Evaluation of Antimicrobial Efficacy and Permeability of Various Sealing Materials at the Implant–Abutment Interface—A Pilot In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
- GapSeal gel (Hager and Werken, Duisburg, Germany);
- Oxysafe gel (Hager and Werken, Duisburg, Germany);
- Flow.sil (Bredent GmbH and Co.KG, Senden, Germany).
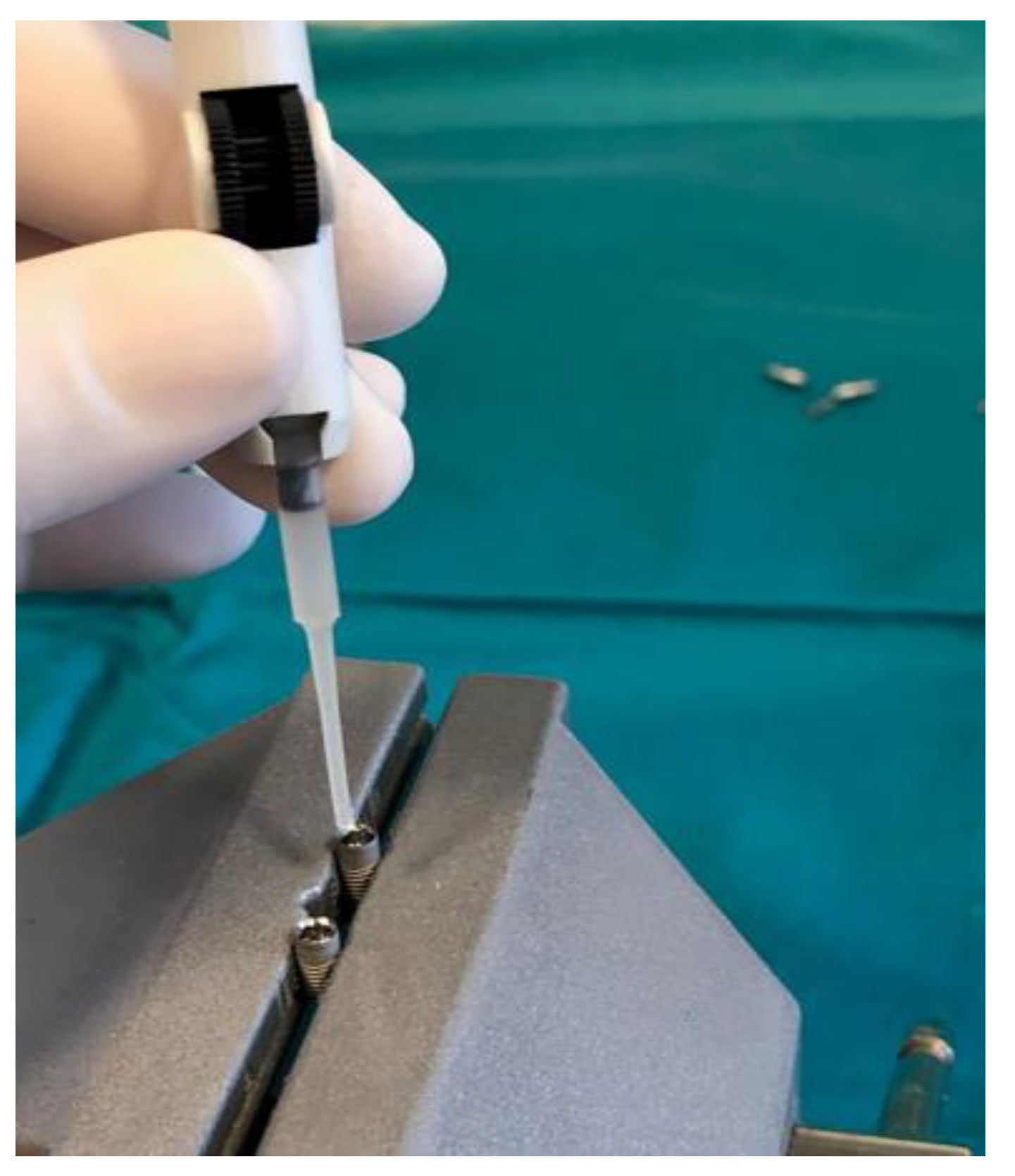
2.1. Preparation of Dental Implants
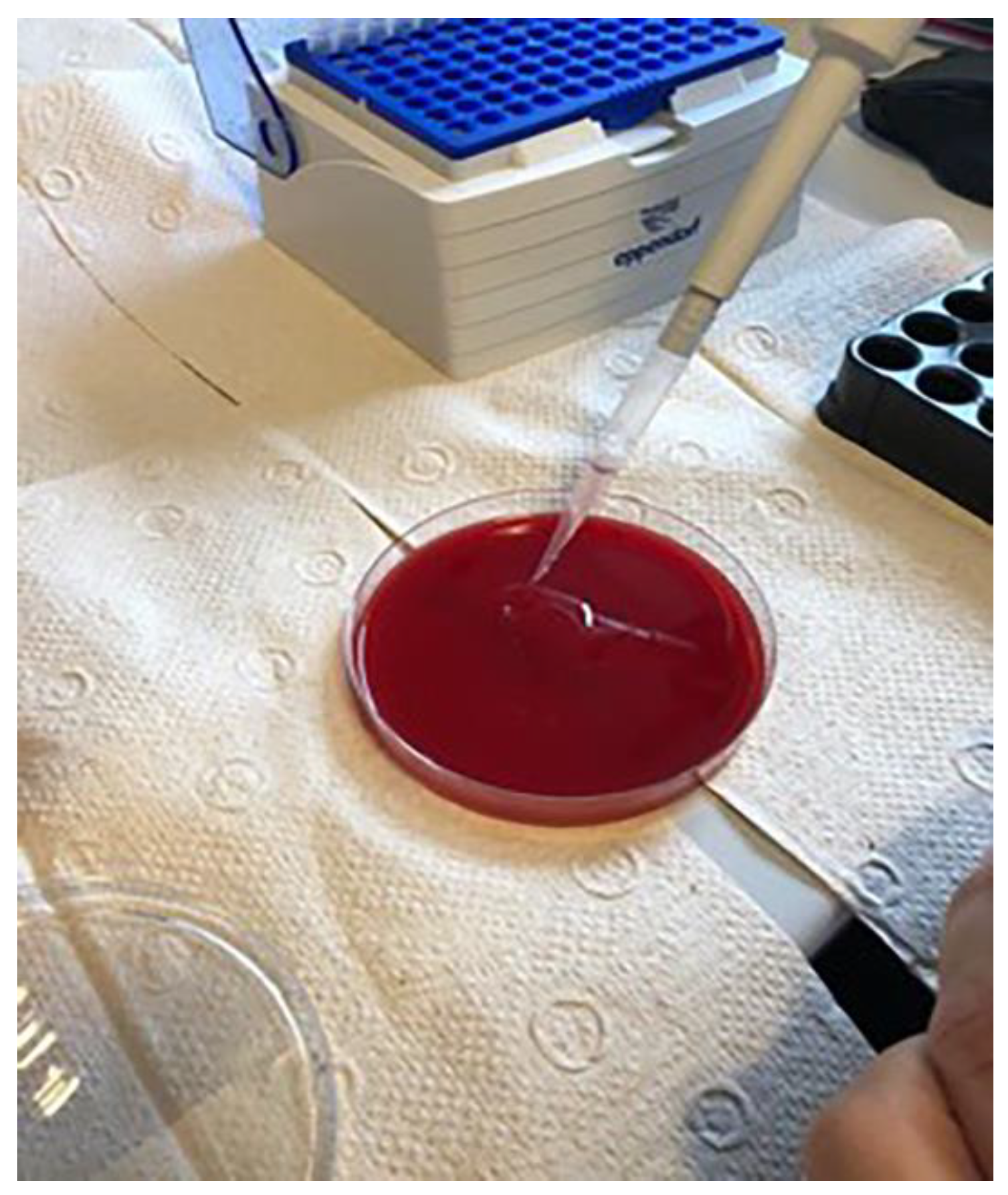
2.2. Contamination of Dental Implants
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogle, O.E. Implant surface material, design, and osseointegration. Dent. Clin. 2015, 59, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Periodontol. 2018, 89, 249–256. [Google Scholar] [CrossRef]
- Suárez-López Del Amo, F.; Yu, S.H.; Wang, H.L. Non-surgical therapy for peri-implant diseases: A systematic review. J. Oral Maxillofac. Res. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Proff, P.; Steinmetz, I.; Bayerlin, T.; Dietze, S.; Fanghanel, J.; Gedrange, T. Bacterial colonization of interior implant threads with and without sealing. Folia Morphol. 2006, 65, 75–77. [Google Scholar]
- Gross, M.; Ambromovich, I.; Weiss, E.L. Microleakage at the abutment-implant interface of osseointegrated implants: A comparative study. Int. J. Oral Maxillofac. Implant. 1999, 14, 94–100. [Google Scholar]
- Dias, E.C.; Bisognin, E.D.; Harari, N.D.; Machado, S.J.; da Silva, C.P.; Soares, G.D.; Vidigal, G.M., Jr. Evaluation of implant-abutment microgap and bacterial leakage in five external-hex implant systems: An in vitro study. Int. J. Oral Maxillofac. Implants. 2012, 27, 346–351. [Google Scholar]
- Quirynen, M.; Bolen, C.M.; Essyen, H.; Van Steenberghe, D. Microbial penetration along the implant components of the Branemark system. Clin. Oral Implant. Res. 1994, 5, 239–244. [Google Scholar] [CrossRef]
- Duarte, A.R.; Rossetti, P.H.; Rossetti, L.M.; Torres, S.S.; Bonachela, W. In Vitro sealing ability of two materials at five different implant abutment surfaces. J. Periodontol. 2006, 77, 1828–1832. [Google Scholar] [CrossRef]
- Nayak, A.G.; Fernandes, A.; Kulkarni, R.; Ajantha, G.S.; Lekha, K.; Nadiger, R. Efficacy of antibacterial sealing gel and O-ring to prevent microleakage at the implant abutment interface: An in vitro study. J. Oral Implantol. 2014, 40, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, A.; Renvert, S.; Dahlen, G. Microbial findings at failing implants. Clin. Oral Implant. Res. 1999, 10, 339–345. [Google Scholar] [CrossRef]
- Mombelli, A.; Decaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38, 203–213. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Rahn, S.; Hegewald, A.; Pfeffer, K.; Henrich, B. Real-time PCR analysis of fungal organisms and bacterial species at peri-implantitis sites. Int. J. Implant. Dent. 2015, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Albertini, M.; López-Cerero, L.; O’Sullivan, M.G.; Chereguini, C.F.; Ballesta, S.; Ríos, V.; Herrero-Climent, M.; Bullón, P. Assessment of periodontal and opportunistic flora in patients with peri-implantitis. Clin. Oral Implant. Res. 2015, 26, 937–941. [Google Scholar] [CrossRef]
- Jankovic, S.; Aleksic, Z.; Dimitrijevic, B.; Lekovic, V.; Camargo, P.; Kenney, B. Prevalence of human cytomegalovirus and Epstein-Barr virus in subgingival plaque at peri-implantitis, mucositis and healthy sites. A pilot study. Int. J. Oral Maxillofac. Surg. 2011, 40, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Grusovin, M.G.; Canullo, L. The microbiologic profile associated with peri-implantitis in humans: A systematic review. Int. J. Oral Maxillofac. Implant. 2016, 31, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Padial-Molina, M.; Lopez-Martinez, J.; O’Valle, F.; Galindo-Moreno, P. Microbial profiles and detection techniques in peri-implant diseases: A systematic review. J. Oral Maxillofac. Res. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reynaud, A.H.; Nygaard-Østby, B.; Bøygard, G.K.; Eribe, E.R.; Olsen, I.; Gjermo, P. Yeasts in periodontal pockets. J. Clin. Periodontol. 2001, 28, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, G.; Wikstrom, M. Occurrence of enteric rods, staphylococci and Candida in subgingival samples. Oral Microbiol. Immunol. 1995, 10, 42–46. [Google Scholar] [CrossRef]
- Blankenship, J.R.; Mitchell, A.P. How to build a biofilm: A fungal perspective. Curr. Opin. Microbiol. 2006, 9, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; van Steenberghe, D. Bacterial colonization of the internal part of two-stage implants. An in vivo study. Clin. Oral Implant. Res. 1993, 4, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.K.; Conrads, G.; Richter, E.J. Microbial leakage and marginal fit of the implant-abutment interface. Int. J. Oral Maxillofac. Implant. 1997, 12, 527–540. [Google Scholar]
- Steinebrunner, L.; Wolfart, S.; Bössmann, K.; Kern, M. In Vitro evaluation of bacterial leakage along the implant-abutment interface of different implant systems. Int. J. Oral Maxillofac. Implant. 2005, 20, 875–881. [Google Scholar]
- Koutouzis, T.; Wallet, S.; Calderon, N.; Lundgren, T. Bacterial colonization of the implant-abutment interface using an in vitro dynamic loading model. J. Periodontol. 2011, 82, 613–618. [Google Scholar] [CrossRef]
- Listgarten, M.A. Microorganisms and dental implants. J. Periodontol. 1999, 70, 220–222. [Google Scholar] [CrossRef]
- Koutouzis, T.; Gadalla, H.; Lundgren, T. Bacterial colonization of the implant-abutment interface (IAI) of dental implants with a sloped marginal design: An in-vitro study. Clin. Implant. Dent. Relat. Res. 2016, 18, 161–167. [Google Scholar] [CrossRef]
- Podhorsky, A.; Biscoping, S.; Rehmann, P.; Streckbein, P.; Domann, E.; Wostmann, B. Transfer of bacteria into the internal cavity of dental implants after application of disinfectant or sealant agents in vitro. Int. J. Oral Maxillofac. Implant. 2016, 31, 563–570. [Google Scholar] [CrossRef]









| Microbe | Sealant Material | Controls | |||
|---|---|---|---|---|---|
| S1 | S2 | S3 | Positive (CHX) | Negative (No Seal) | |
| Staphylococcus spp. | 66.7 | 83.3 | 33.3 | 66.7 | 100.0 |
| Candida spp. | 0.0 | 83.3 | 66.7 | 33.3 | 91.7 |
| Seal | Controls | |||||
|---|---|---|---|---|---|---|
| Positive (CHX) | Negative (No Seal) | |||||
| z | 95% CI | p | z | 95% CI | p | |
| S1 | 0.0 | 34.9; 90.1 | 1.00 | 36.4 | 34.9; 90.1 | <0.0001 * |
| S2 | 1.2 | 51.6; 97.9 | 0.22 | 18.2 | 51.6; 97.9 | <0.0001 * |
| S3 | 2.5 | 9.9; 65.1 | 0.01 * | 73.0 | 9.9; 65.1 | <0.0001 * |
| Seal | Controls | |||||
|---|---|---|---|---|---|---|
| Positive (CHX) | Negative (No Seal) | |||||
| z | 95% CI | p | z | 95% CI | p | |
| S1 | 2.4 | 0.0; 26.6 | 0.02 * | 11.5 | 0.0; 26.6 | <0.0001 * |
| S2 | 3.7 | 51.6; 97.9 | 0.0002 * | 1.1 | 51.6; 97.9 | 0.29 |
| S3 | 2.5 | 34.9; 90.1 | 0.01 * | 3.1 | 34.9; 90.1 | 0.002 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smojver, I.; Vuletić, M.; Gerbl, D.; Budimir, A.; Sušić, M.; Gabrić, D. Evaluation of Antimicrobial Efficacy and Permeability of Various Sealing Materials at the Implant–Abutment Interface—A Pilot In Vitro Study. Materials 2021, 14, 385. https://doi.org/10.3390/ma14020385
Smojver I, Vuletić M, Gerbl D, Budimir A, Sušić M, Gabrić D. Evaluation of Antimicrobial Efficacy and Permeability of Various Sealing Materials at the Implant–Abutment Interface—A Pilot In Vitro Study. Materials. 2021; 14(2):385. https://doi.org/10.3390/ma14020385
Chicago/Turabian StyleSmojver, Igor, Marko Vuletić, Dražena Gerbl, Ana Budimir, Mato Sušić, and Dragana Gabrić. 2021. "Evaluation of Antimicrobial Efficacy and Permeability of Various Sealing Materials at the Implant–Abutment Interface—A Pilot In Vitro Study" Materials 14, no. 2: 385. https://doi.org/10.3390/ma14020385
APA StyleSmojver, I., Vuletić, M., Gerbl, D., Budimir, A., Sušić, M., & Gabrić, D. (2021). Evaluation of Antimicrobial Efficacy and Permeability of Various Sealing Materials at the Implant–Abutment Interface—A Pilot In Vitro Study. Materials, 14(2), 385. https://doi.org/10.3390/ma14020385






