Effect of Heating Modes on Reactive Sintering of Ca3Co4O9 Ceramics
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid-State Reaction Method
2.2. Consolidation Using Conventional and Spark Plasma Sintering Techniques
3. Results
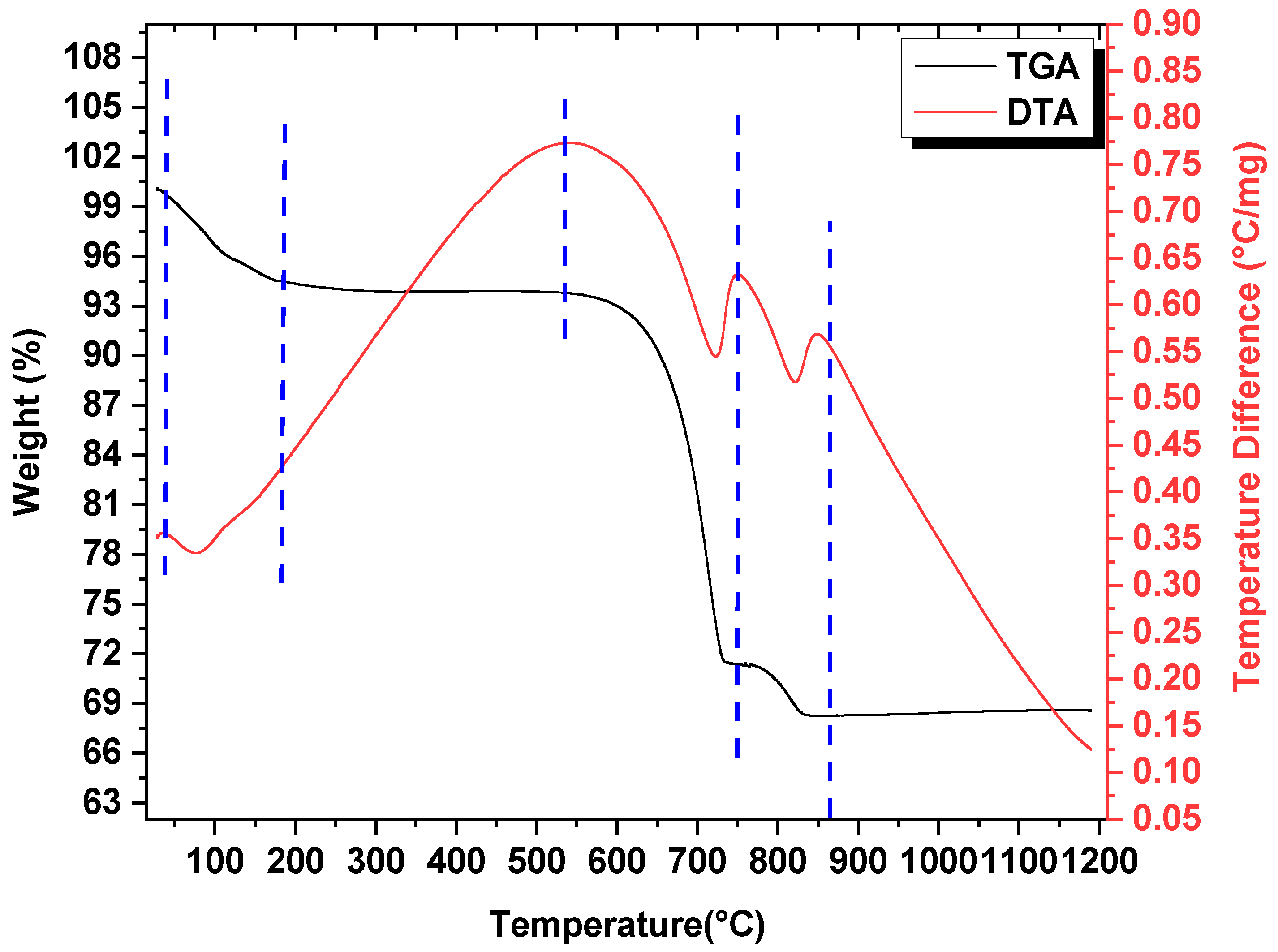
3.1. Thermo-Gravimetric Analysis (TGA) and Differential Thermal Analysis (DTA)
3.2. Relative Density Analysis
3.3. Material Characterization
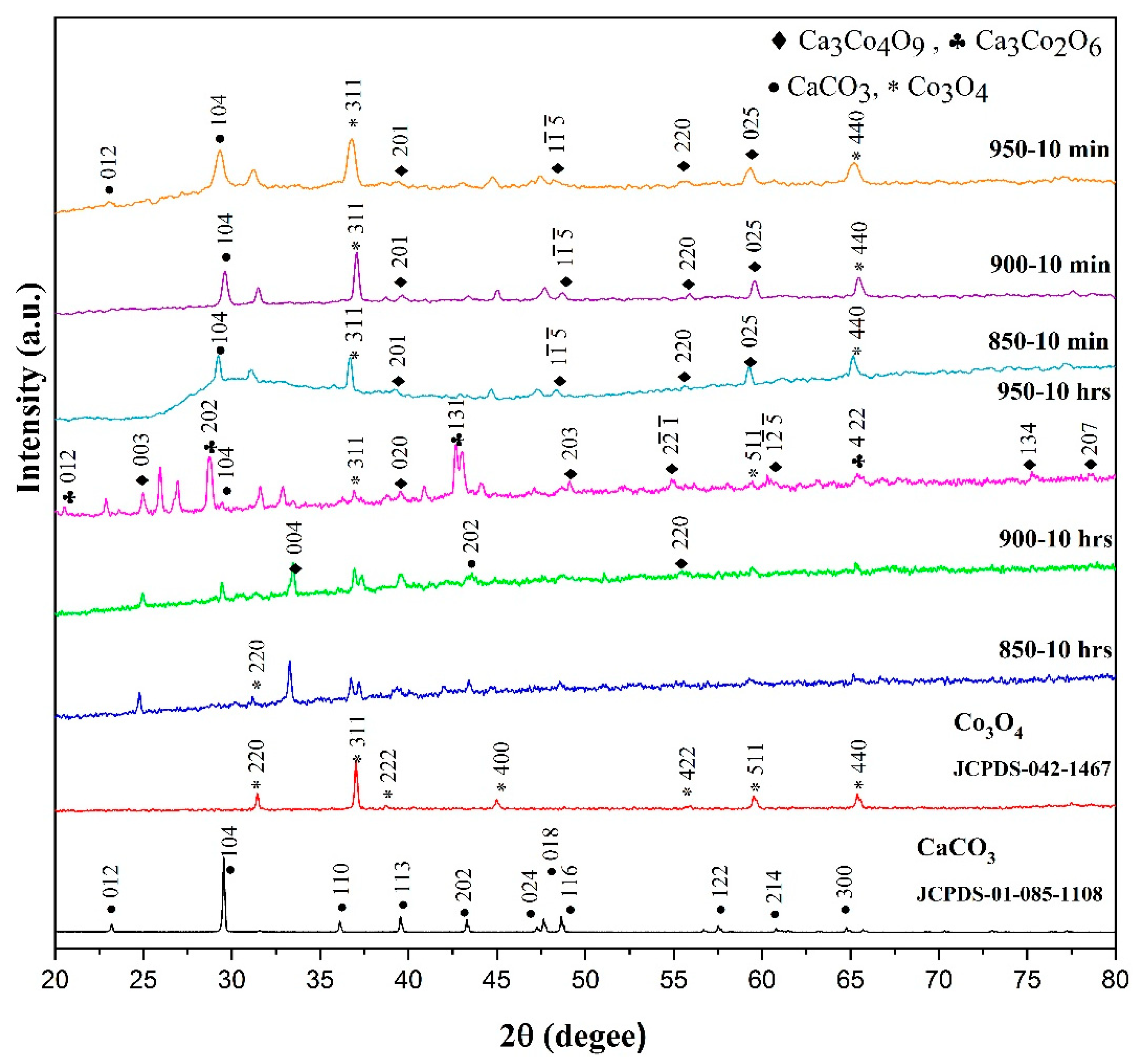
3.3.1. Phase Analysis
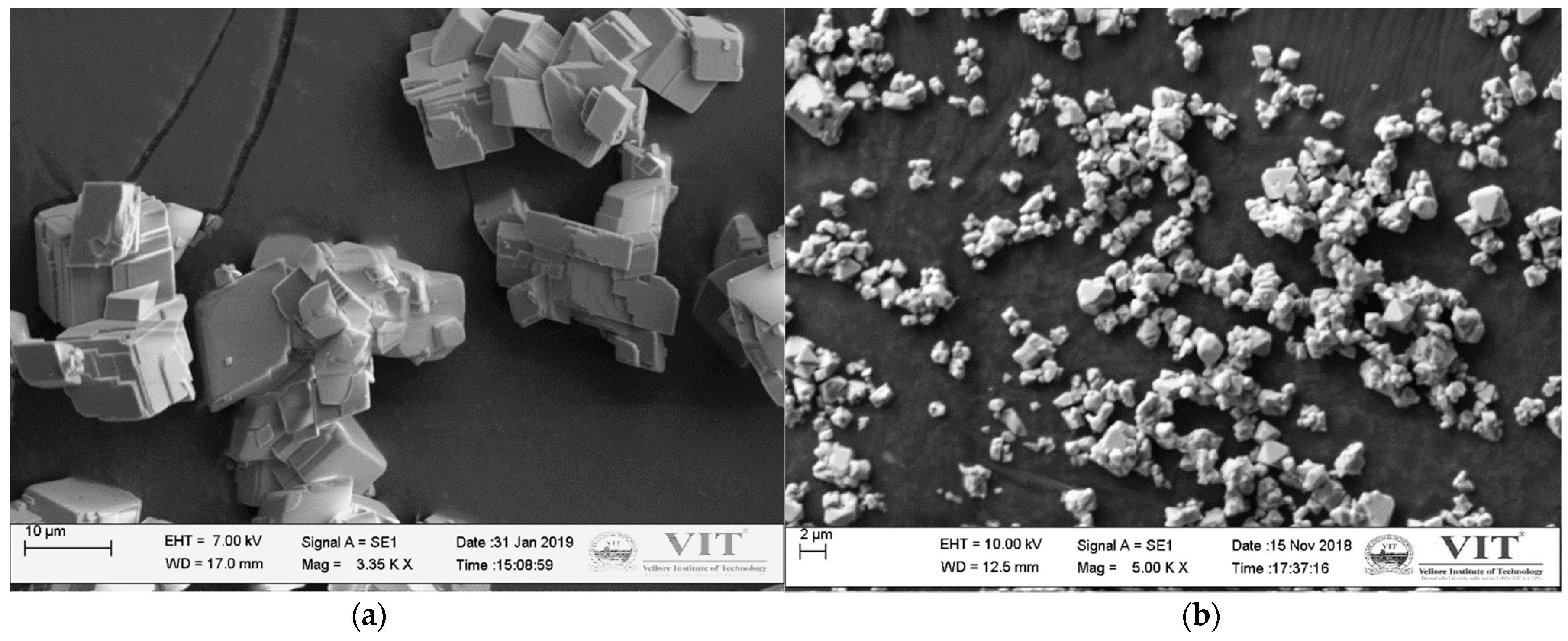
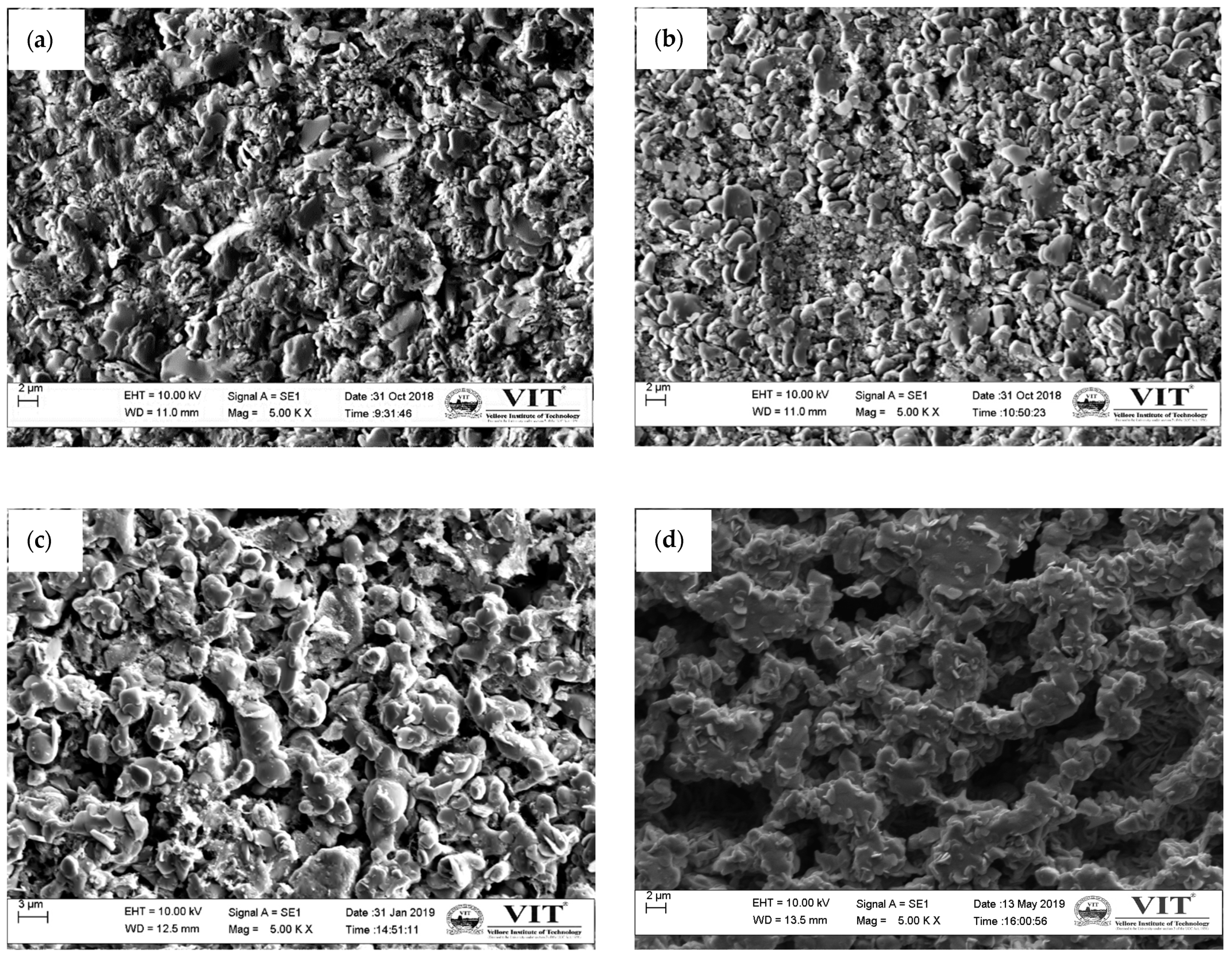
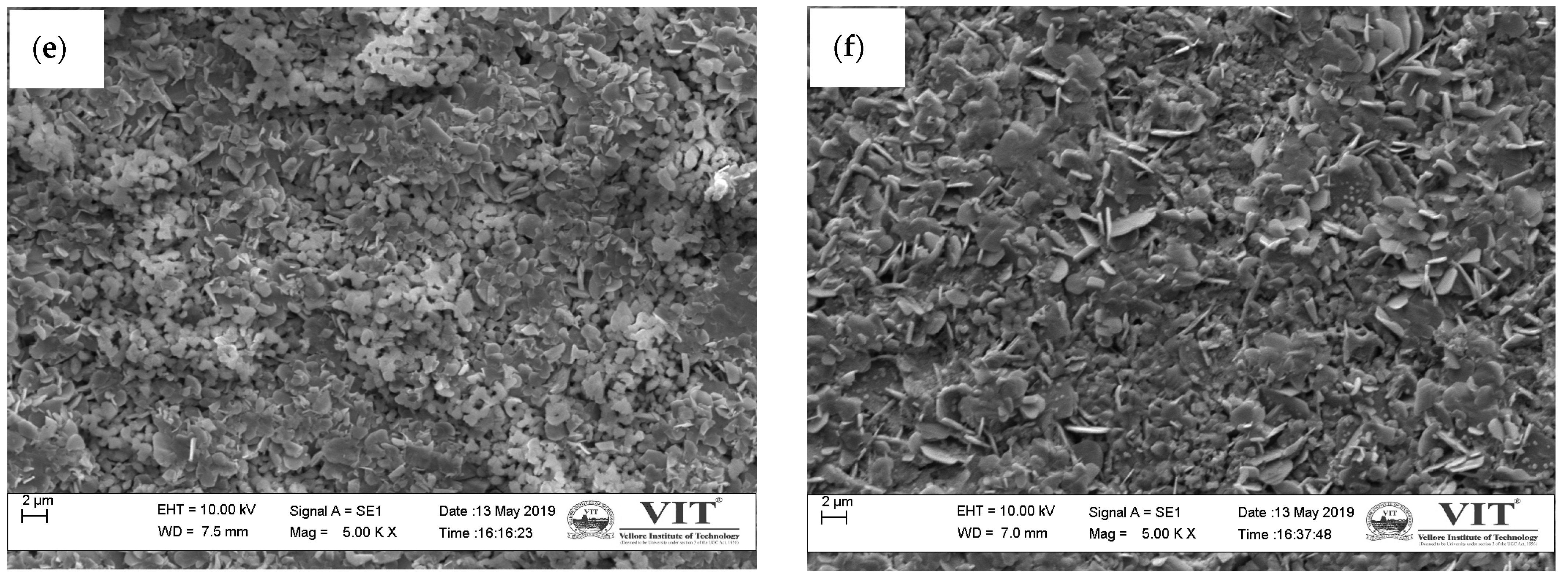
3.3.2. Microstructure Analysis
3.4. Electrical Properties
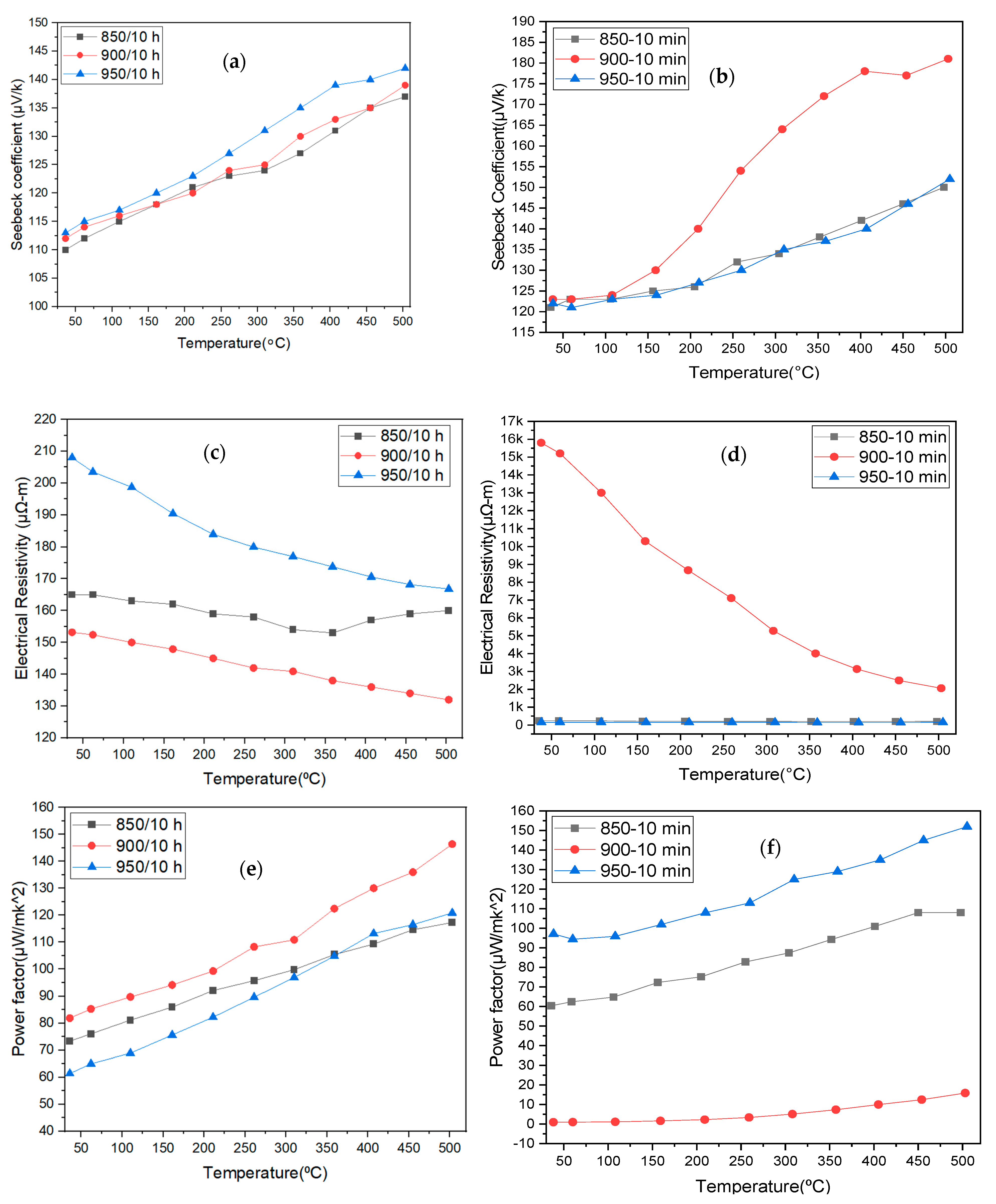
3.4.1. Seebeck Coefficient
3.4.2. Electrical Resistivity
3.4.3. Power Factor
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohta, H.; Sugiura, K.; Koumoto, K. Recent Progress in Oxide Thermoelectric Materials: P-Type Ca3Co4O9 and n-Type SrTiO3. Inorg. Chem. 2008, 47, 8429–8436. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Gou, J.; Hu, J.; Gao, F. A Novel TE-Material Based Thermal Protection Structure and Its Performance Evaluation for Hypersonic Flight Vehicles. Aerosp. Sci. Technol. 2018, 77, 458–470. [Google Scholar] [CrossRef]
- Gong, C.; Shi, Z.; Zhang, Y.; Chen, Y.; Hu, J.; Gou, J. Fabrication and Thermoelectric Properties of Ca-Co-O Ceramics with Negative Seebeck Coefficient. Results Phys. 2018, 9, 1233–1238. [Google Scholar] [CrossRef]
- Schulz, T.; Töpfer, J. Thermoelectric Properties of Ca3Co4O9 Ceramics Prepared by an Alternative Pressure-Less Sintering/Annealing Method. J. Alloys Compd. 2016, 659, 122–126. [Google Scholar] [CrossRef]
- Ying, S.; Nan, C.-W. Preparation of Ca3Co4O9 by Polyacrylamide Gel Processing and Its Thermoelectric Properties. J. Sol-Gel Sci. Technol. 2007, 44, 139–144. [Google Scholar] [CrossRef]
- Fedotov, A.S.; Fedotov, A.K.; Koltunowicz, T.N. Effect of NaF Doping on the Thermoelectric Properties of Ca3Co4O9. J. Alloys Compd. 2017, 695, 2844–2849. [Google Scholar] [CrossRef]
- Eduard, W.; Muan, A. Phase Equilibria in the System CaO-Cobalt Oxide in Air. J. Inorg. Nucl. Chem. 1970, 32, 1455–1459. [Google Scholar] [CrossRef]
- Sopicka-Lizer, M.; Smaczyński, P.; Kozłowska, K.; Bobrowska-Grzesik, E.; Plewa, J.; Altenburg, H. Preparation and Characterization of Calcium Cobaltite for Thermoelectric Application. J. Eur. Ceram. Soc. 2005, 25, 1997–2001. [Google Scholar] [CrossRef]
- Zhang, F.; Qingmei, L.U.; Tingxian, L.I.; Xin, Z. Preparation and Thermoelectric Transport Properties of Ba-, La- and Ag-doped Ca3Co4O9 Oxide Materials. J. Rare Earths 2013, 31, 778–783. [Google Scholar] [CrossRef]
- Yuheng, L.; Lin, Y.; Shi, Z.; Nan, C.; Shen, Z. Preparation of Ca3Co4O9 and Improvement of Its Thermoelectric Properties by Spark Plasma Sintering. J. Am. Ceram. Soc. 2005, 88, 1337–1340. [Google Scholar] [CrossRef]
- Kahramana, F.; Madre, M.A.; Rasekh, S.; Salvador, C.; Bosque, P.; Torres, M.A.; Diez, J.C.; Sotelo, A. Enhancement of Mechanical and Thermoelectric Properties of Ca3Co4O9 by Ag Addition. J. Eur. Ceram. Soc. 2015, 35, 3835–3841. [Google Scholar] [CrossRef]
- Irina, M.; Andrei, K. Processes Occurring at Preparation of Ca3Co4O9 +delta Ceramics by Means of Different Solution Methods, and Its Properties. In Proceedings of the International Conference—Nanomaterials: Applications and Properties NAP-2013, Alushta, Ukraine, 16–21 September 2013. [Google Scholar]
- Yin, T.; Liu, D.; Ou, Y.; Ma, F.; Xie, S.; Li, J.; Li, J. Nanocrystalline Thermoelectric Ca3Co4O9 Ceramics by Sol-Gel Based Electrospinning and Spark Plasma Sintering. J. Phys. Chem. C 2010, 114, 10061–10065. [Google Scholar] [CrossRef]
- Agilandeswari, K.; Ruban Kumar, A. Synthesis, Characterization, Temperature-Dependent Electrical and Magnetic Properties of Ca3Co4O9 by a Starch Assisted Sol-Gel Combustion Method. J. Magn. Magn. Mater. 2014, 364, 117–124. [Google Scholar] [CrossRef]
- Murat, G.; Ozenbas, M. Effect of Grain Size and Porosity on Phonon Scattering Enhancement of Ca3Co4O9. J. Alloys Compd. 2015, 626, 360–367. [Google Scholar] [CrossRef]
- Bresch, S.; Mieller, B.; Selleng, C.; Stöcker, T.; Moos, R.; Rab, T. Influence of the Calcination Procedure on the Thermoelectric Properties of Calcium Cobaltite Ca3Co4O9. J. Electroceram. 2018, 40, 225–234. [Google Scholar] [CrossRef]
- Kenfaui, D.; Bonnefont, G.; Chateigner, D.; Fantozzi, G.; Gomina, M.; Noudem, J.G. Ca3Co4O9 Ceramics Consolidated by SPS Process: Optimisation of Mechanical and Thermoelectric Properties. Mater. Res. Bull. 2010, 45, 1240–1249. [Google Scholar] [CrossRef]
- Sotelo, A.; Costa, F.M.; Ferreira, N.M.; Kovalevsky, A.; Ferro, M.C.; Amaral, J.S.; Amaral, S. Tailoring Ca3Co4O9 Microstructure and Performances Using a Transient Liquid Phase Sintering Additive. J. Eur. Ceram. Soc. 2016, 36, 1025–1032. [Google Scholar] [CrossRef]
- Kenfaui, D.; Gomina, M.; Chateigner, D.; Noudem, J.G. Mechanical Properties of Ca3Co4O9 Bulk Oxides Intended to Be Used in Thermoelectric Generators. Ceram. Int. 2014, 40, 10237–10246. [Google Scholar] [CrossRef]
- Sopicka-lizer, M.; Koz, K.; Plewa, J. Low-Temperature Synthesis of Calcium Cobaltites in a Solid-State Reaction. J. Electroceram. 2007, 18, 255–260. [Google Scholar] [CrossRef]
- Xu, L.; Li, F.; Wang, Y. High-Temperature Transport and Thermoelectric Properties of Ca3Co4−xTixO9. J. Alloys Compd. 2010, 501, 115–119. [Google Scholar] [CrossRef]
- Liou, Y.-C.; Tsai, W.C.; Lin, W.Y.; Lee, U.R. Synthesis of Ca3Co4O9 and CuAlO2 ceramics of the thermoelectric application using a reaction- Sintering process. J. Aust. Ceram. Soc. 2008, 44, 17–22. [Google Scholar]
- Kenfaui, D.; Chateigner, D.; Gomina, M.; Noudem, J.G. Texture, Mechanical and Thermoelectric Properties of Ca3Co4O9 Ceramics. J. Alloys Compd. 2010, 490, 472–479. [Google Scholar] [CrossRef]
- Omori, M. Sintering, Consolidation, Reaction, and Crystal Growth by the Spark Plasma System (SPS). Mater. Sci. Eng. A 2000, 287, 183–188. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The Effect of Electric Field and Pressure on the Synthesis and Consolidation of Materials: A Review of the Spark Plasma Sintering Method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Lu, J.F.; Shen, X.B.; Wang, F.; Wang, Y.D. The Sintering Mechanism in Spark Plasma Sintering—Proof of the Occurrence of Spark Discharge. Scr. Mater. 2014, 81, 56–59. [Google Scholar] [CrossRef]
- Presečnik, M.; Boor, J.D.; Bernik, S. Synthesis of Single-Phase Ca3Co4O9 Ceramics and Their Processing for a Microstructure-Enhanced Thermoelectric Performance. Ceram. Int. 2016, 42, 7315–7327. [Google Scholar] [CrossRef]
- Sotelo, A.S.; Rasekh, M.A.; Torres, P.; Bosque, M.; Madre, A.; Diez, J.C. Effect of Synthesis Methods on the Ca3Co4O9 Thermoelectric Ceramic Performances. J. Solid State Chem. 2015, 221, 247–254. [Google Scholar] [CrossRef]
- Bittner, M.; Helmich, L.; Nietschke, F.; Geppert, B.; Oeckler, O.; Feldhoff, A. Porous Ca3Co4O9 with Enhanced Thermoelectric Properties Derived from Sol-Gel Synthesis. J. Eur. Ceram. Soc. 2017, 37, 3909–3915. [Google Scholar] [CrossRef]
- Delorme, F.; Fernandez Martin, C.; Marudhachalam, P.; Guzman, G.; Ovono Ovono, D.; Fraboulet, O. Synthesis of Thermoelectric Ca3Co4O9 Ceramics with High ZT Values from a CoIICoIII-Layered Double Hydroxide Precursor. Mater. Res. Bull. 2012, 47, 3287–3291. [Google Scholar] [CrossRef]
| Sintering Temperature (°C) | CS | SPS |
|---|---|---|
| 850 | 47.81 | 79.43 |
| 900 | 49.71 | 80.52 |
| 950 | 50.76 | 81.18 |
| Elements | Conventional Sintering Weight % | Spark Plasma Sintering Weight % | ||||
|---|---|---|---|---|---|---|
| 850 °C—10 h | 900 °C—10 h | 950 °C—10 h | 850 °C—10 min | 900 °C—10 min | 950 °C—10 min | |
| C (K) | 1.44 | 2.31 | 1.76 | 1.80 | 5.28 | 3.38 |
| Ca (K) | 21.37 | 21.34 | 25.74 | 22.80 | 27.95 | 23.57 |
| Co (L) | 45.45 | 47.37 | 38.71 | 46.23 | 31.37 | 40.93 |
| O (K) | 31.74 | 28.98 | 33.79 | 29.17 | 35.40 | 32.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teja, P.R.; Annamalai, A.R.; Evangeline T., G.; Srikanth, M.; Agrawal, D.K.; Jen, C.-P. Effect of Heating Modes on Reactive Sintering of Ca3Co4O9 Ceramics. Materials 2021, 14, 273. https://doi.org/10.3390/ma14020273
Teja PR, Annamalai AR, Evangeline T. G, Srikanth M, Agrawal DK, Jen C-P. Effect of Heating Modes on Reactive Sintering of Ca3Co4O9 Ceramics. Materials. 2021; 14(2):273. https://doi.org/10.3390/ma14020273
Chicago/Turabian StyleTeja, P. Ravi, A. Raja Annamalai, Gecil Evangeline T., Muthe Srikanth, Dinesh K. Agrawal, and Chun-Ping Jen. 2021. "Effect of Heating Modes on Reactive Sintering of Ca3Co4O9 Ceramics" Materials 14, no. 2: 273. https://doi.org/10.3390/ma14020273
APA StyleTeja, P. R., Annamalai, A. R., Evangeline T., G., Srikanth, M., Agrawal, D. K., & Jen, C.-P. (2021). Effect of Heating Modes on Reactive Sintering of Ca3Co4O9 Ceramics. Materials, 14(2), 273. https://doi.org/10.3390/ma14020273









