Influence of Tranexamic Acid on Elution Characteristics and Compressive Strength of Antibiotic-Loaded PMMA-Bone Cement with Gentamicin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Gentamicin Release Test
2.4. Antimicrobial Activity Test
2.5. Compression Test
2.6. Statistical Analysis
3. Results
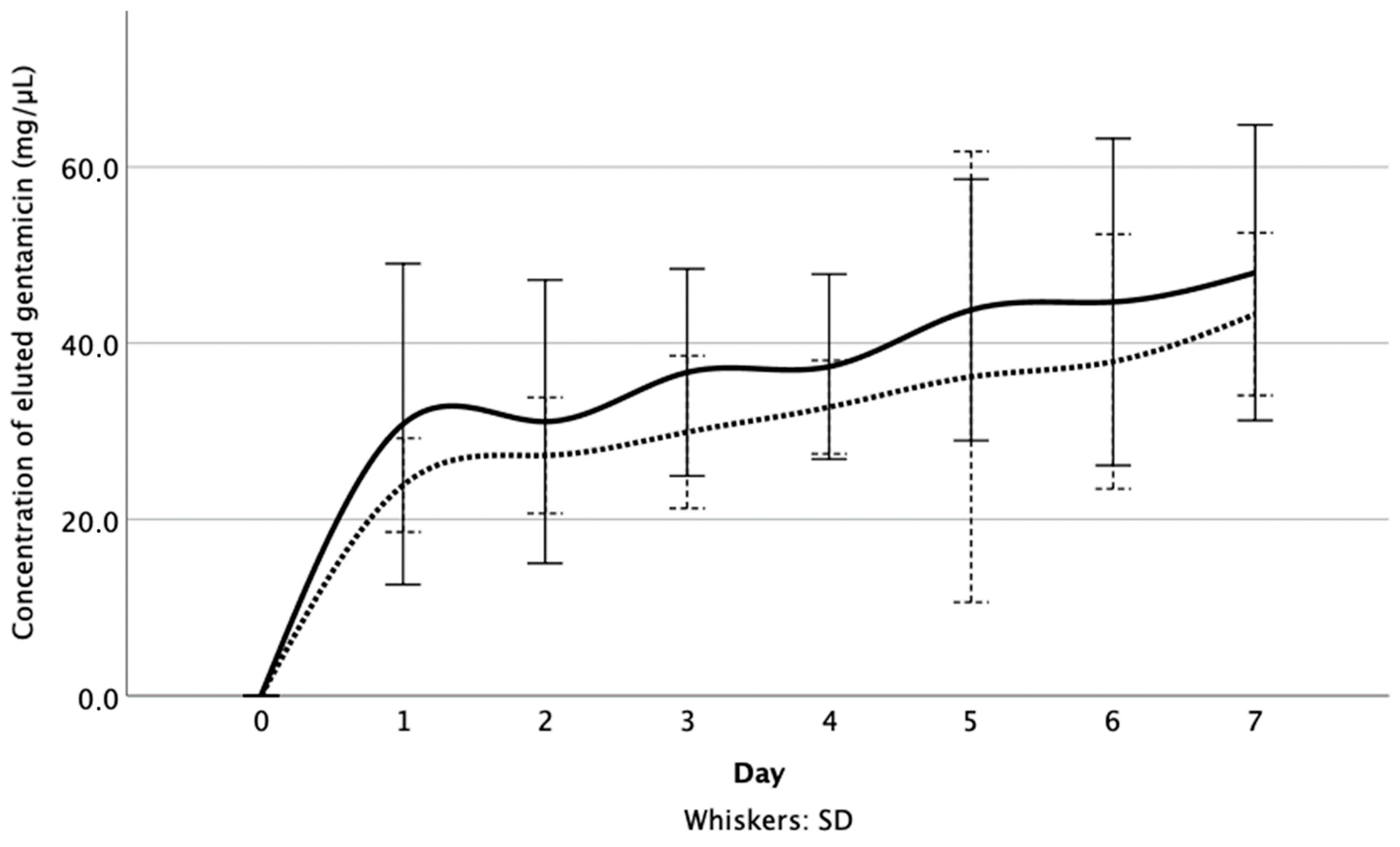
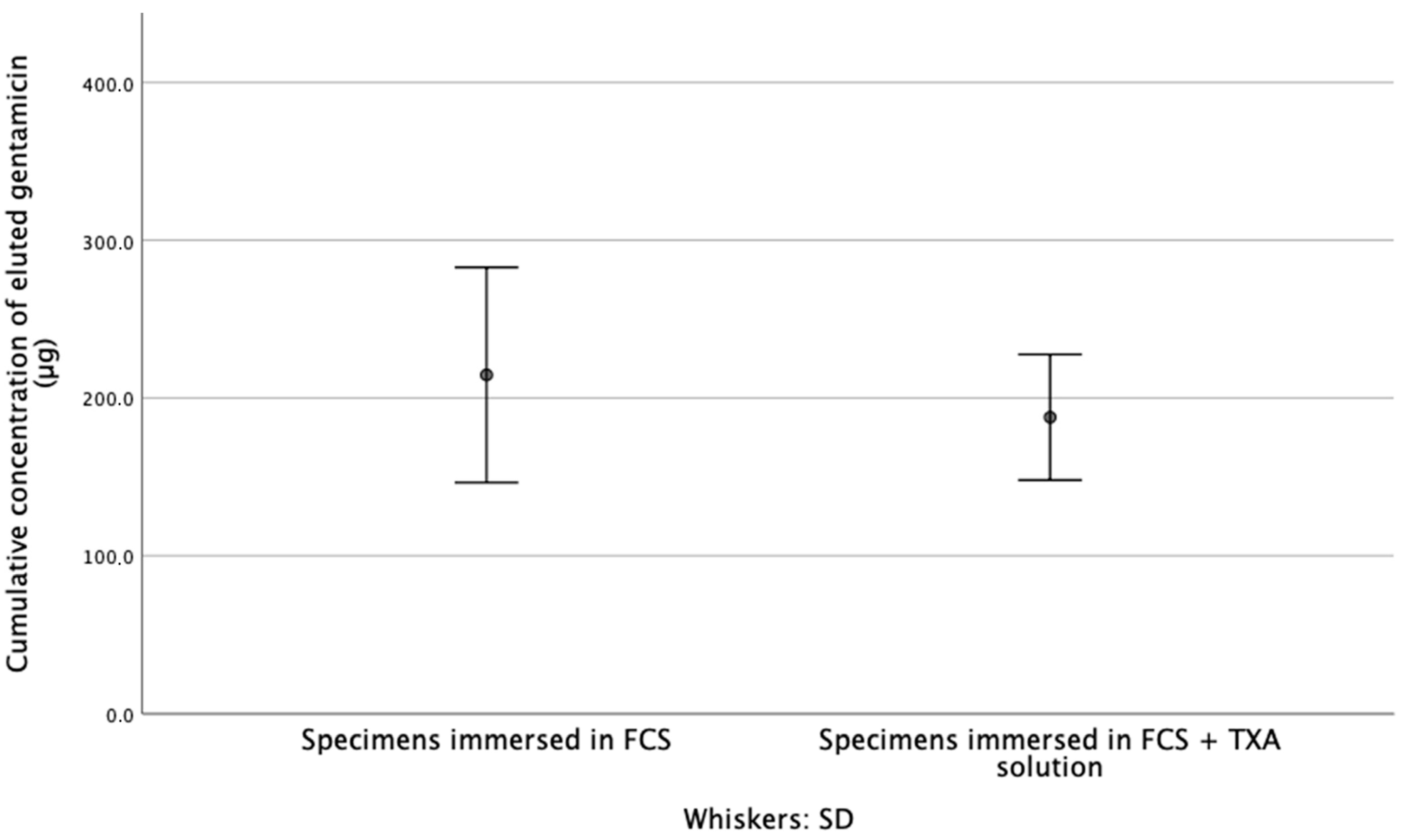
3.1. Released Gentamicin Concentration
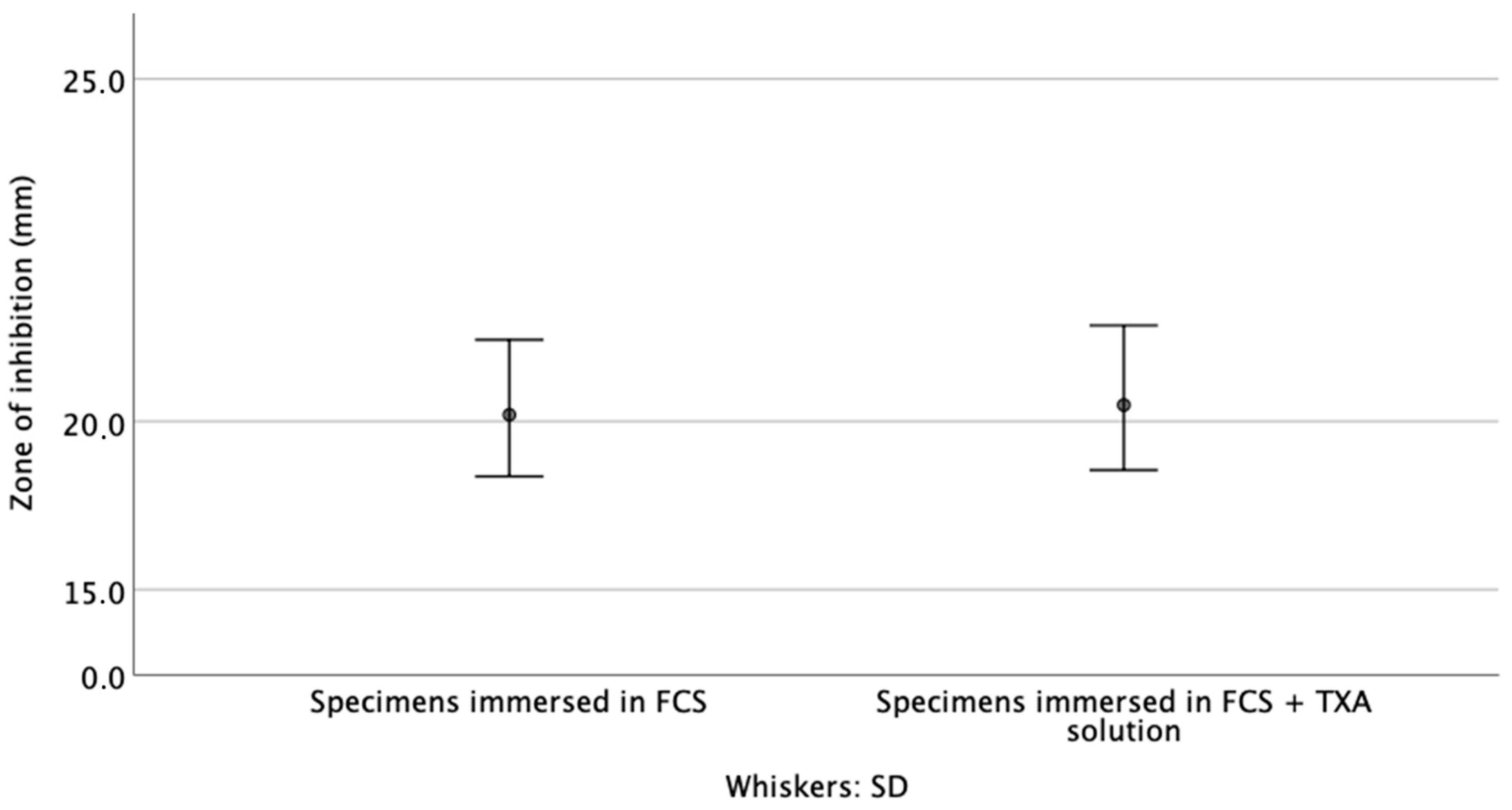
3.2. Zone of Inhibition
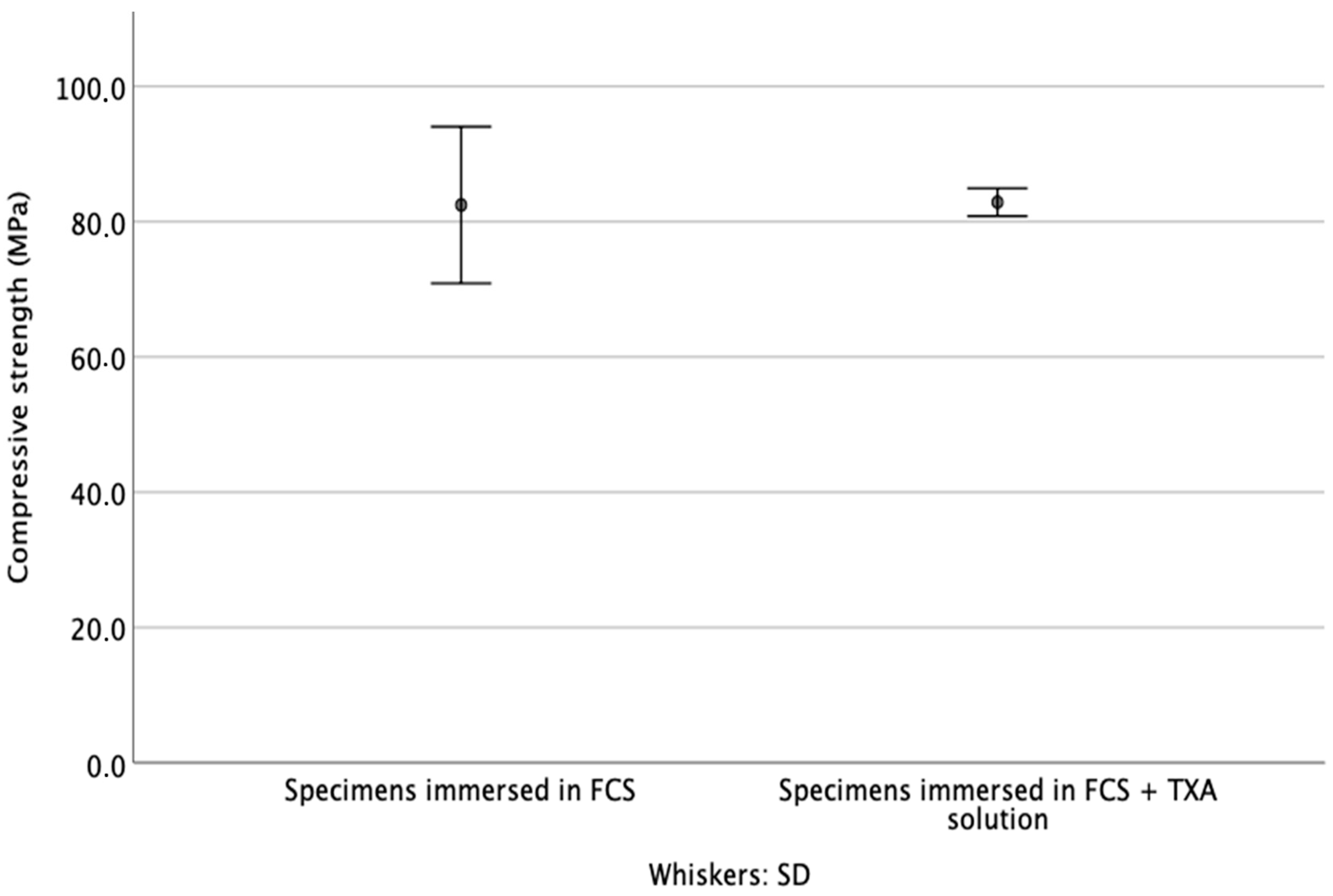
3.3. Compressive Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charnley, J. The Bonding Of Prostheses To Bone By Cement. J. Bone Joint Surg. Br. 1964, 46, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Parvizi, J.; Saleh, K.J.; Ragland, P.S.; Pour, A.E.; Mont, M.A. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008, 79, 335–341. [Google Scholar] [CrossRef]
- Kuhn, K.-D. PMMA Cements; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Hailer, N.P.; Garellick, G.; Kärrholm, J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop. 2010, 81, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Niemeläinen, M.J.; Mäkelä, K.; Robertsson, O.; W-Dahl, A.; Furnes, O.; Fenstad, A.M.; Pedersen, A.B.; Schrøder, H.M.; Reito, A.; Eskelinen, A. The effect of fixation type on the survivorship of contemporary total knee arthroplasty in patients younger than 65 years of age: A register-based study of 115,177 knees in the Nordic Arthroplasty Register Association (NARA) 2000–2016. Acta Orthop. 2020, 91, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klika, A.K.; Small, T.J.; Saleh, A.; Szubski, C.; Pillai, A.L.P.C.; Barsoum, W.K. Primary Total Knee Arthroplasty Allogenic Transfusion Trends, Length of Stay, and Complications: Nationwide Inpatient Sample 2000–2009. J. Arthroplast. 2014, 29, 2070–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, A.; Small, T.; Pillai, A.L.P.C.; Schiltz, N.; Klika, A.K.; Barsoum, W.K. Allogenic Blood Transfusion Following Total Hip Arthroplasty: Results from the Nationwide Inpatient Sample, 2000 to 2009. J. Bone Jt. Surg. Am. Vol. 2014, 96, e155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.G.; Cassatt, K.B.; Kincaid-Cinnamon, K.A.; Westendorf, D.S.; Garton, A.S.; Lemke, J.H. Topical Administration of Tranexamic Acid in Primary Total Hip and Total Knee Arthroplasty. J. Arthroplast. 2014, 29, 889–894. [Google Scholar] [CrossRef]
- Johansson, T.; Pettersson, L.-G.; Lisander, B. Tranexamic acid in total hip arthroplasty saves blood and money: A randomized, double-blind study in 100 patients. Acta Orthop. 2005, 76, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Boelch, S.P.; Jordan, M.C.; Arnholdt, J.; Rudert, M.; Luedemann, M.; Steinert, A.F. Loading with vancomycin does not decrease gentamicin elution in gentamicin premixed bone cement. J. Mater. Sci. Mater. Med. 2017, 28, 104. [Google Scholar] [CrossRef]
- Standardization IOf. ISO 5833 Implants for Surgery—Acrylic Resins Cements; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Microgenics Corporation. CEDIA® Gentamicin II Assay; Microgenics Corporation: Fremont, CA, USA, 2018. [Google Scholar]
- Vorndran, E.; Geffers, M.; Ewald, A.; Lemm, M.; Nies, B.; Gbureck, U. Ready-to-use injectable calcium phosphate bone cement paste as drug carrier. Acta Biomater. 2013, 9, 9558–9567. [Google Scholar] [CrossRef]
- Liu, T.; Hua, X.; Yu, W.; Lin, J.; Zhao, M.; Liu, J.; Zeng, X. Long-term follow-up outcomes for patients undergoing primary total hip arthroplasty with uncemented versus cemented femoral components: A retrospective observational study with a 5-year minimum follow-up. J. Orthop. Surg. Res. 2019, 14, 371–376. [Google Scholar] [CrossRef]
- Morales Santias, M.; Mas Martinez, J.; Sanz-Reig, J.; Martinez Gimenez, E.; Verdu Roman, C.; Bustamante Suarez de Puga, D. Topical tranexamic acid in cemented primary total knee arthroplasty without tourniquet: A prospective randomized study. Eur. J. Orthop Surg Traumatol. 2020, 30, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Jaeblon, T. Polymethylmethacrylate: Properties and Contemporary Uses in Orthopaedics. J. Am. Acad. Orthop. Surg. 2010, 18, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.B.; Gie, G.A.; Lee, A.J.; Ling, R.S.; Volz, R.G. Cementing technique and the effects of bleeding. J. Bone Jt. Surg. 1987, 69, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Li, H.; Zhao, H.; Wang, J.; Liu, S.; Song, Y.; Wu, H.-F. Combined use of intravenous and topical versus intravenous tranexamic acid in primary total knee and hip arthroplasty: A meta-analysis of randomised controlled trials. J. Orthop. Surg. Res. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petsatodis, G.; Parziali, M.; Christodoulou, A.G.; Hatzokos, I.; Chalidis, B.E. Prognostic value of suction drain tip culture in determining joint infection in primary and non-infected revision total hip arthroplasty: A prospective comparative study and review of the literature. Arch. Orthop. Trauma Surg. 2009, 129, 1645–1649. [Google Scholar] [CrossRef]
- Fillingham, Y.A.; Ramkumar, D.B.; Jevsevar, D.S.; Yates, A.J.; Bini, S.A.; Clarke, H.D.; Schemitsch, E.; Johnson, R.L.; Memtsoudis, S.G.; Sayeed, S.A.; et al. Tranexamic Acid Use in Total Joint Arthroplasty: The Clinical Practice Guidelines Endorsed by the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J. Arthroplast. 2018, 33, 3065–3069. [Google Scholar] [CrossRef]
- Exela Pharma Sciences, Tranexamic Acid Package Insert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212020lbl.pdf (accessed on 9 May 2021).
- Kuehn, K.-D.; Ege, W.; Gopp, U. Acrylic bone cements: Composition and properties. Orthop. Clin. N. Am. 2005, 36, 17–28. [Google Scholar] [CrossRef]
- Nottrott, M.; Mølster, A.O.; Gjerdet, N.R. Time dependent mechanical properties of bone cement. Anin vitro study over one year. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2007, 83, 416–421. [Google Scholar] [CrossRef]
- Lidgren, L.; Bodelind, B.; Moller, J. Bone cement improved by vacuum mixing and chilling. Acta Orthop. Scand. 1987, 58, 27–32. [Google Scholar] [CrossRef]
- Lee, A.J.C.; Ling, R.S.M.; Vangala, S.S. Some clinically relevant variables affecting the mechanical behaviour of bone cement. Arch. Orthop. Trauma Surg. 1978, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kjellson, F.; Brudeli, B.; McCarthy, I.D.; Lidgren, L. Water uptake and release from iodine-containing bone cement. J. Biomed. Mater. Res. 2004, 71, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Brock, H.S.; Moodie, P.G.; Hendricks, K.J.; McIff, T.E. Compression strength and porosity of single-antibiotic cement vacuum-mixed with vancomycin. J. Arthroplast. 2010, 25, 990–997. [Google Scholar] [CrossRef]
- Meyer, J.P.G.; Spiegel, C.A.; Hetzel, S.; Squire, M. Vacuum-Mixing Significantly Changes Antibiotic Elution Characteristics of Commercially Available Antibiotic-Impregnated Bone Cements. J. Bone Joint Surg. Am. 2011, 93, 2049–2056. [Google Scholar] [CrossRef]
- Lu, F.; Sun, X.; Wang, W.; Zhang, Q.; Guo, W. What is the ideal route of administration of tranexamic acid in total knee arthroplasty? A meta-analysis based on randomized controlled trials. Ann. Palliat. Med. 2021, 10, 1880–1894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Dong, W.; Wang, F.; Yu, J.; Jiang, F.; Tang, J.; Qian, Y.; Shen, H. The Topical Tranexamic Acid Have Potential Hazard of Promoting Biofilm Formation of Staphylococcus aureus in Microenvironment of the Prosthetic Joint. BioMed Res. Int. 2021, 2021, 5748069. [Google Scholar] [CrossRef]
- Wagenbrenner, M.; Heinz, T.; Horas, K.; Jakuscheit, A.; Arnholdt, J.; Mayer-Wagner, S.; Rudert, M.; Holzapfel, B.M.; Weißenberger, M. Impact of Tranexamic Acid on Chondrocytes and Osteogenically Differentiated Human Mesenchymal Stromal Cells (hMSCs) In Vitro. J. Clin. Med. 2020, 9, 3880. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lüdemann, M.; Jakuscheit, A.; Ewald, A.; Frühmann, L.; Hölscher-Doht, S.; Rudert, M.; von Hertzberg-Boelch, S.P. Influence of Tranexamic Acid on Elution Characteristics and Compressive Strength of Antibiotic-Loaded PMMA-Bone Cement with Gentamicin. Materials 2021, 14, 5639. https://doi.org/10.3390/ma14195639
Lüdemann M, Jakuscheit A, Ewald A, Frühmann L, Hölscher-Doht S, Rudert M, von Hertzberg-Boelch SP. Influence of Tranexamic Acid on Elution Characteristics and Compressive Strength of Antibiotic-Loaded PMMA-Bone Cement with Gentamicin. Materials. 2021; 14(19):5639. https://doi.org/10.3390/ma14195639
Chicago/Turabian StyleLüdemann, Martin, Axel Jakuscheit, Andrea Ewald, Leena Frühmann, Stefanie Hölscher-Doht, Maximilian Rudert, and Sebastian Philipp von Hertzberg-Boelch. 2021. "Influence of Tranexamic Acid on Elution Characteristics and Compressive Strength of Antibiotic-Loaded PMMA-Bone Cement with Gentamicin" Materials 14, no. 19: 5639. https://doi.org/10.3390/ma14195639
APA StyleLüdemann, M., Jakuscheit, A., Ewald, A., Frühmann, L., Hölscher-Doht, S., Rudert, M., & von Hertzberg-Boelch, S. P. (2021). Influence of Tranexamic Acid on Elution Characteristics and Compressive Strength of Antibiotic-Loaded PMMA-Bone Cement with Gentamicin. Materials, 14(19), 5639. https://doi.org/10.3390/ma14195639






