Thermally Induced Silane Dehydrocoupling: Hydrophobic and Oleophilic Filter Paper Preparation for Water Separation and Removal from Organic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Spectroscopic Analysis
2.4. Preparation of ODS-Coated Cellulose Filter Paper (OCFP)
2.5. Separation Efficiency of the Oil-Water Mixture Analysis
2.6. Solvent Drying Efficiency Analysis
2.7. Statistical Analysis
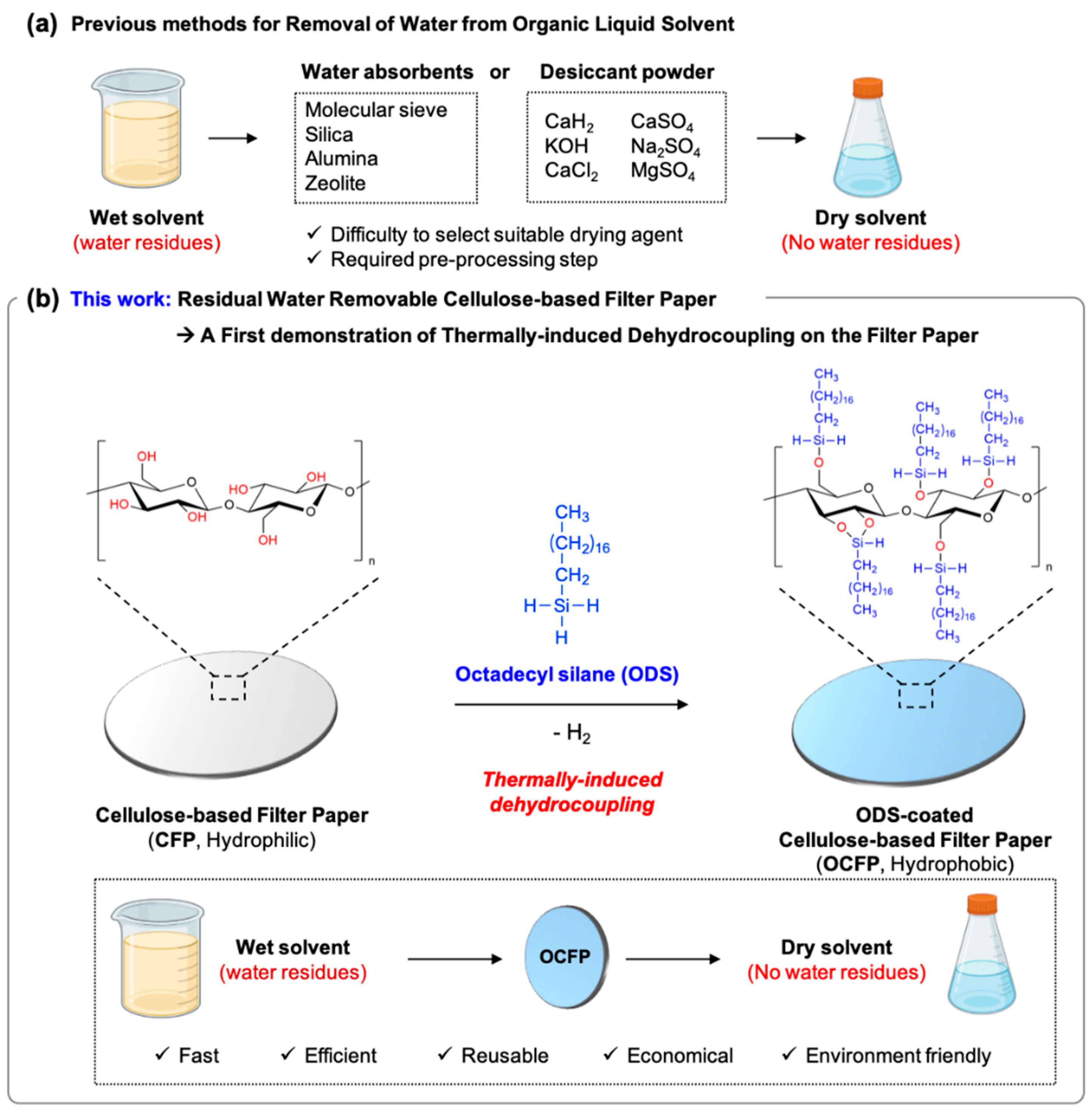
3. Results and Discussion
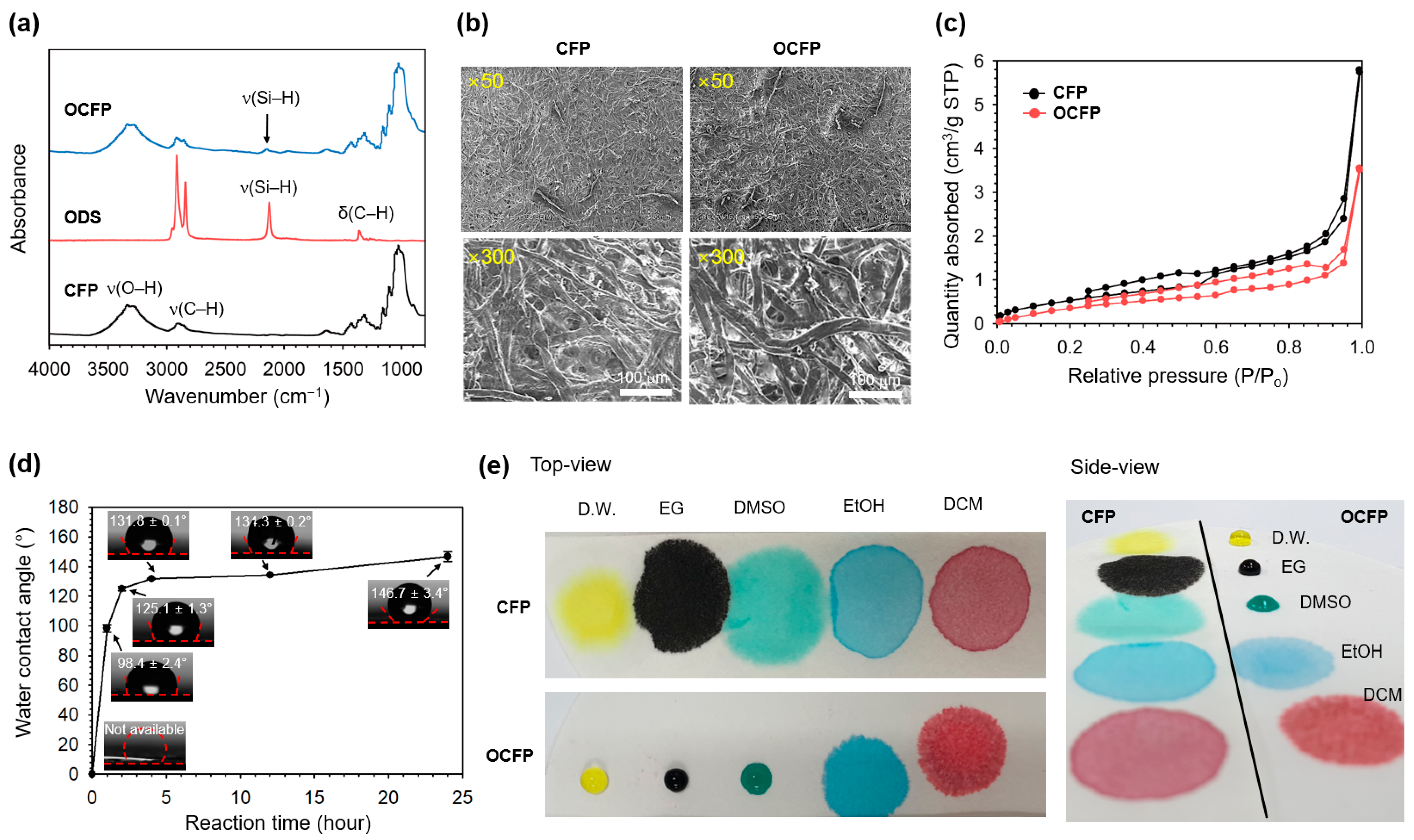
3.1. Preparation and Characterization of OCFP
3.2. Surface Wettability of OCFP
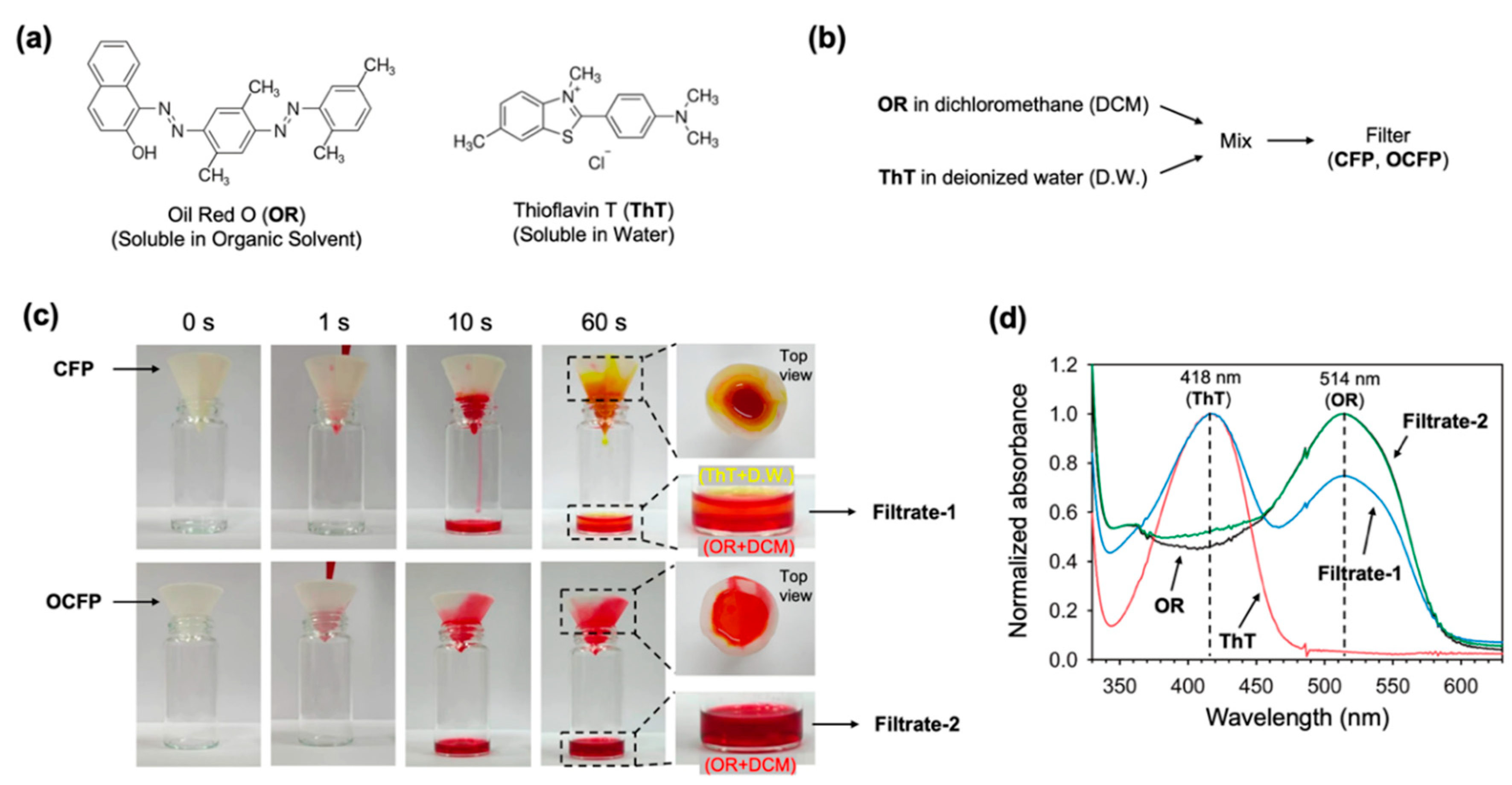
3.3. Separation Efficiency of the Oil-Water Mixtures and Recyclability
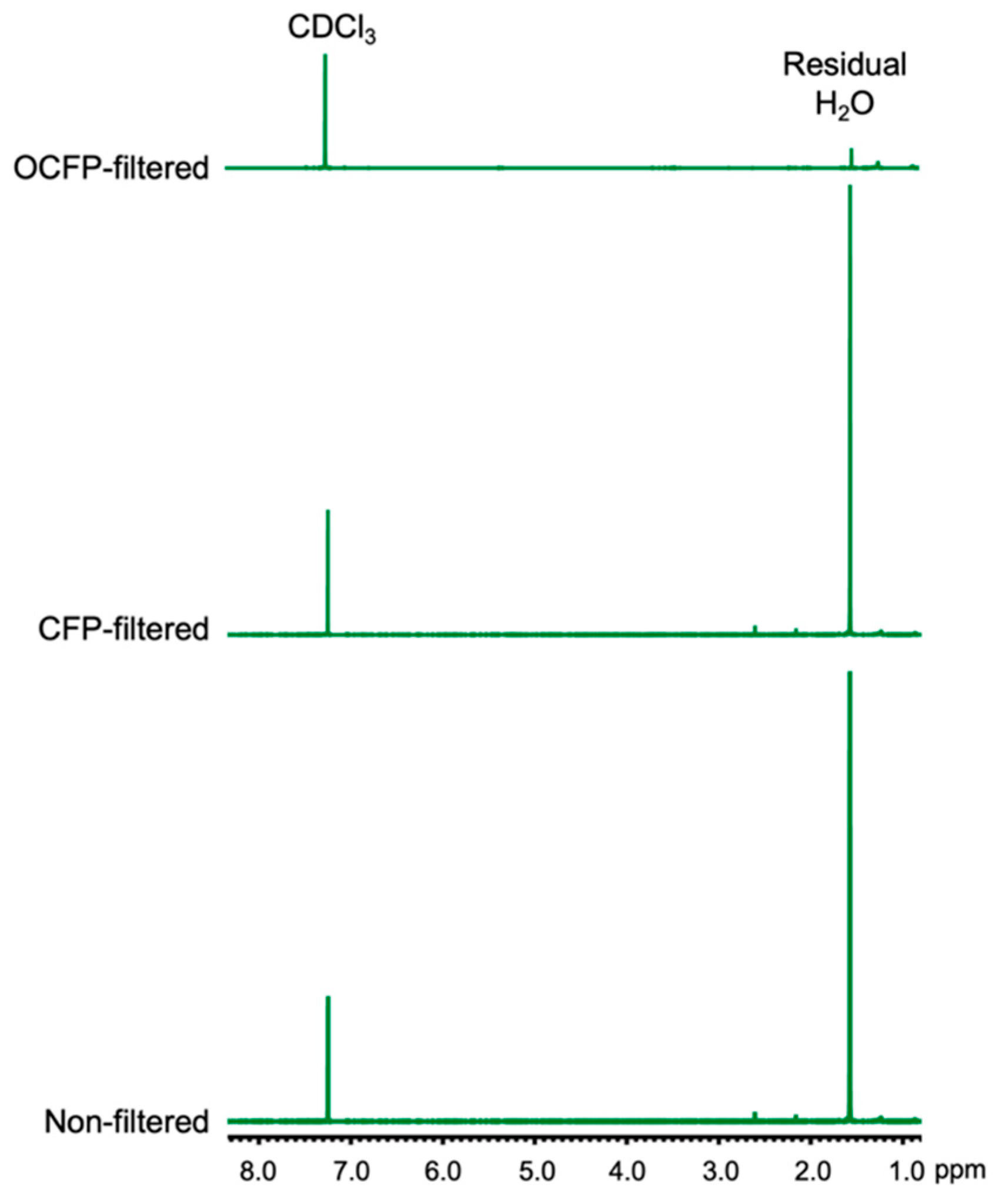
3.4. Wet Solvent Drying Efficiency of OCFP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugisaki, M.; Fujiwara, M.; Kosumi, D.; Fujii, R.; Nango, M.; Cogdell, R.J.; Hashimoto, H. Comparison of transient grating signals from spheroidene in an organic solvent and in pigment-protein complexes from Rhodobacter sphaeroides 2.4.1. Phys. Rev. B 2010, 81, 245112. [Google Scholar] [CrossRef] [Green Version]
- Covington, A.K.; Dickinson, T. Physical Chemistry of Organic Solvent Systems; Springer Science & Business Media: Berlin, Germany, 1973; pp. 4–6. [Google Scholar]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- Peeva, L.; da Silva Burgal, J.; Valtcheva, I.; Livingston, A.G. Continuous purification of active pharmaceutical ingredients using multistage organic solvent nanofiltration membrane cascade. Chem. Eng. Sci. 2014, 116, 183–194. [Google Scholar] [CrossRef]
- Buonomenna, M.; Bae, J. Organic solvent nanofiltration in pharmaceutical industry. Sep. Purif. Rev. 2015, 44, 157–182. [Google Scholar] [CrossRef]
- Zuehlsdorff, T.; Haynes, P.D.; Hanke, F.; Payne, M.; Hine, N.D. Solvent effects on electronic excitations of an organic chromophore. J. Chem. Theory Comput. 2016, 12, 1853–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Chung, T.-S. Pharmaceutical concentration using organic solvent forward osmosis for solvent recovery. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Mittal, A.; Robichaud, D.J.; Pilath, H.M.; Etz, B.D.; St. John, P.C.; Johnson, D.K.; Kim, S. Prediction of Hydroxymethylfurfural Yield in Glucose Conversion through Investigation of Lewis Acid and Organic Solvent Effects. ACS Catal. 2020, 10, 14707–14721. [Google Scholar] [CrossRef]
- Loópez-Salas, N.; Vicent-Luna, J.M.; Posada, E.; Imberti, S.; Madero-Castro, R.; Calero, S.; Ania, C.O.; Jiménez-Riobóo, R.; Gutierrez, M.C.; Ferrer, M.L. Further Extending the Dilution Range of the “Solvent-in-DES” Regime upon the Replacement of Water by an Organic Solvent with Hydrogen Bond Capabilities. ACS Sustain. Chem. Eng. 2020, 8, 12120–12131. [Google Scholar] [CrossRef]
- Salim, A.A.; Bakhtiar, H.; Ghoshal, S.K.; Huyop, F. Customised structural, optical and antibacterial characteristics of cinnamon nanoclusters produced inside organic solvent using 532 nm Q-switched Nd: YAG-pulse laser ablation. Opt. Laser Technol. 2020, 130, 106331. [Google Scholar] [CrossRef]
- Slang, S.; Palka, K.; Jancalek, J.; Kurka, M.; Vlcek, M. Deposition and characterization of solution processed Se-rich Ge-Se thin films with specular optical quality using multi-component solvent approach. Opt. Mater. Express 2020, 10, 2973–2986. [Google Scholar] [CrossRef]
- Imura, S.; Kobayashi, T.; Tokunaga, E. More Than 50-Fold Enhanced Nonlinear Optical Response of Porphyrin Molecules in Aqueous Solution Induced by Mixing Base and Organic Solvent. Appl. Sci. 2021, 11, 4892. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.; Dewé, W.; Flament, A.; Gibella, M.; Ceccato, A. A new validation approach applied to the GC determination of impurities in organic solvents. J. Pharm. Biomed. Anal. 2006, 40, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; Amicis, C.V.D.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry. Org. Process. Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Iordan, H.H.; Roger, L.D.; Grant, D.W.A.; Inigo, G.; Stefan, M.; Tom, Z. Possible Side Reactions Due to Water in Emulsion Polymerization by Late Transition Metal Complexes. 1. Water Complexation and Hydrolysis of the Growing Chain. Inorg. Chem. 2005, 44, 7806–7818. [Google Scholar]
- National Research Council (US) Safe Drinking Water Committee. Drinking Water and Health; National Academies Press (US): Washington, DC, USA, 1980; Volume 2, pp. 1–392. [Google Scholar]
- Kieboom, A.P.G. Purification of Laboratory Chemicals, 3rd ed.; Perrin, D.D., Armarego, W.L.F., Eds.; Pergamon Press: Oxford, UK, 1988; Volume 107, p. 685. [Google Scholar] [CrossRef]
- Sabane, D.G.; Mohite, M.T.; Pratapwar, M.N.; Patil, R.S.; Jagtap, T.C. Study of The Effect of Anhydrous Solvent on Methotrexate by uisng UV-Spectrophotometer. Int. J. Chem. Pharm. Anal. 2017, 4, 1–6. [Google Scholar]
- Rubino, A.; Camellini, A.; Kriegel, I. Stable solution emission of 2, 3, 5, 6-Tetrafluoro-7, 7, 8, 8-tetracyanoquinodimethane. Opt. Mater. 2021, 11, 100081. [Google Scholar]
- Honda, K.; Ono, T.; Okano, K.; Miyake, R.; Dekishima, Y.; Kawabata, H. Expression of engineered carbonyl reductase from Ogataea minuta in Rhodococcus opacus and its application to whole-cell bioconversion in anhydrous solvents. J. Biosci. Bioeng. 2019, 127, 145–149. [Google Scholar] [CrossRef]
- Pahl, C.; Pasel, C.; Luckas, M.; Bathen, D. Adsorptive Water Removal from Organic Solvents in the ppm-Region. Chem. Ing. Tech. 2011, 83, 177–182. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Lawton, M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef] [PubMed]
- Burfield, D.R.; Lee, K.-H.; Smithers, R.H. Desiccant efficiency in solvent drying. A reappraisal by application of a novel method for solvent water assay. J. Org. Chem. 1977, 42, 3060–3065. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Pearson: Harlow, UK, 1989; pp. 165–168. [Google Scholar]
- Burfield, D.R.; Smithers, R.H. Desiccant efficiency in solvent and reagent drying. 7. Alcohols. J. Org. Chem. 1983, 48, 2420–2422. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Beshkar, F.; Salavati-Niasari, M.; Amiri, O. Superhydrophobic–superoleophilic copper–graphite/styrene–butadiene–styrene based cotton filter for efficient separation of oil derivatives from aqueous mixtures. Cellulose 2020, 27, 4691–4705. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Jedvert, K.; Heinze, T. Cellulose modification and shaping—A review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules 2019, 24, 2782. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Li, J.; Wang, G.; Liang, W.; Zhang, Y.; Shi, L.; Guo, Z.; Liu, W. Methodology for Robust Superhydrophobic Fabrics and Sponges from In Situ Growth of Transition Metal/Metal Oxide Nanocrystals with Thiol Modification and Their Applications in Oil/Water Separation. ACS Appl. Mater. Interfaces 2013, 5, 1827–1839. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Bordes, R.; Köhnke, T. Wet Spinning of Flame-Retardant Cellulosic Fibers Supported by Interfacial Complexation of Cellulose Nanofibrils with Silica Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 39069–39077. [Google Scholar] [CrossRef]
- Chen, Q.; de Leon, A.; Advincula, R.C. Inorganic–Organic Thiol–ene Coated Mesh for Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 18566–18573. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Liu, Y.; Hu, H.; Li, Y.; Yan, P.; Yu, B.; Zhou, F. One-Step Modification of Fabrics with Bioinspired Polydopamine@Octadecylamine Nanocapsules for Robust and Healable Self-Cleaning Performance. Small 2015, 11, 426–431. [Google Scholar] [CrossRef]
- Tursi, A.; De Vietro, N.; Beneduci, A.; Milella, A.; Chidichimo, F.; Fracassi, F.; Chidichimo, G. Low pressure plasma functionalized cellulose fiber for the remediation of petroleum hydrocarbons polluted water. J. Hazard. Mater. 2019, 373, 773–782. [Google Scholar] [CrossRef]
- Feng, X.; Shi, Y.; Liu, J.; Yang, W. Fabrication of filter paper with tunable wettability and its application in oil-water separation. J. Sol.-Gel Sci. Technol. 2015, 76, 129–137. [Google Scholar] [CrossRef]
- Oyola-Reynoso, S.; Tevis, D.I.; Chen, J.; Chang, S.B.; Çinar, S.; Bloch, J.-F.; Thuo, M.M. Recruiting physisorbed water in surface polymerization for bio-inspired materials of tunable hydrophobicity. J. Mater. Chem. A 2016, 4, 14729–14738. [Google Scholar] [CrossRef] [Green Version]
- Naik, V.V.; Crobu, M.; Nenkataraman, N.V.; Spencer, N.D. Multiple Transmission-Reflection IR Spectroscopy Shows that Surface Hydroxyls Play Only a Minor Role in Alkylsilane Monolayer Formation on Silica. J. Phys. Chem. Lett. 2013, 4, 2745–2751. [Google Scholar] [CrossRef]
- Kim, D.; Joo, J.; Pan, Y.; Boarino, A.; Jun, Y.W.; Ahn, K.H.; Arkles, B.; Sailor, M.J. Thermally Induced Silane Dehydrocoupling on Silicon Nanostructures. Angew. Chem. Int. Ed. 2016, 55, 6423–6427. [Google Scholar] [CrossRef]
- Owens, D.; Wendt, R. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Luo, C.; Liu, Q. Oxidant-induced high-efficient musselinspired modification on PVDF membrane with superhydrophilicity and underwater superoleophobicity characteristics for oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 8297–8307. [Google Scholar] [CrossRef]
- Hong, S.K.; Bae, S.; Jeon, H.; Kim, M.; Cho, S.J.; Lim, G. An underwater superoleophobic nanofibrous cellulosic membrane for oil/water separation with high separation flux and high chemical stability. Nanoscale 2018, 10, 3037–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Analyt. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Abdul Hadi, N.; Wiege, B.; Stabenau, S.; Marefati, A.; Rayner, M. Comparison of Three Methods to Determine the Degree of Substitution of Quinoa and Rice Starch Acetates, Propionates, and Butyrates: Direct Stoichiometry, FTIR, and 1H-NMR. Foods 2020, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Belgacem, M.N.; Czeremuszkin, G.; Sapieha, S.; Gandini, A. Surface characterization of cellulose fibres by XPS and inverse gas chromatography. Cellulose 1995, 2, 145–157. [Google Scholar] [CrossRef]
- Tang, X.; Wang, X.; Tang, C.; Ma, J.; Zhang, S.; Li, Z.; Dong, F. PDA-assisted one-pot fabrication of bioinspired filter paper for oil-water separation. Cellulose 2019, 26, 1355–1366. [Google Scholar] [CrossRef]
- Jensen, D.S.; Kanyal, S.S.; Madaan, N. Silicon (100)/SiO2 by XPS. Surf. Sci. Spectra 2014, 20, 36. [Google Scholar] [CrossRef]
- Marinkovic, F.S.; Popovic, D.M.; Jovanovic, J.D.; Stankovic, B.S.; Adnadjevic, B.K. Methods for quantitative determination of filler weight fraction and filler dispersion degree in polymer composites: Example of low-density polyethylene and NaA zeolite composite. Appl. Phys. A 2019, 125, 611. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, R.H.; Kim, D. Thermally Induced Silane Dehydrocoupling: Hydrophobic and Oleophilic Filter Paper Preparation for Water Separation and Removal from Organic Solvents. Materials 2021, 14, 5775. https://doi.org/10.3390/ma14195775
Kang RH, Kim D. Thermally Induced Silane Dehydrocoupling: Hydrophobic and Oleophilic Filter Paper Preparation for Water Separation and Removal from Organic Solvents. Materials. 2021; 14(19):5775. https://doi.org/10.3390/ma14195775
Chicago/Turabian StyleKang, Rae Hyung, and Dokyoung Kim. 2021. "Thermally Induced Silane Dehydrocoupling: Hydrophobic and Oleophilic Filter Paper Preparation for Water Separation and Removal from Organic Solvents" Materials 14, no. 19: 5775. https://doi.org/10.3390/ma14195775
APA StyleKang, R. H., & Kim, D. (2021). Thermally Induced Silane Dehydrocoupling: Hydrophobic and Oleophilic Filter Paper Preparation for Water Separation and Removal from Organic Solvents. Materials, 14(19), 5775. https://doi.org/10.3390/ma14195775






