Metal-Organic Frameworks: Synthetic Methods and Potential Applications
Abstract
1. Introduction
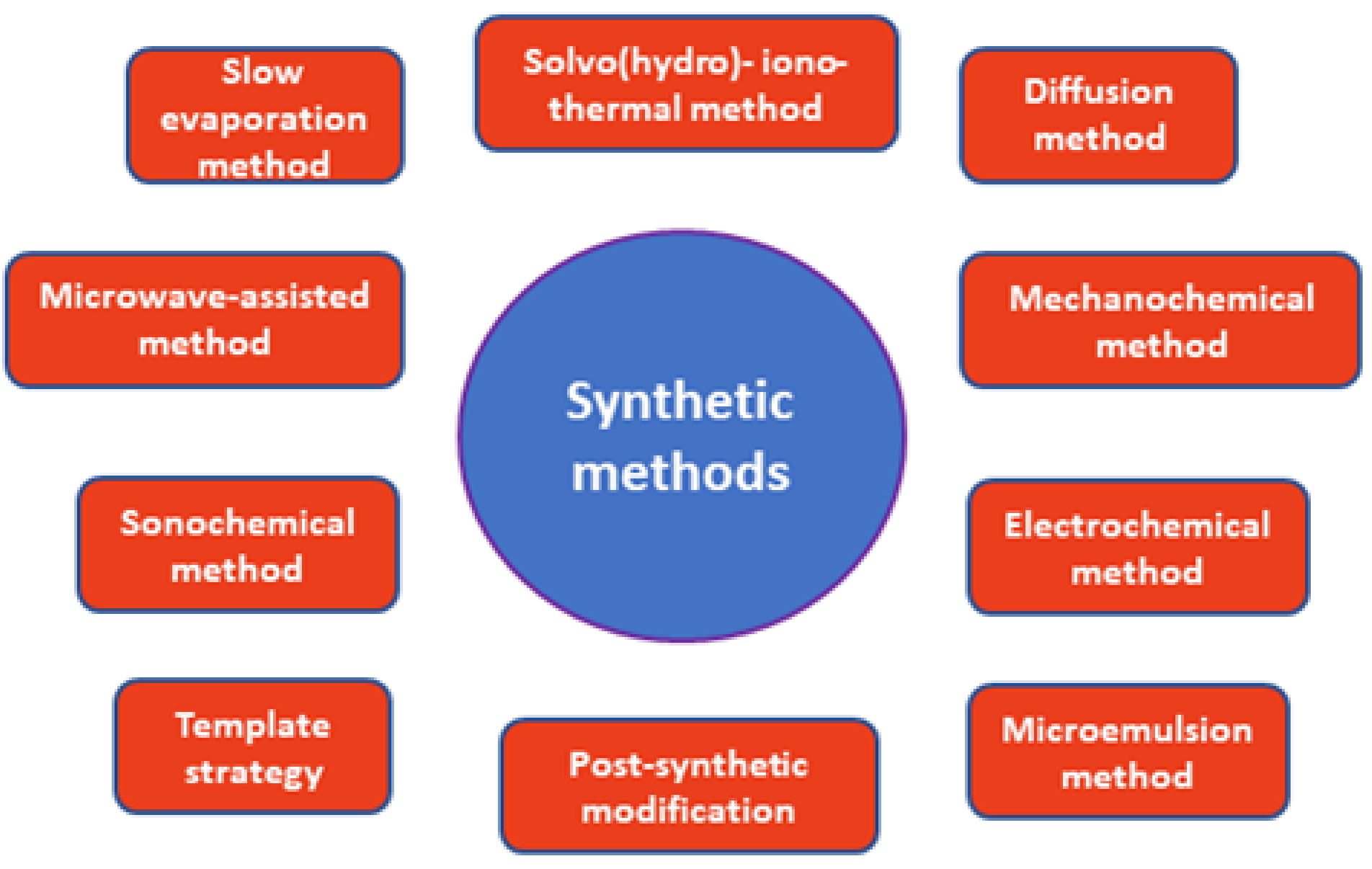
2. Synthesis of MOFs
2.1. Slow Evaporation and Diffusion Methods
2.2. Solvo(Hydro)-Thermal and Iono-Thermal Method
2.3. Microwave-Assisted Method
2.4. Mechanochemical Method
2.5. Electrochemical Method
2.6. Sonochemical Method
2.7. Microemulsion Method
2.8. Post-Synthetic Modification
2.9. Template Strategies
3. Applications of MOFs
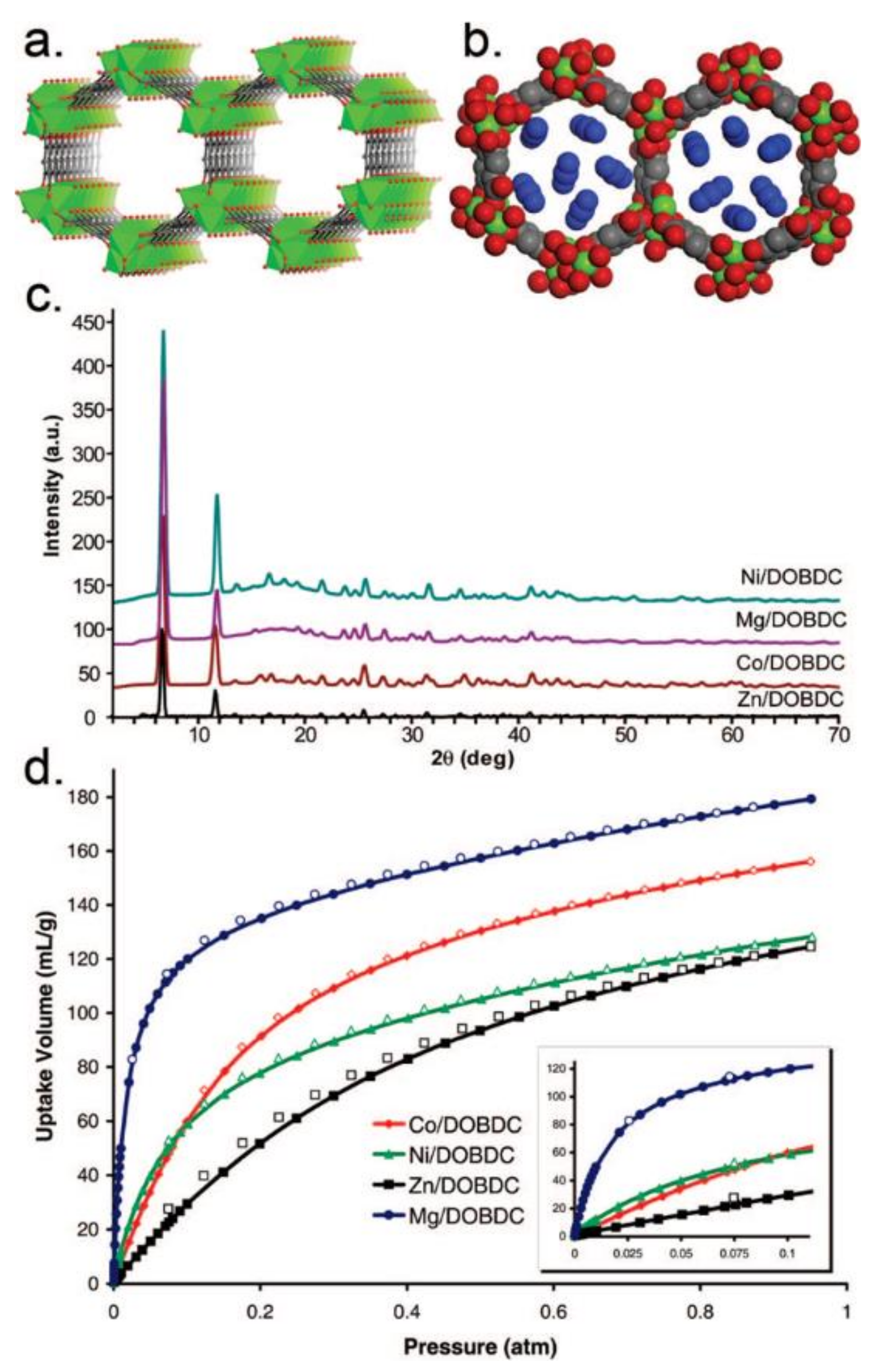
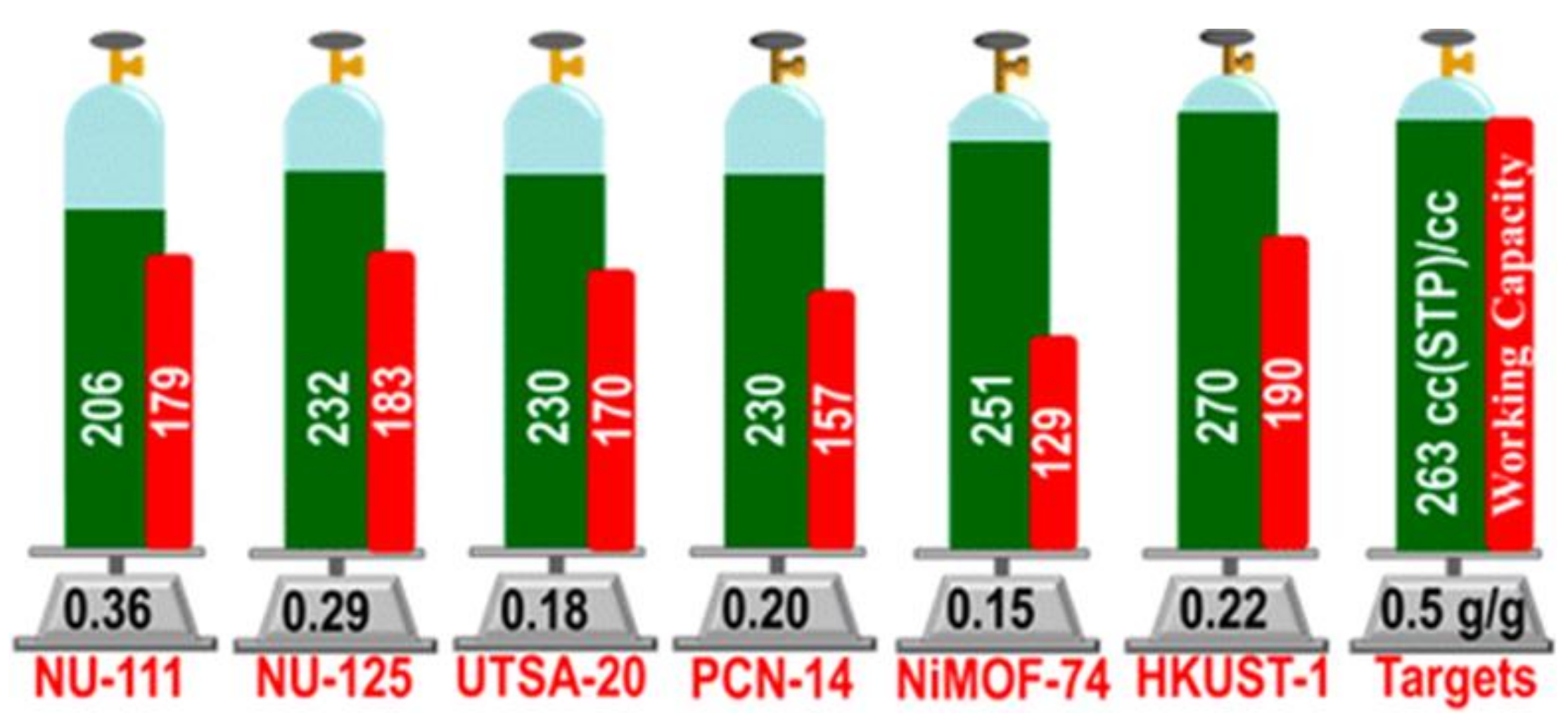
3.1. Gas Adsorption/Separation/Storage for Energy and Environmental Applications
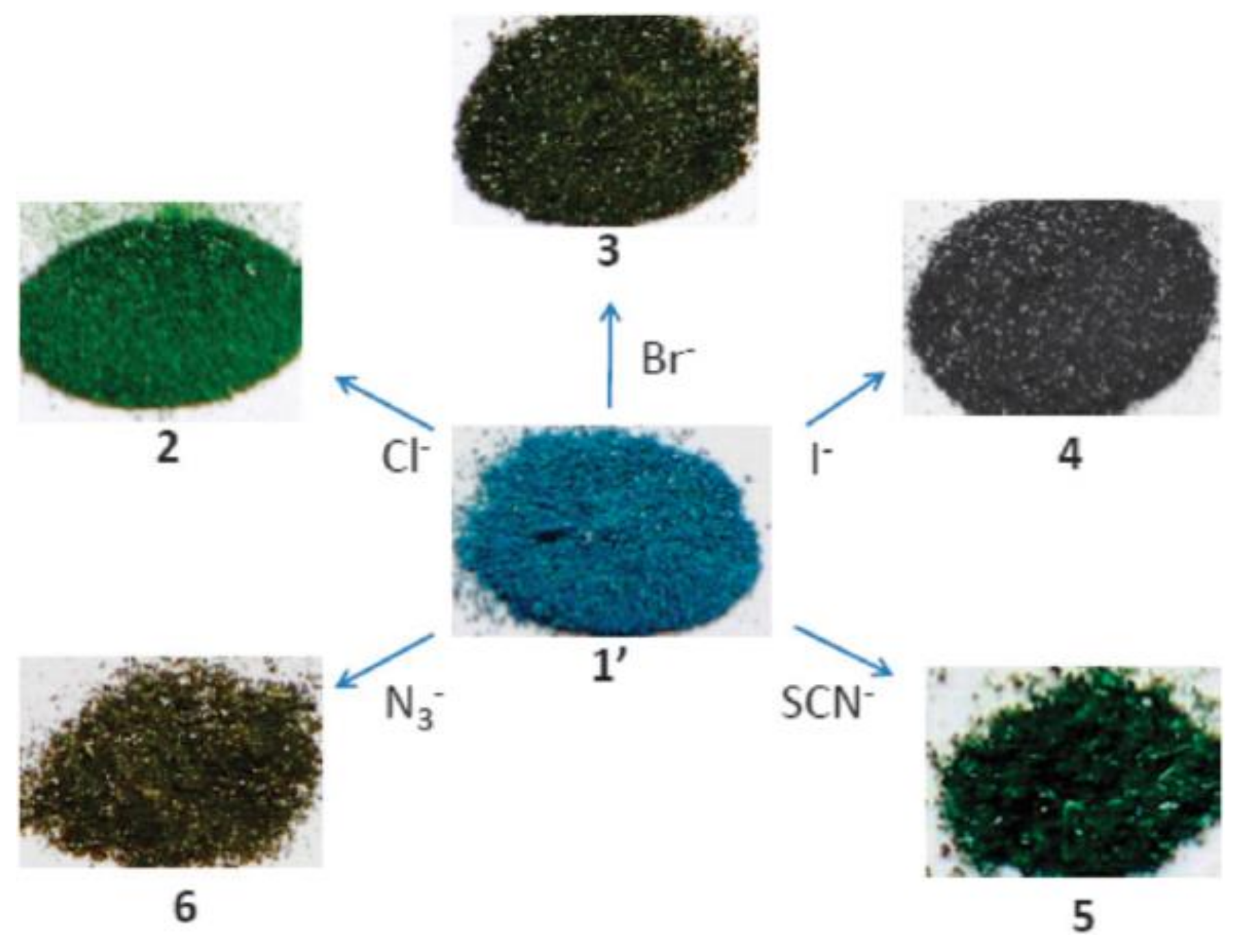
3.2. Sensing Applications
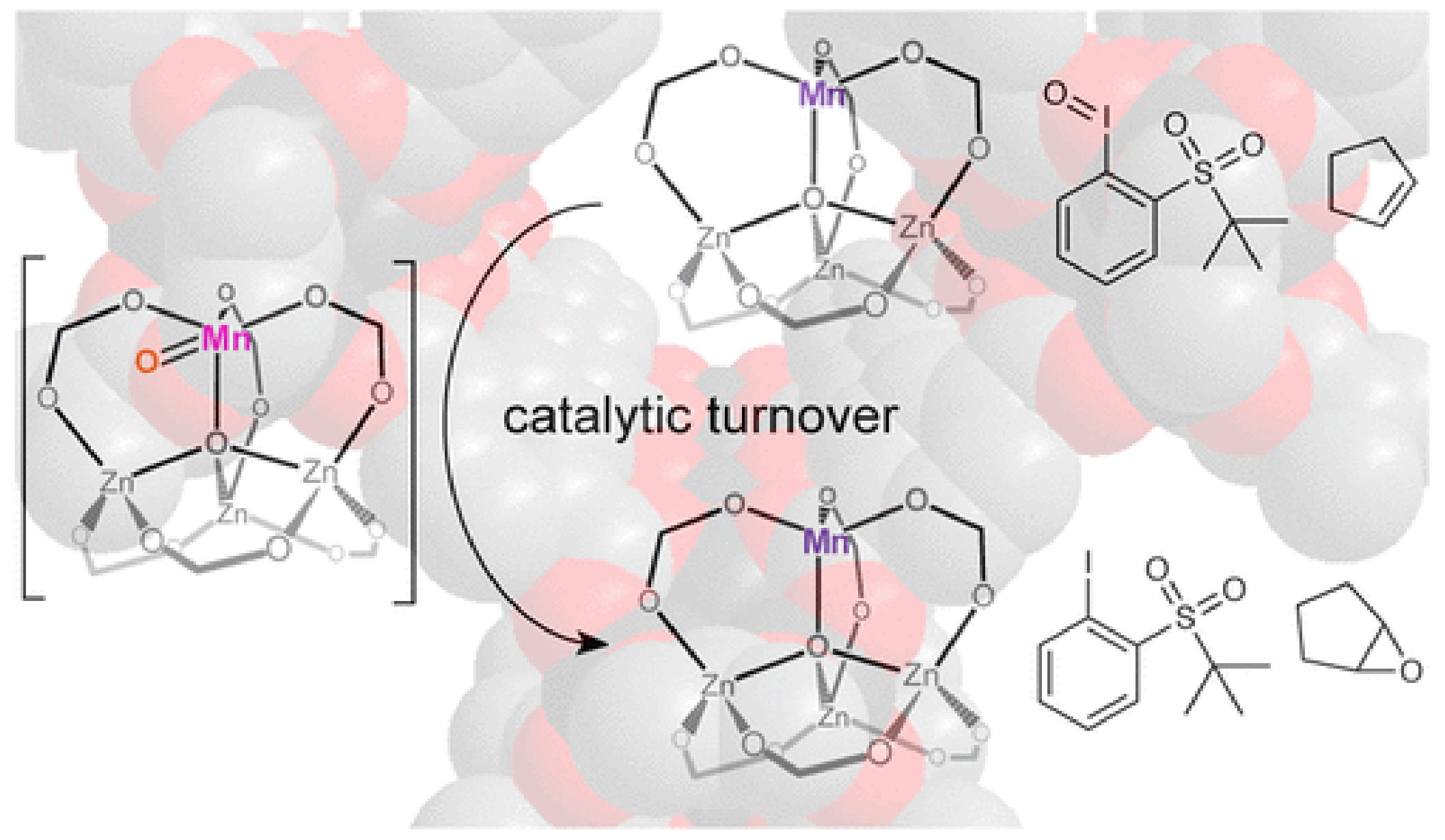
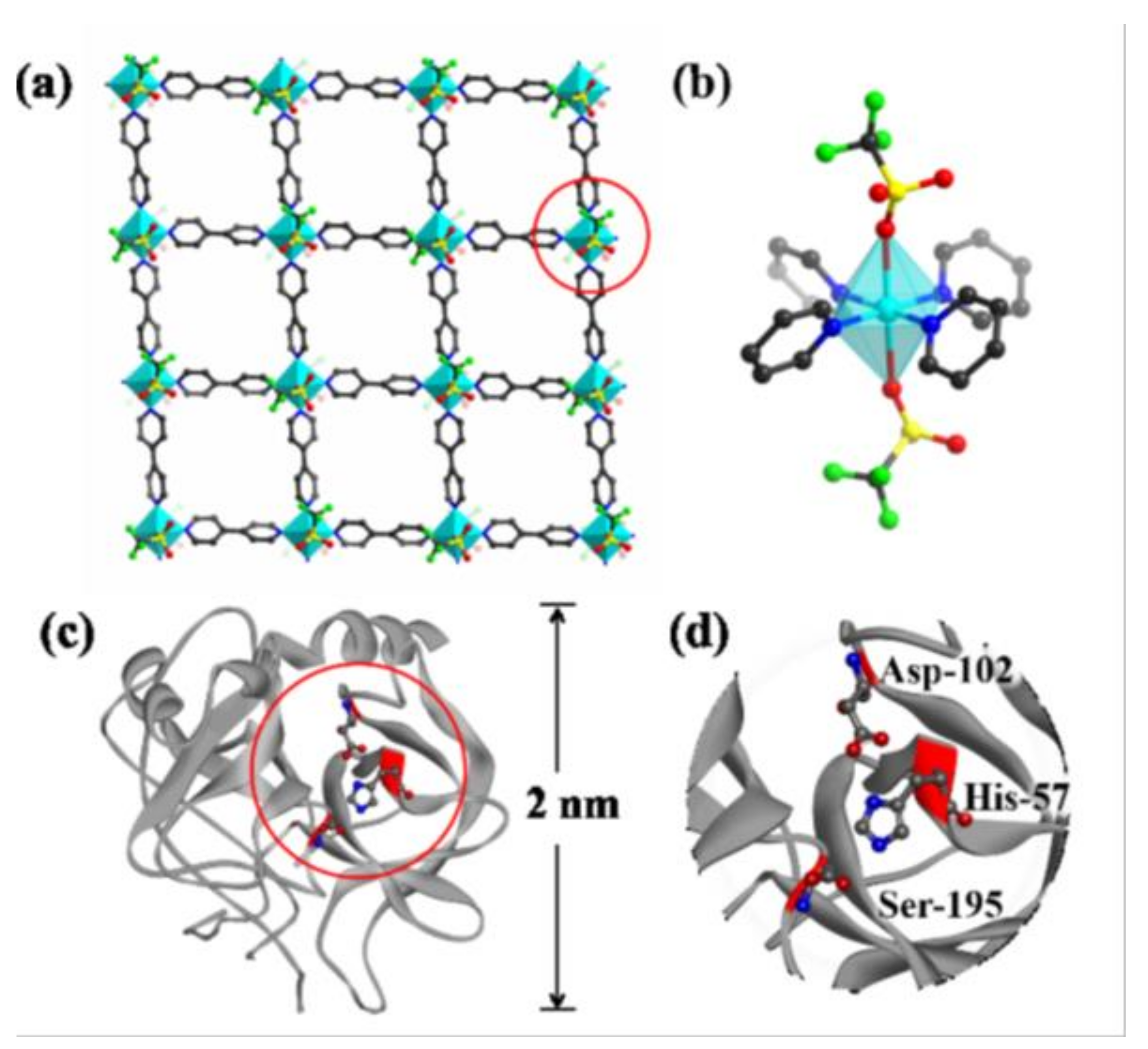
3.3. Catalytic Applications
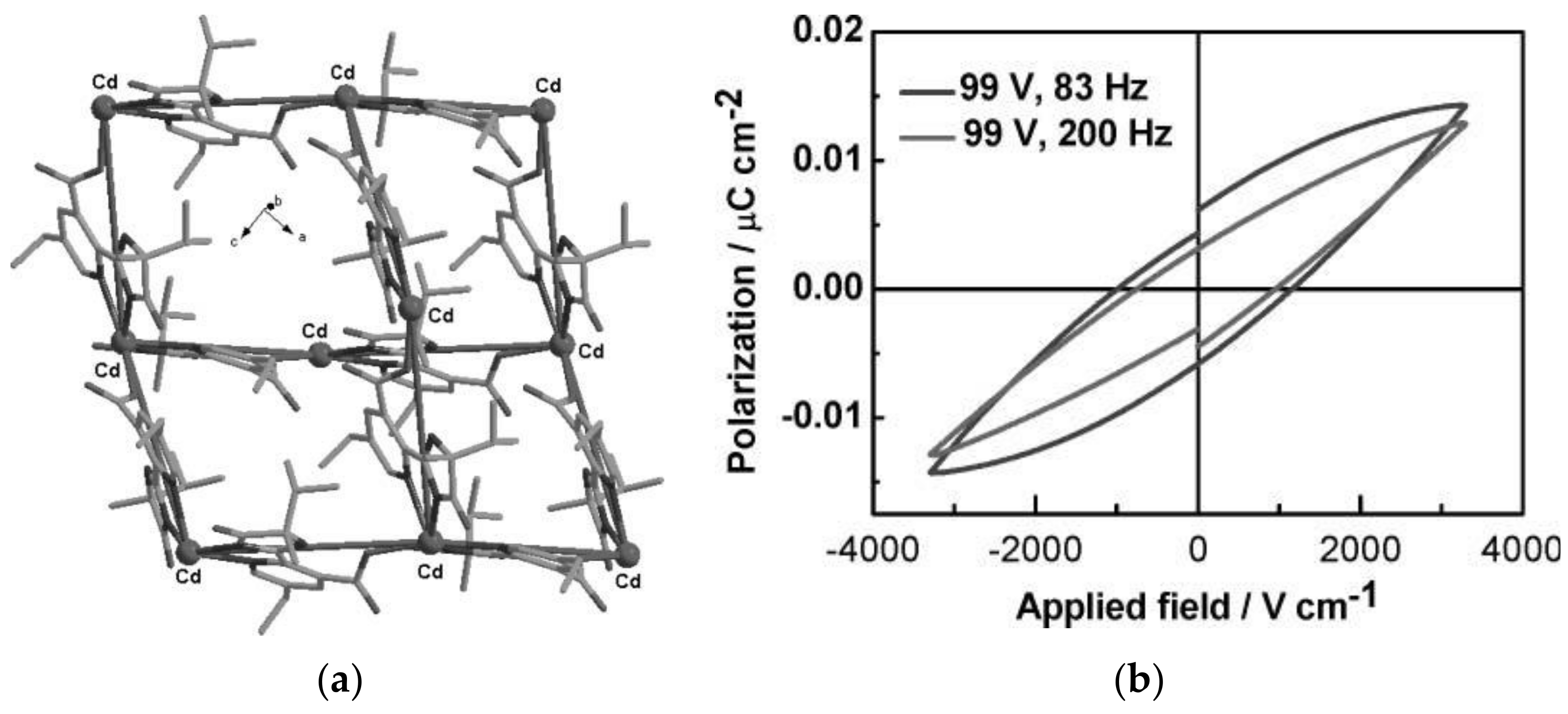
3.4. Piezo/Ferroelectric, Thermoelectric, and Dielectric Applications
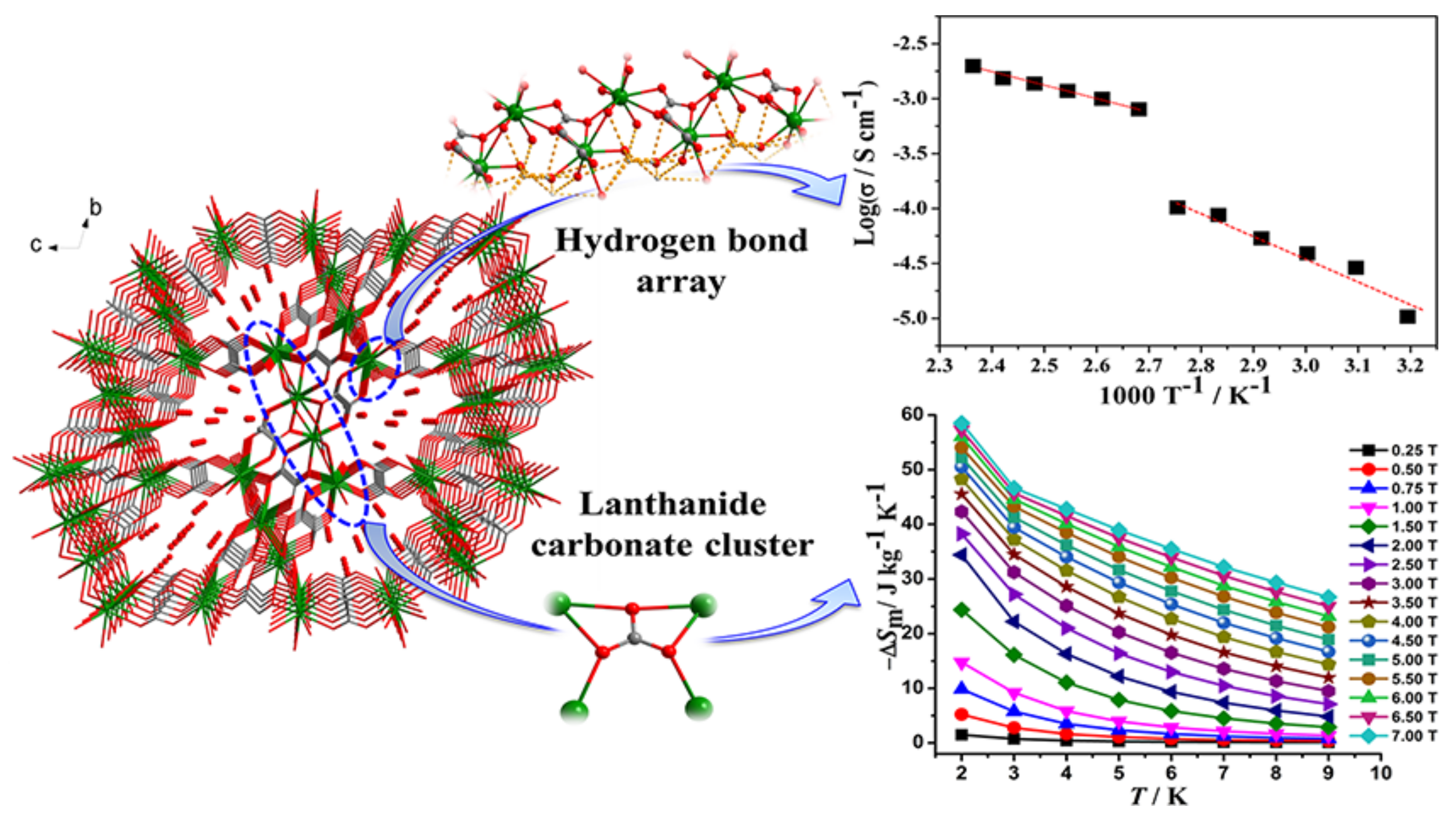
3.5. Proton-Conducting and Magnetic Materials
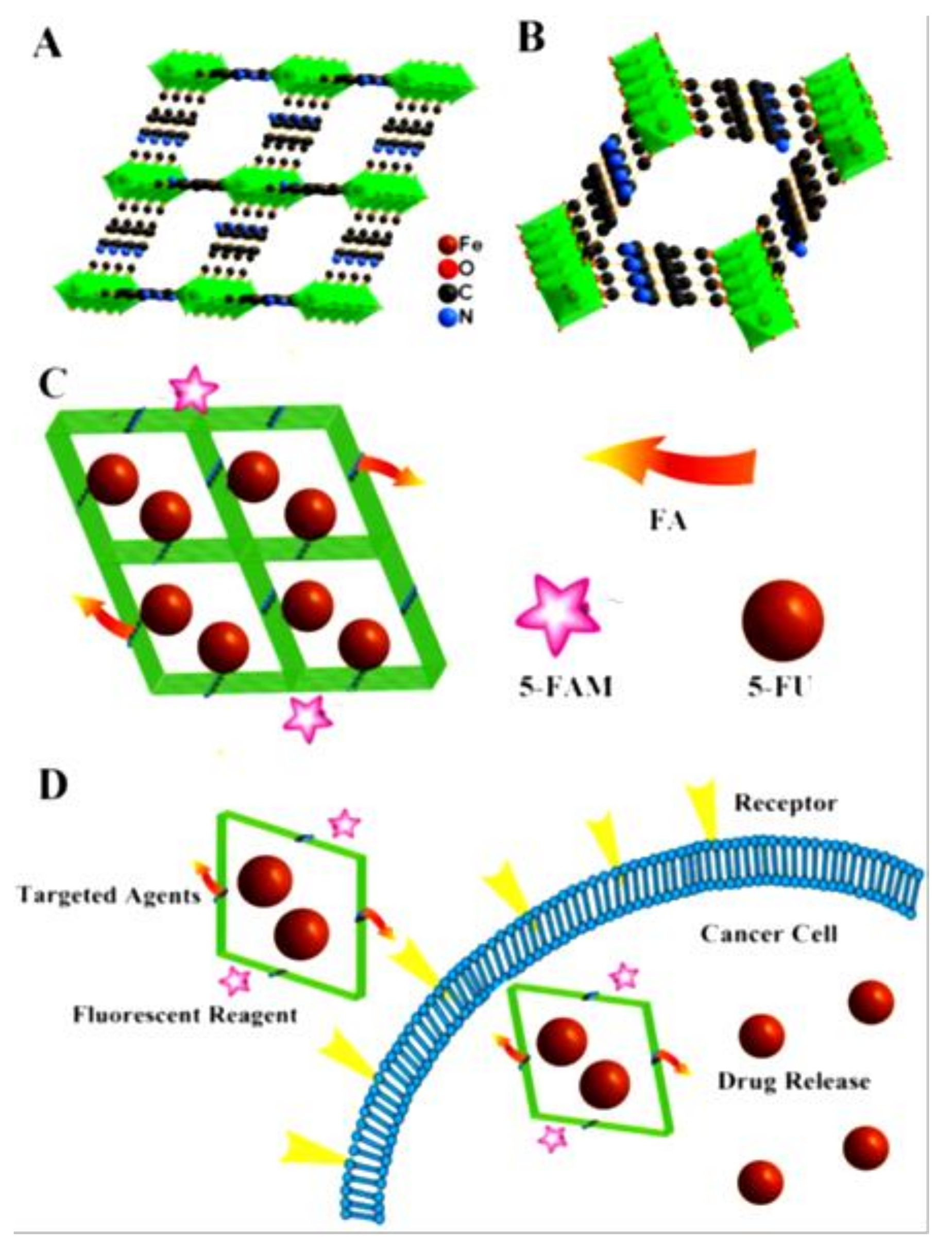
3.6. Biomedical Applications
3.7. Analytical Applications
4. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc. Natl. Acad. Sci. USA 2008, 105, 11623–11627. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Champness, N.R.; Janiak, C. Recent advances in crystal engineering. CrystEngComm 2010, 12, 22–43. [Google Scholar] [CrossRef]
- Kato, M.; Fujihara, T.; Yano, D.; Nagasawa, A. Anion influence on the coordination polymer structures of silver(I) complexes with 2-methylisothiazol-3(2H)-one. CrystEngComm 2008, 10, 1460–1466. [Google Scholar] [CrossRef]
- Sague, J.L.; Meuwly, M.; Fromm, K.M. Counterion effect on the formation of coordination polymer networks between AgNO3 and L (2,2′-oxybis(ethane-2,1-diyl) diisonicotinate). CrystEngComm 2008, 10, 1542–1549. [Google Scholar] [CrossRef]
- Noro, S.-I.; Horike, S.; Tanaka, D.; Kitagawa, S.; Akutagawa, T.; Nakamura, T. Flexible and shaphe-selective guest binding at CuII axial sites in 1-dimensional CuII-1,2-bis(4-pyridyl-ethane) coordination polymers. Inorg. Chem. 2006, 45, 9290–9300. [Google Scholar] [CrossRef]
- Paz, F.A.A.; Klinowski, J.; Vilela, S.M.F.; Tome, J.P.C.; Cavaleiro, J.A.S.; Rocha, J. Ligand design for functional metal-organic frameworks. Chem. Soc. Rec. 2012, 41, 1088–1110. [Google Scholar]
- Lazarou, K.N.; Psycharis, V.; Terzis, A.; Raptopoulou, C.P. Network diversity and supramolecular isomerism in copper(II)/1,2-bis(4-pyridyl)ethane coordination polymers. Polyhedron 2011, 30, 963–970. [Google Scholar] [CrossRef]
- Noro, S.-I.; Kitaura, R.; Kondo, M.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Yamashita, M. Framework engineering by anions and porous functionalities of Cu(II)/4,4′-bpy coordination polymers. J. Am. Chem. Soc. 2002, 124, 2568–2583. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-triggered drug release from a porous zinc-adeninate metal-organic framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.-G.; Yuan, B.; Shao, C.-Y.; Chen, Y.-Y.; Zhou, B.-b.; Li, M.-S.; An, X.-M.; Cheng, P.; Zhao, X.-J. Cation-exchange porosity tuning in a dynamic 4d-4f-3d framework for NiII ion-selective luminescent probe. Inorg. Chem. 2015, 54, 4456–4465. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Haouas, M.; Loiseau, T.; Taulelle, F. Nanoporous solids: How do they form? An in situ approach. Chem. Mater. 2014, 26, 299–309. [Google Scholar] [CrossRef]
- Cronin, L.; Kögerler, P.; Müller, A. Controlling growth of novel solid-state materials via discrete molybdenum-oxide-based building blocks as synthons. J. Solid State Chem. 2000, 152, 57–67. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline metal-organic frameworks (MOFs): Synthesis, structure and function. Acta Cryst. 2014, B70, 3–10. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Halper, S.R.; Do, L.; Stork, J.R.; Cohen, S.M. Topological control in heterometallic metal-organic frameworks by anion templating and metalloligand design. J. Am. Chem. Soc. 2006, 128, 15255–15268. [Google Scholar] [CrossRef]
- Du, M.; Li, C.-P.; Zhao, X.-J. Metal-controlled assembly of coordination polymers with the flexible building block 4-pyridylacetic acid (Hpya). Cryst. Growth Des. 2006, 6, 335–341. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Rabenau, A. The role of hydrothermal synthesis in preparative chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal synthesis of metal-organic frameworks containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Dybtsev, D.N.; Nuzhdin, A.L.; Chun, H.; Bryliakov, K.; Talsi, E.P.; Fedin, V.P.; Kim, K. A homochiral metal-organic material with permanent porosity, enantioselective sorption properties, and catalytic activity. Angew. Chem. Int. Ed. Engl. 2006, 45, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology, Materials and Processing Technology; Noyes Publications: New York, NY, USA, 2002. [Google Scholar]
- Biemmi, E.; Christian, S.; Stock, N.; Bein, T. High-throughput screening of synthesis parameters in the formation of the metal-organic frameworks MOF-5 and HKUST-1. Microporous Mesoporous Mater. 2009, 117, 111–117. [Google Scholar] [CrossRef]
- Millange, F.; Osta, R.E.; Medina, M.E.; Walton, R.I. A time-resolved diffraction study of a window of stability in the synthesis of a copper carboxylate metal-organic framework. CrystEngComm 2011, 13, 103–108. [Google Scholar] [CrossRef]
- Li, P.; Cheng, F.-F.; Xiong, W.-W.; Zhang, Q. New synthetic strategies to prepare metal-organic frameworks. Inorg. Chem. Front. 2018, 5, 2693–2708. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, C.; Zhang, Y.; Ren, N.; Yang, Y. Microwave-assisted hydrothermal synthesis of nanozeolites with controllable size. Microporous Mesoporous Mater. 2009, 119, 306–314. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Song, Y.; Liang, H.; Zeng, M.-H. Microwave-assisted synthesis, crystal structure and properties of a disc-like heptanuclear Co(II) cluster and a heterometallic cubanic Co(II) cluster. CrystEngComm 2009, 11, 865–872. [Google Scholar] [CrossRef]
- Klinowski, J.; Almeida Paz, F.A.; Silva, P.; Rocha, J. Microwave-assisted synthesis of metal-organic frameworks. Dalton Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Yang, Z.; Liu, T.-F.; Huang, Y.-B.; Cao, R. Microwave-assisted synthesis of a series of lanthanide metal-organic frameworks and gas sorption properties. Inorg. Chem. 2012, 51, 1813–1820. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Hundal, G.; Jang, I.T.; Hwang, Y.K.; Jun, C.-H.; Chang, J.-S. Microwave synthesis of hybrid inorganic-organic materials including porous Cu3(BTC)2 from Cu(II)-trimesate mixture. Microporous Mesoporous Mater. 2009, 119, 331–337. [Google Scholar] [CrossRef]
- Ni, Z.; Masel, R.I. Rapid production of metal-organic frameworks via microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Kimber, S.A.J.; Honkimäki, V.; Dinneier, R.E. Real-time and in situ monitoring of mechanochemical milling reactions. Nat. Chem. 2012, 5, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ganay, A.L.; Pichon, A.; James, S.L. Solvent-free synthesis of metal complexes. Chem. Soc. Rev. 2007, 36, 846–855. [Google Scholar]
- Joaristi, A.M.; Juan-Alcaniz, J.; Serra-Crespo, P.; Kapteijn, F.; Gascon, J. Electrochemical synthesis of some archetypical Zn2+, Cu2+, and Al3+ metal organic frameworks. Cryst. Growth Des. 2012, 12, 3489–3498. [Google Scholar] [CrossRef]
- Aslani, A.; Morsali, A. Sonochemical synthesis of nano-sized metal-organic lead(II) polymer: A precursor for the preparation of nano-structured lead(II) iodide and lead(II) oxide. Inorg. Chim. Acta 2009, 362, 5012–5016. [Google Scholar] [CrossRef]
- Casrson, C.G.; Brown, A.J.; Sholl, D.S.; Nair, S. Sonochemical synthesis and characterization of submicrometer crystals of the metal-organic framework Cu[(hfipbb)(H2hfipbb)0.5]. Cryst. Growth Des. 2011, 11, 4505–4510. [Google Scholar] [CrossRef]
- Kim, J.; Yang, S.-T.; Choi, S.B.; Sim, J.; Kim, J.; Ahn, W.-S. Control of catenation in CuTATB-n-metal-organic frameworks by sonochemical synthesis and its effect on CO2 absorption. J. Mater. Chem. 2011, 21, 3070–3076. [Google Scholar] [CrossRef]
- Son, W.-J.; Kim, J.; Kim, J.; Ahn, W.-S. Sonochemical synthesis of MOF-5. Chem. Commun. 2008, 6336–6338. [Google Scholar] [CrossRef]
- Jung, D.-W.; Yang, D.-A.; Kim, J.; Kim, J.; Ahn, W.-S. Facile synthesis of MOF-177 by a sonochemical method using 1-methyl-2-pyrrolidinone as a solvent. Dalton Trans. 2010, 39, 2883–2887. [Google Scholar] [CrossRef]
- Ye, R.; Ni, M.; Xu, Y.; Chen, H.; Li, S. Synthesis of Zn-based metal-organic frameworks in ionic liquid microemulsions at room temperature. RSC Adv. 2018, 8, 26237–26242. [Google Scholar] [CrossRef]
- Zheng, W.; Hao, X.; Zhao, L.; Sun, W. Controllable preparation of nanoscale metal-organic frameworks by ionic liquid microemulsions. Int. Eng. Chem. Res. 2017, 56, 5899–5905. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal-organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Tanabe, K.K.; Cohen, S.M. Postsynthetic modification of metal-organic frameworks—A progress report. Chem. Soc. Rev. 2011, 40, 498–519. [Google Scholar] [CrossRef]
- Xamera, F.L.I.; Gascon, J.; Burrows, A.D. Metal Organic Frameworks as Heterogeneous Catalysts’; The Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Song, Y.-F.; Cronin, L. Postsynthetic covalent modification of metal-organic framework (MOF) materials. Angew. Chem. Int. Ed. Engl. 2008, 47, 4635–4637. [Google Scholar] [CrossRef]
- Garibay, S.J.; Wang, Z.; Tanabe, K.K.; Cohen, S.M. Postsynthetic modification: A versatile approach toward multifunctional metal-organic frameworks. Inorg. Chem. 2009, 48, 7341–7349. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Bury, W.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Solvent-assisted linker exchange: An alternative to the De Novo synthesis of unattainable metal-organic frameworks. Angew. Chem. Int. Ed. Engl. 2014, 53, 4530–4540. [Google Scholar] [CrossRef]
- Lalonde, M.; Bury, W.; Karagiaridi, O.; Brown, Z.; Hupp, J.T.; Farha, O.K. Transmetalation: Routes to metal exchange within metal-organic frameworks. J. Mater. Chem. A 2013, 1, 5453–5468. [Google Scholar] [CrossRef]
- Yanai, N.; Granick, S. Directional self-assembly of a colloidal metal-organic framework. Angew. Chem. Int. Ed. Engl. 2012, 51, 5638–5641. [Google Scholar] [CrossRef]
- Song, X.; Jeong, S.; Kim, D.; Lah, M.S. Transmetalations in two metal-organic frameworks with different framework flexibilities: Kinetics and core-shell heterostructure. CrystEngComm 2012, 14, 5753–5756. [Google Scholar] [CrossRef]
- Song, X.; Kim, T.K.; Kim, H.; Kim, D.; Jeong, S.; Moon, H.R.; Lah, M.S. Post-synthetic modifications of framework metal ions in isostructural metal-organic frameworks: Core-shell heterostructures via selective transmetalations. Chem. Mater. 2012, 24, 3065–3073. [Google Scholar] [CrossRef]
- Zhang, Z.; Bueken, B.; De Vos, E.E.; Fischer, R.A. Defect-engineered metal organic frameworks. Angew. Chem. Int. Ed. Engl. 2015, 54, 7234–7254. [Google Scholar]
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-metal MOFs: Unique opportunities in metal-organic framework (MOF) functionality and design. Angew. Chem. Int. Ed. Engl. 2019, 58, 15188–15205. [Google Scholar] [CrossRef]
- Zhao, N.; Cai, K.; He, H. The synthesis of metal-organic frameworks with template strategies. Dalton Trans. 2020, 49, 11467–11479. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffee, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Wong-Foy, A.G.; Matzger, A.J.; Yaghi, O.M. Exceptional H2 saturation uptake in microporous metal-organic frameworks. J. Am. Chem. Soc. 2006, 128, 3494–3495. [Google Scholar] [CrossRef]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volume and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef]
- Jo, H.; Lee, W.R.; Kim, N.W.; Jung, H.; Lim, K.S.; Kim, J.E.; Kang, D.W.; Lee, H.; Hiremath, V.; Seo, J.G.; et al. Fine-tuning of the carbon dioxide capture capability of diamine-grafted metal-organic framework adsorbents through amine functionalization. ChemSusChem 2017, 10, 541–550. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. High and selective CO2 uptake in a cobalt adeninate metal-organic framework exhibiting pyrimidine- and amino-decorated pores. J. Am. Chem. Soc. 2010, 132, 38–39. [Google Scholar] [CrossRef]
- Lin, Y.; Yan, Q.; Kong, C.; Chen, L. Polyethyleneimine incorporated metal-organic frameworks adsorbent for highly selective CO2 capture. Sci. Rep. 2013, 3, 1859. [Google Scholar] [CrossRef]
- Xian, S.; Wu, Y.; Wu, J.; Wang, X.; Xiao, J. Enhanced dynamic CO2 adsorption capacity and CO2/CH4 selectivity on polyethyleneimine-impregnated UiO-66. Ind. Eng. Chem. Res. 2015, 54, 11151–11158. [Google Scholar] [CrossRef]
- Fracaroli, A.M.; Furukawa, H.; Suzuki, M.; Dodd, M.; Okajima, S.; Gandara, F.; Reimer, J.A.; Yaghi, O.M. Metal-organic frameworks with precisely designed interion for carbon dioxide capture in the presence of water. J. Am. Chem. Soc. 2014, 136, 8863–8866. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Qi, D.; Xu, Y.; Zhang, L.; Liu, X.; Jiang, J.; Dai, F.; Xiao, X.; Sun, D. A Zn metal-organic framework with high stability and sorption selectivity for CO2. Inorg. Chem. 2015, 54, 10587–10592. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Liu, H.; Wang, Z.; Yu, X.; Yi, P.; Bai, J. Porous NbO-type metal-organic framework with inserted acylamide groups exhibiting highly selective CO2 capture. CrystEngComm 2013, 15, 3517–3520. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Q.; Lu, Z.; Liu, H.; Liu, W.; Bai, J. A nitro-decorated NbO-type metal-organic framework with a highly selective CO2 uptake and CH4 storage capacity. CrystEngComm 2014, 16, 6287–6290. [Google Scholar] [CrossRef]
- Song, C.; He, Y.; Li, B.; Ling, Y.; Wang, H.; Feng, Y.; Krishna, R.; Chen, B. Enhanced CO2 sorption and selectivity by functionalization of a Nb-O type metal-organic framework with polarized benzothiadiazole moieties. Chem. Commun. 2014, 50, 12105–12108. [Google Scholar] [CrossRef]
- Song, C.; Ling, Y.; Jin, L.; Zhang, M.; Chen, D.-L.; He, Y. CO2 adsorption of three isostructural metal-organic frameworks depending on the incorporated highly polarized heterocyclic moieties. Dalton Trans. 2016, 45, 190–197. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, H.; Li, L.; Wang, C.; Wang, Y.; Zhao, X. Enhanced CO2 adsorption affinity in a NbO-type MOF constructed from a low-cost diisophthalate ligand with a piperazine-ring bridge. Chem. Asian J. 2015, 10, 1864–1869. [Google Scholar] [CrossRef]
- Yang, Q.; Vaesen, S.; Ragon, F.; Wiersum, A.D.; Wu, D.; Lago, A.; Devic, T.; Martineau, C.; Taulelle, F.; Llewellyn, P.L.; et al. A water stable metal-organic framework with optimal features for CO2 capture. Angew. Chem. Int. Ed. 2013, 52, 10316–10320. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, H.; Lv, X.-L.; Xie, Y.; Li, M.; Li, J.-R. Tuning CO2 selective adsorption over N2 and CH4 in UiO-67 analogues through ligand functionalization. Inorg. Chem. 2014, 53, 9254–9259. [Google Scholar] [CrossRef] [PubMed]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamis and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Belmabkhout, Y.; Chen, Z.; Guillerm, V.; Cairns, A.; Adil, K.; Eddaoudi, M. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 2014, 55, 4228. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Yy, J.; Lu, W.; Sun, L.-B.; Sculley, J.; Balbuena, P.B.; Zhou, H.-C. Porous materials with pre-designed single-molecule traps for CO2 selective adsorption. Nat. Commun. 2013, 4, 1538. [Google Scholar] [CrossRef]
- Zhai, Q.-G.; Bu, X.; Mao, C.; Zhao, X.; Feng, P. Systematic and dramatic tuning on gas sorption performance in heterometallic metal-organic frameworks. J. Am. Chem. Soc. 2016, 138, 2524–2527. [Google Scholar] [CrossRef]
- Zhou, Z.; Mei, L.; Ma, C.; Xu, F.; Xiao, J.; Xia, Q.; Li, Z. A novel bimetallic MIL-101(Cr,Mg) with high CO2 adsorption capacity and CO2/N2 selectivity. Chem Eng. Sci. 2016, 147, 109–117. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, K.; Zhang, M.; Guo, Z.; Jiang, J.; Zhao, D. A combinatorial approach towards water-stable metal-organic frameworks for highly efficient carbon dioxide separation. ChemSusChem 2014, 7, 2719–2795. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, J.; Ogiwara, N.; Rodriguez, A.; Peng, Y.; Wang, Y.; Horike, S.; Zhao, D. A pH-responsive phase transformation of a sulfonated metal-organic framework from amorphous to crystalline for efficient CO2 capture. CrystEngComm 2016, 18, 2803–2807. [Google Scholar] [CrossRef]
- Hu, Z.; Faucher, S.; Zhuo, Y.; Sun, Y.; Wang, S.; Zhao, D. Combination of optimization and metalated-ligand exchange: An effective approach to functionalize UiO-66(Zr) MOFs for CO2 separation. Chem. Eur. J. 2015, 21, 17246–17255. [Google Scholar] [CrossRef]
- Kozachuk, O.; Meilikhov, M.; Yusenk, K.; Schneemann, A.; Jee, B.; Kuttatheyil, A.V.; Bertmer, M.; Sternemann, C.; Pöppl, A.; Fischer, R.A. A solid-solution approach to mixed-metal metal-organic frameworks—Detailed characterization of local structures, defects and breathing behavior of Al/V frameworks. Eur. J. Inorg. Chem. 2013, 4546–4557. [Google Scholar] [CrossRef]
- Xiang, S.-C.; Zhang, Z.; Zhao, C.-G.; Hong, K.; Zhao, X.; Ding, D.-R.; Xie, M.-H.; Wu, C.-D.; Das, M.C.; Gill, R.; et al. Rationally tuned micropores within enantiopure metal-organic frameworks for highly selective separation of acetylene and ethylene. Nat. Commun. 2011, 2, 204. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.-L.; Wang, H.; Li, B.; Krishna, R.; Wu, H.; Zhou, W.; Zhao, Y.; Han, Y.; Wang, X.; Zhu, W.; et al. Microporous metal-organic framework with dual functionalities for highly efficient removal of acetylene from ethylene/acetylene mixtures. Nat. Commun. 2015, 6, 7328. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-B.; Li, L.; Wu, H.; Arman, H.; Li, B.; Lin, R.-G.; Zhou, W.; Chen, B. Optimized separation of acetylene from carbon dioxide and ethylene in a microporous material. J. Am. Chem. Soc. 2017, 139, 8022–8028. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Chen, K.; Xing, H.; Yang, Q.; Krishna, R.; Bao, Z.; Wu, H.; Zhou, W.; Dong, X.; Han, Y.; et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Hazra, A.; Jana, S.; Bonakala, S.; Balasubramanian, S.; Maji, T.K. Separation/purification of ethylene from an acetylene/ethylene mixtrure in a pillared-layer porous metal-organic framework. Chem. Commun. 2017, 53, 4907–4910. [Google Scholar] [CrossRef]
- Yang, S.; Ramirez-Cuesta, A.J.; Newby, R.; Garcia-Sakai, V.; Manuel, P.; Callear, S.K.; Campbell, S.I.; Tang, C.C.; Schröder, M. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal-organic framework. Nat. Chem. 2015, 7, 121–129. [Google Scholar] [CrossRef]
- Li, L.; Lin, R.-B.; Krishna, R.; Wang, X.; Li, B.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Flexible-robust metal-organic framework for efficient removal of propyne from propylene. J. Am. Chem. Soc. 2017, 139, 7733–7736. [Google Scholar] [CrossRef]
- Uchida, S.; Kawamoto, R.; Tagami, H.; Nakagawa, Y.; Mizuno, N. Highly selective sorption of small unsaturated hydrocarbons by nonporous flexible framework with silver ion. J. Am. Chem. Soc. 2008, 130, 12370–12376. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Krishna, R.; Wu, Z.; Ma, D.; Shi, Z.; Pham, T.; Forrest, K.; Space, B.; Ma, S. Highly selective adsorption of ethylene over ethane in a MOF featuring the combination of open metal site and π-complexation. Chem. Commun. 2015, 51, 2714–2717. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.O.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Cadiau, A.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Eddaoudi, M. A metal-organic framework-based splitter for separating prolylene from propane. Science 2016, 353, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Manna, B.; Desai, A.V.; Yin, Y.; Krishna, R.; Babarao, R.; Ghosh, S.K. Harnessing Lewis acidic open metal sites of metal-organic frameworks: The foremost route to achieve highly selective benzene sorption over cyclohexane. Chem. Commun. 2016, 52, 8215–8218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-Y.; Wang, P.; Ma, J.-P.; Liu, Q.-K.; Dong, Y.-B. A nanoporous Ag(I)-MOF showing unique selective adsorption of benzene among its organic analogues. Chem. Commun. 2014, 50, 13672–13675. [Google Scholar] [CrossRef] [PubMed]
- Manna, B.; Mukherjee, S.; Desai, A.V.; Sharma, S.; Krishna, R.; Ghosh, S.K. A π-electron deficient diaminotriazine functionalized MOF for selective sorption of benzene over cyclohexane. Chem. Commun. 2015, 51, 15386–15389. [Google Scholar] [CrossRef]
- Joarder, B.; Mukherjee, S.; Chaudhari, A.K.; Desai, A.V.; Manna, B.; Ghosh, S.K. Guest-responsive function of a dynamic metal-organic framework with a π Lewis acidic pore surface. Chem. Eur. J. 2014, 20, 15303–15308. [Google Scholar] [CrossRef]
- Gygi, D.; Bloch, E.D.; Mason, J.A.; Hudson, M.R.; Gonzalez, M.I.; Siegelman, R.L.; Darwish, T.A.; Queen, W.L.; Brown, C.M.; Long, J.R. Hydrogen storage in the expanded pore metal-organic frameworks M2(dobpdc) (M = Mg, Mn, Fe, Co, Ni, Zn). Chem. Mater. 2016, 28, 1128–1138. [Google Scholar] [CrossRef]
- Chae, H.K.; Seberio-Perez, D.Y.; Kim, J.; Go, Y.-B.; Eddaoudi, M.; Matzger, A.J.; O’Keefee, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef]
- Abid, H.R.; Tian, H.; Ang, H.-M.; Tade, M.O.; Buckley, C.E.; Wang, S. Nanosize Zr-metal organic framework (UiO-66) for hydrogen and carbon dioxide storage. Chem. Eng. J. 2012, 187, 415–420. [Google Scholar] [CrossRef]
- Qin, W.; Cao, W.; Liu, H.; Li, Z.; Li, Y. Metal-organic framework MIL-101 doped with palladium for toluene adsorption and hydrogen storage. RSC Adv. 2014, 4, 2414–2420. [Google Scholar] [CrossRef]
- Kim, J.; Yeo, S.; Jeon, J.-D.; Kwak, S.-Y. Enhancement of hydrogen storage capacity and hydrostability of metal-organic frameworks (MOFs) with surface-loaded platinum nanoparticles and carbon black. Microporous Mesoporous Mater. 2015, 202, 8–15. [Google Scholar] [CrossRef]
- Barman, S.; Khutia, A.; Koitz, R.; Blacque, O.; Furukawa, H.; Iannuzzi, M.; Yaghi, O.M.; Janiak, C.; Hutter, J.; Berke, H. Synthesis and hydrogen adsorption properties of internally polarized 2,6-azulenedicarboyxlate based metal-organic frameworks. J. Mater. Chem. A 2014, 44, 18823–18830. [Google Scholar] [CrossRef]
- Li, J.-S.; Tang, Y.-J.; Li, S.-L.; Zhang, S.-R.; Dai, Z.-H.; Si, L.; Lan, Y.-Q. Carbon nanodots functional MOFs composites by a stepwise synthetic approach: Enhanced H2 storage and fluorescent sensing. CrystEngComm 2015, 17, 1080–1085. [Google Scholar] [CrossRef]
- Spanopoulos, I.; Tsangarakis, C.; Klontzas, E.; Tylianakis, E.; Froudakis, G.; Adil, K.; Belmabkhout, Y.; Eddaoudi, M.; Trikalitis, P.N. Reticular synthesis of HKUST-like tbo-MOFs with enhanced CH4 storage. J. Am. Chem. Soc. 2015, 138, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane storage in metal-organic frameworks: Current records, surprise findings, and challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.-M.; Wang, H.; Wu, H.; Tyagi, M.; Yildirim, T.; Zhou, W.; Chen, B. A porous metal-organic framework with dynamic pyrimidine groups exhibiting record high methane storage working capacity. J. Am. Chem. Soc. 2014, 136, 6207–6210. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, H.; Jiao, J.; Bai, D.; Zhou, W.; Yildirim, T.; He, Y. High methane storage and working capacities in a NbO-type metal-organic framework. Dalton Trans. 2016, 45, 7559–7562. [Google Scholar] [CrossRef]
- Peng, J.; Xian, S.; Xiao, J.; Huang, Y.; Xia, Q.; Wang, H.; Li, Z. A supported Cu(I)@MIL-100(Fe) adsorbent with high CO adsorption capacity and CO/N2 selectivity. Chem. Eng. J. 2015, 270, 282–289. [Google Scholar] [CrossRef]
- Kozachuk, O.; Luz, I.; Xamena, F.X.L.I.; Noei, H.; Kauer, M.; Albada, H.S.; Bloch, E.D.; Marler, B.; Qang, Y.; Muhler, M.; et al. Multifunctional, defect-engineered metal-organic frameworks with ruthenium centers: Sorption and catalytic properties. Angew. Chem. Int. Ed. Engl. 2014, 53, 7058–7062. [Google Scholar] [CrossRef]
- Rieth, A.J.; Tulchinsky, Y.; Dincă, M. High and reversible ammonia uptake in mesoporous azolate metal-organic frameworks with open Mn, Co and Ni sites. J. Am. Chem. Soc. 2016, 138, 9401–9404. [Google Scholar] [CrossRef]
- He, W.-W.; Yang, G.-S.; Tang, Y.-J.; Li, S.-L.; Zhang, S.-R.; Su, Z.-M.; Lan, Y.-Q. Phenyl groups result in the highest benzene storage and most efficient desulfurization in a series of isostructural metal-organic frameworks. Chem. Eur. J. 2015, 21, 9784–9789. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.W.; Mahle, J.J.; DeCoste, J.B.; Gordon, W.O.; Rossin, J.A. Extraordinary NO2 removal by the metal-organic framework UiO-66-NH2. Angew. Chem. Int. Ed. Engl. 2016, 55, 6235–6238. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A.M.; Bandosz, T.J. Effect of amine modification on the properties of zirconium-carboxylic acid based materials and their applications as NO2 adsorbents at ambient conditions. Microporous Mesoporous Mater. 2014, 188, 149–162. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Demasky, T.J.; Katz, M.J.; Farha, O.K.; Hupp, J.T. A UiO-66 analogue with uncoordinated carboxylic acids for the broad-spectrum removal of toxic chemicals. New J. Chem. 2015, 39, 2396–2399. [Google Scholar] [CrossRef]
- Tan, K.; Canepa, R.; Gong, Q.; Liu, J.; Johnson, D.H.; Dyevoich, A.; Thallapally, P.K.; Thonhauser, T.; Li, J.; Chabal, Y.J. Mechanism of preferential adsorption of SO2 into two microporous paddle wheel frameworks M(bdc)(ted)0.5. Chem. Mater. 2013, 23, 4653–4662. [Google Scholar] [CrossRef]
- Yang, C.; Liu, L.; Sun, J.; Thomas, K.M.; Davies, A.J.; George, M.W.; Blake, A.J.; Hill, A.H.; Fitch, A.N.; Tang, C.C.; et al. Irreversible network transformation in a dynamic porous host catalyzed by sulfur dioxide. J. Am. Chem. Soc. 2013, 135, 4954–4957. [Google Scholar] [CrossRef]
- Savage, M.; Cheng, Y.; Easun, T.L.; Eyley, J.E.; Argent, S.P.; Warren, M.R.; Lewis, W.; Murray, C.; Tang, C.C.; Frogley, M.D.; et al. Selective adsorption of sulfur dioxide in a robust metal-organic framework material. Adv. Mater. 2016, 28, 8705–8711. [Google Scholar] [CrossRef]
- Cui, X.; Yang, Q.; Yang, L.; Krishna, R.; Zhang, Z.; Bao, Z.; Wu, H.; Ren, Q.; Zhou, W.; Chen, B.; et al. Ultrahigh and selective SO2 uptake in inorganic anion-pillared hybrid porous materials. Adv. Mater. 2017, 29, 1606929. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Rillai, R.S.; Alezi, D.; Shekhah, O.; Bhatt, P.M.; Chen, Z.; Adil, K.; Vaesen, S.; DeWeireld, G.; Pang, M.; et al. Metal-organic frameworks to satisfy gas uprading demands: Fine-tuning the soc-MOF platform for the operative removal of H2S. J. Mater. Chem. A 2017, 5, 3293–3303. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Belmabkhout, Y.; Assen, A.H.; Weseliński, L.J.; Jiang, H.; Cadiau, A.; Xue, D.-X.; Eddaoudi, M. Isoreticular rare earth fsu-MOFs for the selective removal of H2S from CO2 containing gases. Chem. Eng. J. 2017, 324, 392–396. [Google Scholar] [CrossRef]
- Mohideen, M.I.H.; Pillari, R.S.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Shkurenko, A.; Maurin, G.; Eddaoudi, M. A fine-tuned MOF for gas and vapor separation: A multipurpose adsorbent for acid gas removal, dehydration, and BTX sieving. Chemistry 2017, 3, 822–833. [Google Scholar] [CrossRef]
- Ebrahim, A.M.; Jagiello, J.; Bandosz, T.J. Enhanced reactive adsorption of H2S on Cu-BTC/S- and N-doped GO composites. J. Mater. Chem. A 2015, 3, 8194–8204. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Sun, W.; Liu, Z. A robust microporous metal-organic framework as a highly selective and sensitive, instantaneous and colorimetric sensor for Eu3+ ions. Dalton Trans. 2015, 44, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Tian, D.; Gao, Q.; Sun, H.-W.; Xu, J.; Bu, X.-H. A chiral lanthanide metal-organic framework for selective sensing of Fe(III) ions. Dalton Trans. 2016, 45, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, Y.; Zhang, J.; Hu, H.; Wu, X.; Long, Z.; Hou, Z. Facile colorimetric sensing of Pb2+ using bimetallic lanthanide metal-organic frameworks as luminescent probe for field screen analysis of lead-polluted environmental water. Microchem. J. 2017, 134, 140–145. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Y.; Liu, Y.; Wang, J.; Li, Y.; Liu, W.; Wang, J. Cadmium-based metal-organic framework as a highly selective and sensitive ratiometric luminescent sensor for mercury(II). Inorg. Chem. 2015, 54, 11046–11048. [Google Scholar] [CrossRef]
- Xiong, J.; Fan, Y.; Luo, F. Grafting functional groups in metal-organic frameworks for U(IV) sorption from aqueous solutions. Dalton Trans. 2020, 49, 12536–12545. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical sensors based on metal-organic frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Shi, Z.-Q.; Ji, N.-N.; Hu, H.-L. Luminescent triphenylamine-based metal-organic frameworks: Recent advances in nitroaromatics detection. Dalton Trans. 2020, 49, 12929–12939. [Google Scholar] [CrossRef]
- Kaur, R.; Paul, A.K.; Deep, A. Nanocomposite of europium organic framework and quantum dots for highly sensitive chemosensing of trinitrotoluene. Forensic Sci. Int. 2014, 242, 88–93. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Shi, W.; Xu, N.; Cheng, P. Highly selective luminescent sensing of fluoride and organic small-molecule pollutants based on novel lanthanide metal-organic frameworks. Inorg. Chem. 2013, 52, 8082–8090. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.-F.; Hu, H.-C.; Zhang, Z.-Y.; Xiong, G.; Zhao, B. Heterometal-organic frameworks as highly sensitive and highly selective luminescent probes to detect I− ions in aqueous solutions. Chem. Commun. 2015, 51, 3985–3988. [Google Scholar] [CrossRef]
- Wong, K.-L.; Law, G.-L.; Yang, Y.-Y.; Wong, W.-T. A highly porous luminescent terbium-organic framework for reversible anion sensing. Adv. Mater. 2006, 18, 1051–1054. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Chu, T.; Yu, M.; Yang, Y. An electrodeposited lanthanide MOF thin film as a luminescent sensor for carbamate detection in aqueous solution. J. Mater. Chem. C 2014, 41, 8683–8690. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Shi, W.; Li, H.-M.; Li, H.; Cheng, P. Experimental studies and mechanism analysis of high-sensitivity luminescent sensing of pollutional small molecules and ions in Ln4O4 cluster based microporous metal-organic frameworks. J. Phys. Chem. C 2014, 118, 416–426. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, L.; Liu, Z.-Y.; Wang, X.-G.; Ding, B.; Yin, L.; Zhou, B.-B.; Li, M.-S.; Wang, J.-X.; Zhao, X.-J. An ideal detector composed of two-dimensional Cd(II)-triazole frameworks for nitro-compound explosives and potassium dichromate. Chem. Eur. J. 2015, 21, 14171–14178. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-P.; Yu, Y.; Dong, Y.-B. Fluorene-based Cu(II)-MOF: A visual colorimetric anion sensor and separator based on an anion-exchange approach. Chem. Commun. 2012, 48, 2946–2948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ying, Y.; Feng, M.; Wu, L.; Wang, D.; Li, C. Two isostructural Ln3+-based heterometallic MOFs for the detection of nitro-aromatics and Cr2O72−. New J. Chem. 2020, 44, 12748–12754. [Google Scholar] [CrossRef]
- Zhao, D.; Wan, X.; Song, H.; Hao, L.; Su, Y.; Lv, Y. Metal-organic frameworks (MOFs) combined with ZnO quantum dots as a fluorescent sensing platform for phosphate. Sens. Actuators B Chem. 2014, 197, 50–57. [Google Scholar] [CrossRef]
- Falcaro, P.; Hill, A.J.; Nairn, K.M.; Jasieniak, J.; Mardel, J.I.; Bastow, T.J.; Mayo, S.C.; Gimona, M.; Gomez, D.; Whitfield, H.J.; et al. A new method to position and functionalize metal-organic framework crystals. Nat. Commun. 2011, 2, 237. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, X.-M.; Ma, J.-P.; Liu, Q.-K.; Wang, P.; Dong, Y.-B. Cu(I)-MOF: Naked-eye colorimetric sensor for humidity and formaldehyde in single-crystal-to-single-crystal fashion. Chem. Commun. 2014, 50, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ma, J.-P.; Dong, Y.-B. Luminescent humidity sensors based on porous Ln3+-MOFs. CrystEngComm 2012, 14, 7157–7160. [Google Scholar] [CrossRef]
- Liu, J.; Sun, F.; Zhang, F.; Wang, Z.; Zhang, R.; Wang, C.; Qiu, S. In situ growth of continuous thin metal-organic framework film for capacitive humidity sensing. J. Mater. Chem. 2011, 21, 3775–3778. [Google Scholar] [CrossRef]
- Robinsin, A.L.; Stavila, V.; Zeitler, T.R.; White, M.I.; Thornberg, S.M.; Greathouse, J.A.; Allendorf, M.D. Ultrasensitive humidity detection using metal-organic framework-coated microsensors. Anal. Chem. 2012, 84, 7043–7051. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhang, Y.; Cong, H.; Fu, B.; Wen, S.; Ruan, S. A novel humidity sensor based on NH2-MIL-125(Ti) metal organic framework with high responsiveness. J. Nanoparticle Res. 2013, 15, 2014. [Google Scholar] [CrossRef]
- Harbuzaru, B.V.; Corma, A.; Rey, F.; Jorda, J.L.; Ananias, D.; Carlos, L.D.; Rocha, J. A miniaturized linear pH sensor based on a highly photoluminescent self-assembled europium(III) metal-organic framework. Angew. Chem. Int. Ed. Engl. 2009, 48, 6476–6479. [Google Scholar] [CrossRef]
- Aguilera-Sigalat, H.; Bradshaw, D. A colloidal water-stable MOF as a broad-range fluorescent pH sensor via post-synthetic modification. Chem. Commun. 2014, 50, 4711–4713. [Google Scholar] [CrossRef]
- Sun, L.-N.; Yu, J.; Peng, H.; Zhang, J.Z.; Shi, L.-Y.; Wolfbeis, O.S. Temperature-sensitive luminescent nanoparticles and films based on a terbium(III) complex probe. J. Phys. Chem. C 2010, 114, 12642–12648. [Google Scholar] [CrossRef]
- Ma, D.; Li, B.; Zhou, X.; Zhou, Q.; Liu, K.; Zeng, G.; Li, G.; Shi, Z.; Feng, S. A dual functional MOF as a luminescent sensor for quantitatively detecting the concentration of nitrobenzene and temperature. Chem. Commun. 2013, 49, 8964–8966. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, J.; Ma, F.; Ling, P.; Liu, J.; Ju, H. A porphyrin photosensitized metal-organic framework for cancer cell apoptosis and caspase responsive theranostics. Chem. Commun. 2015, 51, 10831–10834. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wang, Y.; Guo, Z. Terbium(III) complex as a luminescent sensor for human serum albumin in aqueous solution. Chem. Commun. 2011, 47, 8127–8129. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Lei, J.; Zhang, L.; Ju, H. Porphyrin-encapsulated metal-organic frameworks as mimetic catalysts for electrochemical DNA sensing via allosteric switch of hairpin DNA. Anal. Chem. 2015, 87, 3957–3963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C.-F.; Lv, Y.-K. Luminescent switch sensors for the detection of biomolecules based on metal-organic frameworks. Analyst 2018, 143, 4221–4229. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Ma, J.G.; Li, H.; Chen, D.M.; Gu, W.; Yang, G.M.; Cheng, P. Metal-organic framework biosensor with high stability and selectivity in a biomimic environment. Chem. Commun. 2015, 51, 9161–9164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, H.L.; Ye, G.Q.; Chen, H.H.; Hu, X.Y. Carbon functionalized metal organic framework/Nafion composites as novel electrode materials for ultrasensitive determination of dopamine. J. Mater. Chem. B 2015, 3, 3747–3753. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, W.; Wang, L.Y.; Zhuang, Q.F.; Ni, Y.N. Electrochemical determination of 2,4,6-trinitrophenol using a hybrid film composed of a copper-based metal organic framework and electroreduced graphene oxide. Microchim. Acta 2018, 185, 315. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, X.B.; He, X.W.; Zhang, Y.K. Electrochemistry and electrochemiluminescence from a redox-active metal–organic framework. Biosens. Bioelectron. 2015, 68, 197–203. [Google Scholar] [CrossRef]
- Xiong, C.Y.; Wang, H.J.; Liang, W.B.; Yuan, Y.L.; Yuan, R.; Chai, Y.Q. Luminescence-functionalized metal-organic frameworks based on a ruthenium(II) complex: A signal amplification strategy for electrogenerated chemiluminescence immunosensors. Chem. Eur. J. 2015, 21, 9825–9832. [Google Scholar] [CrossRef]
- Iskierko, Z.; Sharma, P.S.; Prochowicz, D.; Fronc, K.; D’Souza, F.; Toczydłowska, D.; Stefaniak, F.; Noworyta, K. Molecularly imprinted polymer (MIP) film with improved surface area developed by using metal-organic framework (MOF) for sensitive lipocalin (NGAL) determination. ACS Appl. Mater. Interfaces 2016, 8, 19860–19865. [Google Scholar] [CrossRef]
- Tchalala, M.R.; Bhatt, P.M.; Chappanda, K.N.; Tavares, S.R.; Adil, K.; Belmabkhout, Y.; Shkurenko, A.; Cadiau, A.; Heymans, N.; Weireld, G.D.; et al. Fluorinated MOF platform for selective removal and sensing of SO2 from flue gas and air. Nat. Commun. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Abuzalat, O.; Wong, D.; Park, S.S.; Kim, S. High-performance, room temperature hydrogen sensing with a Cu-BTC/polyaniline nanocomposite film on a quartz crystal microbalance. IEEE Sens. J. 2019, 13, 4789–4795. [Google Scholar] [CrossRef]
- Haghighi, E.; Zeinali, S. Nanoporous MIL-101(Cr) as a sensing layer coated on a quartz crystal microbalance (QCM) nanosensor to detect volatile organic compounds (VOCs). RSC Adv. 2019, 9, 24460–24470. [Google Scholar] [CrossRef]
- Wen, L.L.; Zhou, L.; Zhang, B.G.; Meng, X.G.; Qu, H.; Li, D.F. Multifunctional amino-decorated metal–organic frameworks: Nonlinear-optic, ferroelectric, fluorescence sensing and photocatalytic properties. J. Mater. Chem. 2012, 22, 22603–22609. [Google Scholar] [CrossRef]
- Stubbs, A.W.; Braglia, L.; Borfecchia, E.; Meyer, R.J.; Roman-Leshkov, Y.; Lamberti, C.; Dinca, M. Selective Catalytic Olefin Epoxidation with MnII-Exchanged MOF-5. ACS Catal. 2018, 8, 596–601. [Google Scholar] [CrossRef]
- Yuan, K.; Song, T.; Wang, D.; Zou, Y.; Li, J.; Zhang, X.; Tang, Z.; Hu, W. Bimetal–organic frameworks for functionality optimization: MnFe-MOF-74 as a stable and efficient catalyst for the epoxidation of alkenes with H2O2. Nanoscale 2018, 10, 1591–1597. [Google Scholar] [CrossRef]
- Tabatabaeian, K.; Zanjanchi, M.A.; Mahmoodi, N.O.; Eftekhari, T.; Shafiei, S.M. Diimino Nickel Complex Anchored into the MOF Cavity as Catalyst for Epoxidation of Chalcones and Bischalcones. J. Clust. Sci. 2017, 28, 949–962. [Google Scholar] [CrossRef]
- Kaposi, M.; Cokoja, M.; Hutterer, C.H.; Hauser, S.A.; Kaposi, T.; Klappenberger, F.; Pöthig, A.; Barth, J.V.; Herrmanna, W.A.; Kühn, F.E. Immobilisation of a molecular epoxidation catalyst on UiO-66 and -67: The effect of pore size on catalyst activity and recycling. Dalton Trans. 2015, 44, 15976–15983. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Tang, H.; Ramella, D.; Luan, Y. Modification of Cu2+ into Zr-based metal–organic framework (MOF) with carboxylic units as an efficient heterogeneous catalyst for aerobic epoxidation of olefins. Mol. Catal. 2018, 456, 57–64. [Google Scholar] [CrossRef]
- Akbari, S.; Mokhtari, J.; Mirjafari, Z. Solvent-free and melt aerobic oxidation of benzyl alcohols using Pd/Cu2(BDC)2DABCO–MOF prepared by one-step and through reduction by dimethylformamide. RSC Adv. 2017, 7, 40881–40886. [Google Scholar] [CrossRef]
- Wang, J.-S.; Jin, F.-Z.; Ma, H.-C.; Li, X.-B.; Liu, M.-Y.; Kan, J.-L.; Chen, G.-J.; Dong, Y.-B. Au@Cu(II)-MOF: Highly efficient bifunctional heterogeneous catalyst for successive oxidation–condensation reactions. Inorg. Chem. 2016, 55, 66856691. [Google Scholar] [CrossRef]
- Lu, B.-B.; Yang, J.; Che, G.-B.; Pei, W.-Y.; Ma, J.-F. Highly Stable Copper(I)-Based Metal–Organic Framework Assembled with Resorcin [4] arene and Polyoxometalate for Efficient Heterogeneous Catalysis of Azide–Alkyne “Click” Reaction. ACS Appl. Mater. Interfaces 2018, 10, 2628–2636. [Google Scholar] [CrossRef]
- Li, P.; Regati, S.; Huang, H.; Arman, H.; John, D.; Zhao, C.-G.; Chen, B. A metal-organic framework as a highly efficient and reusable catalyst for the solvent-free 1, 3-dipolar cycloaddition of organic azides to alkynes. Inorg. Chem. Front. 2015, 2, 42–46. [Google Scholar] [CrossRef]
- Liu, X.; Qi, W.; Wang, Y.; Su, R.; He, Z. Exploration of Intrinsic Lipase-Like Activity of Zirconium-Based Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2018, 4579–4585. [Google Scholar] [CrossRef]
- Schumacher, W.T.; Mathews, M.J.; Larson, S.A.; Lemmon, C.E.; Campbell, K.A.; Crabb, B.T.; Chicoine, B.J.-A.; Beauvais, L.G.; Perry, M.C. Organocatalysis by site-isolated N-heterocyclic carbenes doped into the UiO-67 framework. Polyhedron 2016, 114, 422–427. [Google Scholar] [CrossRef]
- Tarnowicz-Ligus, S.; Augustyniak, A.; Trzeciak, A.M. Incorporation of PdCl2P2 Complexes in Ni-MOF for Catalyzing Heck Arylation of Functionalized Olefins. Eur. J. Inorg. Chem. 2019, 4282–4288. [Google Scholar] [CrossRef]
- Bai, C.H.; Jian, S.P.; Yao, X.F.; Li, Y.W. Carbonylative Sonogashira coupling of terminal alkynes with aryl iodides under atmospheric pressure of CO using Pd(II)@MOF as the catalyst. Catal. Sci. Technol. 2014, 4, 3261–3267. [Google Scholar] [CrossRef]
- Li, X.; Zeeland, R.V.; Maligal-Ganesh, R.V.; Pei, Y.; Power, G.; Stanley, L.; Huang, W. Impact of linker engineering on the catalytic activity of metal-organic frameworks containing Pd(II)-bipyridine complexes. ACS Catal. 2016, 6, 6324–6328. [Google Scholar] [CrossRef]
- Yan, X.; Wang, K.; Xu, X.; Wang, S.; Ning, Q.; Xiao, W.; Zhang, N.; Chen, Z.; Chen, C. Brönsted Basicity in Metal-organic Framework-808 and Its Application in Base-Free Catalysis. Inorg. Chem. 2018, 57, 8033–8036. [Google Scholar] [CrossRef]
- Sun, D.; Li, Z. Double-Solvent Method to Pd Nanoclusters Encapsulated inside the Cavity of NH2-UiO-66(Zr) for Efficient Visible-Light-Promoted Suzuki Coupling Reaction. J. Phys. Chem. C 2016, 120, 19744–19750. [Google Scholar] [CrossRef]
- Zhao, M.; Yuan, K.; Wang, Y.; Li, G.; Guo, J.; Gu, L.; Hu, W.; Zhao, H.; Tang, Z. Metal-organic frameworks as selectivity regulators for hydrogenation reactions. Nature 2016, 539, 76–80. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, C.; Xiao, W.; Jian, L.; Zhang, N. Ni/MIL-120: An efficient metal-organic framework catalyst for hydrogenation of benzene to cyclohexane. Microporous Mesoporous Mater. 2013, 171, 9–13. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Lu, H.; Hong, X.; Jiang, H.; Wu, Y.; Li, Y. Hollow Zn/CO ZIF particles derived from core-shell ZIF-67@ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew. Chem. Int. Ed. Engl. 2015, 54, 10889–10893. [Google Scholar] [CrossRef]
- Zahmakirna, M. Iridium nanoparticles stabilized by metal organic frameworks (IrNPs@ZIF-8): Synthesis, structural properties and catalytic performance. Dalton Trans. 2012, 41, 12690–12696. [Google Scholar] [CrossRef]
- Noei, H.; Amirjalayer, S.; Müller, M.; Zhang, Z.; Schmid, R.; Muhler, M.; Fischer, R.A.; Wang, Y. Low-temperature CO oxidation over Cu-based metal-organic frameworks monitored by using FTIR spectroscopy. ChemCatChem 2012, 4, 755–759. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Isley III, W.C.; Ghosh, P.; Liao, P.; Bury, W.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W.; et al. Destruction of chemical warfare agents using metal-organic frameworks. Nat. Mater. 2015, 14, 512–516. [Google Scholar] [CrossRef]
- Tan, C.; Liu, G.; Li, H.; Cui, Y.; Liu, Y. Ultrathin two-dimensional metal-organic framework nanosheets-and emerging class of catalytic nanomaterials. Dalton Trans. 2020, 49, 11073–11084. [Google Scholar] [CrossRef]
- Xu, M.; Yuan, S.; Chen, X.-Y.; Chang, Y.-J.; Day, G.; Gu, Z.-Y.; Zhou, H.-C. Two-dimensional metal-organic framework nanosheets as an enzyme inhibitor: Modulation of the α-chymotrypsin activity. J. Am. Chem. Soc. 2017, 139, 8312–8319. [Google Scholar] [CrossRef]
- He, T.; Ni, B.; Zhang, S.; Gong, Y.; Wang, H.; Gu, L.; Zhuang, J.; Hu, W.; Wang, X. Ultrathin 2D zirconium metal-organic framework nanosheets: Preparation and application in photocatalysis. Small 2018, 14, 1703929. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, W.; Chen, H.; Li, H.; Xu, X.; Wu, P.; Shen, Y.; Zheng, B.; Huo, F.; Wei, W.D. Ultrathin 2D Cu-porphyrin MOF nanosheets as a heterogeneous catalyst for styrene oxidation. Mater. Chem. Front. 2019, 3, 1580–1585. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Integration of metal organic frameworks with enzymes as multifunctional solids for cascade catalysis. Dalton Trans. 2020, 49, 11059–11072. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Li, Z.; Huo, J.; Chen, C.; Li, Q.; Niu, S.; Wang, S. Regulating hydrogenation chemoselectivity of α, β-unsaturated aldehydes by combination of transfer and catalytic hydrogenation with ammonia borane and Pt/MOL. ChemSusChrm 2020, 13, 1746–1750. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Wu, Y.; Guan, C.; Cheetham, A.K.; Wang, J. MOF-derived nanohybrids for electrocatalysis and energy storage: Current status and perspectives. Chem. Commun. 2018, 54, 5268–5288. [Google Scholar] [CrossRef]
- Wen, X.; Guan, J. Recent progress on MOF-derived electrocatalysts for hydrogen evolution reaction. Appl. Mater. Today 2019, 16, 146–168. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Jothi, V.R.; Vikraman, D.; Prasanna, K.; Maiyalagan, T.; Sang, B.I.; Yi, S.C.; Kim, H.S. Metal–organic framework derived NiMo polyhedron as an efficient hydrogen evolution reaction electrocatalyst. Appl. Surf. Sci. 2019, 478, 916–923. [Google Scholar] [CrossRef]
- Li, H.; Ke, F.; Zhu, J. MOF-Derived Ultrathin Cobalt Phosphide Nanosheets as Efficient Bifunctional Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts. Nanomaterials 2018, 8, 89. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, H.; Li, A.; Li, Z.; Liu, S.; Zhang, Q.; Gong, Y.; Zheng, L.; Zhu, Y.; Chen, C.; et al. Constructing NiCo/Fe3O4 Heteroparticles within MOF-74 for Efficient Oxygen Evolution Reactions. J. Am. Chem. Soc. 2018, 45, 140–15336. [Google Scholar] [CrossRef]
- Zhou, W.; Dan Huang, D.; Wu, Y.P.; Zhao, J.; Wu, T.; Zhang, J.; Li, D.S.; Sun, C.; Feng, P.; Bu, X. Stable Hierarchical Bimetal-Organic Nanostructures as High Performance Electrocatalysts for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 4227–4231. [Google Scholar] [CrossRef]
- Tripathy, R.K.; Samantara, A.K.; Behera, J.N. A cobalt metal-organic framework (Co-MOF): A bi-functional electro active material for the oxygen evolution and reduction reaction. Dalton Trans. 2019, 48, 10557–10564. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zheng, S.; Xue, H.; Pang, H. Regulation of the Ni2+ Content in a Hierarchical Urchin-Like MOF for High-Performance Electrocatalytic Oxygen Evolution. Front. Chem. 2019, 7, 411. [Google Scholar] [CrossRef]
- Hinogami, R.; Yotsuhashi, S.; Deguchi, M.; Zenitani, Y.; Hashiba, H.; Yamada, Y. Electrochemical reduction of carbon dioxide using a copper rubeanate metal organic framework. Ecs Electrochem. Lett. 2012, 1, H17–H19. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Senthil Kumar, S.; Anbu Kulandainathan, M. Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 2012, 25, 70–73. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, Q.; Sun, X.; Hu, J.; Zhang, J.; Liu, Z.; Han, B. Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal-organic framework cathode. Chem. Sci. 2016, 7, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, Y.; Quan, X.; Chen, S.; Yu, H. CO2 Electroreduction at Low Overpotential on Oxide-Derived Cu/Carbons Fabricated from Metal Organic Framework. Acs Appl. Mater. Interfaces 2017, 9, 5302–5311. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yan, S.; Sun, Y.; Yang, X.; Guo, Z.; Du, J.; Chen, D.; Chen, P.; Xing, H. Enhancement of visible-light-driven CO2 reduction performance using an amine-functionalized zirconium metal–organic framework. Dalton Trans. 2018, 47, 909–915. [Google Scholar] [CrossRef]
- Chen, D.; Xing, H.; Wang, C.; Su, Z. Highly efficient visible-light-driven CO2 reduction to formate by a new anthracene-based zirconium MOF via dual catalytic routes. J. Mater. Chem. A 2016, 4, 2657–2662. [Google Scholar] [CrossRef]
- Wang, X.; Lu, W.; Gu, Z.-Y.; Wei, Z.; Zhou, H.-C. Topology-guided design of an anionic bor-network for photocatalytic [Ru(bpy)3]2+ encapsulation. Chem. Commun. 2016, 52, 1926–1929. [Google Scholar] [CrossRef]
- Cai, J.; Lu, J.-Y.; Chen, Q.-Y.; Qu, L.-L.; Lu, Y.-Q.; Gao, G.-F. Eu-based MOF/graphene oxide composite: A novel photocatalyst for the oxidation of benzyl alcohol using water as oxygen source. New J. Chem. 2017, 41, 3883–3886. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Mu, Y.-F.; Guo, X.-X.; Zhang, W.; Zhang, Z.-M.; Zhang, M.; Bu, T.-B. Encapsulating perovskite quantum dots in iron-based metal-organic frameworks for efficient photocatalytic CO2 reduction. Angew. Chem. Int. Ed. Engl. 2019, 58, 9491–9495. [Google Scholar] [CrossRef]
- Li, R.; Hu, J.; Deng, M.; Wang, H.; Wang, X.; Hu, Y.; Jiang, H.-L.; Jiang, J.; Zhang, Q.; Xie, Y.; et al. Integration of an inorganic semiconductor with a metal-organic framework: A platform for enhanced gaseous photocatalytic reactions. Adv. Mater. 2014, 26, 4783–4788. [Google Scholar] [CrossRef]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. CdS-decorated UiO-66(NH2) nanocomposites fabricated by a facile photodeposition process: And efficient and stable visible-light-driven photocatalyst for selective oxidation of alcohols. J. Mater. Chem. A 2013, 1, 11473–11482. [Google Scholar] [CrossRef]
- Ke, F.; Wang, L.; Zhu, J. Facile fabrication of CdS-metal-organic framework nanocomposites with enhanced visible-light photocatalytic activity for organic transformation. Nano Res. 2015, 8, 1834–1846. [Google Scholar] [CrossRef]
- Saha, S.; Das, G.; Thote, J.; Banerjee, R. Photocatalytic metal-organic framework from CdS quantum dots incubated luminescent methallohydrogel. J. Am. Chem. Soc. 2014, 136, 14845–14851. [Google Scholar] [CrossRef]
- Sharma, V.K.; Feng, M. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2019, 372, 3–16. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Yu, Z.; Zhang, L.; Wang, D.; Pang, B.; Sun, W. Application of Fe-MOFs in advanced oxidation processes. Res. Chem. Intermed. 2019, 45, 3777–3793. [Google Scholar] [CrossRef]
- Farrokhi, A.; Jafarpour, M.; Alipour, M. Solar-driven advanced oxidation process catalyzed by metal-organic frameworks for water depollution. Polyhedron 2019, 170, 325–333. [Google Scholar] [CrossRef]
- Goswami, S.; Miller, C.E.; Logsdon, J.L.; Buru, C.T.; Wu, Y.-L.; Bowman, D.N.; Islamoglu, T.; Asiri, A.M.; Cramer, C.J.; Wasielewski, M.R.; et al. Atomistic approach toward selective oxidation of a mustard-gas simulant: A case study with heavy-chalcogen-containing PCN-57 analogues. ACS Appl. Mater. Interfaces 2017, 9, 19535–19540. [Google Scholar] [CrossRef]
- Atilgan, A.; Islamoglu, T.; Howarth, A.J.; Hupp, J.T.; Farha, O.K. Detoxification of a sulfur mustard simulant using a BODIPY functionalized zirconium-based metal-organic framework. ACS Appl. Mater. Interfaces 2017, 9, 24555–24560. [Google Scholar] [CrossRef]
- Liu, D.S.; Sui, Y.; Chen, W.T.; Feng, P. Two new nonlinear optical and ferroelectric Zn(ll) compounds based on nicotinic acid and tetrazole derivative ligands. Cryst. Growth Des. 2015, 15, 4020–4025. [Google Scholar] [CrossRef]
- Hua, J.A.; Zhao, Y.; Zhao, D.; Kang, Y.S.; Chen, K.; Sun, W.Y. Functional group effects on structure and topology of cadmium(II) frameworks with mixed organic ligands. RSC Adv. 2015, 5, 43268–43278. [Google Scholar] [CrossRef]
- Pan, L.; Liu, G.; Li, H.; Meng, S.; Han, L.; Shang, J.; Chen, B.; Platero-Prats, A.E.; Lu, W.; Zou, X.; et al. A Resistance-Switchable and Ferroelectric Metal-Organic Framework. J. Am. Chem. Soc. 2014, 136, 17477–17483. [Google Scholar] [CrossRef]
- Fu, D.-W.; Zhang, W.; Xiong, R.-G. The first metal-organic framework (MOF) Imazethapyr and its SHG, piezoelectric and dielectric properties. Dalton Trans. 2008, 3946–3948. [Google Scholar] [CrossRef]
- Knebel, A.; Geppert, B.; Volgmann, K.; Kolokolov, D.I.; Stepanov, A.G.; Twiefel, J.; Heitjans, P.; Volkmer, D.; Caro, J. Defibrillation of soft porous metal-organic Frameworks with electric fields. Science 2017, 358, 347–351. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, Z.; Zhao, D.; Zeng, K. Probing nanoscale functionalities of metal-organic framework nanocrystals. Nanoscale 2017, 9, 12163–12169. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, J.; Cheng, Y.; Zhang, Y.-W.; Zeng, K. Design of the Hybrid Metal-Organic Frameworks (MOFs) as Potential Supramolecular Piezo/Ferro-Electrics. J. Phys. Chem. C 2019, 123, 3122–3129. [Google Scholar] [CrossRef]
- Lu, Y.; Young, D.J. Coordination polymers for n-type thermoelectric applications. Dalton Trans. 2020, 49, 7644–7657. [Google Scholar] [CrossRef]
- Ryder, M.R.; Donà, L.; Vitillo, J.G.; Civalleri, B. Understanding and controlling the dielectric response of metal-organic frameworks. ChemPlusChem 2018, 83, 308–316. [Google Scholar] [CrossRef]
- Warmbier, R.; Quandt, A.; Sefert, G. Dielectric properties of selected metal-organic frameworks. J. Phys. Chem. C 2014, 118, 11799–11805. [Google Scholar] [CrossRef]
- Redel, E.; Wang, Z.; Walheim, S.; Liu, J.; Gliermann, H.; Wöll, C. On the dielectric and optical properties of surface-anchored metal-organic frameworks: A study on epitaxially grown thin films. Appl. Phys. Lett. 2013, 103, 091903. [Google Scholar] [CrossRef]
- Eslava, S.; Zhang, L.; Esconjauregui, S.; Yang, J.; Vanstreels, K.; Baklanov, M.R.; Saiz, E. Metal-organic framework ZIF-8 films as low-κ dielectrics in microelectronics. Chem. Mater. 2013, 25, 27–33. [Google Scholar] [CrossRef]
- Usman, M.; Lee, C.-H.; Hung, D.-S.; Lee, S.-F.; Wang, C.-C.; Luo, T.-T.; Zhao, L.; Wu, M.-K.; Lu, K.-L. Intrinsic low dielectric behavior of a highly thermally stable Sr-based metal-organic framework for interlayer dielectric materials. J. Mater. Chem. C 2014, 2, 3762–3768. [Google Scholar] [CrossRef]
- Mendiratta, S.; Usman, M.; Chang, C.-C.; Lee, Y.-C.; Chen, J.-W.; Wu, M.-K.; Lin, Y.-C.; Hsu, C.-P.; Lu, K.-L. Zn(II)-based metal-organic framework: An exceptionally thermally stable, guest-free low dielectric material. J. Mater. Chem. C 2017, 5, 1508–1513. [Google Scholar] [CrossRef]
- Usman, M.; Mendiratta, S.; Lu, K.-L. Metal-organic frameworks: New interlayer dielectric materials. ChemElectroChem 2015, 2, 786–788. [Google Scholar] [CrossRef]
- Xing, X.-S.; Fu, Z.-H.; Zhang, N.-N.; Yu, X.-Q.; Wang, M.-S.; Guo, G.-C. High proton conduction in an excellent water-stable gadolinium metal-organic framework. Chem. Commun. 2019, 55, 1241–1244. [Google Scholar] [CrossRef]
- Okawa, H.; Shigematsu, A.; Sadakiyo, M.; Miyagawa, T.; Yoneda, K.; Ohba, M.; Kitagawa, H. Oxalate-bridged bimetallic complexes {NH(prol)3}[MCr(ox)3] (M = MnII, FeII, CoII.; NH(prol)3+ = tri(3-hydroxypropyl)ammonium) exhibiting coexistent ferromagnetism and proton conduction. J. Am. Chem. Soc. 2009, 131, 13516–13522. [Google Scholar] [CrossRef]
- Feng, L.; Wang, H.-S.; Xu, H.-L.; Huang, W.-T.; Zeng, T.-Y.; Cheng, Q.-R.; Pan, Z.-Q.; Zhou, H. A water stable layered Tb(III) polycarboxylate with a proton conductivity over 10−2 Scm−1 in a wide temperature range. Chem. Commun. 2019, 55, 1762–1765. [Google Scholar] [CrossRef]
- Bera, S.P.; Mondal, A.; Konar, S. Lanthanide based layer type two dimensional coordination polymers featuring slow magnetic relaxation, magnetocaloric effect and proton conductivity. Chem. Asian J. 2019, 14, 3702–3711. [Google Scholar] [CrossRef]
- Tang, Q.; Yang, Y.-L.; Zhang, N.; Liu, Z.; Zhang, S.-H.; Tang, F.-S.; Hu, J.-Y.; Zheng, Y.-Z.; Liang, F.-P. A multifunctional lanthanide carbonate cluster based metal-organic framework exhibits high proton transport and magnetic exchange change. Inorg. Chem. 2018, 57, 9020–9027. [Google Scholar] [CrossRef]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1153–1179. [Google Scholar] [CrossRef]
- Coronado, E.; Espallargas, G.M. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Natarajan, S.; Vaidhyanathan, R. Metal carboxylates with open architectures. Angew. Chem. Int. Ed. Engl. 2004, 43, 1466–1496. [Google Scholar] [CrossRef]
- Maspoch, D.; Ruiz-Molina, D.; Wurst, K.; Domingo, N.; Cavallini, M.; Biscarini, F.; Tejada, J.; Rovira, C.; Veciana, J. A nanoporous molecular magnet with reversible solvent-induced mechanical and magnetic properties. Nat. Mater. 2003, 2, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Roques, N.; Maspoch, D.; Imaz, I.; Datcu, A.; Sutter, J.-P.; Rovira, C.; Veciana, J. A three-dimensional lanthanide-organic radical open-framework. Chem. Commun. 2008, 3160–3162. [Google Scholar] [CrossRef] [PubMed]
- Roques, N.; Maspoch, D.; Luis, F.; Camón, A.; Wurst, K.; Datcu, A.; Rovira, C.; Ruiz-Molina, D.; Veciana, J. A hexacarboxylic open-shell building block: Synthesis, structure and magnetism of a three-dimensional metal-radical framework. J. Mater. Chem. 2008, 18, 98–108. [Google Scholar] [CrossRef]
- Lu, K.; Aung, T.; Guo, N.; Weichselbaum, R.; Lin, W. Nanoscale metal-organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater. 2018, 30, 1707634. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Tualelle, F.; Férey, G. Metal-organic frameworks as efficient materials for drug-delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- Rieter, W.J.; Taylor, K.M.L.; An, H.; Lin, W.; Lin, W. Nanoscale metal-organic frameworks as potential multimodal contrast enhancing agents. J. Am. Chem. Soc. 2006, 128, 9024–9025. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, X.; Chen, J.; Wang, X.; Wei, L.; Fang, L.; Kumar, A.; Zhuang, S.; Liu, J. Recent advances in MOF-based nanoplatforms generating reactive species for chemodynamic therapy. Dalton Trans. 2020, 49, 11045–11058. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhai, M.; Guan, W.; Liu, J.; Liu, Z.; Damirin, A. Controllable synthesis of a smart multifunctional nanoscale metal-organic framework for magnetic resonance/optical imaging and targeted drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 3455–3462. [Google Scholar] [CrossRef]
- Imaz, I.; Rubio-Martinez, M.; Garcia-Fernández, L.; Garcia, F.; Ruiz-Molina, D.; Hernando, J.; Puntesa, V.; Maspoch, D. Coordination polymer particles as potential drug delivery systems. Chem. Commun. 2010, 46, 4737–4739. [Google Scholar] [CrossRef]
- Bieniek, A.; Wiśniewski, M.; Roszek, K.; Bolibok, P.; Terzyk, A.P.; Ferrer, P.; da Silva, I. New strategy of controlled, stepwise release from novel MBioF and its potential application for drug delivery systems. Adsorption 2019, 25, 383–391. [Google Scholar] [CrossRef]
- Chen, W.; Wu, C. Synthesis, functionalization, and applications of metal-organic frameworks in biomedicine. Dalton Trans. 2018, 47, 2114–2133. [Google Scholar] [CrossRef]
- Filippousi, M.; Turner, S.; Leys, K.; Siafaka, P.I.; Tseligka, E.D.; Vandichel, M.; Nanaki, S.G.; Vizirianakis, I.S.; Bikiaris, D.N.; Van Der Voort, P.; et al. Biocompatible Zr-based nanoscale MOFs coated with modified poly(ε-caprolactone) as anticancer drug carriers. Int. J. Pharmaceut. 2016, 509, 208–218. [Google Scholar] [CrossRef]
- Cuhna, D.; Ben-Yahia, M.; Hall, S.; Miller, S.R.; Chevreau, H.; Elkaim, E.; Maurin, G.; Horcajada, P.; Serre, C. Rationale of drug encapsulation and release from biocompatible porous metal-organic framework. Chem. Mater. 2013, 25, 2767–2776. [Google Scholar]
- Devautor-Vinot, S.; Martineau, C.; Diaby, S.; Ben-Yahia, M.; Miller, S.; Serre, C.; Horcajada, P.; Cuhna, D.; Taulelle, F.; Maurin, G. Caffeine confinement into a series of functionalized porous zirconium MOFs: A joint experimental/modeling exploration. J. Phys. Chem. 2013, 117, 11694–11704. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Q.; Zhang, Q.; Jiang, K.; Lin, W.; Yang, Y.; Cui, Y.; Qian, G. A large capacity cationic metal-organic framework for physiological pH responsive drug delivery. Mol. Pharm. 2016, 13, 2782–2786. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Chen, X. Bioengineering of metal-organic frameworks for nanomedicine. Theranostics 2019, 9, 3122–3133. [Google Scholar] [CrossRef]
- Morris, W.; Briley, W.E.; Auyeung, E.; Cabezas, M.D.; Mirkin, C.A. Nucleic Acid-Metal Organic Framework (MOF) Nanoparticle Conjugates. J. Am. Chem. Soc. 2014, 136, 7261–7264. [Google Scholar] [CrossRef]
- Röder, R.; Preiss, T.; Hirschle, P.; Steinborn, B.; Zimpel, A.; Höhn, M.; Rädler, J.O.; Bein, T.; Wagner, E.; Wuttke, S.; et al. Multifunctional nanoparticles by coordinative self-assemble of his-tagged units with metal-organic frameworks. J. Am. Chem. Soc. 2017, 139, 2359–2368. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Imbuluzqueta, E.; Guillou, N.; Serre, C.; Miller, S.; Elkaïm, E.; Horcajada, P.; Blanco-Prieto, M. A Zn azelate MOF: Combining antibacterial effect. CrystEngComm 2015, 17, 456–462. [Google Scholar] [CrossRef]
- Restrepo, J.; Serroukh, Z.; Santiago-Morales, J.; Aguado, S.; Gomez-Sal, P.; Mosquera, M.E.G.; Rosal, R. An antibacterial Zn-MOF with hydrazinebenzoate linkers. Eur. J. Inorg. Chem. 2017, 574–580. [Google Scholar] [CrossRef]
- Berchel, M.; Le Gall, T.; Denis, C.; Le Hir, S.; Quentel, F.; Elleouet, C.; Montier, T.; Rueff, J.; Salaun, J.; Haelters, J.; et al. A silver-based metal–organic framework material as a ‘reservoir’ of bactericidal metal ions. New J. Chem. 2011, 35, 1000–1003. [Google Scholar] [CrossRef]
- Zhuang, W.; Yuan, D.; Li, J.R.; Luo, Z.; Zhou, H.C.; Bashir, S.; Liu, J. Highly potent bactericidal activity of porous metal–organic frameworks. Adv. Healthc. Mater. 2012, 1, 225–238. [Google Scholar] [CrossRef]
- Chiericatti, C.; Basilico, J.C.; Basilico, M.L.Z.; Zamaro, J.M. Novel application of HKUST-1 metal–organic framework as antifungal: Biological tests and physicochemical characterizations. Microporous Mesoporous Mater. 2012, 162, 60–63. [Google Scholar] [CrossRef]
- Bouson, S.; Krittayavathananon, A.; Phattharasupakun, N.; Siwayaprahm, P.; Sawangphruk, M. Antifungal activity of water-stable copper-containing metal-organic frameworks. R. Soc. Open Sci. 2017, 4, 170654. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Li, H.; Bai, Y.; Liu, H. Metal-organic frameworks as advanced sorbents in sample preparation for small organic analytes. Coord. Chem. Rev. 2019, 397, 1–13. [Google Scholar] [CrossRef]
- Ma, W.; Li, X.; Bai, Y.; Liu, H. Applications of metal-organic frameworks as advanced sorbents in biomacromolecules sample preparation. Trends Anal. Chem. 2018, 109, 154–162. [Google Scholar] [CrossRef]
- Pérez-Cejuela, H.M.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Recent advances in affinity MOF-based sorbents with sample preparation purposes. Molecules 2020, 25, 4216. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A.; Samanidou, V.F. Applications of metal-organic frameworks in food sample preparation. Molecules 2018, 23, 2896. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. https://doi.org/10.3390/ma14020310
Raptopoulou CP. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials. 2021; 14(2):310. https://doi.org/10.3390/ma14020310
Chicago/Turabian StyleRaptopoulou, Catherine P. 2021. "Metal-Organic Frameworks: Synthetic Methods and Potential Applications" Materials 14, no. 2: 310. https://doi.org/10.3390/ma14020310
APA StyleRaptopoulou, C. P. (2021). Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials, 14(2), 310. https://doi.org/10.3390/ma14020310




