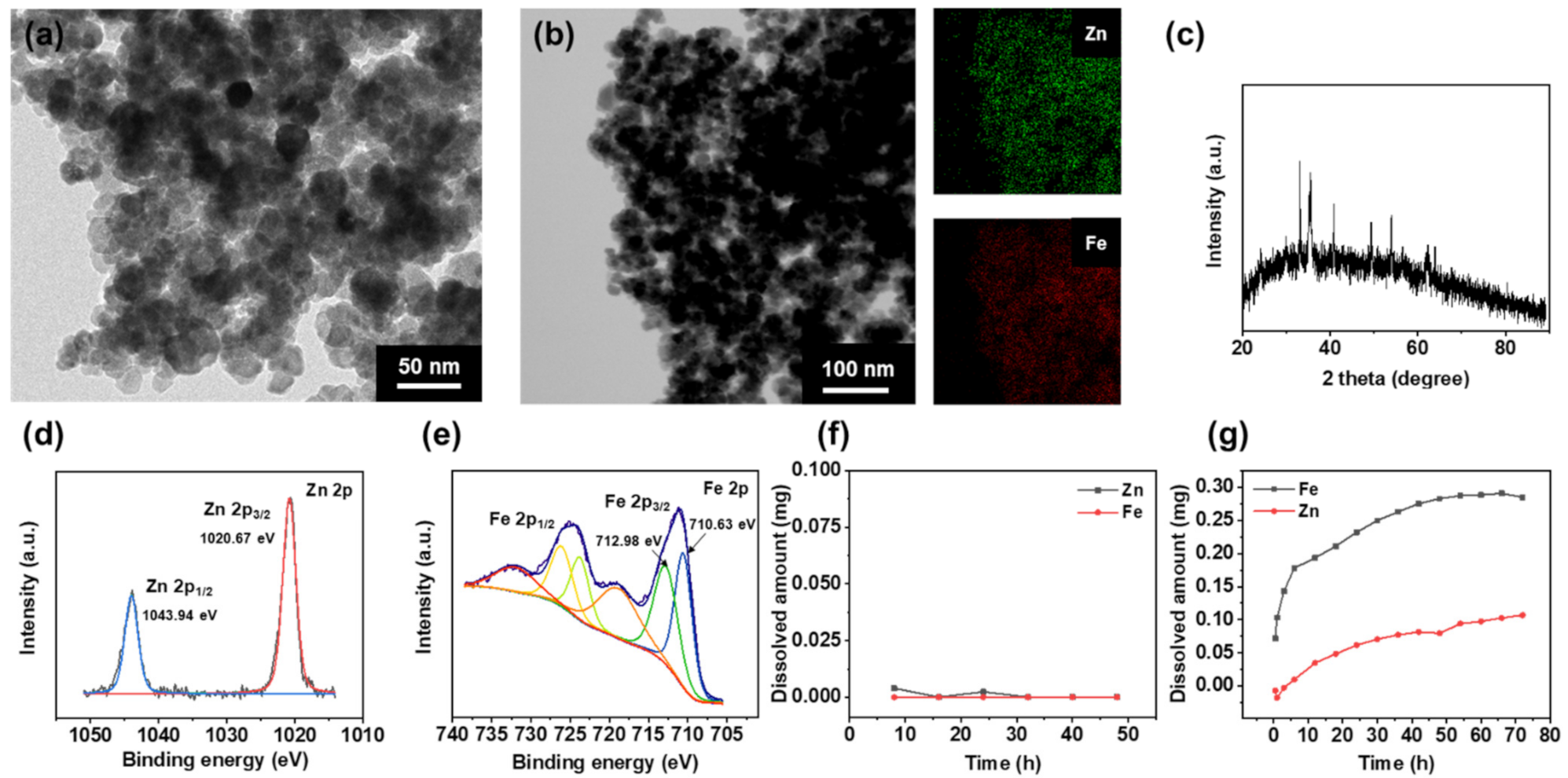
3.1. Characterization of ZIOs
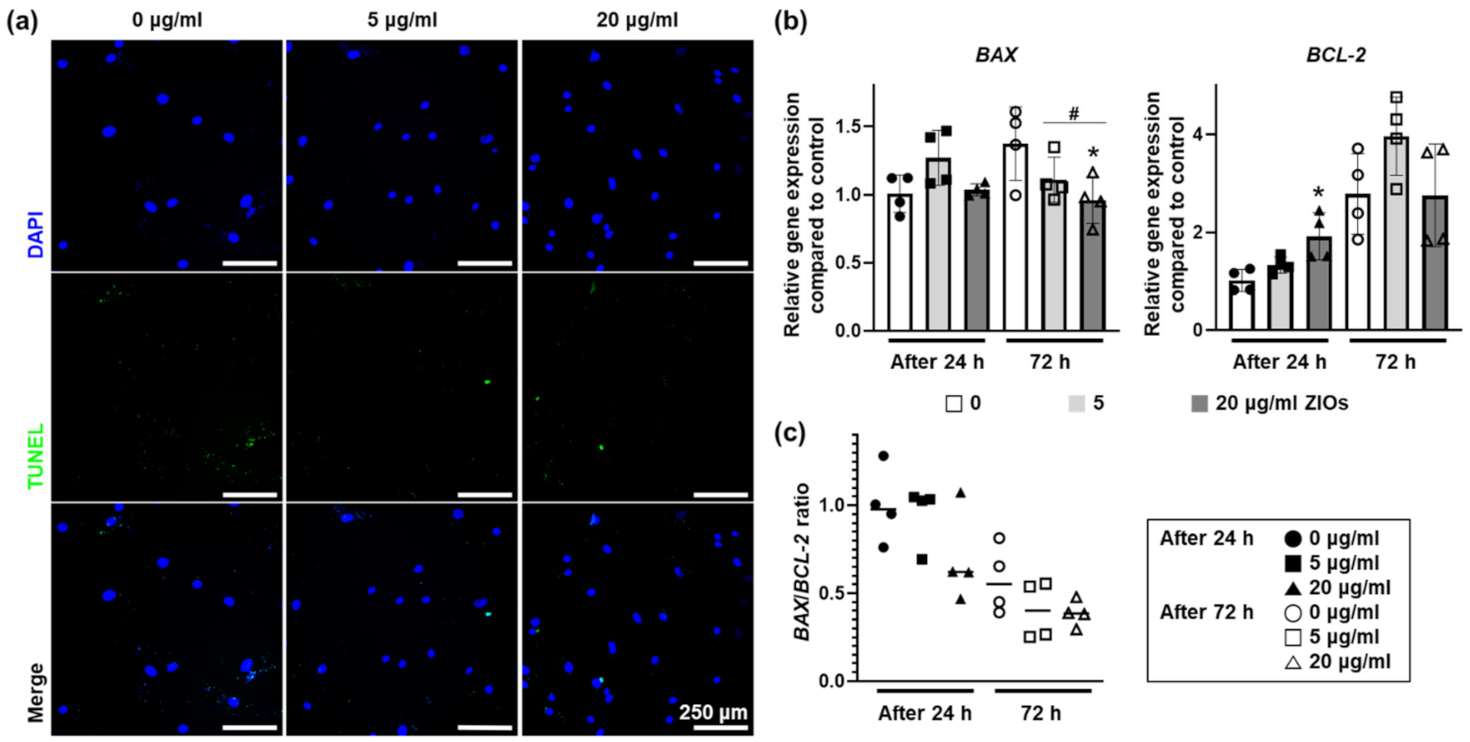
3.2. Cytotoxicity According to ZIOs Concentration on hMSCs
3.3. Effects of ZIOs on hMSCs Viability
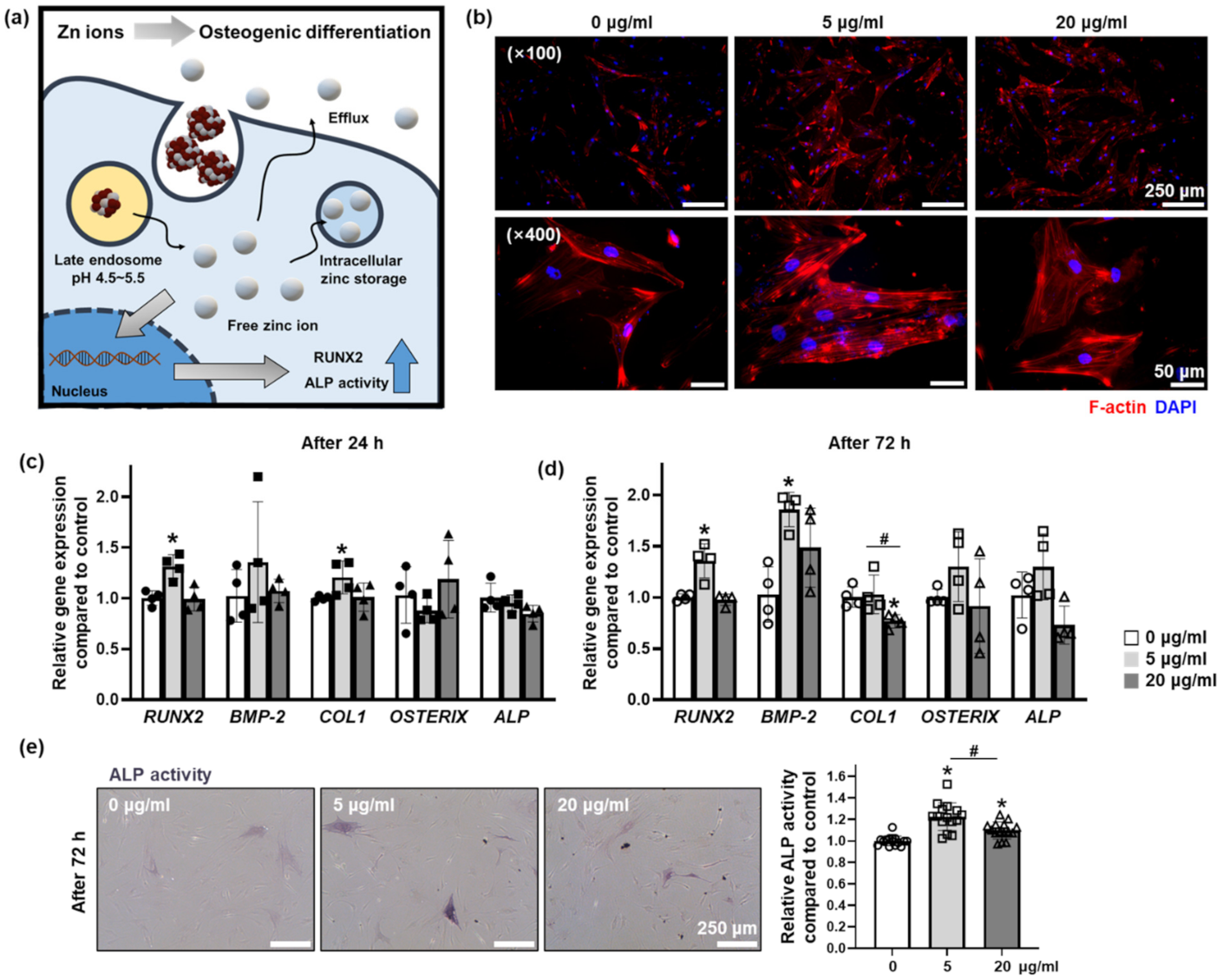
3.4. Effect of Zn Ions Released from ZIOs on Osteogenic Differentiation of hMSCs
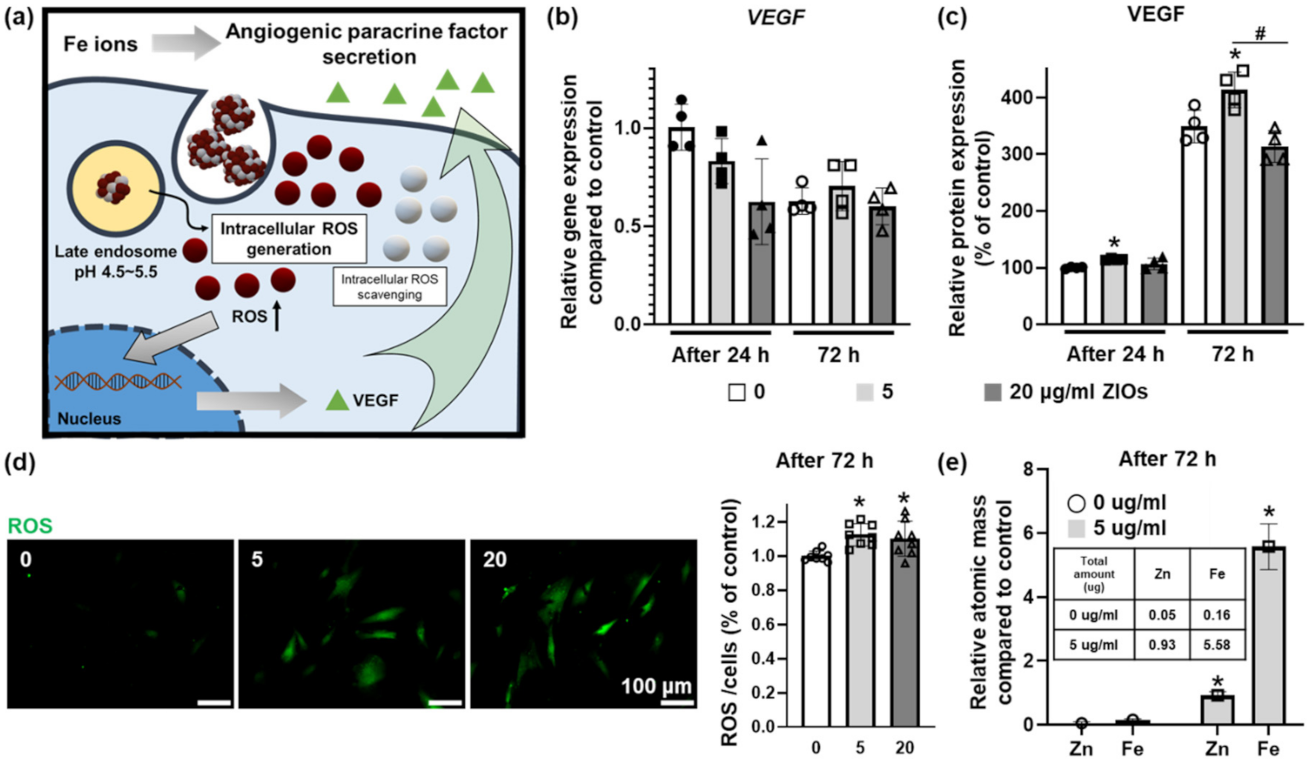
3.5. Effect of Fe Ions Released from ZIOs on Angiogenic Paracrine Factors Secretion from hMSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobo, D.N. Fluid, electrolytes and nutrition: Physiological and clinical aspects. Proc. Nutr. Soc. 2004, 63, 453–466. [Google Scholar] [CrossRef]
- Allison, S. Fluid, electrolytes and nutrition. Clin. Med. 2004, 4, 573. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status for diagnosis and therapy. Magnes. Res. 2010, 23, 194–198. [Google Scholar]
- Gupta, C. Role of iron (Fe) in body. IOSR J. Appl. Chem. 2014, 7, 38–46. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Kozlowski, H.; Janicka-Klos, A.; Brasun, J.; Gaggelli, E.; Valensin, D.; Valensin, G. Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coord. Chem. Rev. 2009, 253, 2665–2685. [Google Scholar] [CrossRef]
- Wataha, J.C.; Hanks, C.T.; Sun, Z. Effect of cell line on in vitro metal ion cytotoxicity. Dent. Mater. 1994, 10, 156–161. [Google Scholar] [CrossRef]
- Wataha, J.C.; Hanks, C.; Craig, R.G. In vitro effect of metal ions on cellular metabolism and the correlation between these effects and the uptake of the ions. J. Biomed. Mater. Res. 1994, 28, 427–433. [Google Scholar] [CrossRef]
- Puleo, D.A.; Huh, W.W. Acute toxicity of metal ions in cultures of osteogenic cells derived from bone marrow stromal cells. J. Appl. Biomater. 1995, 6, 109–116. [Google Scholar] [CrossRef]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. The biochemical effects of extracellular Zn 2+ and other metal ions are severely affected by their speciation in cell culture media. Metallomics 2015, 7, 102–111. [Google Scholar] [CrossRef]
- Haider, W.; Munroe, N.; Tek, V.; Gill, P.; Tang, Y.; McGoron, A. Cytotoxicity of metal ions released from nitinol alloys on endothelial cells. J. Mater. Eng. Perform. 2011, 20, 816. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, S.-H.; Lee, S.; Lee, D.-K.; Han, Y.; Jeon, S.; Cho, W.-S. Differential contribution of constituent metal ions to the cytotoxic effects of fast-dissolving metal-oxide nanoparticles. Front. Pharmacol. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. 1), 51. [Google Scholar]
- Kim, Y.H.; Jung, E.; Im, G.-B.; Kim, Y.-J.; Kim, S.-W.; Jeong, G.-J.; Jang, Y.C.; Park, K.M.; Kim, D.-I.; Yu, T. Regulation of intracellular transition metal ion level with a pH-sensitive inorganic nanocluster to improve therapeutic angiogenesis by enriching conditioned medium retrieved from human adipose derived stem cells. Nano Converg. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Im, G.-B.; Jung, E.; Kim, Y.H.; Kim, Y.-J.; Kim, S.-W.; Jeong, G.-J.; Lee, T.-J.; Kim, D.-I.; Kim, J.; Hyeon, T. Endosome-triggered ion-releasing nanoparticles as therapeutics to enhance the angiogenic efficacy of human mesenchymal stem cells. J. Control. Release 2020. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, Y.; Huang, T.; Yu, Z.; Ma, K.; Yang, M.; Liu, Q.; Pan, H.; Wang, H.; Wang, J. Runx2/osterix and zinc uptake synergize to orchestrate osteogenic differentiation and citrate containing bone apatite formation. Adv. Sci. 2018, 5, 1700755. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-E.; Kwun, I.-S. Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts. J. Nutr. Health 2018, 51, 23–30. [Google Scholar] [CrossRef]
- Safarova, Y.; Umbayev, B.; Hortelano, G.; Askarova, S. Mesenchymal stem cells modifications for enhanced bone targeting and bone regeneration. Regen. Med. 2020. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef]
- Wang, C.; Tan, X.; Yan, J.; Chai, B.; Li, J.; Chen, S. Electrospinning direct synthesis of magnetic ZnFe2O4/ZnO multi-porous nanotubes with enhanced photocatalytic activity. Appl. Surf. Sci. 2017, 396, 780–790. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Li, Q.; Li, Y.; Li, Y.; Zhang, X.; Huang, Q. Effects of serum proteins on intracellular uptake and cytotoxicity of carbon nanoparticles. Carbon 2009, 47, 1351–1358. [Google Scholar] [CrossRef]
- Cukalevski, R.; Ferreira, S.A.; Dunning, C.J.; Berggård, T.; Cedervall, T. IgG and fibrinogen driven nanoparticle aggregation. Nano Res. 2015, 8, 2733–2743. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D. Bioabsorbable zinc ion induced biphasic cellular responses in vascular smooth muscle cells. Sci. Rep. 2016, 6, 26661. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Rachlin, A.; Otey, C.A.; Loboa, E.G. Human adipose-derived adult stem cells upregulate palladin during osteogenesis and in response to cyclic tensile strain. Am. J. Physiol.-Cell Physiol. 2007, 293, C1532–C1538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tong, Z.; Liu, Y.; Xia, R.; Chang, Y.; Hu, Y.; Liu, P.; Zhai, Z.; Zhang, J.; Li, H. F-actin Regulates Osteoblastic Differentiation of Mesenchymal Stem Cells on TiO 2 Nanotubes Through MKL1 and YAP/TAZ. Nanoscale Res. Lett. 2020, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. Off. Publ. Int. Soc. Trace Elem. Res. Hum. 1998, 11, 119–135. [Google Scholar] [CrossRef]
- Yusa, K.; Yamamoto, O.; Iino, M.; Takano, H.; Fukuda, M.; Qiao, Z.; Sugiyama, T. Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering. Arch. Oral Biol. 2016, 71, 162–169. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, K.; Wen, Z.; Liu, W.; Zhang, L.; Su, J. Double-edged effects and mechanisms of Zn2+ microenvironments on osteogenic activity of BMSCs: Osteogenic differentiation or apoptosis. RSC Adv. 2020, 10, 14915–14927. [Google Scholar] [CrossRef]
- Yusa, K.; Yamamoto, O.; Fukuda, M.; Koyota, S.; Koizumi, Y.; Sugiyama, T. In vitro prominent bone regeneration by release zinc ion from Zn-modified implant. Biochem. Biophys. Res. Commun. 2011, 412, 273–278. [Google Scholar] [CrossRef]
- Wang, D.; Cui, L.; Chang, X.; Guan, D. Biosynthesis and characterization of zinc oxide nanoparticles from Artemisia annua and investigate their effect on proliferation, osteogenic differentiation and mineralization in human osteoblast-like MG-63 Cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111652. [Google Scholar] [CrossRef]
- Oikonomopoulos, A.; Van Deen, W.K.; Manansala, A.-R.; Lacey, P.N.; Tomakili, T.A.; Ziman, A.; Hommes, D.W. Optimization of human mesenchymal stem cell manufacturing: The effects of animal/xeno-free media. Sci. Rep. 2015, 5, 16570. [Google Scholar] [CrossRef]
- Khang, D.; Choi, J.; Im, Y.-M.; Kim, Y.-J.; Jang, J.-H.; Kang, S.S.; Nam, T.-H.; Song, J.; Park, J.-W. Role of subnano-, nano- and submicron-surface features on osteoblast differentiation of bone marrow mesenchymal stem cells. Biomaterials 2012, 33, 5997–6007. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, S.; Hantash, B.M. Transforming growth factor β1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng. Part A 2010, 16, 725–733. [Google Scholar] [CrossRef]
- Yun, W.S.; Aryal, S.; Ahn, Y.J.; Seo, Y.J.; Key, J. Engineered iron oxide nanoparticles to improve regenerative effects of mesenchymal stem cells. Biomed. Eng. Lett. 2020, 10, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.D.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; De Oliveira, A.R.S. Zinc and oxidative stress: Current mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Lee, S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Yu, S.P.; Canzoniero, L.M.; Choi, D.W. Ion homeostasis and apoptosis. Curr. Opin. Cell Biol. 2001, 13, 405–411. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Zhang, Y.; Ma, L.; Song, E.; Song, Y. “Iron free” zinc oxide nanoparticles with ion-leaking properties disrupt intracellular ROS and iron homeostasis to induce ferroptosis. Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| Human GAPDH | Forward | GTC GGA GTC AAC GGA TTT GG |
| Reverse | GGG TGG AAT CAA TTG GAA CAT | |
| Human BAX | Forward | GCA ACT TCA ACT GGG GCC GGG |
| Reverse | GAT CCA GCC CAA CAG CCG CTC | |
| Human BCL-2 | Forward | CAA CAT CGC CCT GTG GAT GA |
| Reverse | GGG CCA AAC TGA GCA GAG TC | |
| Human RUNX2 | Forward | TCA CTA CCA GCC ACC GAG AC |
| Reverse | ACG CCA TAG TCC CTC CTT TT | |
| Human BMP-2 | Forward | TGT ATC GCA GGC ACT CAG GTC A |
| Reverse | CCA CTC GTT TCT GGT AGT TCT TC | |
| Human COL 1 | Forward | TGC GAT GAC GTG ATC TGT GA |
| Reverse | TTG GTC GGT GGG TGA CTC TG | |
| Human OSTERIX | Forward | TAA TGG GCT CCT TTC ACC TG |
| Reverse | CAC TGG GCA GAC AGT CAG AA | |
| Human ALP | Forward | CCT CCT CGG AAG ACA CTC TG |
| Reverse | GCA GTG AAG GGC TTC TTG TC | |
| Human VEGF | Forward | GAG GGC AGA ATC ATC ACG AAG T |
| Reverse | CAC CAG GGT CTC GAT TGG AT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J.; Lee, J.; Im, G.-B.; Song, J.; Song, J.; Chung, J.; Yu, T.; Bhang, S.H. Dual Ion Releasing Nanoparticles for Modulating Osteogenic Cellular Microenvironment of Human Mesenchymal Stem Cells. Materials 2021, 14, 412. https://doi.org/10.3390/ma14020412
Kim Y-J, Lee J, Im G-B, Song J, Song J, Chung J, Yu T, Bhang SH. Dual Ion Releasing Nanoparticles for Modulating Osteogenic Cellular Microenvironment of Human Mesenchymal Stem Cells. Materials. 2021; 14(2):412. https://doi.org/10.3390/ma14020412
Chicago/Turabian StyleKim, Yu-Jin, Jaeyoung Lee, Gwang-Bum Im, Jihun Song, Jiwoo Song, Jiyong Chung, Taekyung Yu, and Suk Ho Bhang. 2021. "Dual Ion Releasing Nanoparticles for Modulating Osteogenic Cellular Microenvironment of Human Mesenchymal Stem Cells" Materials 14, no. 2: 412. https://doi.org/10.3390/ma14020412
APA StyleKim, Y.-J., Lee, J., Im, G.-B., Song, J., Song, J., Chung, J., Yu, T., & Bhang, S. H. (2021). Dual Ion Releasing Nanoparticles for Modulating Osteogenic Cellular Microenvironment of Human Mesenchymal Stem Cells. Materials, 14(2), 412. https://doi.org/10.3390/ma14020412







