Physical and Dielectric Properties of Ni-Doped In2S3 Powders for Optical Windows in Thin Film Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
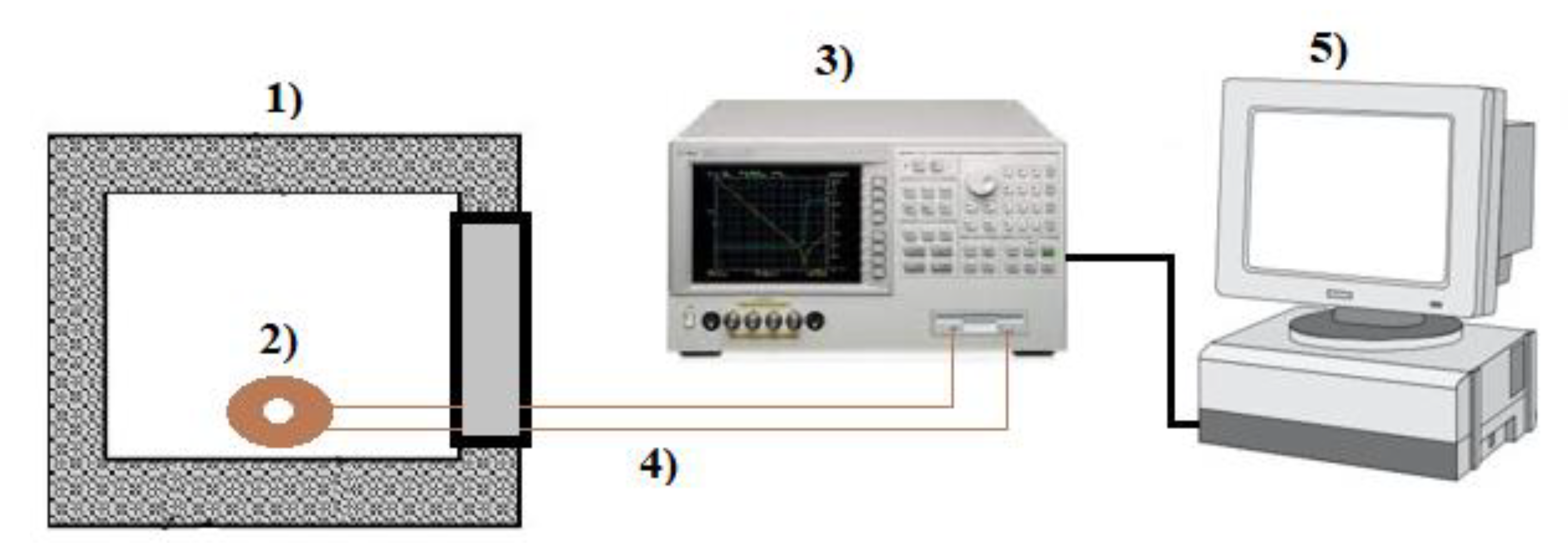
2.2. Experimental Methods
3. Results and Discussion
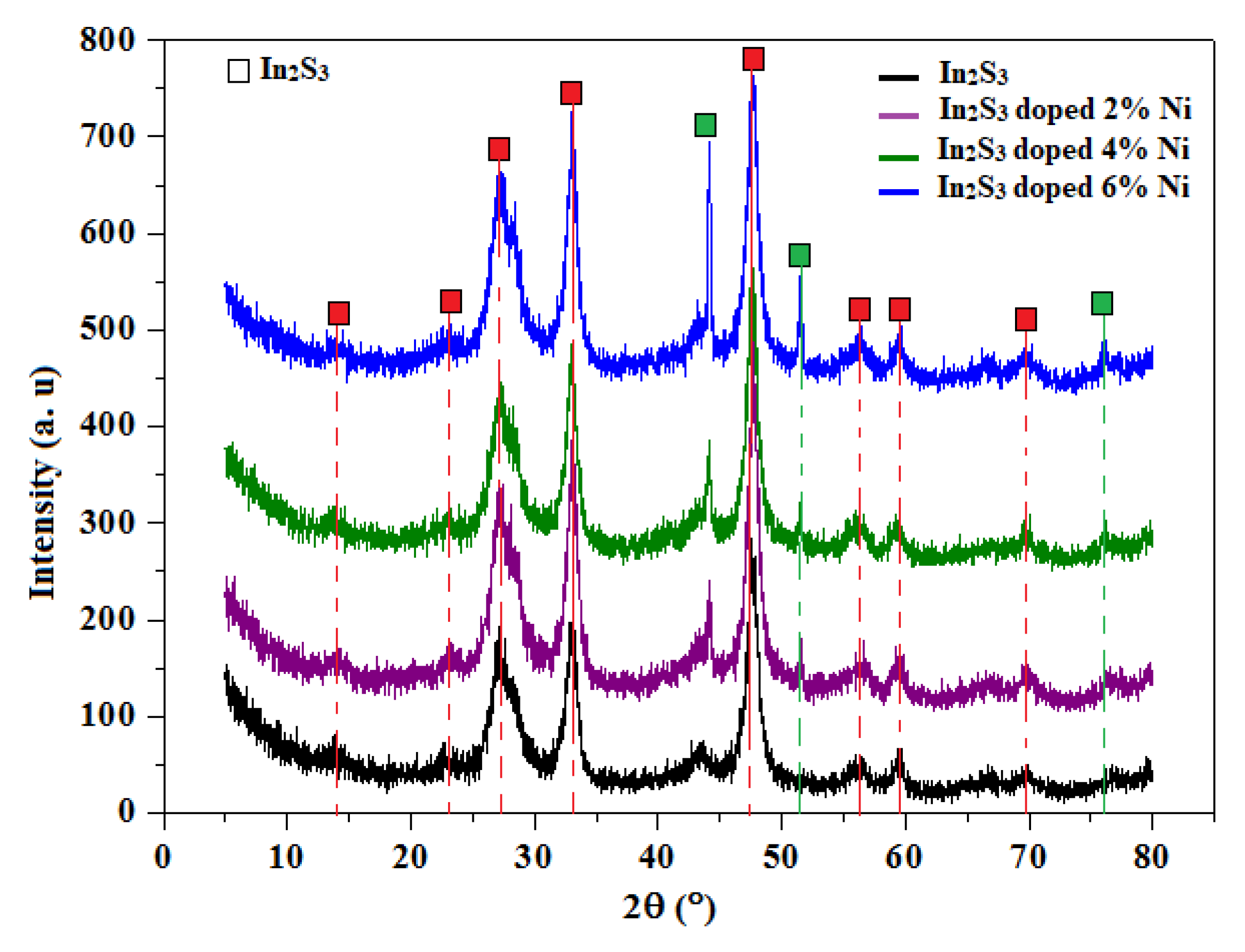
3.1. XRD and EDS Analysis
3.2. Thermal Analysis
3.3. Raman Analysis
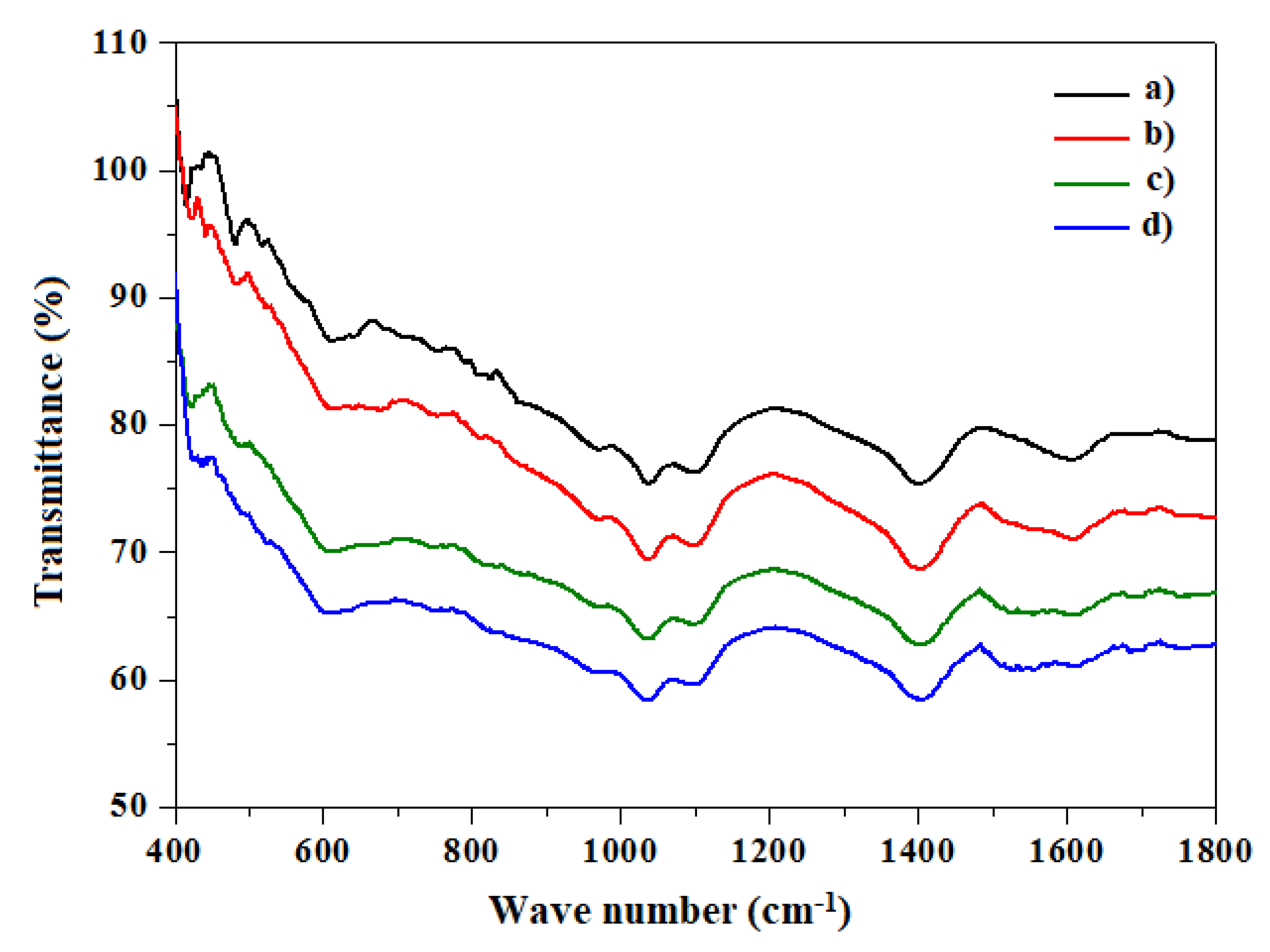
3.4. FTIR
3.5. SEM
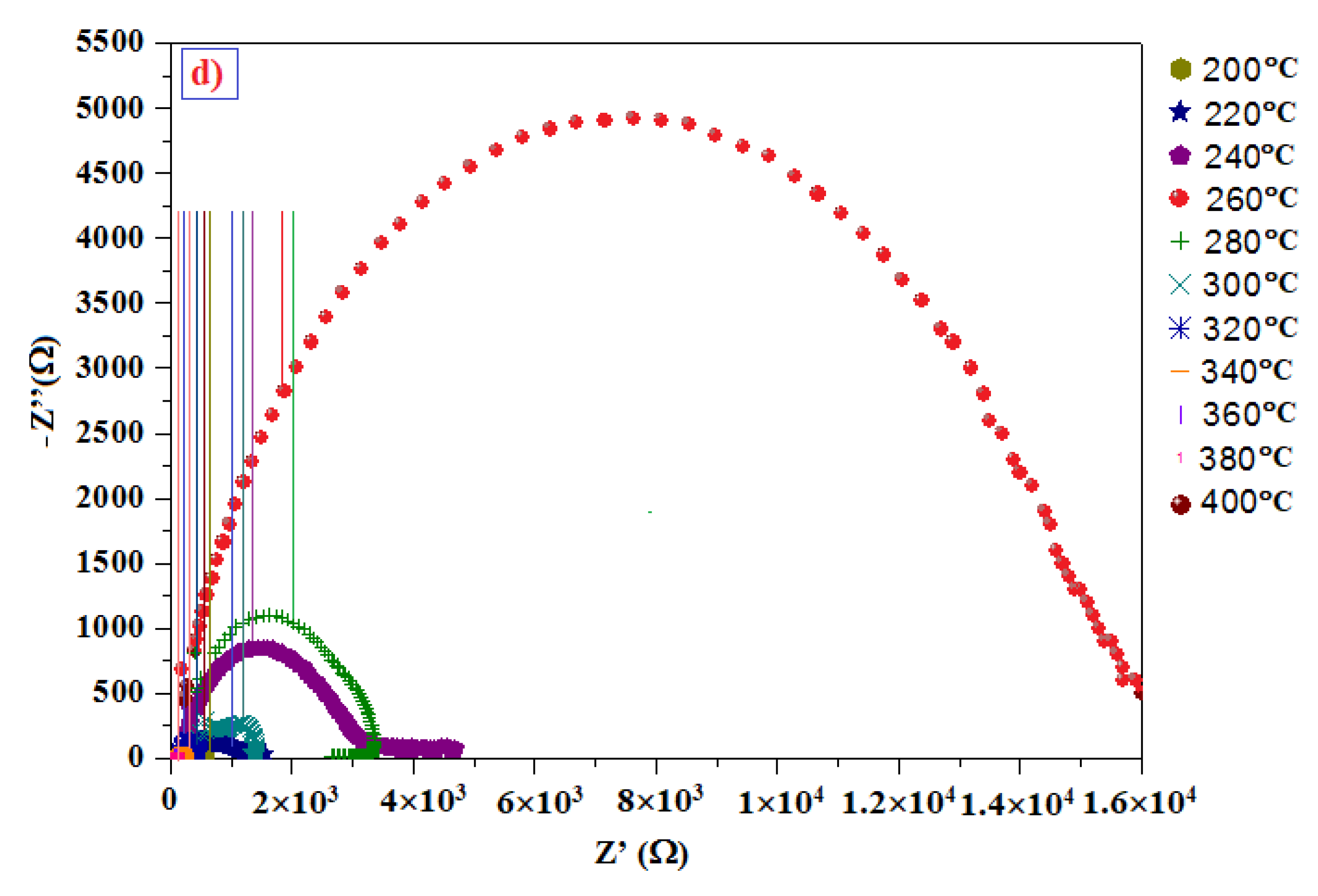
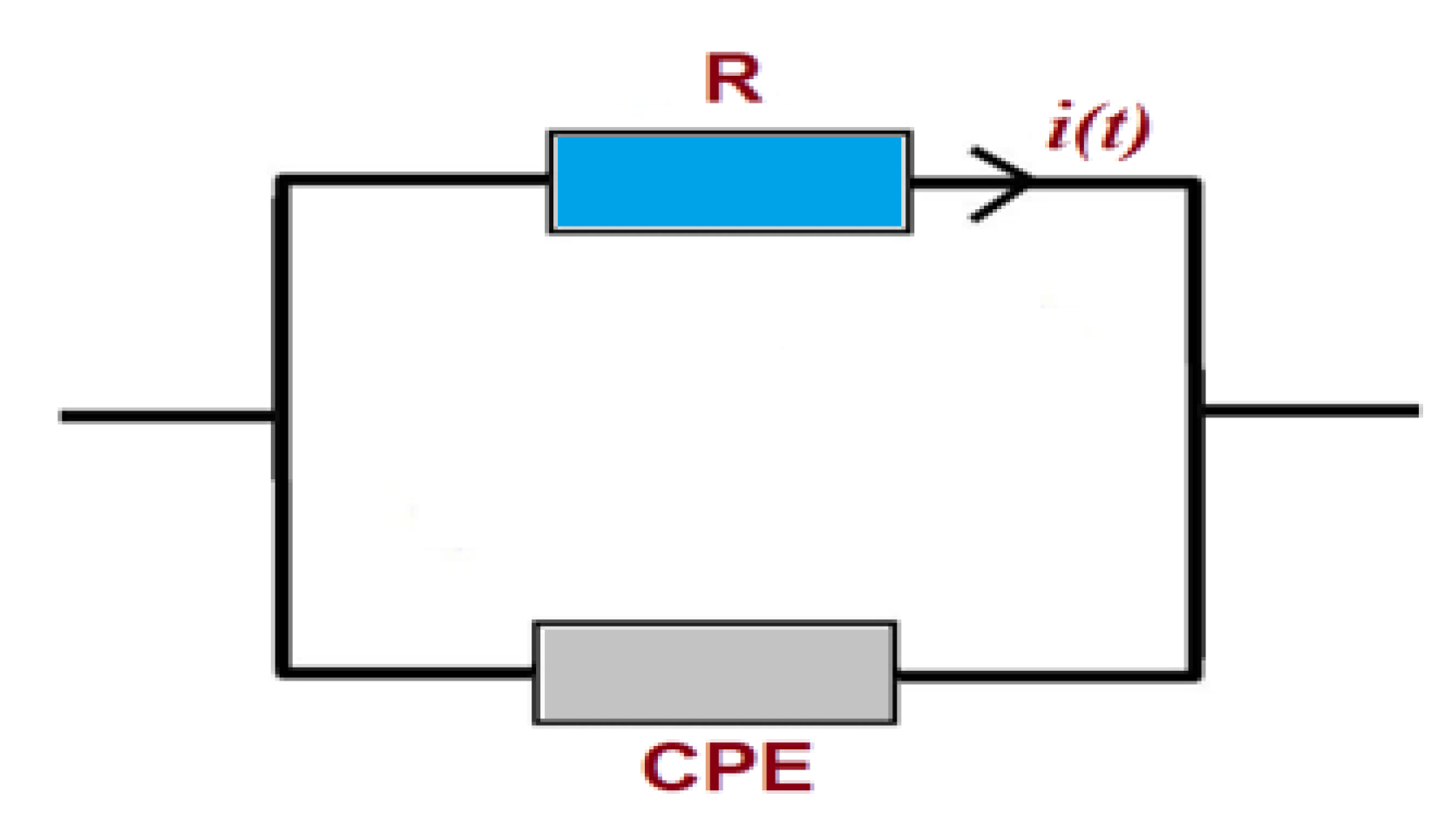
3.6. Electrical Impedance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revathi, N.; Prathap, P.; Miles, R.; Reddy, K.R. Annealing effect on the physical properties of evaporated In2S3 films. Sol. Energy Mater. Sol. Cells 2010, 94, 1487–1491. [Google Scholar] [CrossRef]
- Hsiao, Y.-J.; Lu, C.-H.; Ji, L.-W.; Meen, T.-H.; Chen, Y.-L.; Chi, H.-P. Characterization of photovoltaics with In2S3 nanoflakes/p-Si heterojunction. Nanoscale Res. Lett. 2014, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasool, S.; Saritha, K.; Reddy, K.R.; Bychto, L.; Patryn, A.; Maliński, M.; Tivanov, M.; Gremenok, V. Optical properties of thermally evaporated In 2 S 3 thin films measured using photoacoustic spectroscopy. Mater. Sci. Semicond. Process. 2017, 72, 4–8. [Google Scholar] [CrossRef]
- Sim, Y.; Kim, J.; Chang, S.H.; Choi, C.-J.; Seong, M.-J. Highly luminescent In2S3 thin films with preferred growth direction of [1 0 3]. Appl. Surf. Sci. 2021, 555, 149706. [Google Scholar] [CrossRef]
- De Moure-Flores, F.; Nieto-Z, K.E.; Guillén, C.A.; Gallardo, S.; Quiñones, G.J.G.; Hernández, H.A.; Olvera, L.M.; Zapata, T.M.; Kundriavtsev, Y.; Meléndez, L.M. Effect of the immersion in CdCl2 and annealing on physical properties of CdS:F films grown by CBD. J. Phys. Chem. Solids 2013, 74, 611. [Google Scholar] [CrossRef]
- Hashemi, M.; Heidariramsheh, M.; Ghorashi, S.M.B.; Taghavinia, N.; Mahdavi, S.M. Study on spray-pyrolyzed In2S3 thin films, targeted as electron transport layer in solar energy. J. Photon-Energy 2020, 10, 024001. [Google Scholar] [CrossRef]
- Gao, W.; Liu, W.; Leng, Y.; Wang, X.; Wang, X.; Hu, B.; Yu, D.; Sang, Y.; Liu, H. In2S3 nanomaterial as a broadband spectrum photocatalyst to display significant activity. Appl. Catal. B Environ. 2015, 176–177, 83–90. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Kim, J.Y.; Goldstein, D.N.; Neale, N.R.; Zhu, K.; Elliott, C.M.; Frank, A.J.; George, S.M. In2S3 atomic layer deposition and its application as a sensitizer on TiO2 nanotube arrays for solar energy conversion. J. Phys. Chem. C 2010, 114, 17, 8032. [Google Scholar] [CrossRef]
- Nehra, S.; Chander, S.; Sharma, A.; Dhaka, M. Effect of thermal annealing on physical properties of vacuum evaporated In2S3 buffer layer for eco-friendly photovoltaic applications. Mater. Sci. Semicond. Process. 2015, 40, 26–34. [Google Scholar] [CrossRef]
- Pulipaka, S.; Koushik, A.K.S.; Deepa, M.; Meduri, P. Enhanced photoelectrochemical activity of Co-doped β-In2S3nanoflakes as photoanodes for water splitting. RSC Adv. 2019, 9, 1335–1340. [Google Scholar] [CrossRef] [Green Version]
- Kwoka, M.; Ottaviano, L.; Szuber, J. AFM study of the surface morphology of L-CVD SnO2 thin films. Thin Solid Film. 2007, 515, 8328–8331. [Google Scholar] [CrossRef]
- Timoumi, A.; Bouzouita, H.; Kanzari, M.; Rezig, B. Fabrication and characterization of In2S3 thin films deposited by thermal evaporation technique. Thin Solid Films 2005, 480–481, 124–128. [Google Scholar] [CrossRef]
- Timoumi, A.; Bouzouita, H.; Brini, R.; Kanzari, M.; Rezig, B. Optimization of annealing conditions of In2S3 thin films deposited by vacuum thermal evaporation. Appl. Surf. Sci. 2006, 253, 306–310. [Google Scholar] [CrossRef]
- Timoumi, A.; Bouzouita, H.; Rezig, B. Optical constants of Na–In2S3 thin films prepared by vacuum thermal evaporation technique. Thin Solid Film. 2011, 519, 7615–7619. [Google Scholar] [CrossRef]
- Bouguila, N.; Timoumi, A.; Bouzouita, H.; Lacaze, E.; Bouchriha, H.; Rezig, B. Molar ratio S/In effect on properties of sprayed In2S3films. Eur. Phys. J. Appl. Phys. 2013, 63, 20301. [Google Scholar] [CrossRef]
- Bouguila, N.; Timoumi, A.; Bouzouita, H. Vacuum annealing temperature on spray In2S3layers. Eur. Phys. J. Appl. Phys. 2014, 65, 20304. [Google Scholar] [CrossRef]
- Bouguila, N.; Kraini, M.; Timoumi, A.; Halidou, I.; Vazquez-Vazquez, C.; López-Quintela, M.A.; Alaya, S. Substrate temperature effect on properties of sprayed In2S3 films. J. Mater. Sci. Mater. Electron. 2015, 26, 7639–7648. [Google Scholar] [CrossRef]
- Timoumi, A.; Bouguila, N.; Chaari, M.; Kraini, M.; Matoussi, A.; Bouzouita, H. Electrical and dielectric properties of In2S3 synthesized by solid state reaction. J. Alloys Compd. 2016, 679, 59–64. [Google Scholar] [CrossRef]
- Abassi, H.; Bouguila, N.; Timoumi, A. Theoretical Study of the AC Conduction in β-In2S3. J. Electron. Mater. 2018, 47, 2519–2525. [Google Scholar] [CrossRef]
- Timoumi, A.; Belhadj, W.; Alamri, S.; Al Turkestani, M. Experimental studies and new theoretical modeling on the properties of In2S3 thin films. Opt. Mater. 2021, 118, 111238. [Google Scholar] [CrossRef]
- Hamici, M.; Guessoum, K.; Vaillant, L.; Gagou, Y.; Saint-Grégoire, P. Study of the Oxidation Process of Crystalline Powder of In2S3 and Thin Films Obtained by Dr Blade Method. J. Electron. Mater. 2019, 48, 4715–4725. [Google Scholar] [CrossRef] [Green Version]
- Gorai, S.; Guha, P.; Ganguli, D.; Chaudhuri, S. Chemical synthesis of β-In2S3 powder and its optical characterization. Mater. Chem. Phys. 2003, 82, 974–979. [Google Scholar] [CrossRef]
- Alamri, S.N. Structure and optical properties of chunk and powder CdS film prepared by electron beam deposition. Indian J. Phys. 2013, 88, 259–264. [Google Scholar] [CrossRef]
- Masikane, S.C.; McNaughter, P.D.; Lewis, D.J.; Vitorica-Yrezabal, I.; Doyle, B.P.; Carleschi, E.; O’Brien, P.; Revaprasadu, N. Important Phase Control of Indium Sulfide Nanomaterials by Choice of Indium(III) Xanthate Precursor and Thermolysis Temperature. Eur. J. Inorg. Chem. 2019, 2019, 1421–1432. [Google Scholar] [CrossRef]
- Ghosh, S.; Saha, M.; Ashok, V.D.; Chatterjee, A.; De, S.K. Excitation dependent multicolor emission and photoconductivity of Mn, Cu doped In2S3monodisperse quantum dots. Nanotechnology 2016, 27, 155708. [Google Scholar] [CrossRef] [PubMed]
- Lucena, R.; Conesa, J.C.; Aguilera, I.; Palacios, P.; Wahnón, P. V-substituted In2S3: An intermediate band material with photocatalytic activity in the whole visible light range. J. Mater. Chem. A 2014, 2, 8236–8245. [Google Scholar] [CrossRef] [Green Version]
- Timoumi, A.; Zayoud, W.; Sharma, A.; Kraini, M.; Bouguila, N.; Hakamy, A.; Revaprasadu, N.; Alaya, S. Impact of thermal annealing inducing oxidation process on the crystalline powder of In2S3. J. Mater. Sci. Mater. Electron. 2020, 31, 13636–13645. [Google Scholar] [CrossRef]
- Kraini, M.; Bouguila, N.; Moutia, N.; El Ghoul, J.; Khirouni, K.; Vázquez-Vázquez, C. Properties of nickel doped In2S3 thin films deposited by spray pyrolysis technique. J. Mater. Sci. Mater. Electron. 2017, 29, 1888–1906. [Google Scholar] [CrossRef]
- Aldon, L.; Uhrmacher, M.; Branci, C.; Ziegler, L.; Roth, J.; Schaaf, P.; Metzner, H.; Olivier, F.J.; Jumas, J.C. Perturbed angular correlation study of the thiospinel β−In2S3. Phys. Rev. B. 1998, 58, 11303. [Google Scholar] [CrossRef]
- Liu, P.; Ng, V.M.H.; Yao, Z.; Zhou, J.; Lei, Y.; Yang, Z.; Lv, H.; Kong, L.B. Facile Synthesis and Hierarchical Assembly of Flowerlike NiO Structures with Enhanced Dielectric and Microwave Absorption Properties. ACS Appl. Mater. Interfaces 2017, 9, 16404–16416. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yao, Z.; Zhou, J.; Yang, Z.; Kong, L.B. Small magnetic Co-doped NiZn ferrite/graphene nanocomposites and their dual-region microwave absorption performance. J. Mater. Chem. C 2016, 4, 9738–9749. [Google Scholar] [CrossRef]
- Liu, P.; Yao, Z.; Ng, V.M.H.; Zhou, J.; Kong, L.B.; Yue, K. Facile synthesis of ultrasmall Fe3O4 nanoparticles on MXenes for high microwave absorption performance. Compos. Part A 2018, 115, 371. [Google Scholar] [CrossRef]
- Koaib, J.; Bouguila, N.; Abassi, H.; Moutia, N.; Kraini, M.; Timoumi, A.; Vázquez-Vázquez, C.; Khirouni, K.; Alaya, S. Dielectric and electrical properties of annealed ZnS thin films. The appearance of the OLPT conduction mechanism in chalcogenides. RSC Adv. 2020, 10, 9549–9562. [Google Scholar] [CrossRef]
- Chakroun, R.; Jamoussi, B.; Al-Mur, B.; Timoumi, A.; Essalah, K. Impedance Spectroscopy and Dielectric Relaxation of Imidazole-Substituted Palladium(II) Phthalocyanine (ImPdPc) for Organic Solar Cells. ACS Omega 2021, 6, 10655–10667. [Google Scholar] [CrossRef]
- Timoumi, A.A.; Bouguila, N.; Koaib, J.; Al Turkestani, M.K.; Jamoussi, B.; Timoumi, A.; Koiab, J. Study of electrical and dielectric properties of palladium-phthalocyanine (PdPc) in pellet form. Mater. Res. Express 2019, 6, 055103. [Google Scholar] [CrossRef]
- Timoumi, A.; Wederni, M.A.; Bouguila, N.; Jamoussi, B.; AL Turkestani, M.K.; Chakroun, R.; Al-Mur, B. Electrical impedance spectroscopy study of unsubstituted palladium (II) phthalocyanine. Synth. Met. 2021, 272, 116659. [Google Scholar] [CrossRef]
- Tao, H.; Zang, H.; Dong, G.; Zeng, J.; Zhao, X. Raman and infrared spectroscopic study of the defect spinel In21.333S32. Optoelectron. Adv. Mater. 2008, 2, 356. [Google Scholar]
- Aakansha; Deka, B.; Ravi, S.; Pamu, D. Impedance spectroscopy and ac conductivity mechanism in Sm doped Yttrium Iron Garnet. Ceram. Int. 2017, 43, 10468–10477. [Google Scholar] [CrossRef]
- Khosravi, S.H.; Veerapanduyan, V.K.; Vallant, R.; Reichmann, K. Effect of processing conditions on the structural properties and corrosion behavior of TiO2–SiO2 multilayer coatings derived via the sol-gel method. Ceram. Int. 2020, 46, 17741. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Hasan, M.M. Impedance spectroscopic investigation of electro active regions, conduction mechanism and origin of colossal dielectric constant in Nd1−xSrxFeO3 (0.1 ≤ x ≤ 0.5). Mater. Res. Bull. 2014, 60, 474. [Google Scholar] [CrossRef]
- Tan, F.K.; Hassan, J.; Wahab, Z.A.; Azis, R.S. Electrical conductivity and dielectric behaviour of manganese and vanadium mixed oxide prepared by conventional solid state method. Eng. Sci. Technol. Int. J. 2016, 19, 2081–2087. [Google Scholar] [CrossRef] [Green Version]
- Trabelsi, I.; Jebali, A.; Kanzari, M. Electrical characterization of SnSb4S7 thin films by impedance spectroscopy. J. Mater. Sci. Mater. Electron. 2016, 27, 4326–4335. [Google Scholar] [CrossRef]
- Pistor, P.; Merino, Á.J.M.; León, M.; Michiel, M.; Schorr, S.; Klenk, R.; Lehmann, S. Structure reinvestigation of α-, β- and γ-In2S3. Acta Crystallogr. B. 2016, 72, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoltowski, P. On the electrical capacitance of interfaces exhibiting constant phase element behaviour. J. Electroanal. Chem. 1998, 443, 149–154. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2005, 51, 1473–1479. [Google Scholar] [CrossRef]





| Ni:In Ratio in Powder Samples | Elements | ||
|---|---|---|---|
| In (at.%) | S (at.%) | Ni (at.%) | |
| Undoped | 40.54 | 59.46 | 0 |
| 2% | 39.47 | 58.50 | 2.04 |
| 4% | 40.12 | 57.08 | 2.80 |
| 6% | 39.63 | 56.70 | 3.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timoumi, A.; Belhadj, W.; Alamri, S.N.; Al-Turkestani, M.K. Physical and Dielectric Properties of Ni-Doped In2S3 Powders for Optical Windows in Thin Film Solar Cells. Materials 2021, 14, 5779. https://doi.org/10.3390/ma14195779
Timoumi A, Belhadj W, Alamri SN, Al-Turkestani MK. Physical and Dielectric Properties of Ni-Doped In2S3 Powders for Optical Windows in Thin Film Solar Cells. Materials. 2021; 14(19):5779. https://doi.org/10.3390/ma14195779
Chicago/Turabian StyleTimoumi, Abdelmajid, Walid Belhadj, Salah Noaiman Alamri, and Mohamed Khalil Al-Turkestani. 2021. "Physical and Dielectric Properties of Ni-Doped In2S3 Powders for Optical Windows in Thin Film Solar Cells" Materials 14, no. 19: 5779. https://doi.org/10.3390/ma14195779






