Improving Photocatalytic Performance Using Nanopillars and Micropillars
Abstract
:1. Introduction
2. Nanopillars for Water Splitting—Improving the Efficiency due to Doping Silicon or Titanium Dioxide Nanopillars
2.1. Titanium Dioxide—TiO2
2.2. Hematite—Fe2O3
3. Nanopillars for Removing Organic Pollutants
3.1. Improving the Efficiency of Oxides due to Larger Surface Area
3.2. Degradation of Organic Pollutants due to Photocatalytically Enhanced TiO2 Nanostructures
3.3. Improving the Efficiency with Decorated TiO2 Nanopillars
3.4. Improving the Efficiency with Decorated Hematite Nanopillars
4. Nanopillars for Breaking Organic Chemicals for Analysis
5. Nanopillars for Photoswitching Achieved by Varying Surface Hydrophobicity and Hydrophilicity
6. Nanopillars for Photocatalytic Soot Oxidation
7. Nanopillars for Photothermalization
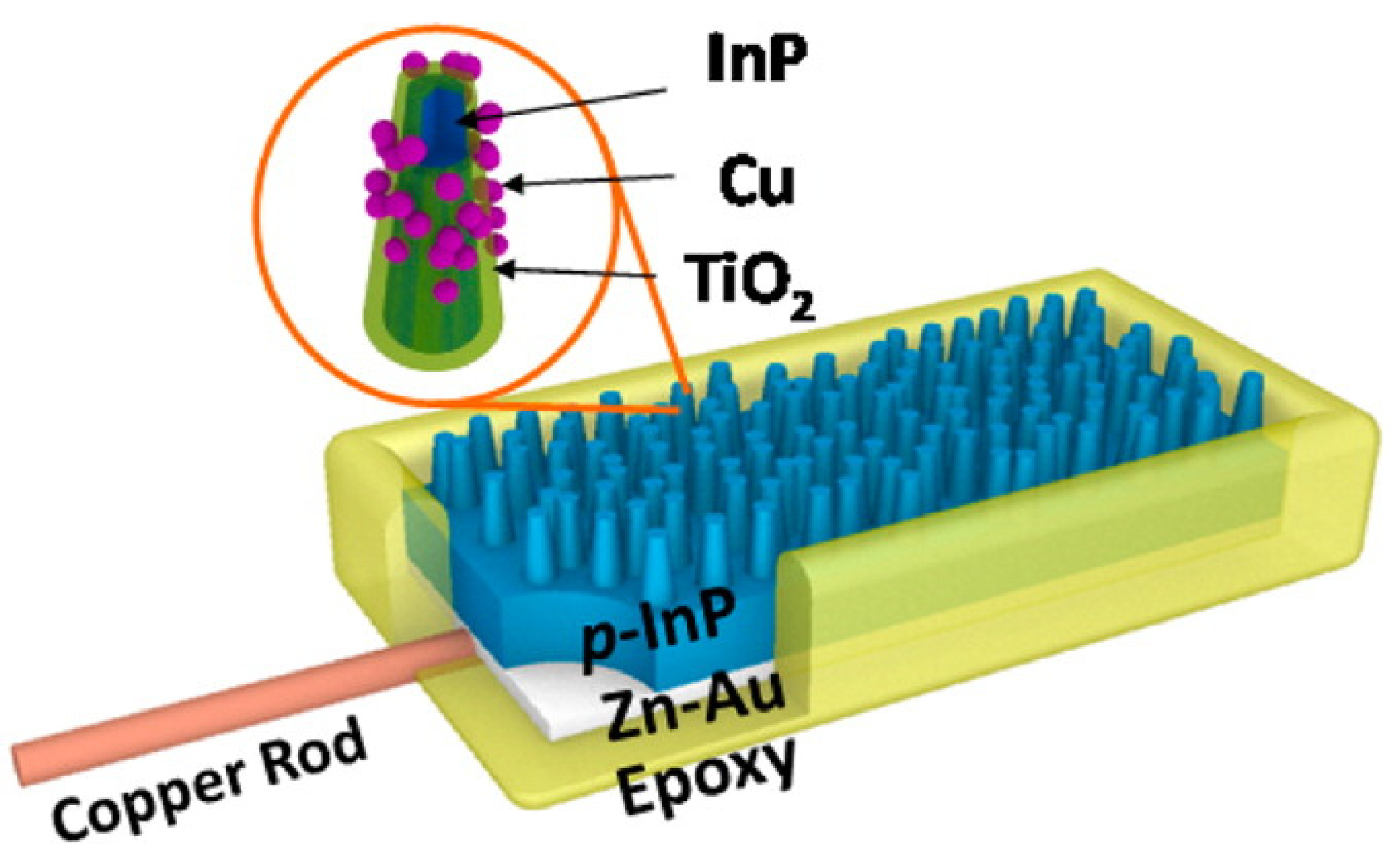
8. Nanopillars for Conversion of Carbon Dioxide to Energy—Increasing Photosynthetic Efficiency through Doping of InP Nanopillars with TiO2
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kertmen, A.; Barbé, E.; Szkoda, M.; Siuzdak, K.; Babačić, V.; Torruella, P.; Iatsunskyi, I.; Kotkowiak, M.; Rytel, K.; Estradé, S.; et al. Photoelectrochemically Active N-Adsorbing Ultrathin TiO2 Layers for Water-Splitting Applications Prepared by Pyrolysis of Oleic Acid on Iron Oxide Nanoparticle Surfaces under Nitrogen Environment. Adv. Mater. Interfaces 2018, 1801286. [Google Scholar] [CrossRef]
- Shuang, S.; Lv, R.; Xie, Z.; Zhang, Z. Surface Plasmon Enhanced Photocatalysis of Au/Pt-decorated TiO2 Nanopillar Arrays. Sci. Rep. 2016, 6, 26670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.-E.; Lin, C.-Y.; Hsu, C.-M. Fabrication of SnO2-TiO2 core-shell nanopillar-array films for enhanced photocatalytic activity. Appl. Surf. Sci. 2017, 396, 393–399. [Google Scholar] [CrossRef]
- Gao, H.; Liu, C.; Jeong, H.E.; Yang, P. Plasmon-Enhanced Photocatalytic Activity of Iron Oxide on Gold Nanopillars. ACS Nano 2011, 6, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-Z.; Lin, B.-Z.; Chen, Y.-L.; Xu, B.-H.; Pian, X.-T.; Kuang, J.-D.; Li, B. Fe-doped and ZnO-pillared titanates as visible-light-driven photocatalysts. J. Colloid Interface Sci. 2011, 358, 360–368. [Google Scholar] [CrossRef]
- Lalhriatpuia, C.; Tiwari, A.; Shukla, A.; Tiwari, D.; Lee, S.-M. Nanopillars TiO2 thin film photocatalyst application in the remediation of aquatic environment. Korean J. Chem. Eng. 2016, 33, 3367–3373. [Google Scholar] [CrossRef]
- Mirabedini, P.S.; Truskowska, A.; Ashby, D.Z.; Rao, M.P.; Greaney, P.A. Coupled Light Capture and Lattice Boltzmann Model of TiO2 Micropillar Array for Water Purification. MRS Adv. 2019, 4, 2689–2698. [Google Scholar] [CrossRef]
- Pavlenko, M.; Siuzdak, K.; Coy, E.; Załęski, K.; Jancelewicz, M.; Iatsunskyi, I. Enhanced solar-driven water splitting of 1D core-shell Si/TiO2/ZnO nanopillars. Int. J. Hydrog. Energy 2020, 45, 26426–26433. [Google Scholar] [CrossRef]
- Liao, A.; He, H.; Tang, L.; Li, Y.; Zhang, J.; Chen, J.; Chen, L.; Zhang, C.; Zhou, Y.; Zou, Z. Quasi-Topotactic Transformation of FeOOH Nanorods to Robust Fe2O3 Porous Nanopillars Triggered with a Facile Rapid Dehydration Strategy for Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 10141–10146. [Google Scholar] [CrossRef]
- Xing, Z.; Li, Z.; Wu, X.; Wang, G.; Zhou, W. In-situ S-doped porous anatase TiO2 nanopillars for high-efficient visible-light photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2016, 41, 1535–1541. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Ren, Z.; Tian, C.; LI, J.; Fu, H. Heterojunction Ag–TiO2 Nanopillars for Visible-LightDriven Photocatalytic H2 Production. ChemPlusChem 2014, 79, 995–1000. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Yoon, K.-Y.; Kwak, M.-J.; Jang, J.-H. A Titanium-Doped SiOxPassivation Layer for Greatly Enhanced Performance of a Hematite-Based Photoelectrochemical System. Angew. Chem. 2016, 128, 10076–10080. [Google Scholar] [CrossRef]
- Kimura, T.; Yamauchi, Y.; Miyamoto, N. Highly Photoactive Porous Anatase Films Obtained by Deformation of 3D Mesostructures. Chem. Eur. J. 2011, 17, 4005–4011. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-H.; Sonkaria, S.; Lee, B.; Kim, Y.G.; Ahn, S.-H.; Lee, C.S.; Khare, V. Tuning Intercrystalline Void-like Defects in Nanowire Clusters to TiO2 Quantum Wires with Enhanced Photocatalytic Performance. ACS Appl. Energy Mater. 2019, 2, 5643–5655. [Google Scholar] [CrossRef]
- Shuang, S.; Zhang, Z. The Effect of Annealing Treatment and Atom Layer Deposition to Au/Pt Nanoparticles-Decorated TiO2 Nanorods as Photocatalysts. Molecules 2018, 23, 525. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Shukla, A.; Lalliansanga; Tiwari, D.; Lee, S.-M. Au-nanoparticle/nanopillars TiO2 meso-porous thin films in the degradation of tetracycline using UV-A light. J. Ind. Eng. Chem. 2019, 69, 141–152. [Google Scholar] [CrossRef]
- Jiang, W.; Ullah, N.; Divitini, G.; Ducati, C.; Kumar, R.V.; Ding, Y.; Barber, Z.H. Vertically Oriented TiOxNy Nanopillar Arrays with Embedded Ag Nanoparticles for Visible-Light Photocatalysis. Langmuir 2012, 28, 5427–5431. [Google Scholar] [CrossRef]
- Shuang, S.; Xie, Z.; Zhang, Z. Enhanced photocatalytic properties of CdS nanoparticles decorated α-Fe2O3 nanopillar arrays under visible light. J. Colloid Interface Sci. 2017, 494, 107–113. [Google Scholar] [CrossRef]
- Nissilä, T.; Sainiemi, L.; Karikko, M.-M.; Kemell, M.; Ritala, M.; Franssila, S.; Kostiainen, R.; Ketola, R.A. Integrated photocatalytic micropillar nanoreactor electrospray ionization chip for mimicking phase I metabolic reactions. Lab Chip 2011, 11, 1470–1476. [Google Scholar] [CrossRef]
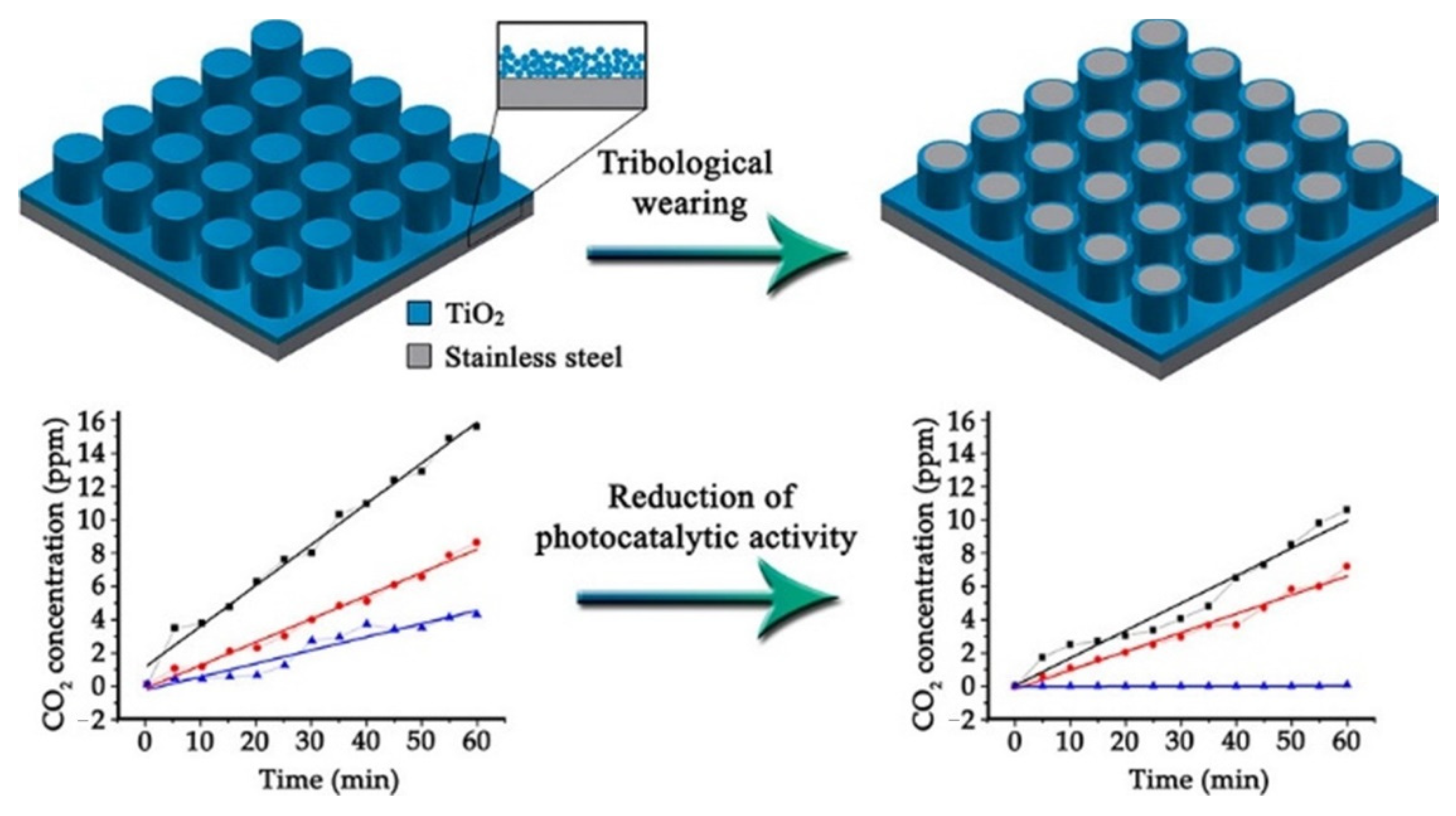
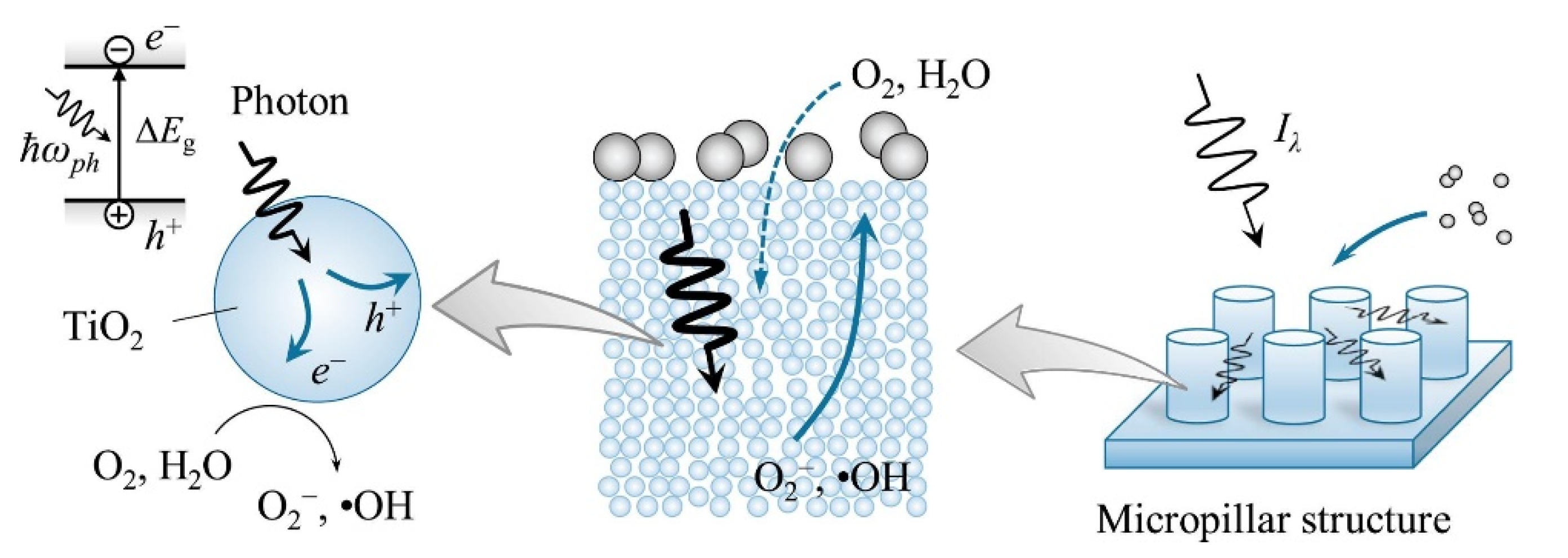
- Temerov, F.; Ammosova, L.; Haapanen, J.; Mäkelä, J.M.; Suvanto, M.; Saarinen, J.J. Protective stainless steel micropillars for enhanced photocatalytic activity of TiO2 nanoparticles during wear. Surf. Coat. Technol. 2020, 381, 125201. [Google Scholar] [CrossRef]
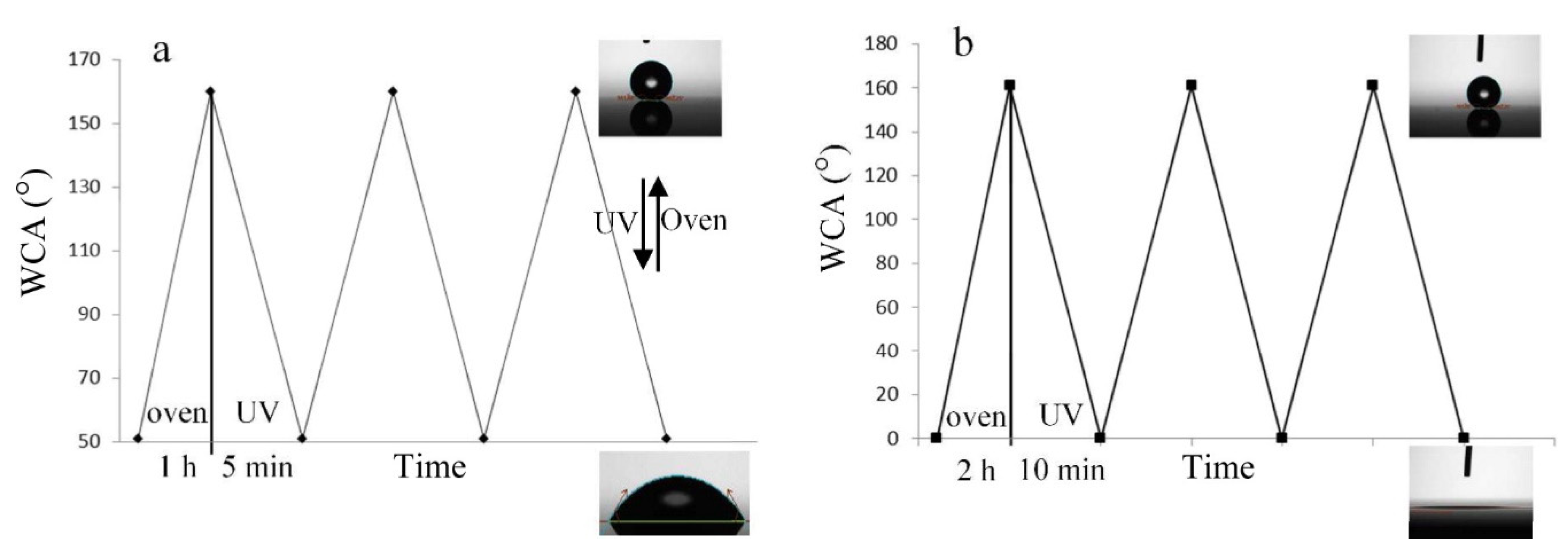
- Hoshian, S.; Jokinen, V.P.; Hjort, K.; Ras, R.H.; Franssila, S. Amplified and Localized Photoswitching of TiO2 by Micro- and Nanostructuring. ACS Appl. Mater. Interfaces 2015, 7, 15593–15599. [Google Scholar] [CrossRef] [PubMed]
- Kameya, Y.; Torii, K.; Hirai, S.; Kaviany, M. Photocatalytic soot oxidation on TiO2 microstructured substrate. Chem. Eng. J. 2017, 327, 831–837. [Google Scholar] [CrossRef]
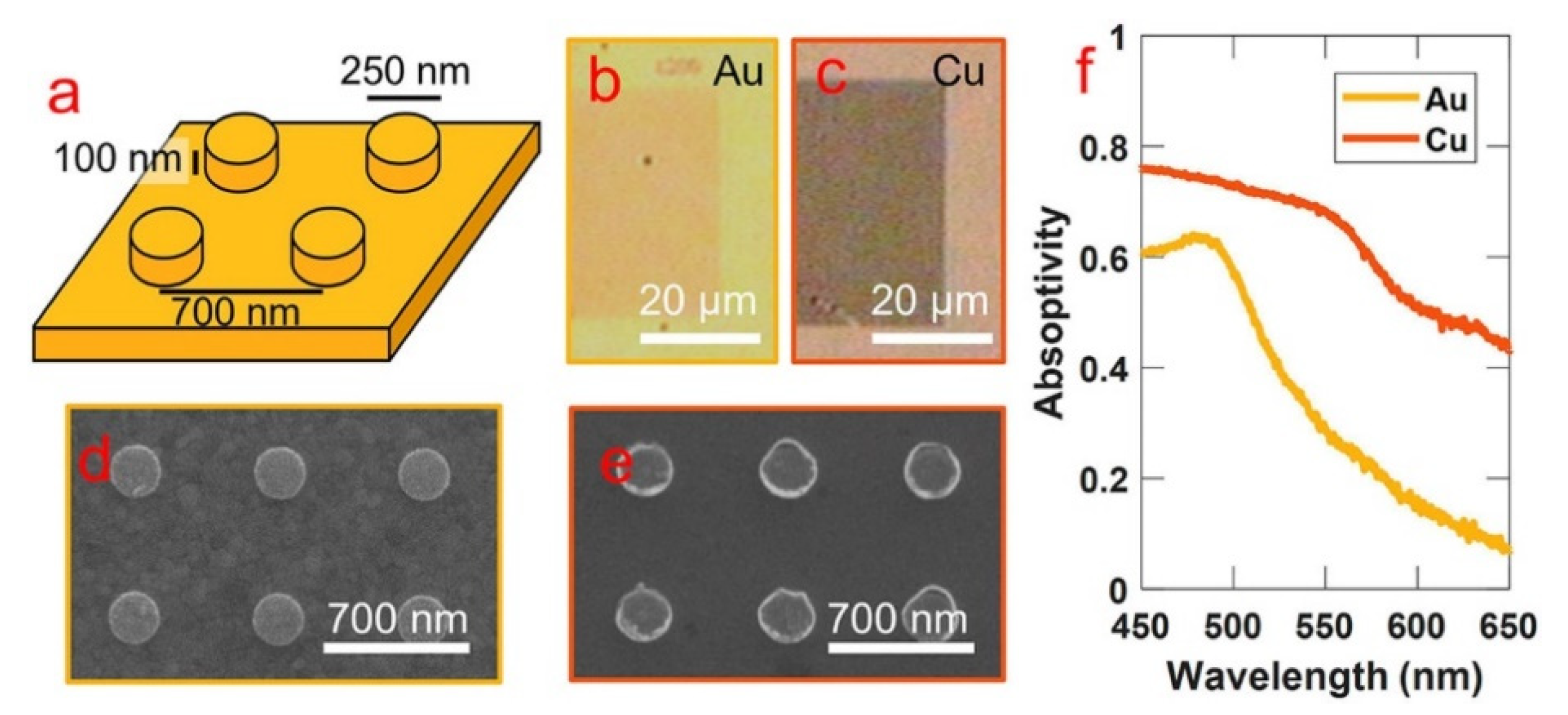
- Hogan, N.; Sheldon, M. Comparing steady state photothermalization dynamics in copper and gold nanostructures. J. Chem. Phys. 2020, 152, 061101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Zeng, G.; Ha, M.-A.; Ge, M.; Lin, Y.; Hettick, M.; Hou, B.; Alexandrova, A.N.; Javery, A.; Cronin, S.B. Artificial Photosynthesis on TiO2-Passivated InP Nanopillars. Nano Lett. 2015, 15, 6177–6181. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Nanomaterial for Enhanced Photocatalytic Performance | Planar Comparison Sample | Improved Photocatalysis Compared to Planar Sample |
|---|---|---|
| S-doped TiO2 with hydrogenation evolution value of 163.9 at a calcination temperature of 700 °C. [10] | No quantitative comparison to pure TiO2, but when compared to S-doped samples calcinated at 500, 600, and 800 °C, H2 evolution values are significantly lower: 35.09, 77.39, and 44.79 . | N/A |
| AT-20: 72.1 greater AT-10: 38.2 AT-40: 44.2 [11] | Pt-loaded TiO2: irradiation values of 5.4 | Raman spectra values increase from 147.7 for TiO2 comparison to 152.7 for Ag-TiO2 nanopillars. |
| SiNP/TiO2 SiNP/ZnO SiNP/TiO2/ZnO [8] | SiNP | SiNP/TiO2/ZnO shows photocurrent densities 60 times higher than SiNP/TiO2 and 4 times higher than SiNP/ZnO. An increase in intensity from 100 to 1200 a.u. is found compared to the planar SiNP sample. |
| Rapid dehydration sample RD-: 2.0 mA/cm2 (1.23 V vs. RHE) and 3.5 mA/cm2 (1.71 V vs. photoanodes RHE) [9] | Conventional temperature sample : 0.75 mA/cm2 (1.23 V vs. RHE) and 1.48 mA/cm2 157 (1.71 V vs. photoanodes RHE). | Photocurrent density improved by 270%. |
| Patterned AuNPAs [4] | Planar Au substrate | 30% surface area increase; 50% photocurrent enhancement over solar spectrum. |
| Ti-doped SiOx photocurrent density of 2.44 mA cm−2 at 1.23 VRHE, [12] | Ti-Fe2O3: photocurrent density of 1.23 mA at 1.01 VRHE | Surface area enhancement of 2.5 times compared to planar sample, 200% photocurrent density enhancement. |
| Nanopillar for Removing Organic Pollutants | Comparison Sample | Overall Improved Efficiency Compared to Planar Sample |
|---|---|---|
| 3D hexagonal mesostructured titania thin film 3D mesostructured film [13] | Commercial anatase film | Surface area is compared through Seff-MB values. Values were re-ported as 0.0095, 0.0148, and 0.0016 for the hexagonal mesostructured, mesostructured, and commercial samples, respectively. |
| SnO2/TiO2 core-shell nanopillar array [3] | Flat TiO2 | 180% (with SnO2) and 300% (without SnO2) enhancement in photocatalytic properties compared to flat TiO2. |
| S2 (with PEG filler) S1 (without PEG filler) [8] | UV light treatment | UV light shows 16% degradation, 20% for S1, and 30% for S2. |
| SMoQW (surface-modified quantum wires) [14] | NCNW (nanoclustered nanowires) | 15.8% improvement in photocatalytic performance compared to NCNW |
| Au/Pt–TiO2 with atomic layer deposition [15] | Au/Pt–TiO2 without atomic layer deposition | Dye degradation rates of 75.3% and 49.3% for samples with and without ALD, respectively. |
| Au/Pt–TiO2 [2] | Pure TiO2 NPA | Au/Pt–TiO2 showed an enhanced photocatalytic efficiency of 21 times greater than that of pure TiO2 under UV–VIS light, and 13 times greater under visible light. |
| Au/TiO2 (A) (nontemplated without PEG solid) Au/TiO2 (B) (templated with PEG solid) [16] | Simple UV-A | Sample Au/TiO2-B maximum percent tetracycline degradation of 68% compared to 41% of the simple UV sample. |
| Oxidized TiOxNy [17] | TiN | Approximately 50% degradation compared to plane TiN. |
[5] | Bare ZnO | Bare ZnO degraded MB at 4% efficiency, while had degrading rate of 25.8, and showed a degradation rate of 67.7%. |
| CdS NP/α-Fe2O3NPAs [18] | Pure hematite NPAs | Dye degradation with and without CdS NP was 94% and 78%, respectively. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waite, J.L.; Hunt, J.; Ji, H. Improving Photocatalytic Performance Using Nanopillars and Micropillars. Materials 2021, 14, 299. https://doi.org/10.3390/ma14020299
Waite JL, Hunt J, Ji H. Improving Photocatalytic Performance Using Nanopillars and Micropillars. Materials. 2021; 14(2):299. https://doi.org/10.3390/ma14020299
Chicago/Turabian StyleWaite, Jessica L., Julianna Hunt, and Haifeng Ji. 2021. "Improving Photocatalytic Performance Using Nanopillars and Micropillars" Materials 14, no. 2: 299. https://doi.org/10.3390/ma14020299
APA StyleWaite, J. L., Hunt, J., & Ji, H. (2021). Improving Photocatalytic Performance Using Nanopillars and Micropillars. Materials, 14(2), 299. https://doi.org/10.3390/ma14020299






