Nutrients Recovery from Dairy Wastewater by Struvite Precipitation Combined with Ammonium Sorption on Clinoptilolite
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Basic Equipment
2.2. Sample Preparation
2.3. Preparation of Zeolite Material
2.4. Ammonium Sorption Experiment
2.5. Struvite Precipitation Experiments
2.6. Effect of Zeolite on Precipitation Process
2.6.1. Gradient Batch Experiments
2.6.2. Combining Sorption and Desirability Approach
2.7. Germination Tests
3. Results
3.1. Characteristics of Zeolite Material
3.2. Sorption and Precipitation Results
3.3. Thermodynamic Aspect
3.4. Characterization of Recovered P Products
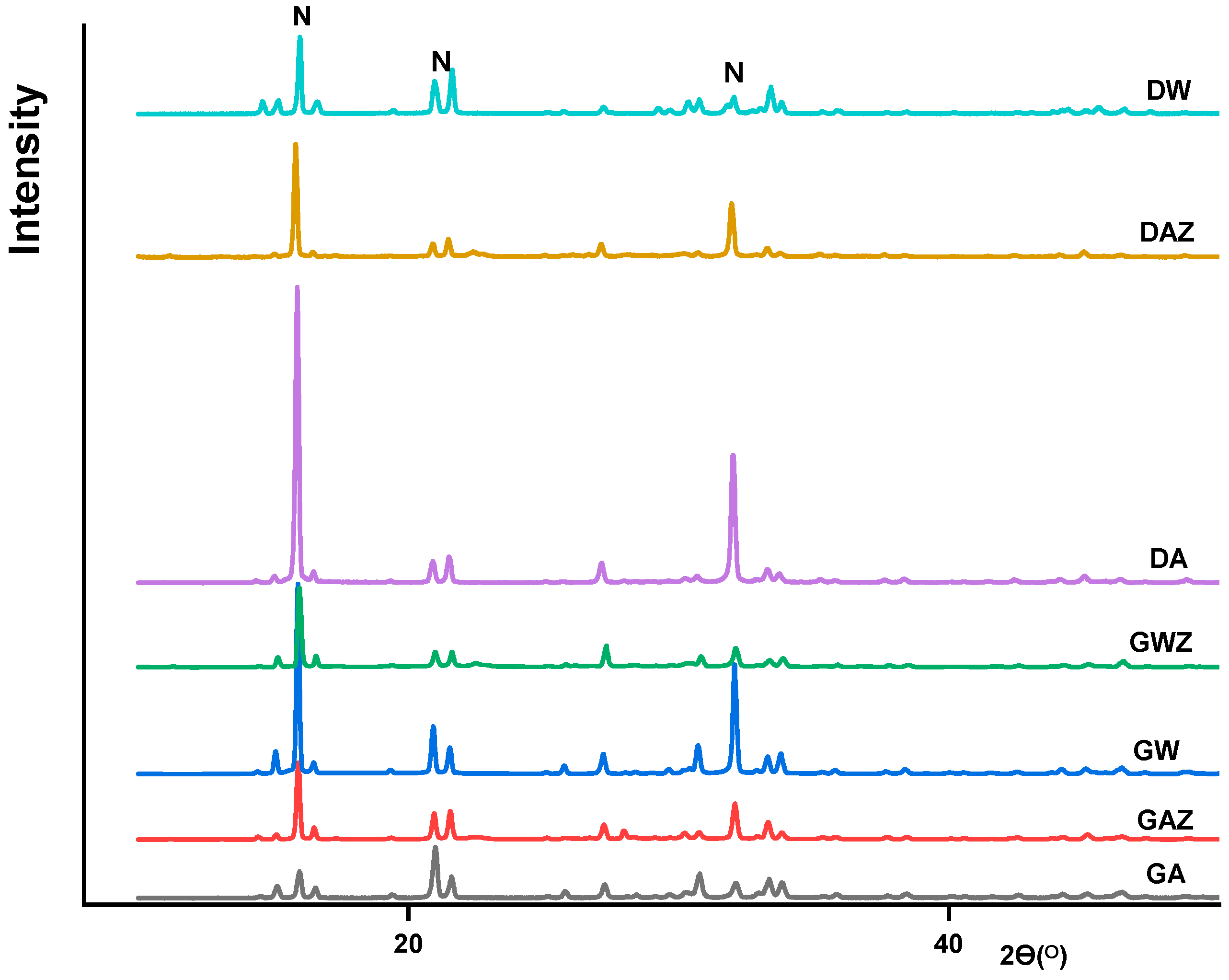
3.4.1. XRD and SEM Results
3.4.2. Evaluation of Fertilizer Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Batch no | Mg:P | NH4+:P | Mg | P | NH4+ |
|---|---|---|---|---|---|
| 1 | 1.21 | 2.69 | 150 | 5.819 | 1278 |
| 2 | 1.81 | 1.0 | 311 | 0.792 | 320.5 |
| 1.21 | 1.0 | 179 | 2.057 | 338.7 | |
| 1.51 | 1.0 | 281 | 1.479 | 316.5 | |
| 3 | 1.81 | 1.0 | 251 | 5 | 149.51 |
| 1.21 | 1.0 | 108 | 15 | 170.27 | |
| 1.51 | 1.0 | 194 | 5 | 143.28 | |
| 4 | 1.81 | 1.0 | 261 | 4 | 118.61 |
| 1.21 | 1.0 | 132 | 66 | 112.25 | |
| 1.51 | 1.0 | 231 | 8 | 103.78 | |
| 5 | 1.81 | 1.0 | 281 | 5 | 82.60 |
| 1.21 | 1.0 | 80 | 63 | 78.37 | |
| 1.51 | 1.0 | 238 | 12 | 61.42 | |
| f(x) | y = 80.54 + 5908.25exp(−1.597x) | ||||
| f’(x) | Y’ = 11453.44exp(−1.597x) | ||||
| #iteration | CNH4+ | C’NH4+ | nNH4+ | nP | nMg |
| 0.104* | 0.039* | 0.044* | |||
| 1 | 1276.455 | 2318.328 | 0.071 | 0.071 | 0.107 |
| 2 | 322.613 | 469.260 | 0.018 | 0.018 | 0.027 |
| 3 | 129.543 | 94.984 | 0.007 | 0.007 | 0.011 |
| 4 | 90.463 | 19.226 | 0.005 | 0.005 | 0.008 |
| 5 | 82.552 | 3.892 | 0.005 | 0.005 | 0.007 |
| 6 | 80.951 | 0.159 | 0.004 | 0.004 | 0.007 |
| 7 | 80.627 | 0.032 | 0.004 | 0.004 | 0.007 |
| 8 | 80.562 | 0.007 | 0.004 | 0.004 | 0.007 |
| 9 | 80.548 | 0.001 | 0.004 | 0.004 | 0.007 |
| 10 | 80.546 | 0.000 | 0.004 | 0.004 | 0.007 |
| 11 | 3 × 10−4 | ||||
Appendix B
| Run no | Factors | Effluent | Inflow | Outflow | Yields | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mg:P | N:P | Eff (g) | Pi | NH4+(i) | Pout | NH4+(out) | RecN | NH4+ | RecP | |
| [%] | ||||||||||
| 1 | 1.21 | 0.8 | 87.5 | 1252 | 581.76 | 7.28 | 162.68 | 72.8 | 4.8 | 98.6 |
| 2 | 1.81 | 0.8 | 90 | 1252 | 1054.44 | 7.06 | 521.32 | 70 | 5.72 | 99.2 |
| 3 | 1.51 | 0.8 | 77.5 | 1252 | 109.08 | 19.48 | 10.01 | 68 | 6.1 | 99.3 |
| 4 | 1.21 | 1.45 | 81.5 | 1252 | 1054.44 | 4.50 | 815.63 | 45.1 | 5.43 | 99.4 |
| 5 | 1.21 | 0.15 | 90 | 1252 | 581.76 | 9.13 | 157.04 | 92.4 | 1.21 | 97.3 |
| 6 | 1.51 | 0.15 | 77.5 | 1252 | 109.08 | 25.81 | 6.45 | 92.3 | 1.25 | 98 |
| 7 | 1.51 | 1.45 | 98 | 1252 | 1054.44 | 5.28 | 534.85 | 48.2 | 7.3 | 99.6 |
| 8 | 1.81 | 0.15 | 77.5 | 1252 | 109.08 | 19.84 | 6.50 | 88.2 | 0.0778 | 98 |
| 9 | 1.81 | 1.45 | 85.5 | 1252 | 581.76 | 14.97 | 135.50 | 33.1 | 7.35 | 99.6 |
References
- Lee, J.J.; Choi, C.U.; Lee, M.J.; Chung, I.H.; Kim, D.S. A study of NH3-N and P refixation by struvite formation in hybrid anaerobic reactor. Water Sci. Technol. 2004, 49, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Amran, M.; Salleh, M.; Rashid, U.; Ahsan, A.; Mujaffar, M.; Six, C. Production of slow release crystal fertilizer from wastewaters through struvite crystallization—A review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Nongqwenga, N.; Muchaonyerwa, P.; Hughes, J.; Odindo, A.; Bame, I. Possible use of struvite as an alternative phosphate fertilizer. J. Soil Sci. Plant Nutr. 2017, 17, 581–593. [Google Scholar] [CrossRef]
- Gómez-Suárez, A.D.; Nobile, C.; Faucon, M.P.; Pourret, O.; Houben, D. Fertilizer potential of struvite as affected by nitrogen form in the rhizosphere. Sustainability 2020, 12, 2212. [Google Scholar] [CrossRef]
- Sundareshwar, P.V.; Morris, J.T.; Koepfler, E.K. Phosphorus Limitation of Coastal Ecosystem Processes. Science 2003, 299, 563–566. [Google Scholar] [CrossRef]
- Ehmann, A.; Bach, I.; Laopeamthong, S.; Bilbao, J.; Lewandowski, I. Can Phosphate Salts Recovered from Manure Replace Conventional Phosphate Fertilizer? Agriculture 2017, 7, 1. [Google Scholar] [CrossRef]
- EIP-AGRI EIP-AGRI Focus Group. Nutrient Recycling. 2017, pp. 1–24. Available online: https://ec.europa.eu/eip/agriculture/sites/default/files/eip-agri_fg_nutrients_recycling_final_report_2017_en.pdf (accessed on 17 September 2021).
- Ballirano, P.; De Vito, C.; Mignardi, S.; Ferrini, V. Phase transitions in the Mg–CO2–H2O system and the thermal decomposition of dypingite, Mg5(CO3)4(OH)2·5H2O: Implications for geosequestration of carbon dioxide. Chem. Geol. 2013, 340, 59–67. [Google Scholar] [CrossRef]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Wang, J.; Burken, J.G.; Zhang, X.; Surampalli, R. Engineered Struvite Precipitation: Impacts of Component-Ion Molar Ratios and pH. J. Environ. Eng. 2005, 131, 1433–1440. [Google Scholar] [CrossRef]
- Sena, M.; Hicks, A. Life cycle assessment review of struvite precipitation in wastewater treatment. Resour. Conserv. Recycl. 2018, 139, 194–204. [Google Scholar] [CrossRef]
- Gong, W.; Li, Y.; Luo, L.; Luo, X.; Cheng, X.; Liang, H. Application of Struvite-MAP Crystallization Reactor for Treating Cattle Manure Anaerobic Digested Slurry: Nitrogen and Phosphorus Recovery and Crystal Fertilizer Efficiency in Plant Trials. Int. J. Environ. Res. Public Health 2018, 15, 1397. [Google Scholar] [CrossRef]
- Capdevielle, A.; Sýkorová, E.; Biscans, B.; Béline, F.; Daumer, M.L. Optimization of struvite precipitation in synthetic biologically treated swine wastewater-Determination of the optimal process parameters. J. Hazard. Mater. 2013, 244–245, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Daneshgar, S.; Buttafava, A.; Capsoni, D.; Callegari, A.; Capodaglio, A.G. Impact of pH and ionic molar ratios on phosphorous forms precipitation and recovery from different wastewater sludges. Resources 2018, 7, 71. [Google Scholar] [CrossRef]
- Numviyimana, C.; Warchoł, J.; Izydorczyk, G.; Baśladyńska, S.; Chojnacka, K. Struvite production from dairy processing wastewater: Optimizing reaction conditions and effects of foreign ions through multi-response experimental models. J. Taiwan Inst. Chem. Eng. 2020, 117, 182–189. [Google Scholar] [CrossRef]
- González-Morales, C.; Camargo-Valero, M.A.; Molina-Pérez, F.J.; Fernández, B. Effect of the stirring speed on the struvite formation using the centrate from a WWTP. Rev. Fac. Ing. Univ. Antioq. 2019, 92, 42–50. [Google Scholar] [CrossRef]
- Shalaby, M.S.; El-Rafie, S.; Hamzaoui, A.H.; M’nif, A. Modeling and optimization of phosphate recovery from industrial wastewater and precipitation of solid fertilizer using experimental design methodology. Chem. Biochem. Eng. Q. 2015, 29, 35–46. [Google Scholar] [CrossRef]
- EU Commission Implementing Decision (Eu) 2018/1147. Establ. Best Available Tech. Conclus. Waste Treat. 2004, 2001, 20–30.
- Finkbeiner, M.; Inaba, A.; Tan, R.B.H.; Christiansen, K.; Klüppel, H. Commentaries the New International Standards for Life Cycle Assessment: ISO 14040 and ISO 14044; ISO: Geneva, Switzerland, 2006; Volume 11, pp. 80–85. [Google Scholar]
- Tan, Z.; Lal, R.; Wiebe, K. Global Soil Nutrient Depletion and Yield Reduction. J. Sustain. Agric. 2005. [Google Scholar] [CrossRef]
- Kinidi, L.; Tan, I.A.W.; Abdul Wahab, N.B.; Bin Tamrin, K.F.; Hipolito, C.N.; Salleh, S.F. Recent Development in Ammonia Stripping Process for Industrial Wastewater Treatment. Int. J. Chem. Eng. 2018, 2018, 3181087. [Google Scholar] [CrossRef]
- Tang, H.; Xu, X.; Wang, B.; Lv, C.; Shi, D. Removal of ammonium from swine waste water using synthesized zeolite from fly ash. Sustainability 2020, 12, 3423. [Google Scholar] [CrossRef]
- Ban, Z.S.; Dave, G. Laboratory studies on recovery of n and p from human urine through struvite crystallisation and zeolite adsorption. Env. Technol. 2004, 25, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xiao, D.; Pang, R.; Han, C.; Ding, L. Simultaneous removal of nutrients from simulated swine wastewater by adsorption of modified zeolite combined with struvite crystallization. Chem. Eng. J. 2014, 256, 431–438. [Google Scholar] [CrossRef]
- Holub, M.; Balintova, M.; Demcak, S.; Hurakova, M. Characterization of Natural Zeolite and Determination Its Adsorption Properties. J. Civ. Eng. Environ. Arch. 2016, 63. [Google Scholar] [CrossRef]
- Wasielewski, S.; Rott, E.; Minke, R.; Steinmetz, H. Evaluation of different clinoptilolite zeolites as adsorbent for ammonium removal from highly concentrated synthetic wastewater. Water 2018, 10, 584. [Google Scholar] [CrossRef]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.; Tatoulis, T.; Akratos, C.; Tekerlekopoulou, A.; Vayenas, D. Zeolite as a Potential Medium for Ammonium Recovery and Second Cheese Whey Treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef]
- An, S.W.; Jeong, Y.C.; Cho, H.H.; Park, J.W. Adsorption of NH4+-N and E. coli onto Mg2+-modified zeolites. Environ. Earth Sci. 2016, 75, 1–11. [Google Scholar] [CrossRef]
- Shaddel, S.; Grini, T.; Ucar, S.; Azrague, K.; Andreassen, J.P.; Østerhus, S.W. Struvite crystallization by using raw seawater: Improving economics and environmental footprint while maintaining phosphorus recovery and product quality. Water Res. 2020, 173, 115572. [Google Scholar] [CrossRef]
- Vudagandla, S.; Siva Kumar, N.; Dharmendra, V.; Asif, M.; Balaram, V.; Zhengxu, H.; Zhen, Z. Determination of Boron, Phosphorus, and Molybdenum Content in Biosludge Samples by Microwave Plasma Atomic Emission Spectrometry (MP-AES). Appl. Sci. 2017, 7, 264. [Google Scholar] [CrossRef]
- Santos, W.O. Acid Ammonium Citrate as P Extractor for Fertilizers of Varying Solubility. Rev. Bras. Ciênc. Solo 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Thant Zin, M.M.; Kim, D.J. Struvite production from food processing wastewater and incinerated sewage sludge ash as an alternative N and P source: Optimization of multiple resources recovery by response surface methodology. Process Saf. Environ. Prot. 2019, 126, 242–249. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, L.; Ren, H.; Guo, Z.; Tan, J. Thermodynamic modeling of ferric phosphate precipitation for phosphorus removal and recovery from wastewater. J. Hazard. Mater. 2010, 176, 444–450. [Google Scholar] [CrossRef]
- Christensen, J.; Bastien, C. Introduction to General Optimization Principles and Methods. In Nonlinear Optimization of Vehicle Safety Structures; Butterworth-Heinemann: Oxford,UK, 2016; pp. 107–168. ISBN 9780128044247. [Google Scholar]
- Chua, L.H.C.; Tan, S.B.K.; Sim, C.H.; Kumar, M. Treatment of baseflow from an urban catchment by a floating wetland system. Ecol. Eng. 2012, 49, 170–180. [Google Scholar] [CrossRef]
- Meza, J.C. Steepest descent. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 719–722. [Google Scholar] [CrossRef]
- Ramprasad, C.; Alekhya, D.; Bhishmitha, C.; Deepika, C.S. Precipitation of struvite by sustainable waste materials and use as slow release fertilizer—A circular economy approach. IOP Conf. Ser. Mater. Sci. Eng. 2020, 955. [Google Scholar] [CrossRef]
- Himmelbauer, M.L.; Loiskandl, W.; Kastanek, F. Estimating length, average diameter and surface area of roots using two different Image analyses systems. Plant Soil 2004, 260, 111–120. [Google Scholar] [CrossRef]
- Holub, M.; Balintova, M.; Pavlikova, P.; Palascakova, L. Study of sorption properties of zeolite in acidic conditions in dependence on particle size. Chem. Eng. Trans. 2013, 32, 559–564. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Stolzenburg, P.; Capdevielle, A.; Teychené, S.; Biscans, B. Struvite precipitation with MgO as a precursor: Application to wastewater treatment. Chem. Eng. Sci. 2015, 133, 9–15. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Gujer, W. Struvite precipitation thermodynamics in source-separated urine. Water Res. 2007, 41, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Ulex, G.L. CLXIII. On struvite, a new mineral. Mem. Proc. Chem. Soc. 1845, 3, 106–110. [Google Scholar] [CrossRef]
- Whitaker, A.; Jeffery, J.W. The crystal structure of struvite, MgNH4 PO4 6H2O. Acta Cryst. Sect. B 1970, 26, 1429–1440. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- El Diwani, G.; El Rafie, S.; El Ibiari, N.N.; El-Aila, H.I. Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer. Desalination 2007, 214, 200–214. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Kocak, E.; Akbin, H.M.; Özçimen, D. Utilization of struvite recovered from high-strength ammonium-containing simulated wastewater as slow-release fertilizer and fire-retardant barrier. Environ. Technol. 2020, 41, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual effect of nanomaterials on germination and seedling growth: Stimulation vs. phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef]
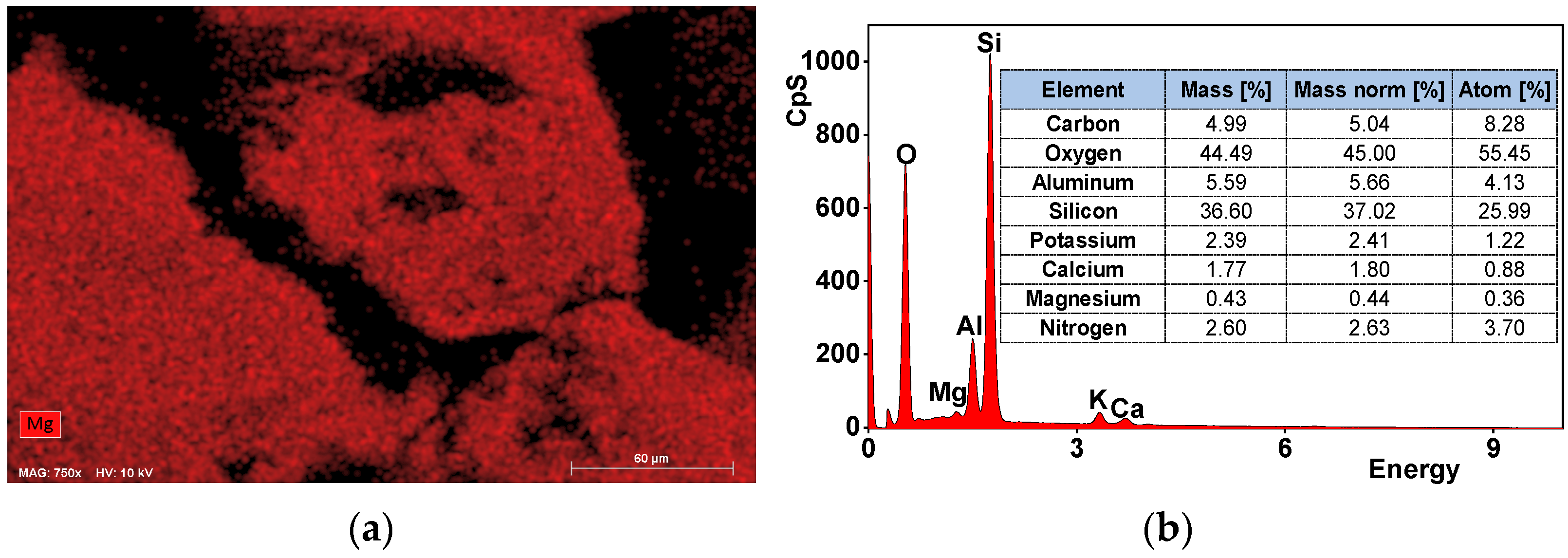

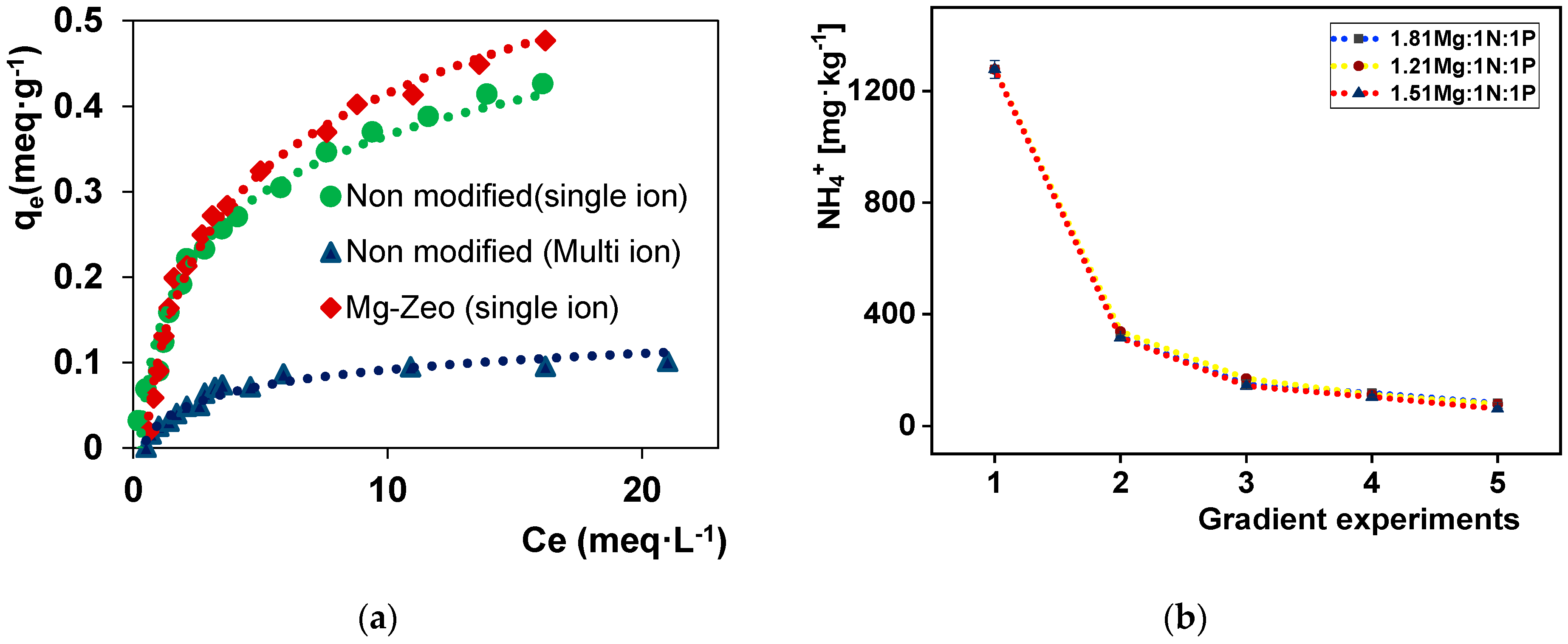
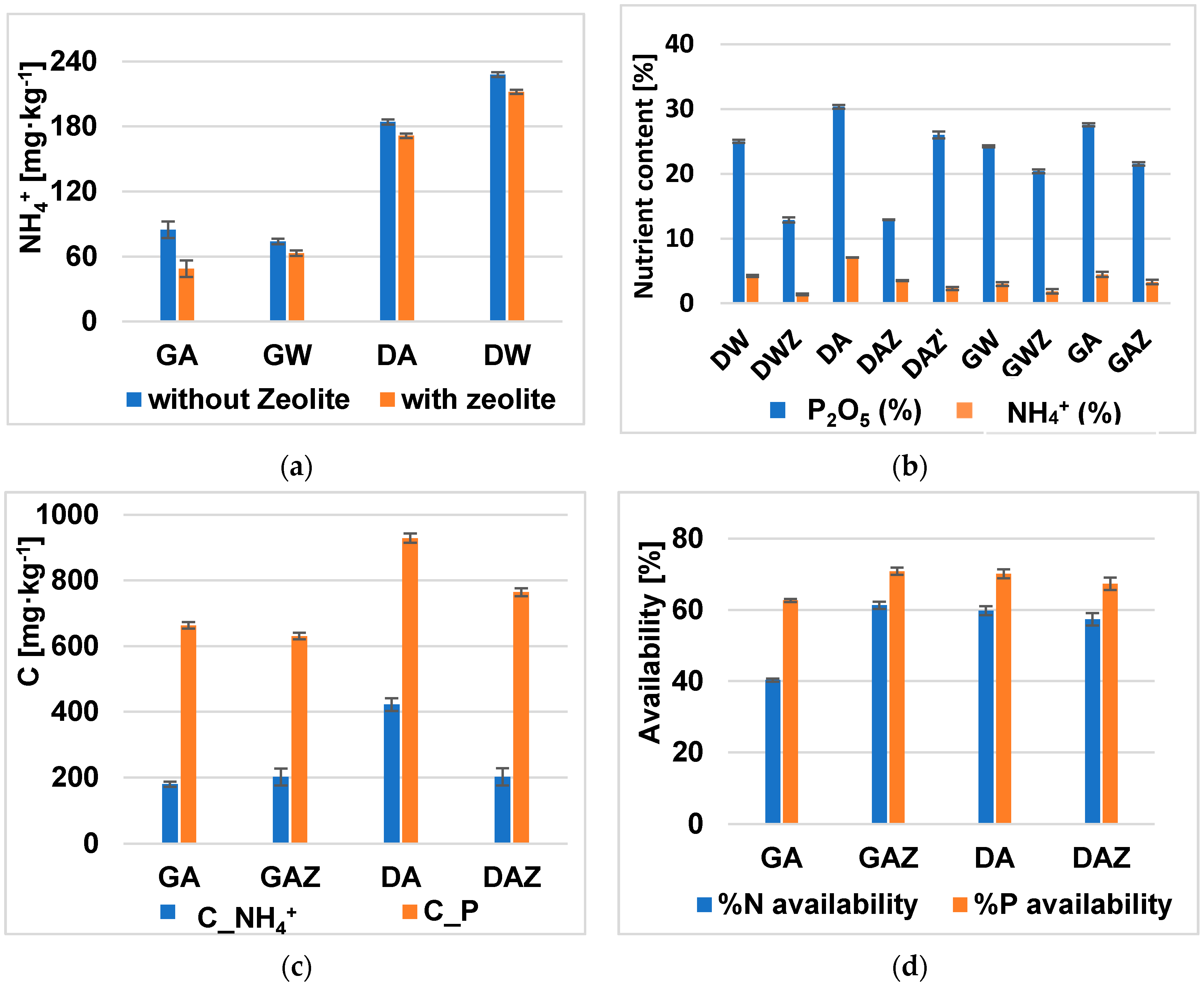
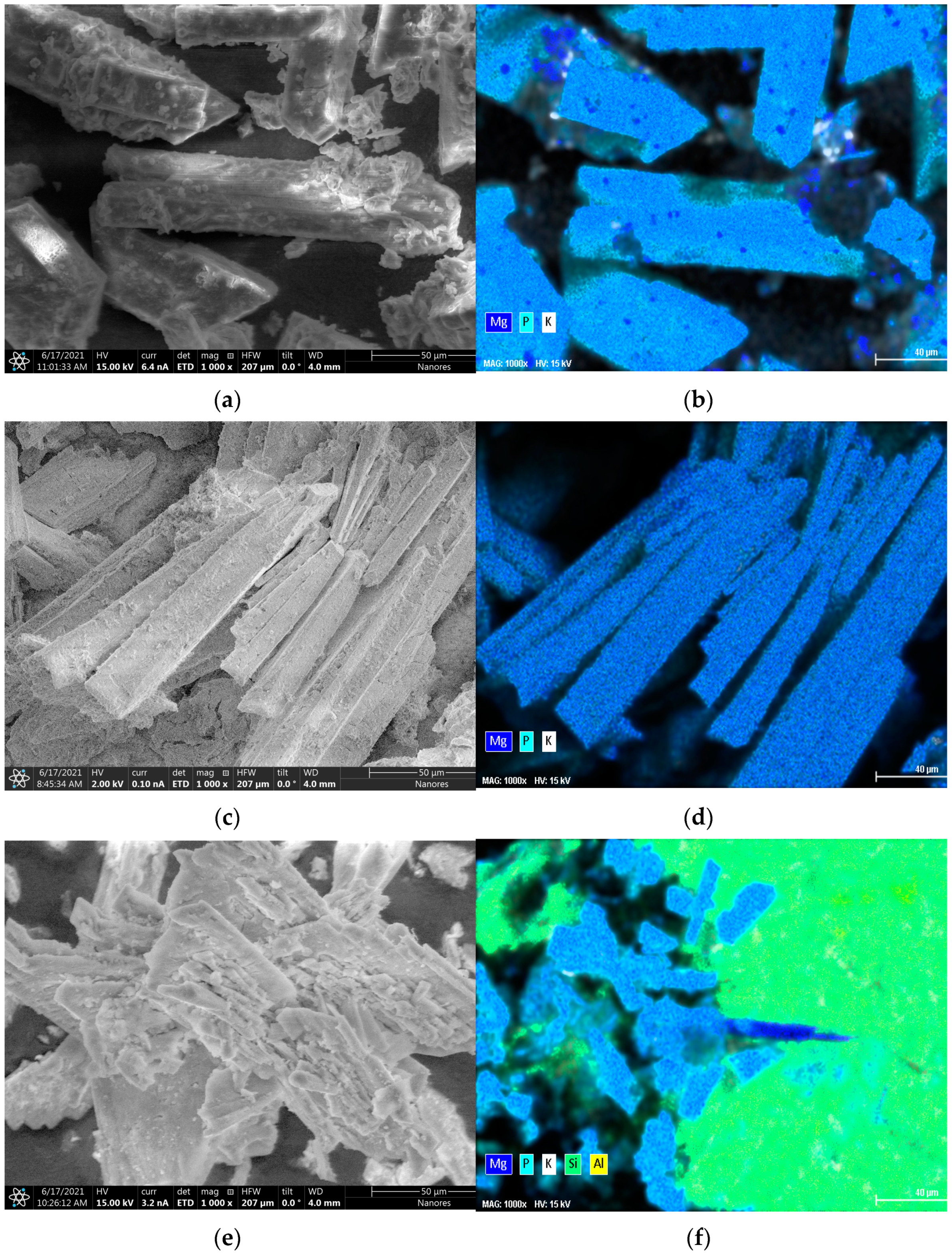
| Concentration [mg∙kg−1] | |||||
|---|---|---|---|---|---|
| Elements | Wastewater | Artificial | Elements | Wastewater | Artificial |
| pH | 4.35 | 8.9 | N [%] | 0.14 | - |
| C [%] | 3.07 | - | Na | 485 | - |
| Cl− [mol·kg−1] | 0.16 | P | 445 | 1203 | |
| B | 10.3 | - | S | 132 | - |
| Ca | 526 | 404 | Si | 99.94 | - |
| K | 1654 | 4524 | NH4+ | 364 | 1876 |
| z-Mg | Raw | after NH4+ Contact | |
|---|---|---|---|
| Compound | m/m% | m/m% | m/m% |
| SiO2 | 64.22 | 68.30 | 59.53 |
| Al2O3 | 8.39 | 14.28 | 8.01 |
| K2O | 8.33 | 4.77 | 11.45 |
| CaO | 7.65 | 6.33 | 9.94 |
| Fe2O3 | 4.96 | 2.70 | 4.90 |
| Effluent Concentration | Product Nutrient Content | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [mg·kg−1] | % | ||||||||
| Sample | Ca | Mg | P | NH4+ | N | P2O5 | K | Ca | Mg |
| GA | 45.2 | 1322 | 4.21 | 84.6 ± 7.63 | 3.42 | 29.5 | 3.05 | 0.65 | 12.8 |
| GAZ | 62.4 | 1348 | 4.32 | 48.6 ± 7.61 | 2.02 | 20.7 | 2.53 | 0.85 | 8.81 |
| GW | 78.2 | 1048 | 5.81 | 73.8 ± 2.55 | 2.21 | 24.7 | 1.95 | 0.25 | 10.1 |
| GWZ | 68.8 | 1235 | 5.54 | 63.0 ± 2.50 | 1.47 | 18.1 | 1.22 | 0.50 | 6.38 |
| DA | 45.2 | 282 | 5.41 | 184 ± 2.10 | 5.49 | 30.3 | 0.76 | 4.27 | 9.80 |
| DAZ | 62.4 | 293 | 4.80 | 176 ± 1.70 | 2.74 | 26.0 | 0.32 | 1.58 | 10.6 |
| DW | 56.7 | 248 | - | 228 ± 2.20 | 3.30 | 25.0 | 0.29 | 2.27 | 9.76 |
| DWZ | 63.5 | 279 | - | 212 ± 1.70 | 1.09 | 12.9 | 0.51 | 1.85 | 2.94 |
| Component | Species | Mg2+ | NH4+ | PO43− | Na+ | K+ | Ca2+ | H+ | CO32− | H2O | Cl− | GA | GAZ αi (%) | DA | DAZ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg2+ | Mg2+ | 1 | 46.72 | 50.43 | 51.53 | 51.52 | |||||||||
| Mg2CO32+ | 2 | 1 | 5.08 | 5.32 | 1.39 | 1.39 | |||||||||
| MgOH+ | −1 | 0.05 | 0.06 | 0.06 | 0.06 | ||||||||||
| MgCl+ | 1 | 1 | 14.74 | 15.87 | 16.59 | 16.60 | |||||||||
| MgHPO4 (aq) | 1 | 0.02 | 0.05 | 0.20 | 0.18 | ||||||||||
| MgCO3 (aq) | 1 | 23.38 | 19.81 | 21.23 | 21.25 | ||||||||||
| MgHCO3+ | 1 | 1 | 1 | 10.01 | 8.47 | 8.99 | 9.00 | ||||||||
| PO43− | PO43− | 1 | 0.07 | 0.06 | 0.09 | 0.09 | |||||||||
| HPO42− | 1 | 1 | 36.20 | 31.59 | 48.06 | 48.13 | |||||||||
| H2PO4− | 1 | 2 | 0.23 | 0.20 | 0.30 | 0.30 | |||||||||
| MgPO4− | 1 | 1.41 | 1.52 | 0.57 | 0.57 | ||||||||||
| MgHPO4 (aq) | 1 | 1 | 1 | 33.56 | 36.34 | 13.81 | 13.84 | ||||||||
| CaHPO4 (aq) | 1 | 1 | 1 | 0.92 | 2.26 | 0.81 | 0.82 | ||||||||
| CaPO4− | 1 | 1 | 3.40 | 8.37 | 2.98 | 2.98 | |||||||||
| NaHPO4− | 1 | 1 | 1 | 18.09 | 14.46 | 25.12 | 25.02 | ||||||||
| KHPO4− | 1 | 1 | 1 | 4.68 | 4.09 | 6.18 | 6.19 | ||||||||
| K2HPO4 (aq) | 1 | 2 | 0.26 | 0.22 | 0.34 | 0.34 | |||||||||
| KH2PO4 (aq) | 1 | 1 | 0.01 | 0.01 | 0.02 | 0.02 | |||||||||
| KPO42− | 1 | 1 | 0.02 | 0.02 | 0.02 | 0.02 | |||||||||
| Na2HPO4 (aq) | 1 | 2 | 1.06 | 0.77 | 1.54 | 1.53 | |||||||||
| Na2PO4− | 1 | 2 | 0.03 | 0.02 | 0.04 | 0.04 | |||||||||
| NaH2PO4 (aq) | 1 | 1 | 0.03 | 0.03 | 0.05 | 0.05 | |||||||||
| NaPO42− | 1 | 1 | 0.04 | 0.04 | 0.06 | 0.06 | |||||||||
| NH4+ | NH4+ | 1 | 76.31 | 76.23 | 76.12 | 76.12 | |||||||||
| NH3 (aq) | 1 | 1 | 23.65 | 23.66 | 23.85 | 23.85 | |||||||||
| CaNH32+ | 1 | 1 | 0.04 | 0.11 | 0.03 | 0.03 | |||||||||
| I (M) | 0.36 | 0.35 | 0.32 | 0.32 | |||||||||||
| ∆G (Kj.mol−1) | −5.87 | −5.42 | −9.66 | −9.56 | |||||||||||
| Sample | Element Content in Dry Biomass (mg·kg−1) | TF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | N | P | K | Ca | Mg | |
| Struvite (DA) | 4.42 | 16.59 | 48.19 | 20.71 | 8547 | 0.44 | 0.07 | 3.47 | 0.27 | 0.23 |
| Str & zeolite (DAZ) | 3.04 | 14.98 | 39.91 | 19.49 | 8423 | 1.04 | 0.12 | 1.09 | 1.58 | 0.33 |
| Control | 2.86 | 12.52 | 0.94 | 14.12 | 8.61 | - | - | - | - | - |
| Group | Fresh Sprouts Mass | Dry Sprouts Mass | Root Area | Root Length | Steam Length | Root Volume |
|---|---|---|---|---|---|---|
| [g] | [g] | [cm2] | [g] | [g] | [cm3] | |
| DA | 45.14 ± 4.62 | 5.62 ± 0.42 | 8.43 ± 0.34 | 6.06 ± 1.44 | 3.03 ± 1.10 | 0.77 ± 0.12 |
| DAZ | 88.7 ± 8.46 | 8.83 ± 1.11 | 13.46 ± 1.15 | 8.32 ± 2.16 | 2.71 ± 0.73 | 1.51 ± 0.24 |
| Control | 26.2 ± 4.61 | 4.56 ± 0.43 | 11.24 ± 0.94 | 4.66 ± 0.14 | 1.82 ± 0.46 | 7.18 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Numviyimana, C.; Warchoł, J.; Ligas, B.; Chojnacka, K. Nutrients Recovery from Dairy Wastewater by Struvite Precipitation Combined with Ammonium Sorption on Clinoptilolite. Materials 2021, 14, 5822. https://doi.org/10.3390/ma14195822
Numviyimana C, Warchoł J, Ligas B, Chojnacka K. Nutrients Recovery from Dairy Wastewater by Struvite Precipitation Combined with Ammonium Sorption on Clinoptilolite. Materials. 2021; 14(19):5822. https://doi.org/10.3390/ma14195822
Chicago/Turabian StyleNumviyimana, Claver, Jolanta Warchoł, Bartosz Ligas, and Katarzyna Chojnacka. 2021. "Nutrients Recovery from Dairy Wastewater by Struvite Precipitation Combined with Ammonium Sorption on Clinoptilolite" Materials 14, no. 19: 5822. https://doi.org/10.3390/ma14195822
APA StyleNumviyimana, C., Warchoł, J., Ligas, B., & Chojnacka, K. (2021). Nutrients Recovery from Dairy Wastewater by Struvite Precipitation Combined with Ammonium Sorption on Clinoptilolite. Materials, 14(19), 5822. https://doi.org/10.3390/ma14195822






