The Use of Anodic Oxides in Practical and Sustainable Devices for Energy Conversion and Storage
Abstract
1. Introduction
2. General Aspects of Anodic Oxide Synthesis for Energy Applications
- The anodic oxides are generally synthesized in environmentally friendly experimental conditions (mild temperature synthesis and low toxicity substances applied);
- They offer facile control of synthesis parameters, such as domain morphology, composition, and structure with the potential to anchor specific catalyst substances to use them as anode or cathode based-materials;
- The materials present a high surface area per volume;
- They allow facile modulation of nanostructure architecture to enhance ion transport;
- The mostly anodic oxides are chemically stable;
- In most cases, they offer excellent adhesion between different layers, avoiding binder agents.
- Substrate: composition, purity, rugosity, and surface defects.
- Electrolyte: composition, temperature, and stirring.
- Electrical parameters: galvanostatic, potentiostatic, potentiodynamic, pulsed, or hybrid methods.
- Synthetic route: one-step, two-step or multi-step anodization.
- Anodizing time.
3. Photovoltaic Devices for Energy Conversion: Solar Cells
3.1. Dye-Sensitized Solar Cells (DSSCs)
3.2. Other Functionalities of Anodic Oxides in Silicon, PSC, and OPV Solar Cells
4. Photoelectrochemical Devices for H2 Production: PEC Water-Splitting Cells
5. Electrochemical Devices for Energy Conversion: Fuel Cells
5.1. Proton-Exchange-Membrane or Polymer-Electrolyte-Membrane Fuel Cells (PEMFC)
5.2. Direct Methanol Fuel Cell (DMFC)
5.3. Direct Formic Acid Fuel Cells (DFAFC)
5.4. Solid Oxide Fuel Cell (SOFC)
5.5. Microbial Fuel Cell (MFC)
6. Energy Storage Devices: Supercapacitors and Batteries
6.1. Supercapacitors
6.2. Rechargeable Batteries
7. General Remarks
8. Technological Aspects and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| AAO | Anodic Aluminum Oxide |
| ABPE | Applied Bias Photon-To-Current Efficiency |
| AC | Activated Carbon |
| ALD | Atomic Layer Deposition |
| APCE | Absorbed Photon-To-Current Efficiency |
| ASC | Asymmetrical Supercapacitors |
| CIGS | Copper Indium Gallium Di-Selenide |
| Co-Pi | Co Phosphate |
| CNT | Carbon nanotubes |
| CVD | Chemical Vapor Deposition |
| CZTS | Cu2ZnSnS4 |
| DFAFC | Direct Formic Acid Fuel Cells |
| DMFC | Direct Methanol Fuel Cell |
| DSSC | Dye-Sensitized Solar Cells |
| EDLC | Electric Double-Layer Capacitor |
| EG | Ethylene glycol |
| EIS | Electrochemical Impedance Spectroscopy |
| FDSSC | Flexible Fiber-type Dye-Sensitized Solar Cell |
| FESEM | Field Emission Scanning Electron Microscopy |
| FTO | Fluorine-doped Tin Oxide |
| HER | Hydrogen Evolution Reaction |
| IPCE | Incident Photon-to-Current Efficiency |
| Jsc | Short-Circuit Current Density |
| LIB | Lithium-Ion Batteries |
| LSV | Linear Stripping Voltammetric |
| MFC | Microbial Fuel Cell |
| MMA-PEG | Monomer methyl ether methacrylate poly (ethylene glycol) |
| NP | Nanoparticle |
| NT | Nanotube |
| OER | Oxygen Evolution Reaction |
| OPV | Organic Photovoltaic |
| ORR | Oxygen Reduction Reaction |
| PEC | Photoelectrochemical |
| PEMFC | Proton-Exchange Membrane Fuel Cell or Polymer-Electrolyte Membrane Fuel Cell |
| PSC | Perovskite Solar Cell |
| PTAA | Poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] |
| PV | Photovoltaic |
| QD | Quantum Dot |
| QDSC | Quantum Dot Solar Cell |
| QE | Quantum Efficiency |
| SIB | Sodium-Ion Battery |
| SOFC | Solid Oxide Fuel Cell |
| SPR | Surface Plasmon Resonance |
| SS | Solar Simulated |
| STH | Solar-to-Hydrogen Conversion Efficiency |
| TEM | Transmission Electron Microscopy |
| TNP | TiO2 nanoparticles |
| TNT | TiO2 nanotubes |
| TRL | Technological Readiness Level |
| Voc | Open-Circuit Voltage |
| XPS | X-Ray Photoelectron Microscopy |
| XRD | X-Ray Diffraction |
| YSZ | Y2O3−stabilized ZrO2 |
| ZIB | Zn-ion battery |
| η | Overall Solar Cell Efficiency |
References
- Smith, Y.R.; Ray, R.S.; Carlson, K.; Sarma, B.; Misra, M. Self-Ordered Titanium Dioxide Nanotube Arrays: Anodic Synthesis and Their Photo/Electro-Catalytic Applications. Materials 2013, 6, 2892–2957. [Google Scholar] [CrossRef] [PubMed]
- Trivinho-Strixino, F.; Santos, J.S.; Souza Sikora, M. 3-Electrochemical Synthesis of Nanostructured Materials. In Nanostructures; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; William Andrew Publishing: Park Ridge, NJ, USA, 2017; pp. 53–103. [Google Scholar] [CrossRef]
- Sulka, G.D. Highly Ordered Anodic Porous Alumina Formation by Self-Organized Anodizing. In Nanostructured Materials in Electrochemistry; Eftekhari, A., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–116. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.Y.; Li, H.Q.; Zhao, A.Z.; Wang, Y.; Zhang, K.Q.; Sun, H.T.; Lai, Y.K. Recent advances on smart TiO2 nanotube platforms for sustainable drug delivery applications. Int. J. Nanomed. 2017, 12, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Awad, N.K.; Edwards, S.L.; Morsi, Y.S. A review of TiO2 NTs on Ti metal: Electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C 2017, 76, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous Anodic Alumina: A Versatile Platform for Optical Biosensors. Materials 2014, 7, 4297–4320. [Google Scholar] [CrossRef]
- Barzegar, S.; Absalan, G.; Moradi, M.; Behaein, S. Constructing geometrically-ordered alumina nanoporous filters and alumina nanowire arrays by using ultrahigh voltage two step anodization. Phys. E Low Dimens. Syst. Nanostruct. 2020, 117, 113789. [Google Scholar] [CrossRef]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A Review of Photocatalysis using Self-organized TiO2 Nanotubes and Other Ordered Oxide Nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Zeng, Y.; Wu, H.; Chen, X.; Fan, Y.; Yang, H.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. Synthesis, surface properties, crystal structure and dye-sensitized solar cell performance of TiO2 nanotube arrays anodized under different parameters. Results Phys. 2019, 15, 102609. [Google Scholar] [CrossRef]
- Ampelli, C.; Tavella, F.; Perathoner, S.; Centi, G. Engineering of photoanodes based on ordered TiO2-nanotube arrays in solar photo-electrocatalytic (PECa) cells. Chem. Eng. J. 2017, 320, 352–362. [Google Scholar] [CrossRef]
- Feng, H.; Liang, Y.; Guo, K.; Chen, W.; Shen, D.; Huang, L.; Zhou, Y.; Wang, M.; Long, Y. TiO2 Nanotube Arrays Modified Titanium: A Stable, Scalable, and Cost-Effective Bioanode for Microbial Fuel Cells. Environ. Sci. Technol. Lett. 2016, 3, 420–424. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Chen, J.-Y.; Li, H.; Wang, C.-R. Recent progress in Li-ion batteries with TiO2 nanotube anodes grown by electrochemical anodization. Rare Met. 2020. [Google Scholar] [CrossRef]
- Auer, A.; Kunze-Liebhäuser, J. Recent Progress in Understanding Ion Storage in Self-Organized Anodic TiO2 Nanotubes. Small Methods 2019, 3, 1800385. [Google Scholar] [CrossRef]
- Auñón, Á.; Esteban, J.; Doadrio, A.L.; Boiza-Sánchez, M.; Mediero, A.; Eguibar-Blázquez, D.; Cordero-Ampuero, J.; Conde, A.; Arenas, M.-Á.; de-Damborenea, J.-J.; et al. Staphylococcus aureus Prosthetic Joint Infection Is Prevented by a Fluorine- and Phosphorus-Doped Nanostructured Ti–6Al–4V Alloy Loaded With Gentamicin and Vancomycin. J. Orthop. Res. 2020, 38, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, C.; Li, S.; Xi, Y.; Cai, C.; Liu, W.; Golosov, D.; Zavadski, S.; Melnikov, S. Preparation of Fe3+ Doped High-Ordered TiO2 Nanotubes Arrays with Visible Photocatalytic Activities. Nanomaterials 2020, 10, 2107. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Kim, J.-Y. Highly Ordered TiO2 Nanotube Electrodes for Efficient Quasi-Solid-State Dye-Sensitized Solar Cells. Energies 2020, 13, 6100. [Google Scholar] [CrossRef]
- Xiao, B.-C.; Lin, L.-Y. Substrate Diameter-Dependent Photovoltaic Performance of Flexible Fiber-Type Dye-Sensitized Solar Cells with TiO2 Nanoparticle/TiO2 Nanotube Array Photoanodes. Nanomaterials 2020, 10, 13. [Google Scholar] [CrossRef]
- Rezaei, B.; Mohammadi, I.; Ensafi, A.A.; Momeni, M.M. Enhanced efficiency of DSSC through AC-electrophoretic hybridization of TiO2 nanoparticle and nanotube. Electrochim. Acta 2017, 247, 410–419. [Google Scholar] [CrossRef]
- Stoll, T.; Zafeiropoulos, G.; Tsampas, M.N. Solar fuel production in a novel polymeric electrolyte membrane photoelectrochemical (PEM-PEC) cell with a web of titania nanotube arrays as photoanode and gaseous reactants. Int. J. Hydrogen Energy 2016, 41, 17807–17817. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.; Pan, D.; Li, G. Nanotube array-like WO3/W photoanode fabricated by electrochemical anodization for photoelectrocatalytic overall water splitting. Chin. J. Catal. 2017, 38, 2132–2140. [Google Scholar] [CrossRef]
- Lu, H.; Yan, Y.; Zhang, M.; Tan, H.; Geng, P.; Le, S.; Yang, Z.; Liu, Y. The effects of adjusting pulse anodization parameters on the surface morphology and properties of a WO3 photoanode for photoelectrochemical water splitting. J. Solid State Electrochem. 2018, 22, 2169–2181. [Google Scholar] [CrossRef]
- Lv, X.; Rodriguez, I.; Hu, C.; Shang, J.; Sit, P.H.L.; Ye, C.; Oskam, G.; Teoh, W.Y. Modulated anodization synthesis of Sn-doped iron oxide with enhanced solar water splitting performance. Mater. Today Chem. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O Nanowire Photocathodes for Efficient and Durable Solar Water Splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, X.; Li, S.; Zhang, B.; Wang, M.; Shen, Y. Carbon coated Cu2O nanowires for photo-electrochemical water splitting with enhanced activity. Appl. Surf. Sci. 2015, 358, 404–411. [Google Scholar] [CrossRef]
- Ho, W.-J.; Hsiao, K.-Y.; Hu, C.-H.; Chuang, T.-W.; Liu, J.-J.; Chen, Y.-H. Characterized plasmonic effects of various metallic nanoparticles on silicon solar cells using the same anodic aluminum oxide mask for film deposition. Thin Solid Films 2017, 631, 64–71. [Google Scholar] [CrossRef]
- Kwon, H.-C.; Kim, A.; Lee, H.; Lee, D.; Jeong, S.; Moon, J. Parallelized Nanopillar Perovskites for Semitransparent Solar Cells Using an Anodized Aluminum Oxide Scaffold. Adv. Energy Mater. 2016, 6, 1601055. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wong, Y.C.; Song, G.; Zhu, D.M.; Wen, C. Improvement on electrochemical performances of nanoporous titania as anode of lithium-ion batteries through annealing of pure titanium foils. J. Energy Chem. 2018, 27, 250–263. [Google Scholar] [CrossRef]
- Ni, J.; Fu, S.; Wu, C.; Maier, J.; Yu, Y.; Li, L. Self-Supported Nanotube Arrays of Sulfur-Doped TiO2 Enabling Ultrastable and Robust Sodium Storage. Adv. Mater. 2016, 28, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Haskul, M.; Ülgen, A.T.; Döner, A. Fabrication and characterization of Ni modified TiO2 electrode as anode material for direct methanol fuel cell. Int. J. Hydrogen Energy 2020, 45, 4860–4874. [Google Scholar] [CrossRef]
- Manikandan, M.; Vedarajan, R.; Kodiyath, R.; Abe, H.; Ueda, S.; Dakshnamoorthy, A.; Rajalakshmi, N.; Dhathathreyan, K.S.; Ramesh, G.V. Pt Decorated Free-Standing TiO2 Nanotube Arrays: Highly Active and Durable Electrocatalyst for Oxygen Reduction and Methanol Oxidation Reactions. J. Nanosci. Nanotechnol. 2016, 16, 8269–8278. [Google Scholar] [CrossRef]
- Ahmed, F.; Pervez, S.A.; Aljaafari, A.; Alshoaibi, A.; Abuhimd, H.; Oh, J.; Koo, B.H. Fabrication of TiO2-Nanotube-Array-Based Supercapacitors. Micromachines 2019, 10, 742. [Google Scholar] [CrossRef]
- Appadurai, T.; Subramaniyam, C.H.; Kuppusamy, R.; Karazhanov, S.; Subramanian, B. Electrochemical Performance of Nitrogen-Doped TiO2 Nanotubes as Electrode Material for Supercapacitor and Li-Ion Battery. Molecules 2019, 24, 2952. [Google Scholar] [CrossRef]
- United-Nations. The Sustainable Development Goals Report 2020; United Nations Publications: New York, NY, USA, 2020. [Google Scholar]
- Giziński, D.; Brudzisz, A.; Santos, J.S.; Trivinho-Strixino, F.; Stępniowski, W.J.; Czujko, T. Nanostructured Anodic Copper Oxides as Catalysts in Electrochemical and Photoelectrochemical Reactions. Catalysts 2020, 10, 1338. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TrAC Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Kumeria, T.; Santos, A.; Losic, D. Nanoporous Anodic Alumina Platforms: Engineered Surface Chemistry and Structure for Optical Sensing Applications. Sensors 2014, 14, 11878–11918. [Google Scholar] [CrossRef] [PubMed]
- AbdElazim, M.M.; Alaa, M.A.-E.; Waleed, A.E.-S.; Tesleem, B.A. Review on the Formation of Anodic Metal Oxides and their Sensing Applications. Curr. Nanosci. 2019, 15, 6–26. [Google Scholar] [CrossRef]
- Li, H.-H.; Chen, R.-F.; Ma, C.; Zhang, S.-L.; An, Z.-F.; Huang, W. Titanium Oxide Nanotubes Prepared by Anodic Oxidation and Their Application in Solar Cells. Acta Phys. Chim. Sin. 2011, 27, 1017–1025. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Cao, S.; Huang, L.; Chen, L. Ordered Organic Nanostructures Fabricated from Anodic Alumina Oxide Templates for Organic Bulk-Heterojunction Photovoltaics. Macromol. Chem. Phys. 2014, 215, 584–596. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Jiang, D.-J.; Gong, Z.-Q.; Li, J.-Y.; Wang, L.-N. Anodized metal oxide nanostructures for photoelectrochemical water splitting. Int. J. Miner. Metall. Mater. 2020, 27, 584–601. [Google Scholar] [CrossRef]
- Cheng, G.; Bai, Q.; Si, C.; Yang, W.; Dong, C.; Wang, H.; Gao, Y.L.; Zhang, Z. Nickel oxide nanopetal-decorated 3D nickel network with enhanced pseudocapacitive properties. RSC Adv. 2015, 5, 15042–15051. [Google Scholar] [CrossRef]
- Stepniowski, W.J.; Misiolek, W.Z. Review of Fabrication Methods, Physical Properties, and Applications of Nanostructured Copper Oxides Formed via Electrochemical Oxidation. Nanomaterials 2018, 8, 379. [Google Scholar] [CrossRef]
- Losic, D.; Santos, A. Nanoporous Alumina Fabrication, Structure, Properties and Applications; Springer: London, UK, 2015; Volume 219. [Google Scholar]
- Macak, J.M.; Schmuki, P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim. Acta 2006, 52, 1258–1264. [Google Scholar] [CrossRef]
- Sulka, G.D. Nanostructured Anodic Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Buyukaksoy, A.; Fürstenhaupt, T.; Birss, V.I. First-time electrical characterization of nanotubular ZrO2 films for micro-solid oxide fuel cell applications. Nanoscale 2015, 7, 8428–8437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Zhang, Y.; Qian, X.; Niu, R.; Hu, R.; Zhu, X.; Wang, X.; Zhu, J. The enhanced adhesion between overlong TiNxOy/MnO2 nanoarrays and Ti substrate: Towards flexible supercapacitors with high energy density and long service life. Nano Energy 2018, 43, 91–102. [Google Scholar] [CrossRef]
- Cheong, Y.L.; Beh, K.P.; Yam, F.K.; Hassan, Z. Performance evaluation of titanium dioxide based dye-sensitized solar cells under the influence of anodization steps, nanotube length and ionic liquid-free redox electrolyte solvents. Superlattices Microstruct. 2016, 94, 74–84. [Google Scholar] [CrossRef]
- Gao, Y.; Lin, Y.; Peng, Z.; Zhou, Q.; Fan, Z. Accelerating ion diffusion with unique three-dimensionally interconnected nanopores for self-membrane high-performance pseudocapacitors. Nanoscale 2017, 9, 18311–18317. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, J.; Lee, M.J.; Son, Y.J.; Jeong, J.; Chung, D.Y.; Lim, A.; Choe, H.; Park, H.S.; Sung, Y.-E. Electrochemical synthesis of nanoporous tungsten carbide and its application as electrocatalysts for photoelectrochemical cells. Nanoscale 2017, 9, 5413–5424. [Google Scholar] [CrossRef]
- Krumpmann, A.; Dervaux, J.; Derue, L.; Douhéret, O.; Lazzaroni, R.; Snyders, R.; Decroly, A. Influence of a sputtered compact TiO2 layer on the properties of TiO2 nanotube photoanodes for solid-state DSSCs. Mater. Des. 2017, 120, 298–306. [Google Scholar] [CrossRef]
- Hossain, M.A.; Oh, S.; Lim, S. Fabrication of dye-sensitized solar cells using a both-ends-opened TiO2 nanotube/nanoparticle hetero-nanostructure. J. Ind. Eng. Chem. 2017, 51, 122–128. [Google Scholar] [CrossRef]
- Saboo, T.; Tavella, F.; Ampelli, C.; Perathoner, S.; Genovese, C.; Marepally, B.C.; Veyre, L.; Quadrelli, E.A.; Centi, G. Water splitting on 3D-type meso/macro porous structured photoanodes based on Ti mesh. Sol. Energy Mater. Sol. Cells 2018, 178, 98–105. [Google Scholar] [CrossRef]
- Abraham, B.G.; Maniam, K.K.; Kuniyil, A.; Chetty, R. Electrocatalytic Performance of Palladium Dendrites Deposited on Titania Nanotubes for Formic Acid Oxidation. Fuel Cells 2016, 16, 656–661. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Li, H.; Wang, J.; Li, M.; Chen, X. Enhanced performance of lithium ion batteries from self-doped TiO2 nanotube anodes via an adjustable electrochemical process. Electrochim. Acta 2019, 326, 134972. [Google Scholar] [CrossRef]
- Salian, G.D.; Koo, B.M.; Lefevre, C.; Cottineau, T.; Lebouin, C.; Tesfaye, A.T.; Knauth, P.; Keller, V.; Djenizian, T. Niobium Alloying of Self-Organized TiO2 Nanotubes as an Anode for Lithium-Ion Microbatteries. Adv. Mater. Technol. 2018, 3, 1700274. [Google Scholar] [CrossRef]
- Pisarek, M.; Kędzierzawski, P.; Andrzejczuk, M.; Hołdyński, M.; Mikołajczuk-Zychora, A.; Borodziński, A.; Janik-Czachor, M. TiO2 Nanotubes with Pt and Pd Nanoparticles as Catalysts for Electro-Oxidation of Formic Acid. Materials 2020, 13, 1195. [Google Scholar] [CrossRef]
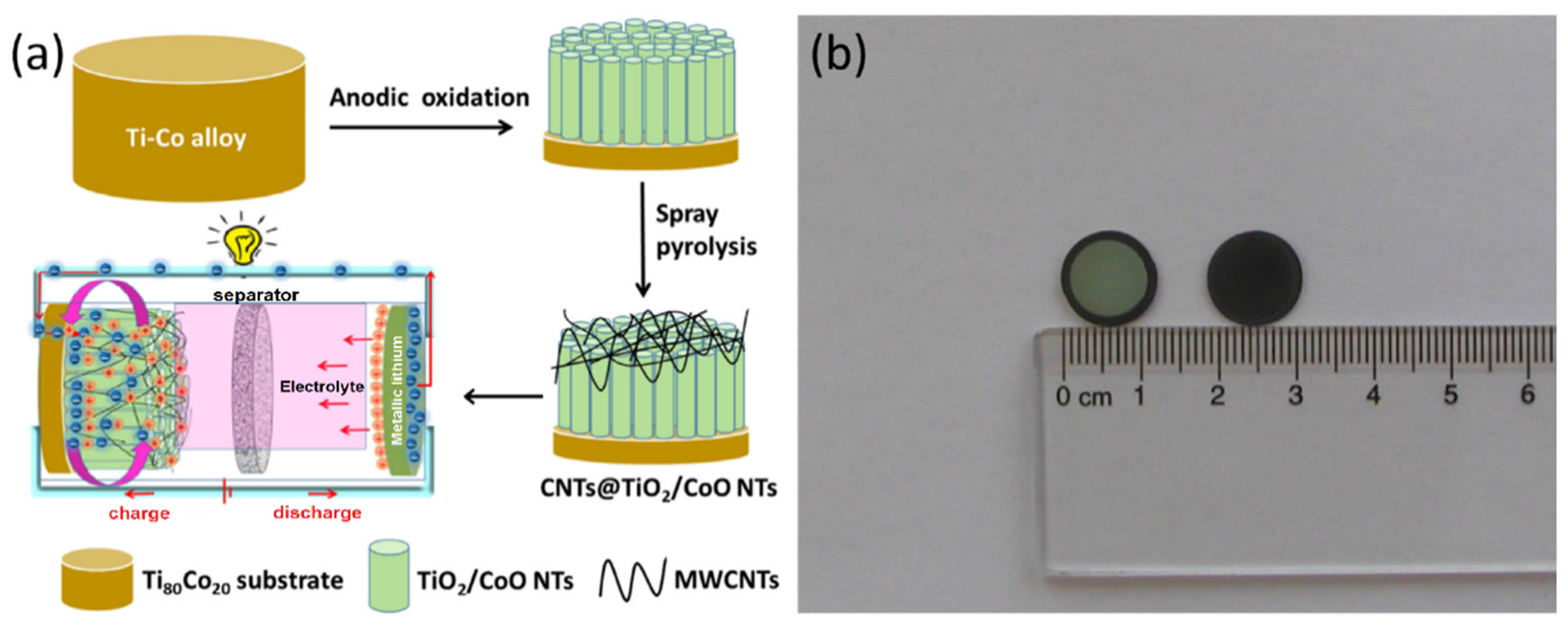
- Madian, M.; Ummethala, R.; El Naga, A.O.A.; Ismail, N.; Rümmeli, M.H.; Eychmüller, A.; Giebele, L. Ternary CNTs@TiO2/CoO Nanotube Composites: Improved Anode Materials for High Performance Lithium Ion Batteries. Materials 2017, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wang, C.; Xu, P.; Feng, C.; Si, S.; Zhang, Y.; Wang, Q.; Shi, M.; Yang, F.; Wang, J.; et al. Effects of the Structure of TiO2 Nanotube Arrays on Its Catalytic Activity for Microbial Fuel Cell. Glob. Chall. 2019, 3, 1800084. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the Photonic Crystal Properties of TiO2 Nanotube Arrays To Enhance Photocatalytic Hydrogen Production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Shen, D.; Li, N.; Ge, Z.; Zhou, Y.; Zhou, M.; Feng, H.; Guo, K. Effect of heat-treatment atmosphere on the current generation of TiO2 nanotube array electrodes in microbial fuel cells. Electrochim. Acta 2017, 257, 203–209. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Chandra Sil, M.; Chen, C.-M. Increasing solar light efficiency by engineering cell structures with modified Ti foil and specific concentrations of electrolyte in liquid dye-sensitized solar cells. Electrochim. Acta 2020, 334, 135631. [Google Scholar] [CrossRef]
- Hong, S.-T.; Lin, L.-Y. Fabrication of TiO2 nanoparticle/TiO2 microcone array photoanode for fiber-type dye-sensitized solar cells: Effect of acid concentration on morphology of microcone. Electrochim. Acta 2020, 331, 135278. [Google Scholar] [CrossRef]
- Ait Ali Yahia, S.; Hamadou, L.; Salar-García, M.J.; Kadri, A.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; Pérez de los Rios, A.; Benbrahim, N. TiO2 nanotubes as alternative cathode in microbial fuel cells: Effect of annealing treatment on its performance. Appl. Surf. Sci. 2016, 387, 1037–1045. [Google Scholar] [CrossRef]
- Ho, W.-J.; Cheng, P.-Y.; Hsiao, K.-Y. Plasmonic silicon solar cell based on silver nanoparticles using ultra-thin anodic aluminum oxide template. Appl. Surf. Sci. 2015, 354, 25–30. [Google Scholar] [CrossRef]
- Mendes, L.F.; Moraes, A.S.; Santos, J.S.; Leite, F.L.; Trivinho-Strixino, F. Investigation of roughness and specular quality of commercial aluminum (6061 alloy) for fabrication of nanoporous anodic alumina films. Surf. Coat. Technol. 2017, 310, 199–206. [Google Scholar] [CrossRef]
- Kim, J.; Yang, W.; Oh, Y.; Kim, J.; Moon, J. Template-directed fabrication of vertically aligned Cu2ZnSnS4 nanorod arrays for photoelectrochemical applications via a non-toxic solution process. J. Alloys Compd. 2017, 691, 457–465. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Shaban, S.A.; El Sayed, H.A.; Alanadouli, B.E.; Allam, N.K. Morphology–photoactivity relationship: WO3 nanostructured films for solar hydrogen production. Int. J. Hydrogen Energy 2016, 41, 866–872. [Google Scholar] [CrossRef]
- Choi, Y.-W.; Kim, S.; Seong, M.; Yoo, H.; Choi, J. NH4-doped anodic WO3 prepared through anodization and subsequent NH4OH treatment for water splitting. Appl. Surf. Sci. 2015, 324, 414–418. [Google Scholar] [CrossRef]
- Das, P.K.; Arunachalam, M.; Seo, Y.J.; Ahn, K.-S.; Ha, J.-S.; Kang, S.H. Functional Blocking Layer of Twisted Tungsten Oxide Nanorod Grown by Electrochemical Anodization for Photoelectrochemical Water Splitting. J. Electrochem. Soc. 2020, 167, 066501. [Google Scholar] [CrossRef]
- Yang, X.; Qi, Y.; Liu, Y.; Zhou, Y.; Chen, K.; Zhou, B.; Chen, W. Self-Assembly Growth of 3D WO3 Framework with Interpenetrated Nanosheets as Binder-Free Anode for Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A2783–A2789. [Google Scholar] [CrossRef]
- Faid, A.Y.; Allam, N.K. Stable solar-driven water splitting by anodic ZnO nanotubular semiconducting photoanodes. RSC Adv. 2016, 6, 80221–80225. [Google Scholar] [CrossRef]
- Batista-Grau, P.; Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-Antón, J. Formation of ZnO nanowires by anodization under hydrodynamic conditions for photoelectrochemical water splitting. Surf. Coat. Technol. 2020, 381, 125197. [Google Scholar] [CrossRef]
- Miles, D.O.; Lee, C.S.; Cameron, P.J.; Mattia, D.; Kim, J.H. Hierarchical growth of TiO2 nanosheets on anodic ZnO nanowires for high efficiency dye-sensitized solar cells. J. Power Source 2016, 325, 365–374. [Google Scholar] [CrossRef]
- Kim, J.Y.; Liu, G.; Shim, G.Y.; Kim, H.; Lee, J.K. Functionalized Zn@ZnO Hexagonal Pyramid Array for Dendrite-Free and Ultrastable Zinc Metal Anodes. Adv. Funct. Mater. 2020, 30, 2004210. [Google Scholar] [CrossRef]
- Lucas-Granados, B.; Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-Antón, J. Influence of electrolyte temperature on the synthesis of iron oxide nanostructures by electrochemical anodization for water splitting. Int. J. Hydrogen Energy 2018, 43, 7923–7937. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Zhou, S.; Zhao, N.; Wong, C.-P. Facile and scalable fabrication of three-dimensional Cu(OH)2 nanoporous nanorods for solid-state supercapacitors. J. Mater. Chem. A 2015, 3, 17385–17391. [Google Scholar] [CrossRef]
- Kim, M.; Choi, I.; Kim, J.J. Facile electrochemical synthesis of heterostructured amorphous-Sn@CuxO nanowire anode for Li-ion batteries with high stability and rate-performance. Appl. Surf. Sci. 2019, 479, 225–233. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Liu, Y.; Li, W.; Lu, W.; Huang, H. Facile synthesis of a mechanically robust and highly porous NiO film with excellent electrocatalytic activity towards methanol oxidation. Nanoscale 2016, 8, 11256–11263. [Google Scholar] [CrossRef]
- Chamanzadeh, Z.; Noormohammadi, M.; Zahedifar, M. Enhanced photovoltaic performance of dye sensitized solar cell using TiO2 and ZnO nanoparticles on top of free standing TiO2 nanotube arrays. Mater. Sci. Semicond. Process. 2017, 61, 107–113. [Google Scholar] [CrossRef]
- Rasheed, M.A.; Rahimullah, R.; Uddin, S.K.; Khaliq, N.; Khan, Y.; Waheed, A.; Shah, A.; Mahmood, A.; Ali, G. Role of temperature and NiO addition in improving photocatalytic properties of TiO2 nanotubes. Appl. Nanosci. 2019, 9, 1731–1742. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Lee, G.; Choi, J. Fast-Charging and High Volumetric Capacity Anode Based on Co3O4/CuO@TiO2 Composites for Lithium-Ion Batteries. Chem. Eur. J. 2018, 24, 19045–19052. [Google Scholar] [CrossRef]
- Kulkarni, S.K. Nanothechnology: Principles and Practices, 3rd ed.; Springer: New York, NY, USA, 2015; p. 418. [Google Scholar]
- Sharma, S.; Jain, K.; Sharma, A. Solar Cells: In Research and Applications—A Review. Mater. Sci. Appl. 2015, 6, 1145–1155. [Google Scholar] [CrossRef]
- Alpay, N.; Benehkohal, N.P.; Côté, M.-P.; Demopoulos, G.P.; Brochu, M. Anodized aluminum–silicon alloy counter electrode substrates for next generation solar cell applications. Appl. Surf. Sci. 2015, 356, 317–324. [Google Scholar] [CrossRef]
- Lee, T.D.; Ebong, A.U. A review of thin film solar cell technologies and challenges. Renew. Sustain. Energy Rev. 2017, 70, 1286–1297. [Google Scholar] [CrossRef]
- Kang, G.; Bae, K.; Nam, M.; Ko, D.-H.; Kim, K.; Padilla, W.J. Broadband and ultrahigh optical haze thin films with self-aggregated alumina nanowire bundles for photovoltaic applications. Energy Environ. Sci. 2015, 8, 2650–2656. [Google Scholar] [CrossRef]
- Bjelajac, A.; Petrovic, R.; Socol, G.; Mihailescu, I.N.; Enculescu, M.; Grumezescu, V.; Pavlovic, V.; Janackovic, D. CdS quantum dots sensitized TiO2 nanotubes by matrix assisted pulsed laser evaporation method. Ceram. Int. 2016, 42, 9011–9017. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, J.-Y.; Kim, W.; Kang, S.H.; Ko, M.J. Comparative Study on the Photoanode Nanoarchitectures for Photovoltaic Application. Int. J. Precis. Eng. Manuf. Green Technol. 2020, 7, 69–76. [Google Scholar] [CrossRef]
- Mohammadpour, F.; Altomare, M.; So, S.; Lee, K.; Mokhtar, M.; Alshehri, A.; Al-Thabaiti, S.A.; Schmuki, P. High-temperature annealing of TiO2nanotube membranes for efficient dye-sensitized solar cells. Semicond. Sci. Technol. 2015, 31, 014010. [Google Scholar] [CrossRef]
- Cao, S.; Yu, D.; Lin, Y.; Zhang, C.; Lu, L.; Yin, M.; Zhu, X.; Chen, X.; Li, D. Light Propagation in Flexible Thin-Film Amorphous Silicon Solar Cells with Nanotextured Metal Back Reflectors. ACS Appl. Mater. Interfaces 2020, 12, 26184–26192. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Tang, X.; Huang, B.; Hu, T.; Tan, L.; Chen, L.; Chen, Y. Recent progress on the long-term stability of perovskite solar cells. Adv. Sci. 2018, 5, 1700387. [Google Scholar] [CrossRef]
- Kwon, H.-C.; Ma, S.; Yun, S.-C.; Jang, G.; Yang, H.; Moon, J. A nanopillar-structured perovskite-based efficient semitransparent solar module for power-generating window applications. J. Mater. Chem. A 2020, 8, 1457–1468. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Rho, W.-Y.; Lee, S.; Kim, S.; Hahn, Y.-B. TiO2 Nanoparticles/Nanotubes for Efficient Light Harvesting in Perovskite Solar Cells. Nanomaterials 2019, 9, 326. [Google Scholar] [CrossRef]
- Heo, J.H.; Shin, D.H.; Lee, M.L.; Kang, M.G.; Im, S.H. Efficient Organic–Inorganic Hybrid Flexible Perovskite Solar Cells Prepared by Lamination of Polytriarylamine/CH3NH3PbI3/Anodized Ti Metal Substrate and Graphene/PDMS Transparent Electrode Substrate. ACS Appl. Mater. Interfaces 2018, 10, 31413–31421. [Google Scholar] [CrossRef]
- Ke, W.; Kanatzidis, M.G. Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 2019, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jain, S.; Ps, V.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Li, R. Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical, and photovoltaic-photoelectrochemical solutions. Chin. J. Catal. 2017, 38, 5–12. [Google Scholar] [CrossRef]
- Grez, P.; Henríquez, R.; Muñoz, E.; Rojas, C.; Moreno, S.; Sessarego, G.; Heyser, C.; Celedón, C.; Schrebler, R. Sonoelectrochemical Synthesis of Nanostructured of p-Cu2O and n-Fe2O3 and Their Application for Photoelectrochemical Splitting of Water. Int. J. Electrochem. Sci. 2019, 14, 5646–5653. [Google Scholar] [CrossRef]
- Dias, P.; Lopes, T.; Meda, L.; Andrade, L.; Mendes, A. Photoelectrochemical water splitting using WO3 photoanodes: The substrate and temperature roles. Phys. Chem. Chem. Phys. 2016, 18, 5232–5243. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Ni-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 443, 321–328. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Facile fabrication of Si-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical hydrogen generation. Appl. Surf. Sci. 2018, 436, 125–133. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Ezati, F. Fabrication, characterization and photoelectrochemical activity of tungsten-copper co-sensitized TiO2 nanotube composite photoanodes. J. Colloid Interface Sci. 2018, 514, 70–82. [Google Scholar] [CrossRef]
- Su, J.Y.; Zhu, L.; Chen, G.H. Ultrasmall graphitic carbon nitride quantum dots decorated self-organized TiO2 nanotube arrays with highly efficient photoelectrochemical activity. Appl. Catal. B Environ. 2016, 186, 127–135. [Google Scholar] [CrossRef]
- Qiu, Y.; Pan, Z.; Chen, H.; Ye, D.; Guo, L.; Fan, Z.; Yang, S. Current progress in developing metal oxide nanoarrays-based photoanodes for photoelectrochemical water splitting. Sci. Bull. 2019, 64, 1348–1380. [Google Scholar] [CrossRef]
- Bendova, M.; Gispert-Guirado, F.; Hassel, A.W.; Llobet, E.; Mozalev, A. Solar water splitting on porous-alumina-assisted TiO2-doped WOx nanorod photoanodes: Paradoxes and challenges. Nano Energy 2017, 33, 72–87. [Google Scholar] [CrossRef]
- Guo, B.; Tian, L.; Xie, W.; Batool, A.; Xie, G.; Xiang, Q.; Jan, S.U.; Boddula, R.; Gong, J.R. Vertically Aligned Porous Organic Semiconductor Nanorod Array Photoanodes for Efficient Charge Utilization. Nano Lett. 2018, 18, 5954–5960. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Cai, Q.; Hong, W.; Li, J.; Liu, W. Enhanced hydrogen evolution reaction of MoOx/Mo cathode by loading small amount of Pt nanoparticles in alkaline solution. Int. J. Hydrogen Energy 2017, 42, 17030–17037. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’Mullane, A.P.; Kalantar-zadeh, K. Nanostructured copper oxide semiconductors: A perspective on materials, synthesis methods and applications. J. Mater. Chem. C 2014, 2, 5247–5270. [Google Scholar] [CrossRef]
- Stepniowski, W.J.; Misiolek, W.Z. 13-Nanostructured anodic films grown on copper: A review of fabrication techniques and applications. In Nanostructured Anodic Metal Oxides; Sulka, G.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 415–452. [Google Scholar] [CrossRef]
- Doménech-Carbó, A. Electrochemistry of Porous Materials; CRC Press: Boca Raton, FL, USA, 2010; p. 340. [Google Scholar]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Roussak, O.V.; Gesser, H.D. Applied Chemistry: A Textbook for Engineers and Technologists, 2nd ed.; Springer: New York, NY, USA, 2013; p. 383. [Google Scholar] [CrossRef]
- Liang, J.; Luo, Y.; Zheng, S.; Wang, D. Enhance performance of micro direct methanol fuel cell by in situ CO2 removal using novel anode flow field with superhydrophobic degassing channels. J. Power Source 2017, 351, 86–95. [Google Scholar] [CrossRef]
- Kwon, C.-W.; Lee, J.-I.; Kim, K.-B.; Lee, H.-W.; Lee, J.-H.; Son, J.-W. The thermomechanical stability of micro-solid oxide fuel cells fabricated on anodized aluminum oxide membranes. J. Power Source 2012, 210, 178–183. [Google Scholar] [CrossRef]
- Yu, X.; Pickup, P.G. Recent advances in direct formic acid fuel cells (DFAFC). J. Power Source 2008, 182, 124–132. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Source 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Tamilselvan, A.; Balakumar, S. Anatase TiO2 nanotube by electrochemical anodization method: Effect of tubes dimension on the supercapacitor application. Ionics 2016, 22, 99–105. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Shaikh, J.S.; Bhat, T.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Hydrothermally grown 3D hierarchical TiO2 based on electrochemically anodized 1D TiO2 nanostructure for supercapacitor. Appl. Phys. A 2018, 124, 592. [Google Scholar] [CrossRef]
- Savilov, S.; Ivanov, A.; Arkhipova, E.; Egorov, A.; Lunin, V. Pseudocapacity of N-doped and polymer modified carbon nanomaterials in non-aqueous media. Mater. Technol. 2014, 29, A98–A106. [Google Scholar] [CrossRef]
- Dou, Q.; Li, Y.; Ming Ng, K. CoO/CoFe2O4 core/shell nanoparticles assembled in carbon sheets as anode materials for lithium ion battery. J. Alloys Compd. 2019, 808, 151691. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, C.; Hao, Y.; Zhang, X.; Agrawal, R.; Zhao, W.; Wang, C.; Yu, H.; Zhu, X.; Yu, Y.; et al. Fabrication of three-dimensional porous ZnMn2O4 thin films on Ni foams through electrostatic spray deposition for high-performance lithium-ion battery anodes. J. Alloys Compd. 2017, 696, 1174–1179. [Google Scholar] [CrossRef]
- Wang, S.; Hu, J.; Jiang, L.; Li, X.; Cao, J.; Wang, Q.; Wang, A.; Li, X.; Qu, L.; Lu, Y. High–performance 3D CuO/Cu flowers supercapacitor electrodes by femtosecond laser enhanced electrochemical anodization. Electrochim. Acta 2019, 293, 273–282. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Z.; Yao, Z.; Meng, Y.; Xia, Q.; Li, D.; Jiang, Z. Ultrahigh capacitance of TiO2 nanotube arrays/C/MnO2 electrode for supercapacitor. J. Alloys Compd. 2019, 805, 396–403. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, X.; Xiao, T.; Zhang, L.; Lv, P.; Zhao, J. Preparation and properties of MnO2–TiO2 nanotube array composite electrodes using titanium foam as the current collector. Int. J. Hydrogen Energy 2018, 43, 8859–8867. [Google Scholar] [CrossRef]
- Qorbani, M.; Khajehdehi, O.; Sabbah, A.; Naseri, N. Ti-rich TiO2 Tubular Nanolettuces by Electrochemical Anodization for All-Solid-State High-Rate Supercapacitor Devices. ChemSusChem 2019, 12, 4064–4073. [Google Scholar] [CrossRef]
- Cao, S.; Huang, W.; Wu, L.; Tian, M.; Song, Y. On the Interfacial Adhesion between TiO2 Nanotube Array Layer and Ti Substrate. Langmuir 2018, 34, 13888–13896. [Google Scholar] [CrossRef]
- Thulasi, K.M.; Manikkoth, S.T.; Paravannoor, A.; Palantavida, S.; Bhagiyalakshmi, M.; Vijayan, B.K. Ceria deposited titania nanotubes for high performance supercapacitors. J. Phys. Chem. Solids 2019, 135, 109111. [Google Scholar] [CrossRef]
- Ray, R.S.; Sarma, B.; Jurovitzki, A.L.; Misra, M. Fabrication and characterization of titania nanotube/cobalt sulfide supercapacitor electrode in various electrolytes. Chem. Eng. J. 2015, 260, 671–683. [Google Scholar] [CrossRef]
- Yu, C.; Wang, Y.; Zhang, J.; Shu, X.; Cui, J.; Qin, Y.; Zheng, H.; Liu, J.; Zhang, Y.; Wu, Y. Integration of mesoporous nickel cobalt oxide nanosheets with ultrathin layer carbon wrapped TiO2 nanotube arrays for high-performance supercapacitors. New J. Chem. 2016, 40, 6881–6889. [Google Scholar] [CrossRef]
- Dong, C.; Bai, Q.; Cheng, G.; Zhao, B.; Wang, H.; Gao, Y.L.; Zhang, Z. Flexible and ultralong-life cuprous oxide microsphere-nanosheets with superior pseudocapacitive properties. RSC Adv. 2015, 5, 6207–6214. [Google Scholar] [CrossRef]
- Zou, Z.-B.; Xiong, X.-B.; Ma, J.; Zeng, X.-R.; Huang, T.; Li, J.-J.; Li, B. In situ two-step electrochemical preparation of fluoride-free nickel-based compound film on nickel plate for supercapacitors. Rare Met. 2016, 35, 930–936. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Altomare, M.; Eugénio, S.; Schmuki, P.; Silva, T.M.; Montemor, M.F. On the Supercapacitive Behaviour of Anodic Porous WO3-Based Negative Electrodes. Electrochim. Acta 2017, 232, 192–201. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Cha, G.; Hildebrand, H.; Schmuki, P.; Silva, T.M.; Montemor, M.F.; Altomare, M. Capacitance response in an aqueous electrolyte of Nb2O5 nanochannel layers anodically grown in pure molten o-H3PO4. Electrochim. Acta 2018, 281, 725–737. [Google Scholar] [CrossRef]
- Jin, B.; Hejazi, S.; Pyczak, F.; Oehring, M.; Mohajernia, S.; Kment, S.; Tomanec, O.; Zboril, R.; Nguyen, N.T.; Yang, M.; et al. Amorphous Mo–Ta Oxide Nanotubes for Long-Term Stable Mo Oxide-Based Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 45665–45673. [Google Scholar] [CrossRef]
- Khalifa, H.; El-Safty, S.A.; Reda, A.; Shenashen, M.A.; Eid, A.I. Anisotropic alignments of hierarchical Li2SiO3/TiO2 @nano-C anode//LiMnPO4@nano-C cathode architectures for full-cell lithium-ion battery. Natl. Sci. Rev. 2020, 7, 863–880. [Google Scholar] [CrossRef]
- Khalifa, H.; El-Safty, S.A.; Reda, A.; Elmarakbi, A.; Metawa, H.; Shenashen, M.A. Multifaceted geometric 3D mesopolytope cathodes and its directional transport gates for superscalable LIB models. Appl. Mater. Today 2020, 19. [Google Scholar] [CrossRef]
- Hassen, D.; Shenashen, M.A.; El-Safty, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A.; El-Safty, A.; Yamaguchi, H. Nitrogen-doped carbon-embedded TiO2 nanofibers as promising oxygen reduction reaction electrocatalysts. J. Power Source 2016, 330, 292–303. [Google Scholar] [CrossRef]
- Sugiawati, V.A.; Florence, V.; Djenizian, T. All-Solid-State Lithium Ion Batteries Using Self-Organized TiO2 Nanotubes Grown from Ti-6Al-4V Alloy. Molecules 2020, 25, 2121. [Google Scholar] [CrossRef] [PubMed]
- Rhee, O.; Lee, G.; Choi, J. Highly Ordered TiO2 Microcones with High Rate Performance for Enhanced Lithium-Ion Storage. ACS Appl. Mater. Interfaces 2016, 8, 14558–14563. [Google Scholar] [CrossRef] [PubMed]
- Cha, G.; Mohajernia, S.; Nguyen, N.T.; Mazare, A.; Denisov, N.; Hwang, I.; Schmuki, P. Li+ Pre-Insertion Leads to Formation of Solid Electrolyte Interface on TiO2 Nanotubes That Enables High-Performance Anodes for Sodium Ion Batteries. Adv. Energy Mater. 2020, 10, 1903448. [Google Scholar] [CrossRef]
- Ni, J.; Fu, S.; Yuan, Y.; Ma, L.; Jiang, Y.; Li, L.; Lu, J. Boosting Sodium Storage in TiO2 Nanotube Arrays through Surface Phosphorylation. Adv. Mater. 2018, 30, 1704337. [Google Scholar] [CrossRef] [PubMed]
- Kure-Chu, S.-Z.; Sakuyama, H.; Saito, S.; Miura, S.; Yashiro, H.; Hirahara, H.; Segawa, H.; Wada, K.; Inoue, S. Controllable Fabrication of Multi-tiered Nanoporous Anodic TiO2–TiN Composite Films as High-Performance Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2016, 212, 481–491. [Google Scholar] [CrossRef]
- Yoo, H.; Lee, G.; Choi, J. Binder-free SnO2–TiO2 composite anode with high durability for lithium-ion batteries. RSC Adv. 2019, 9, 6589–6595. [Google Scholar] [CrossRef]
- Meng, R.; Hou, H.; Liu, X.; Yan, C.; Duan, J.; Liu, S. High performance binder-free quaternary composite CuO/Cu/TiO2NT/Ti anode for lithium ion battery. Ceram. Int. 2016, 42, 6039–6045. [Google Scholar] [CrossRef]
- Xia, S.; Ni, J.; Savilov, S.V.; Li, L. Oxygen-deficient Ta2O5 nanoporous films as self-supported electrodes for lithium microbatteries. Nano Energy 2018, 45, 407–412. [Google Scholar] [CrossRef]
- Bian, H.; Dong, R.; Shao, Q.; Wang, S.; Yuen, M.-F.; Zhang, Z.; Yu, D.Y.W.; Zhang, W.; Lu, J.; Li, Y.Y. Water-enabled crystallization of mesoporous SnO2 as a binder-free electrode for enhanced sodium storage. J. Mater. Chem. A 2017, 5, 23967–23975. [Google Scholar] [CrossRef]
- Ni, J.; Wang, W.; Wu, C.; Liang, H.; Maier, J.; Yu, Y.; Li, L. Highly Reversible and Durable Na Storage in Niobium Pentoxide through Optimizing Structure, Composition, and Nanoarchitecture. Adv. Mater. 2017, 29, 1605607. [Google Scholar] [CrossRef]
- Wang, B.; Guo, W.; Fu, Y. Anodized Aluminum Oxide Separators with Aligned Channels for High-Performance Li–S Batteries. ACS Appl. Mater. Interfaces 2020, 12, 5831–5837. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Li, Z.; Xiao, X.; Schmuki, P.; Lu, J.; Li, Y.Y. Anodic Synthesis of Hierarchical SnS/SnOx Hollow Nanospheres and Their Application for High-Performance Na-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1901000. [Google Scholar] [CrossRef]
- Hasa, I.; Mariyappan, S.; Saurel, D.; Adelhelm, P.; Koposov, A.Y.; Masquelier, C.; Croguennec, L.; Casas-Cabanas, M. Challenges of today for Na-based batteries of the future: From materials to cell metrics. J. Power Source 2021, 482, 228872. [Google Scholar] [CrossRef]
- Yoo, G.W.; Kim, C.; Jang, B.C.; Yang, S.B.; Son, J.T. Synthesis of Hollow Nanorods of SiO2 Anode Material by AAO Template Synthesis Method for Lithium Ion Battery. J. Nanosci. Nanotechnol. 2015, 15, 8773–8776. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, S.; Ding, X.; Wang, Q.; He, P. Three-Dimensional Honeycomb-Structural LiAlO2-Modified LiMnPO4 Composite with Superior High Rate Capability as Li-Ion Battery Cathodes. ACS Appl. Mater. Interfaces 2018, 10, 10786–10795. [Google Scholar] [CrossRef]
- Fang, D.; Li, L.; Xu, W.; Zheng, H.; Xu, J.; Jiang, M.; Liu, R.; Jiang, X.; Luo, Z.; Xiong, C.; et al. High Capacity Lithium Ion Battery Anodes Using Sn Nanowires Encapsulated Al2O3 Tubes in Carbon Matrix. Adv. Mater. Interfaces 2016, 3, 1500491. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Wen, L.; Wang, C.; Zhao, H.; Mi, Y.; Liang, L.; Fu, Q.; Wu, M.; Lei, Y. Highly Ordered Three-Dimensional Ni-TiO2 Nanoarrays as Sodium Ion Battery Anodes. Chem. Mater. 2015, 27, 4274–4280. [Google Scholar] [CrossRef]
- Hu, G.; Sun, Z.; Shi, C.; Fang, R.; Chen, J.; Hou, P.; Liu, C.; Cheng, H.-M.; Li, F. A Sulfur-Rich Copolymer@CNT Hybrid Cathode with Dual-Confinement of Polysulfides for High-Performance Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1603835. [Google Scholar] [CrossRef]
- Qi, W.; Shapter, J.G.; Wu, Q.; Yin, T.; Gao, G.; Cui, D. Nanostructured anode materials for lithium-ion batteries: Principle, recent progress and future perspectives. J. Mater. Chem. A 2017, 5, 19521–19540. [Google Scholar] [CrossRef]
- Schoonman, J. Nanostructured materials in solid state ionics. Solid State Ion. 2000, 135, 5–19. [Google Scholar] [CrossRef]
- Kim, Y.D.; Choi, S.; Kim, A.; Lee, W. Ionic Current Rectification of Porous Anodic Aluminum Oxide (AAO) with a Barrier Oxide Layer. ACS Nano 2020, 14, 13727–13738. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ma, W.; Xu, L.; Hao, C.; Sun, M.; Wu, X.; Colombari, F.M.; de Moura, A.F.; Silva, M.C.; Carneiro-Neto, E.B.; et al. Self-Assembled Gold Arrays That Allow Rectification by Nanoscale Selectivity. Angew. Chem. Int. Ed. 2019, 58, 17418–17424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, L.; Lei, Y. A mini review: Functional nanostructuring with perfectly-ordered anodic aluminum oxide template for energy conversion and storage. Front. Chem. Sci. Eng. 2018, 12, 481–493. [Google Scholar] [CrossRef]
- Wei, Q.; Fu, Y.; Zhang, G.; Yang, D.; Meng, G.; Sun, S. Rational design of novel nanostructured arrays based on porous AAO templates for electrochemical energy storage and conversion. Nano Energy 2019, 55, 234–259. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow Carbon Nanofiber-Encapsulated Sulfur Cathodes for High Specific Capacity Rechargeable Lithium Batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef]
- Chakraverty, M. A Technological Review on Quantum Ballistic Transport Model Based Silicon Nanowire Field Effect Transistors for Circuit Simulation and Design. J. Nanosci. Nanoeng. Appl. 2015, 5, 20–31. [Google Scholar]
- He, L.; Wang, Y.; He, X.; Li, J.; He, Q.; Wei, D. The combined effects of anodization parameters on morphological of the porous silicon and the ballistic electron emission of PS-based emitter. Vacuum 2020, 171, 108998. [Google Scholar] [CrossRef]
- Chen, H.-F.; Gardner, D.M.; Carmieli, R.; Wasielewski, M.R. Controlling the orientation of spin-correlated radical pairs by covalent linkage to nanoporous anodic aluminum oxide membranes. Chem. Commun. 2013, 49, 8614–8616. [Google Scholar] [CrossRef]
- Nasirpouri, F.; Peighambari-Sattari, S.-M.; Bran, C.; Palmero, E.M.; Berganza Eguiarte, E.; Vazquez, M.; Patsopoulos, A.; Kechrakos, D. Geometrically designed domain wall trap in tri-segmented nickel magnetic nanowires for spintronics devices. Sci. Rep. 2019, 9, 9010. [Google Scholar] [CrossRef]
- Mankins, J.C. Technology Readiness Levels, a White Paper; NASA: Washington, DC, USA, 1995; Volume 6, p. 1995.
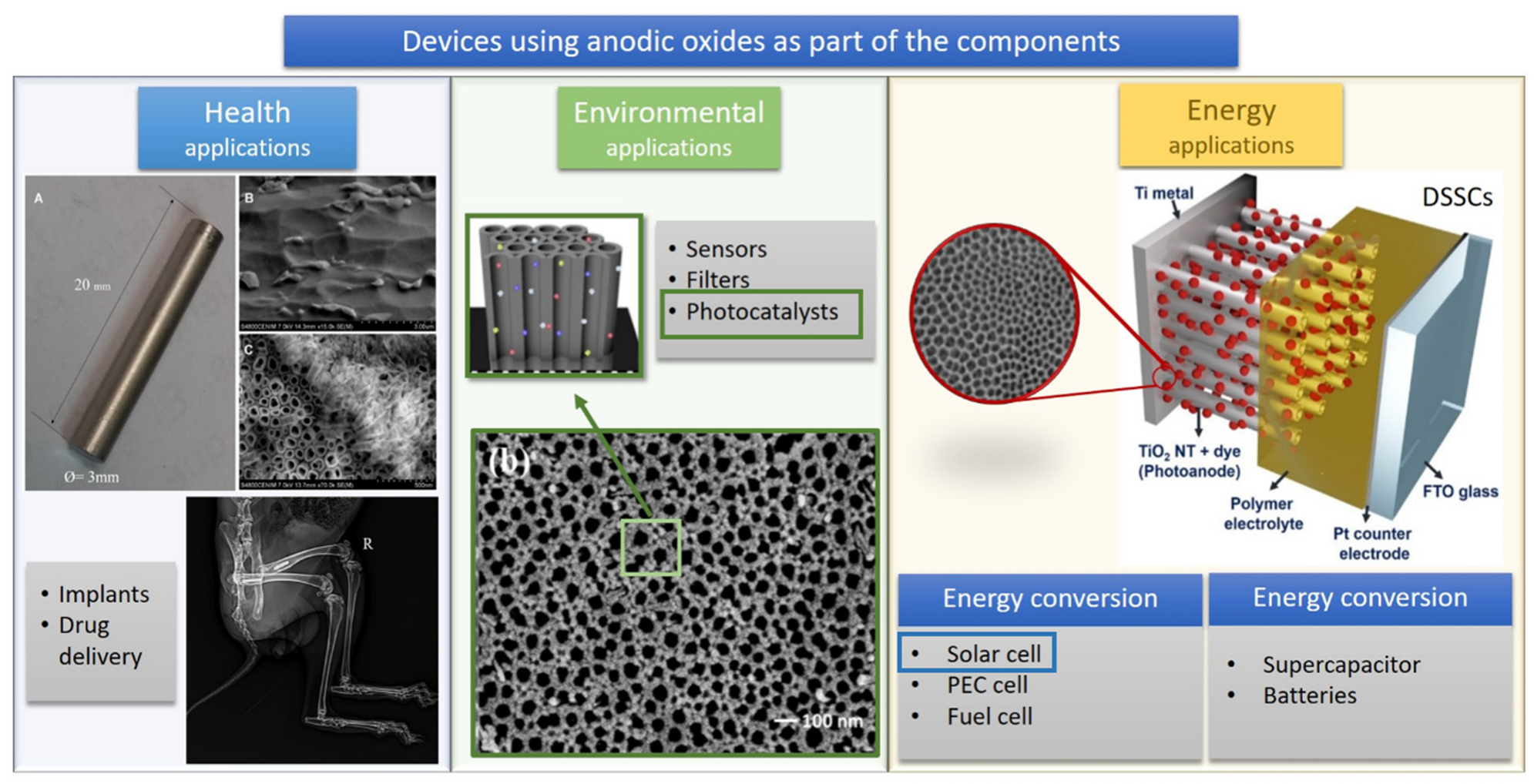
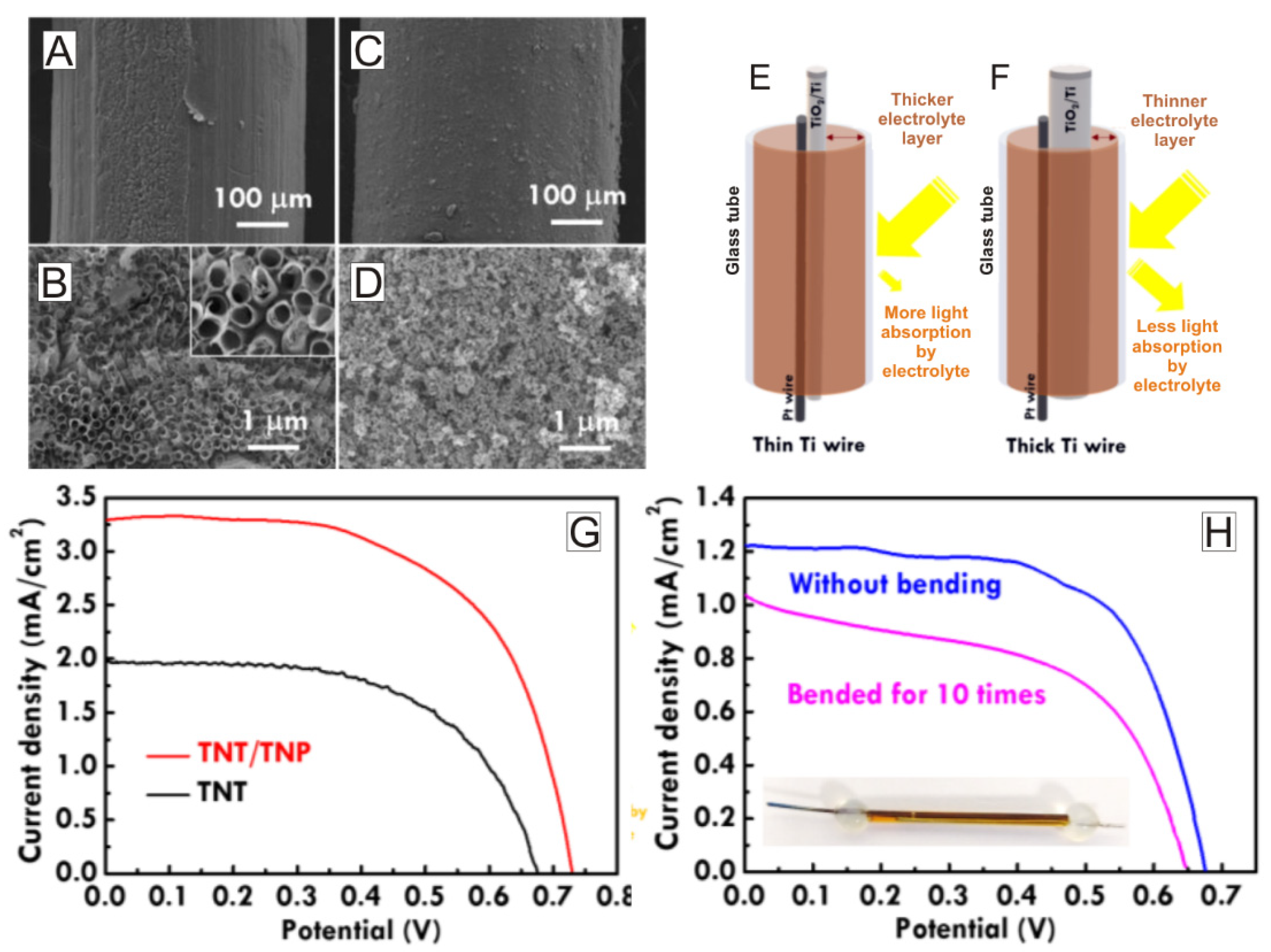
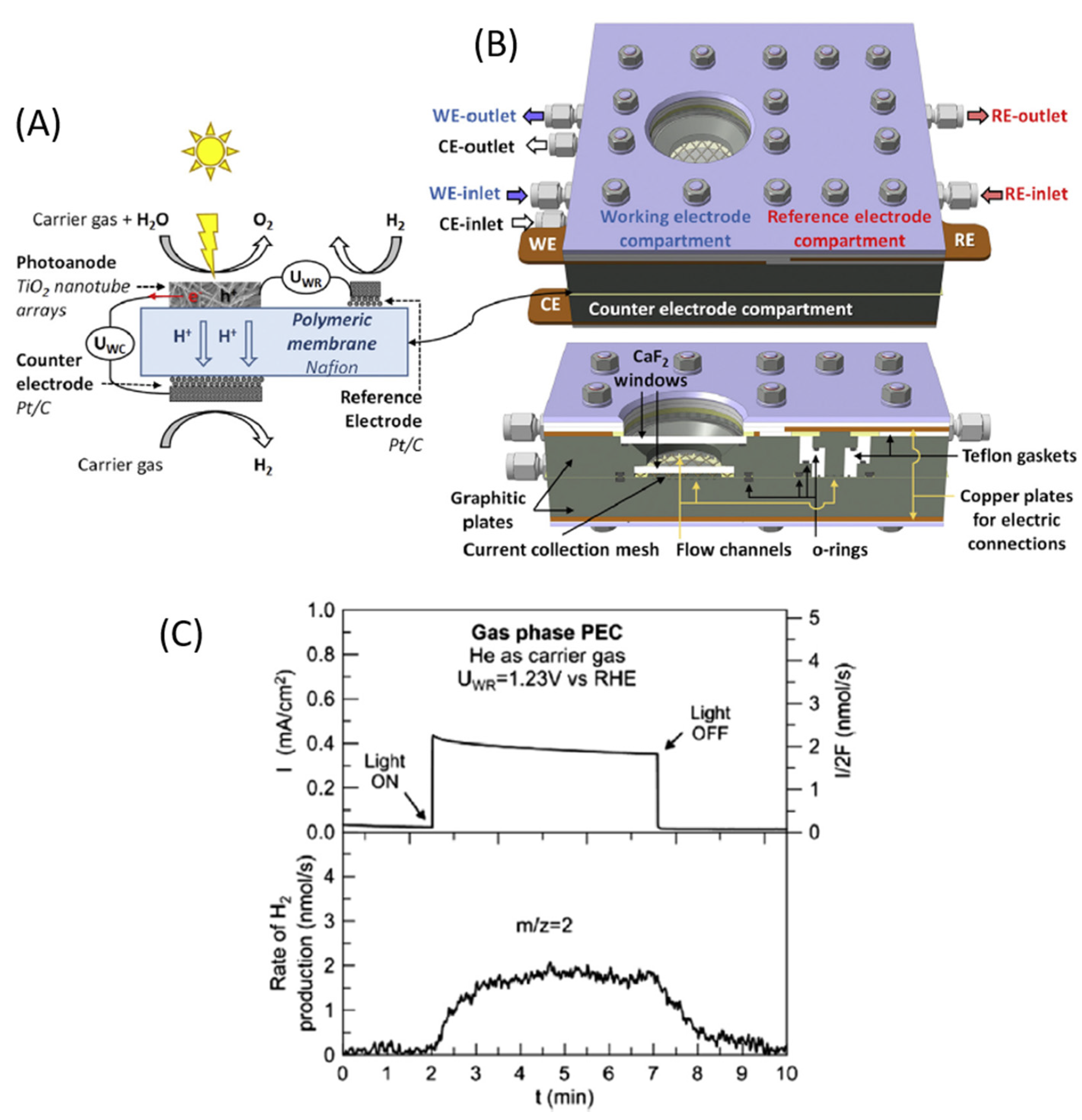
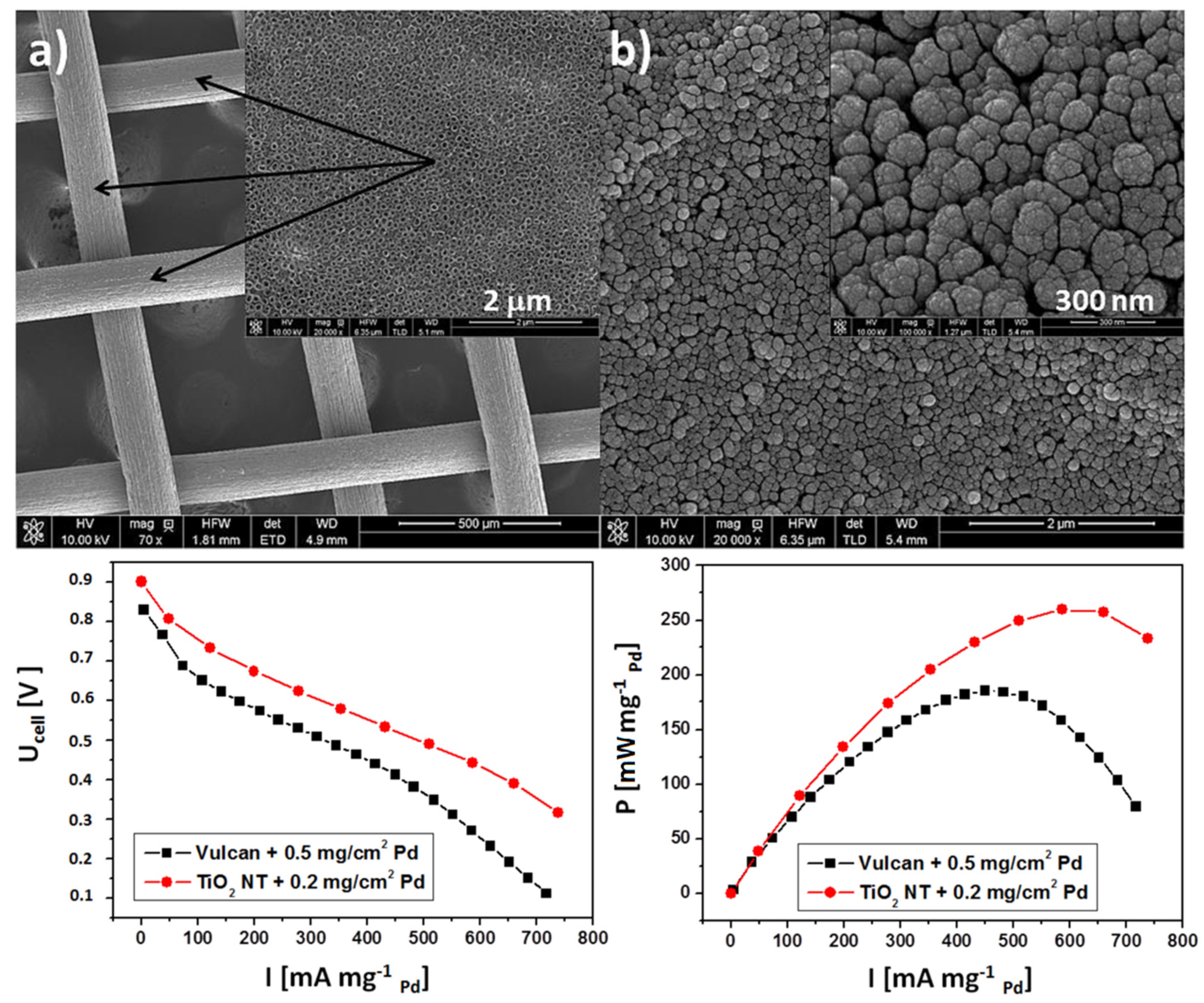

| Solar Cell Type | Photoanode Architecture (Conductive Substrate/Semiconductor/Dye) | Jsc (mA·cm−2) | Voc (V) | η (%) | Ref. |
|---|---|---|---|---|---|
| DSSC | Ti/nanoporous TiO2/N719 | 9.501 | 0.750 | 5.27 | [63] |
| DSSC | Ti/TiO2-NT/Ru(II) complex | 6.477 | 0.590 | 2.040 | [49] |
| DSSC | Pt-FTO/TiO2-NT/N719 | 5.2 | 0.7 | 1.96 | [9] |
| DSSC | FTO/TiO2-NT/C106 | 4.50 | 0.7 | 1.60 | [52] |
| DSSC | FTO/TiO2-NT/TiO2-NP/N719 | 12.70 | 0.69 | 3.62 | [18] |
| DSSC | FTO/TiO2-NP/TiO2-NT/TiO2-NP/N719 | 20.01 | 0.646 | 8.56 | [53] |
| DSSC | FTO/TiO2-NP/TiO2-NT/Z907 | 9.58 | 0.778 | 5.37 | [90] |
| DSSC | FTO/TiO2-NP/TiO2-NT/D719 | 17.56 | 0.80 | 8.05 | [91] |
| DSSC | FTO/TiO2-NT/TiO2-NP/ZnO-NP/N719 | 10.80 | 0.82 | 5.80 | [81] |
| DSSC | FTO/ZnO@TiO2/N719 | 17.36 | 0.72 | 7.46 | [75] |
| FDSSC | Ti/TiO2-NT/TiO2-NP/N719 | 5.95 | 0.75 | 3.31 | [17] |
| FDSSC | Ti/TiO2 microcone/TiO2-NP/N719 | 6.54 | 0.82 | 3.70 | [64] |
| Solar Cell | Active Layer * | Anodic Oxide/Function | Ref. |
|---|---|---|---|
| Plasmonic Si | c-Si wafers/Ag-NPs covered TiO2 | AAO/deposition mask | [66] |
| Plasmonic Si | c-Si wafers/TiO2 covered with Ag-NP, In-NP or Al-NP | AAO/deposition mask | [25] |
| a-Si:H | a-Si:H/Al-doped ZnO/plasmonic metal (Cu, Au or Ag) | TiO2-NT/Ti nanostructured substrate | [92] |
| PSC | CH3NH3PbI3− XClX in AAO scaffold | AAO/scaffold layer | [26,94] |
| PSC | FTO/TiO2-NT-NP/CH3NH3PbI3 | TiO2-NT/charge conduction | [95] |
| Hybrid OPV/PSC | PTAA/CH3NH3PbI3/TiO2/Ti | Anodized TiO2/charge conduction | [96] |
| OPV | PTB7:PC70BM | AAO/haze film | [88] |
| Photoanode | Max. Efficiency | Applied Bias, Electrolyte, Light Irradiation | H2 Production * | Ref. |
|---|---|---|---|---|
| TiO2-NT on Ti foil | 2.5% APCE | No external bias, in 1 M NaOH anolyte + 0.5 M H2SO4 catholyte under SS light | 22.4 µmol·h−1·cm−2 | [10] |
| NiO-modified TiO2-NT on Ti foil | 0.13% ABPE | At 1.0 V vs. Ag/AgCl in distilled water under SS light | − | [82] |
| Ni-doped TiO2-NT on Ti-Ni alloy | 0.67% ABPE | At 0 V vs. Ag/AgCl in 1 M KOH under SS light | − | [102] |
| Si-doped TiO2-NT on Ti-Si alloy | 0.54% ABPE | At −0.65 V vs. Ag/AgCl in 1 M KOH under SS light | − | [103] |
| QD-decorated TiO2-NT on Ti foil | 0.63% STH | At 0.1 V vs. Ag/AgCl in 0.1 M Na2SO4 under SS light | 22 µmol·h−1·cm−2 | [105] |
| WO3-NT on W foil | 5.23% QE | At 0.5 V vs. SCE in 0.5 M Na2SO4 under 420 nm irradiation | 3 µmol·h−1·cm−2 | [20] |
| TiO2-NT on Ti webs | 2.44% ABPE | At 0.6V vs. RHE in 0.1 M H2SO4 under 365 nm irradiation | 2 nmol·s−1 | [19] |
| Bilayered WO3 film on W foil | 2.73% IPCE | At 0.5 V vs. Ag/AgCl in 0.5 M Na2SO4 under 370 nm irradiation | − | [71] |
| WO3 film on W foil | 57.8% IPCE | At 1.2 V vs. SCE in 0.5 M Na2SO4 electrolyte under 350 nm irradiation | − | [21] |
| Co-Pi/Sn-doped Fe2O3 on Fe foil | 24% IPCE | At 1.0 V vs. RHE in 1 M KOH under 400 nm irradiation | 32.5 µmol−1·cm−2 | [22] |
| TiO2-NT on Ti mesh | 0.7% STH | No external bias, in 1 M NaOH anolyte + 0.5 M H2SO4 catholyte under 300 W Xe-arc lamp irradiation | 1.4 L·m−2·h−1 | [54] |
| W-Cu co-sensitized TiO2-NT on Ti foil | 1.03% ABPE | At 0.6 V vs. Ag/AgCl in 1 M KOH + 5 v.% EG under 55 W Xe lamp irradiation (200 mW·cm−2) | 6.10 mL·cm−2·h−1 | [104] |
| Fuel Cell | Anodic Oxide | Function | Ref. |
|---|---|---|---|
| PEMFC | Pt-decorated TiO2-NT | cathode | [30] |
| DMFC | Mesoporous NiO | anode | [80] |
| DMFC | Ni-deposited on TiO2-NT | anode | [29] |
| DMFC | Hydrophobic micro-nano TiO2 | degassing channel in the anode plate | [115] |
| DFAFC | Pd-deposited on TiO2-NT | anode | [55] |
| DFAFC | TiO2-NT decorated with Pt-NP and Pd-NP | anode | [58,59] |
| SOFC | ZrO2-NT | solid electrolyte | [47] |
| SOFC | Pt-deposited on AAO | anode | [116] |
| MFC | TiO2-NT | bioanode | [11,60,62] |
| MFC | TiO2-NT | cathode | [65] |
| Active Material/Current Collector | Specific Capacitance | Energy Density | Long-Term Stability | Ref. |
|---|---|---|---|---|
| MnO2-TiNxOy/Ti foil | 1404.4 F·g−1 at 0.5 A·g−1 | 1.24 μW·h·cm−2 | 93.88% capacitance retention after 10,000 cycles | [48] |
| MnO2-AAO/FTO | 28.9 F·cm−3 at 0.1 mA·cm−2 (VC) | 2.36 mW·h·cm−3 | 85.5 % capacitance retention after 5000 cycles | [50] |
| Cu(OH)2 nanorods/Cu foil (ASC) | 260 F·g−1 at 5 mV·s−1 | 3.68 mW·h·cm−3 | 92.0% capacitance retention after 5000 cycles | [78] |
| NiO nanopetals/Ni foam (ASC) | 415.2 F·g−1 at 2 mV·s−1 | 14.6 W·h·kg−1 | 91.3% capacitance retention after 10,000 cycles | [41] |
| Battery Type | Anodic-Oxide Material | Function | Ref. |
|---|---|---|---|
| LIB | Nanoporous TiO2 | Anode | [27] |
| LIB | TiO2-NT (pristine or doped) | Anode | [32,56,140] |
| LIB | TiO2-NT composites | Anode | [59,83,144,145,146] |
| LIB | Sn@CuxO nanowire | Anode | [79] |
| LIB | WO3 nanosheet arrays | Anode | [72] |
| LIB | Nanoporous Ta2O5 | Cathode | [147] |
| SIB | TiO2-NT (pristine or doped) | Anode | [28,142] |
| SIB | Mesoporous SnO2 | Anode | [148] |
| SIB | Porous Nb2O5 | Anode | [149] |
| ZIB | ZnO Hexagonal Pyramid Array | Anode | [76] |
| Li–S battery | AAO | Separator | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.S.; Araújo, P.d.S.; Pissolitto, Y.B.; Lopes, P.P.; Simon, A.P.; Sikora, M.d.S.; Trivinho-Strixino, F. The Use of Anodic Oxides in Practical and Sustainable Devices for Energy Conversion and Storage. Materials 2021, 14, 383. https://doi.org/10.3390/ma14020383
Santos JS, Araújo PdS, Pissolitto YB, Lopes PP, Simon AP, Sikora MdS, Trivinho-Strixino F. The Use of Anodic Oxides in Practical and Sustainable Devices for Energy Conversion and Storage. Materials. 2021; 14(2):383. https://doi.org/10.3390/ma14020383
Chicago/Turabian StyleSantos, Janaina Soares, Patrícia dos Santos Araújo, Yasmin Bastos Pissolitto, Paula Prenholatto Lopes, Anna Paulla Simon, Mariana de Souza Sikora, and Francisco Trivinho-Strixino. 2021. "The Use of Anodic Oxides in Practical and Sustainable Devices for Energy Conversion and Storage" Materials 14, no. 2: 383. https://doi.org/10.3390/ma14020383
APA StyleSantos, J. S., Araújo, P. d. S., Pissolitto, Y. B., Lopes, P. P., Simon, A. P., Sikora, M. d. S., & Trivinho-Strixino, F. (2021). The Use of Anodic Oxides in Practical and Sustainable Devices for Energy Conversion and Storage. Materials, 14(2), 383. https://doi.org/10.3390/ma14020383










