The Solidification/Stabilization of Wastewater (From a Landfill Leachate) in Specially Designed Binders Based on Coal Ash
Abstract
:1. Introduction
2. Materials and Methods
- Two types of commercially available cements, INERCEM A and INERCEM E, products specially designed to be used for waste/contaminated soils treatment (Holcim Romania SA, Campulung, Romania).
- Fly ash and bottom ash from a Romanian thermal power plant that uses solid fuel, mainly coal, using a controlled combustion at 800 °C for electrical energy production.
- Leachate formed in a solid municipal waste landfill that was treated by a reverse osmosis process. The resulted wastewater was used as the liquid component in the studied materials.
- Natural quartz sand (Societe Nouvelle du Litoral, Leucate, France) used as the aggregate; the sand fulfils the requirements of European norm EN 196-1 [21].
- Shimadzu XRD 6000 (Shimadzu, Kyoto, Japan), (λ = 1.5406 Å).
- Cubix (Malvern PANalytical, Almelo, The Netherlands) (λ = 1.5418 Å, with Rietveld refinement for assessment of the mineralogical composition).
3. Results and Discussion
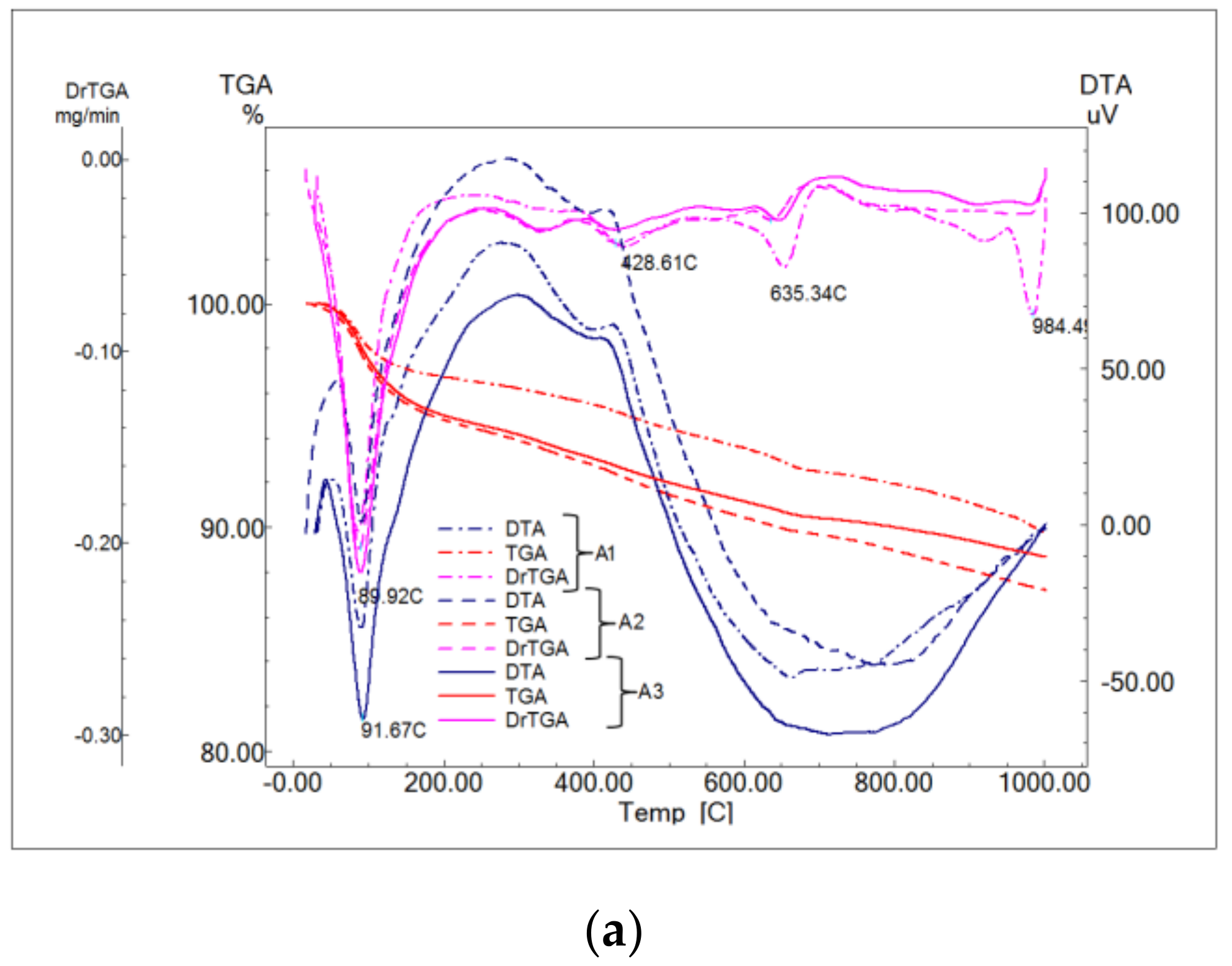
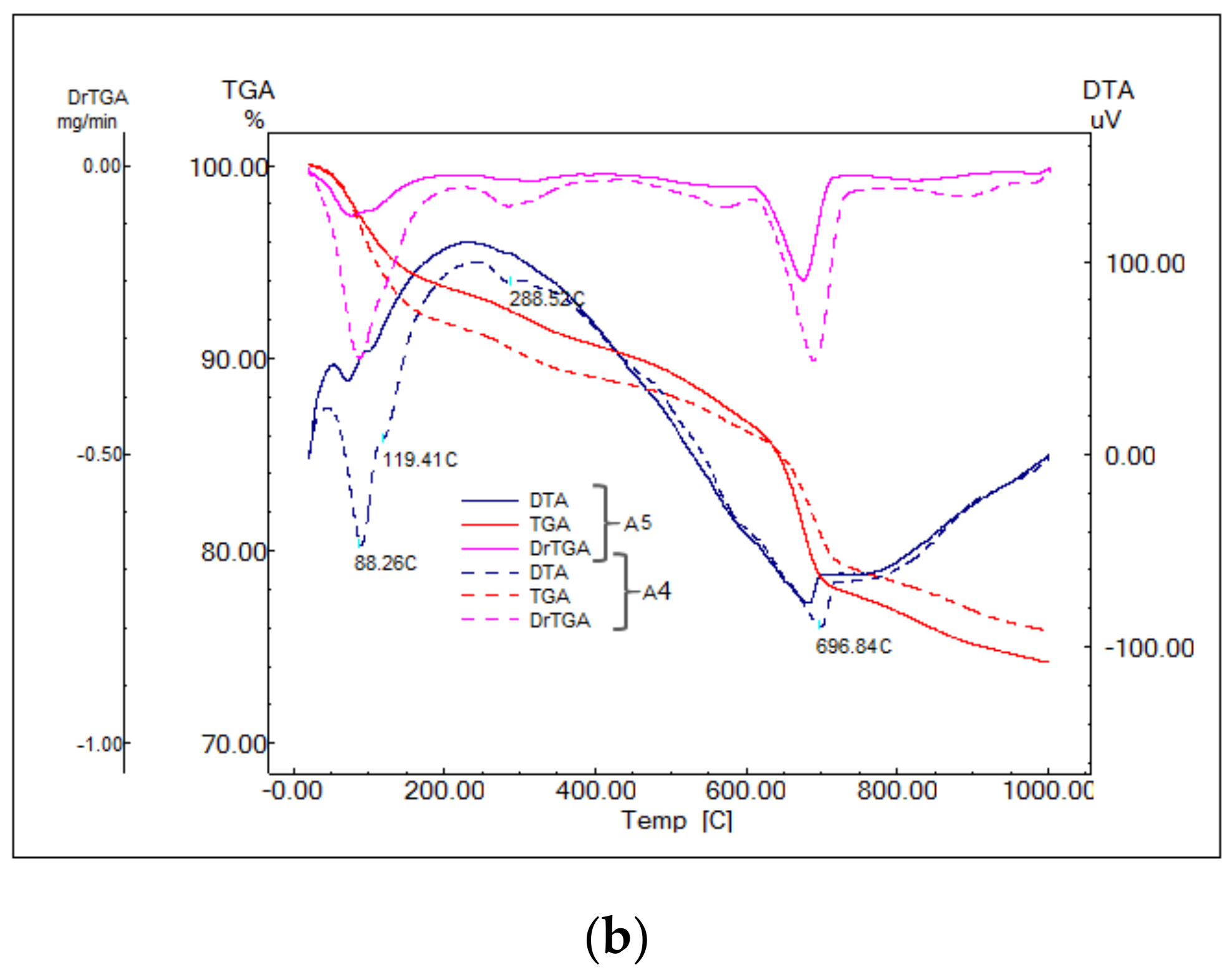
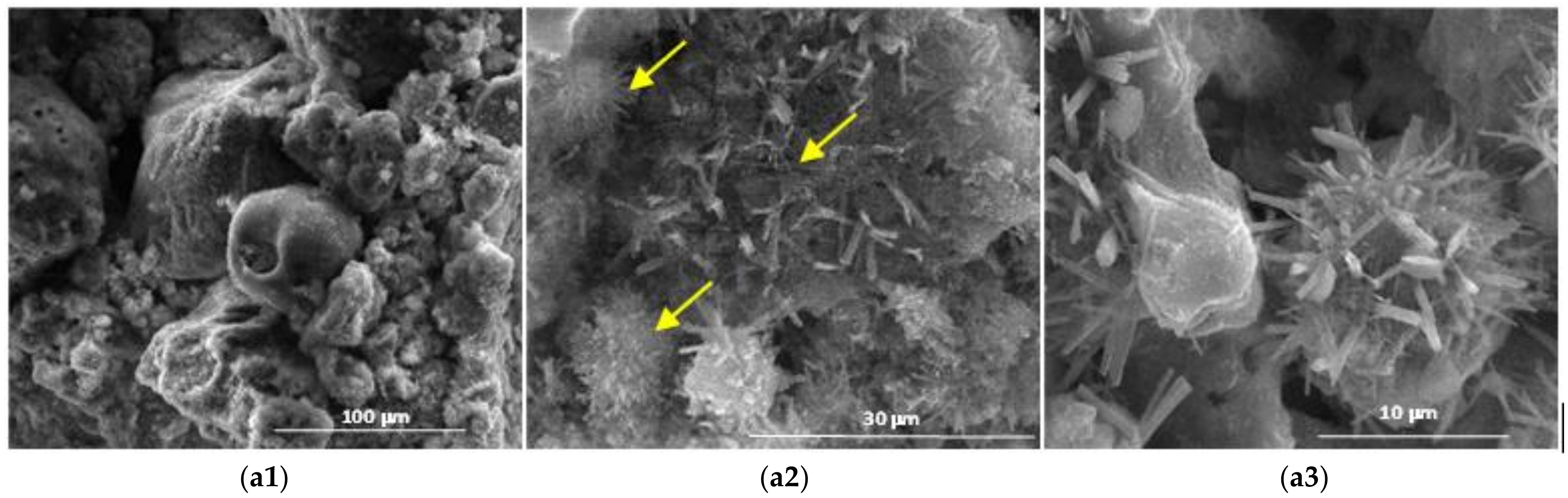
- Endo-effect between 88 and 92 °C determined by the dehydration of ettringite with different degrees of crystallinity;
- Effects at approx. 119, 188 and 288 °C due to the decomposition of calcium silicate hydrates with a low degree of crystallinity;
- Decomposition of calcium hydroxide effects at approx. 428 and 555 °C;
- Effects at approx. 635 and 697 °C from decomposition of calcium carbonate with a low degree of crystallinity;
- Effect at approx. 984 °C of decomposition of calcium carbonate with a higher degree of crystallinity.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LAW No. 211 of 15 November 2011 on the Waste Regime, Published in the Official Gazette no. 837 of 25 November 2011, Bucharest, Romania. Available online: http://www.mmediu.ro/beta/wp-content/uploads/2012/05/2012-05-17_LEGE_211_2011.pdf (accessed on 30 July 2021).
- DECISION No. 349 of 21 April 2005 on Waste Storage, Published in the Official Gazette, Part I No. 394 of 10 May 2005, in Force since 9 June 2005, Bucharest, Romania. Available online: http://legislatie.just.ro/Public/DetaliiDocumentAfis/61498 (accessed on 30 July 2021).
- Available online: https://www.eea.europa.eu/help/glossary/gemet-environmental-thesaurus/landfill-leachate (accessed on 3 August 2021).
- ORDER No. 757 of 26 November 2004 for the Approval of the Technical Norm on Waste Storage, Issued by the Ministry of Environment and Waste Management, Published in the Official Gazette, No. 86 of 26 January 2005, Bucharest, Romania. Available online: http://legislatie.just.ro/Public/DetaliiDocument/58862 (accessed on 30 July 2021).
- Wilk, C.M. Solidification/Stabilization Treatment and Examples of Use at Port Facilities. In Proceedings of the Ports 2004 Conference, Houston, TX, USA, 23–26 May 2004; Curtis, S.A., Ed.; American Society of Civil Engineers: Reston, VA, USA, 2004. [Google Scholar]
- Li, X.D.; Poon, C.S.; Sun, H.; Lo, I.M.C.; Kirk, D.W. Heavy Metal Specifications and Leaching Behaviors in Cement Based Solidified / Stabilized Waste Materials. J. Hazard. Mater. 2001, 82, 215–230. [Google Scholar] [CrossRef]
- Georgescu, M.; Andronescu, C.; Zahanagiu, A. Immobilization of harmful substances in binder matrices. Part I. Immobilization capacity. Influence on binding properties. Rev. Rom. Mater. 2006, 3, 189–197. [Google Scholar]
- Badanoiu, A.; Georgescu, M.; Zahanagiu, A. Properties of blended cements with hazardous waste content. Rev. Roum. Chim. 2008, 53, 229–237. [Google Scholar]
- Zubiate, M.L.; Blasco, I.N.; Fernández, J.M.; Álvarez, J.I. Encapsulation, solid-phases identification and leaching of toxic metals in cement systems modified by natural biodegradable polymers. J. Hazard. Mater. 2012, 7–17, 233–234. [Google Scholar]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics: Twenty-First Century Materials with Diverse Application, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–422. [Google Scholar]
- Vijan, A.C.; Badanoiu, A.; Voicu, G.; Nicoara, A.I. Phosphate cements based on calcined dolomite: Influence of calcination temperature and silica addition. Materials 2021, 14, 3838. [Google Scholar] [CrossRef]
- Singh, N.; Shehnazdeep, A.B. Reviewing the role of coal bottom ash as an alternative of cement. Constr. Build. Mater. 2020, 233, 117276. [Google Scholar] [CrossRef]
- Gadore, V.; Ahmaruzzaman, M. Tailored fly ash materials: A recent progress of their properties and applications for remediation of organic and inorganic contaminants from water. J. Water Process Eng. 2021, 41, 101910. [Google Scholar] [CrossRef]
- Puri, A.; Badanoiu, A.; Voicu, G. Eco-friendly binders based on fly ash. Rev. Roum. Chim. 2008, 53, 1069–1076. [Google Scholar]
- Badanoiu, A.; Voicu, G. Influence of raw materials characteristics and processing parameters on the strength of geopolymer cements based on fly ash. Environ. Eng. Manag. J. 2011, 10, 673–681. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible applications of coal fly ash in wastewater treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Srivastava, S.; Ansari, M.A.; Mehta, P.K.; Srivastava, V. Fly ash as partial re-placement of Portland pozzolana cement concrete: An experimental investigation. Int. J. Civ. Eng. Technol. 2016, 7, 207–214. [Google Scholar]
- Ramjan, S.; Tangchirapat, W.; Jaturapitakkul, C.; Chee Ban, C.; Jitsangiam, P.; Suwan, T. Influence of cement replacement with fly ash and ground sand with different fineness on alkali-silica reaction of mortar. Materials 2021, 14, 1528. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Yadav, K.K.; Tirth, V.; Jangid, A.; Gnanamoorthy, G.; Choudhary, N.; Islam, S.; Gupta, N.; Son, C.T.; Jeon, B.-H. Recent advances in methods for recovery of cenospheres from fly ash and their emerging applications in ceramics, composites, polymers and environmental cleanup. Crystals 2021, 11, 1067. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E. Zero-waste approach in municipal solid waste incineration: Reuse of bottom ash to stabilize fly ash. J. Clean. Prod. 2020, 245, 118779. [Google Scholar] [CrossRef]
- SR EN 196-1: 2006. Test. Methods for Cements. Part. 1: Determination of Mechanical Strength; ASRO: Bucharest, Romania, 2006. [Google Scholar]
- SR EN 196-6: 2010. Test. Methods for Cements. Part. 6: Determination of Fineness; ASRO: Bucharest, Romania, 2010. [Google Scholar]
- SR EN 12457-2:2002. Characterization of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludge’s; ASRO: Bucharest, Romania, 2002. [Google Scholar]
- ORDER No. 95 of 12 February 2005 on the Establishment of Acceptance Criteria and Preliminary Procedures for the Acceptance of Waste for Disposal and the National List of Accepted Wastes in Each Landfill, Bucharest, Romania. 2005. Available online: http://legislatie.just.ro/Public/DetaliiDocumentAfis/59751 (accessed on 3 August 2021).
- NTPA-001/2002, Norm Regarding the Establishment of the Loading Limits with Pollutants of the Industrial and Urban Wastewater at the Discharge in the Natural Receptors. 2002. Available online: http://legislatie.just.ro/Public/DetaliiDocumentAfis/98311 (accessed on 3 August 2021).
- Muttamara, S. Wastewater characteristics. Resour. Conserv. Recycl. 1996, 16, 145–159. [Google Scholar] [CrossRef]
- Amenu, D. Characterization of wastewater and evaluation of the effectiveness of wastewater treatment systems. J. Life Sci. Res. 2014, 1, 1–11. [Google Scholar]
- Sonune, A.; Ghate, R. Development in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Liu, D.H.F.; Lipták, B.G. Wastewater Treatment; Taylor & Francis Group: London, UK; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (U.S. EPA). Guide to Disposal of Chemically Stabilized and Solidified Wastes. U.S.EPA SW872; September 1982. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=2000KAE0.PDF (accessed on 3 August 2021).
- United States Environmental Protection Agency (U.S. EPA). Stabilization/Solidification of CERCLA and RCRA Wastes—Physical Tests, Chemical Testing Procedures, Technology Screening, and Field Activities. EPA/625/6-89/022; May 1989. Available online: https://semspub.epa.gov/work/05/91288.pdf (accessed on 3 August 2021).
- United States Environmental Protection Agency (U.S. EPA). Stabilization/Solidification Processes for Mixed Waste. EPA 402-R-96-014; June 1996. Available online: https://www.epa.gov/sites/default/files/2015-05/documents/402-r-96-014.pdf (accessed on 3 August 2021).
- Pereria, C.F.; Pinero, M.R.; Vale, J. Solidification/stabilization of electric arc furnace dust using coal fly ash analysis of the stabilization process. J. Hazard. Mater. B 2001, 82, 183–195. [Google Scholar] [CrossRef]
- Malviya, R.; Chaudhary, R. Factors affecting hazardous waste solidification/stabilization: A review. J. Hazard. Mater. B 2006, 137, 267–276. [Google Scholar] [CrossRef]
- Badanoiu, A.; Paceagiu, J.; Voicu, G. Hydration and hardening processes of Portland cements obtained from clinkers mineralized with fluoride and oxides. J. Therm. Anal. Calorim. 2011, 103, 879–888. [Google Scholar] [CrossRef]
- Qiao, X.C.; Poon, C.S.; Cheeseman, C.R. Investigation into the Stabilization/Solidification Performance of Portland Cement through Cement Clinker Phases. J. Hazard. Mater. 2007, 139, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Voicu, G.; Georgescu, M.; Zahanagiu, A. Influence of zinc salts on the Portland cement properties with or without slag addition, Univ. Politeh. Buchar. Sci. Bull. Ser. B 2008, 70, 11–20. [Google Scholar]
- Żak, R.; Deja, J. Spectroscopy study of Zn, Cd, Pb and Cr ions immobilization on C–S–H phase, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 614–620. [Google Scholar] [CrossRef]
- Giergiczny, Z.; Król, A. Immobilization of heavy metals (Pb, Cu, Cr, Zn, Cd, Mn) in the mineral additions containing concrete composites. J. Hazard. Mater. 2008, 160, 247–255. [Google Scholar] [CrossRef]
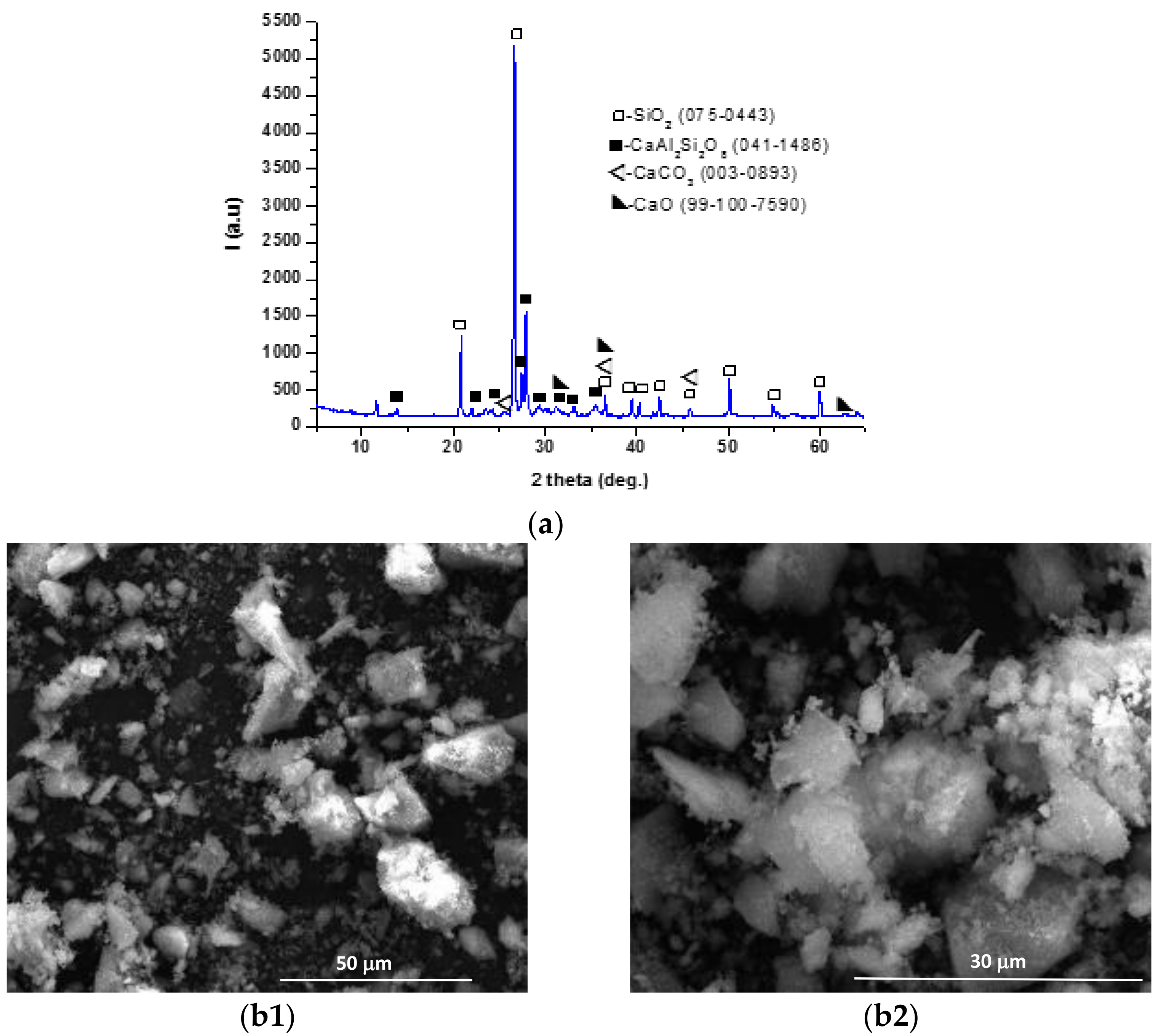


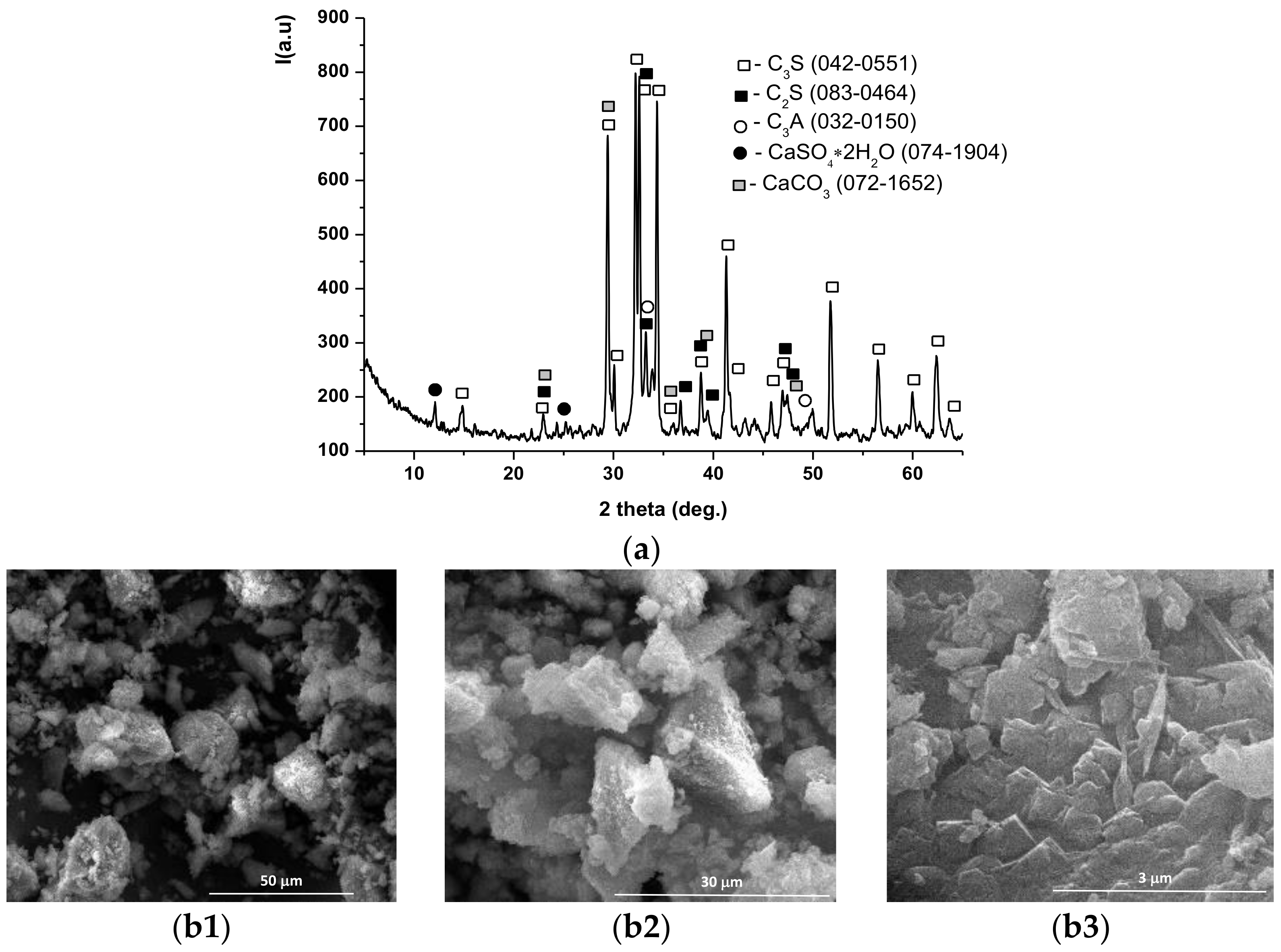
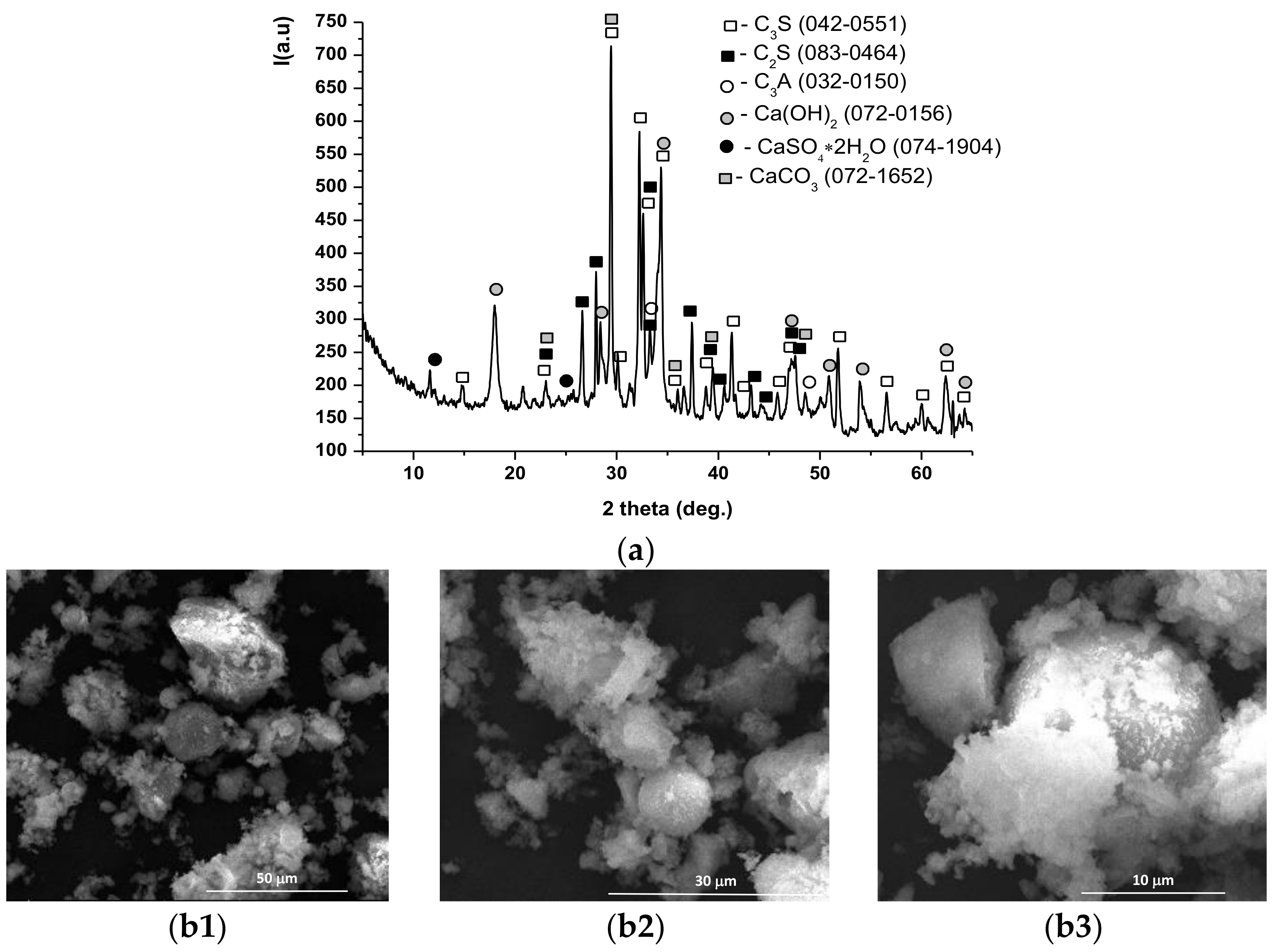
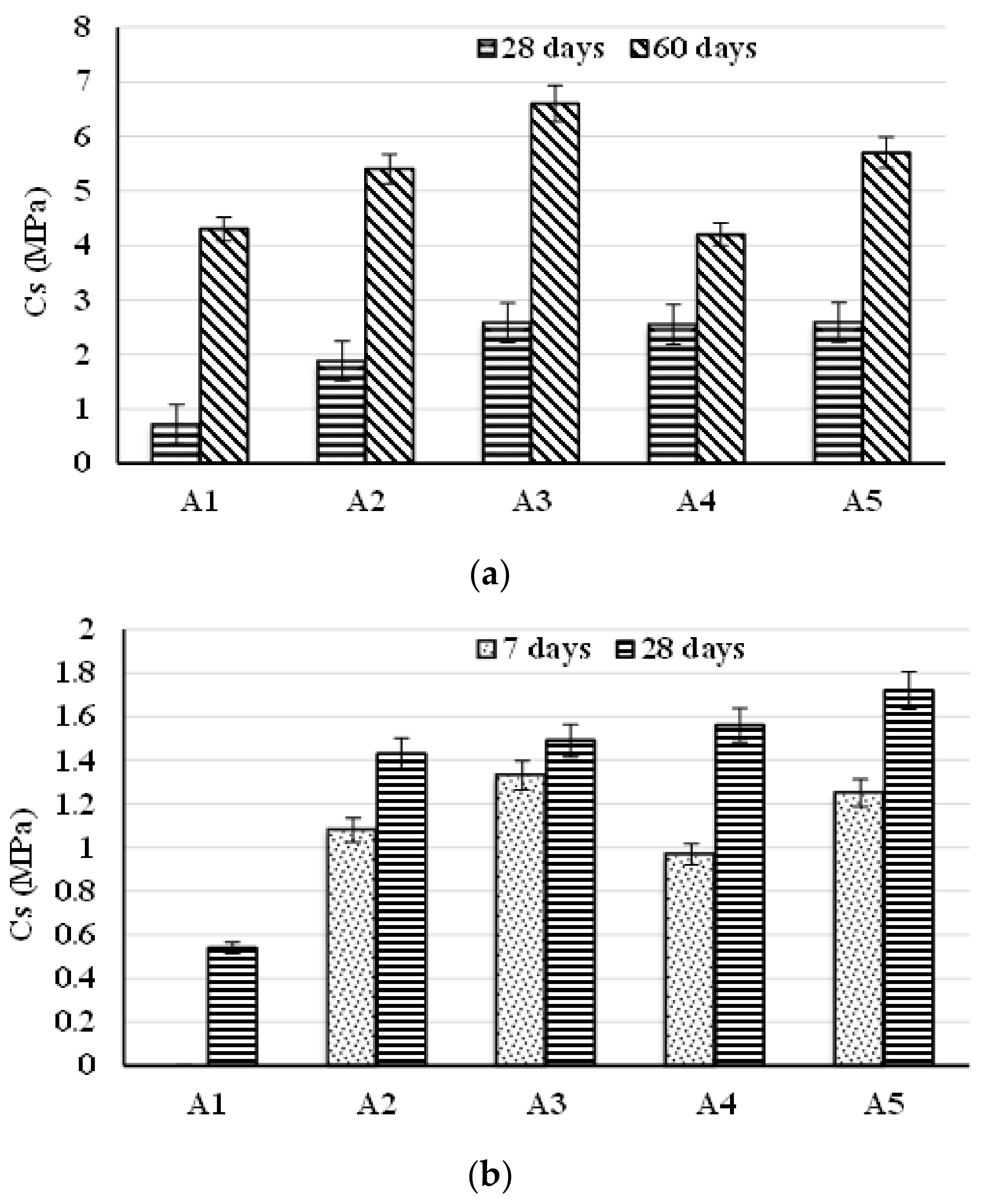
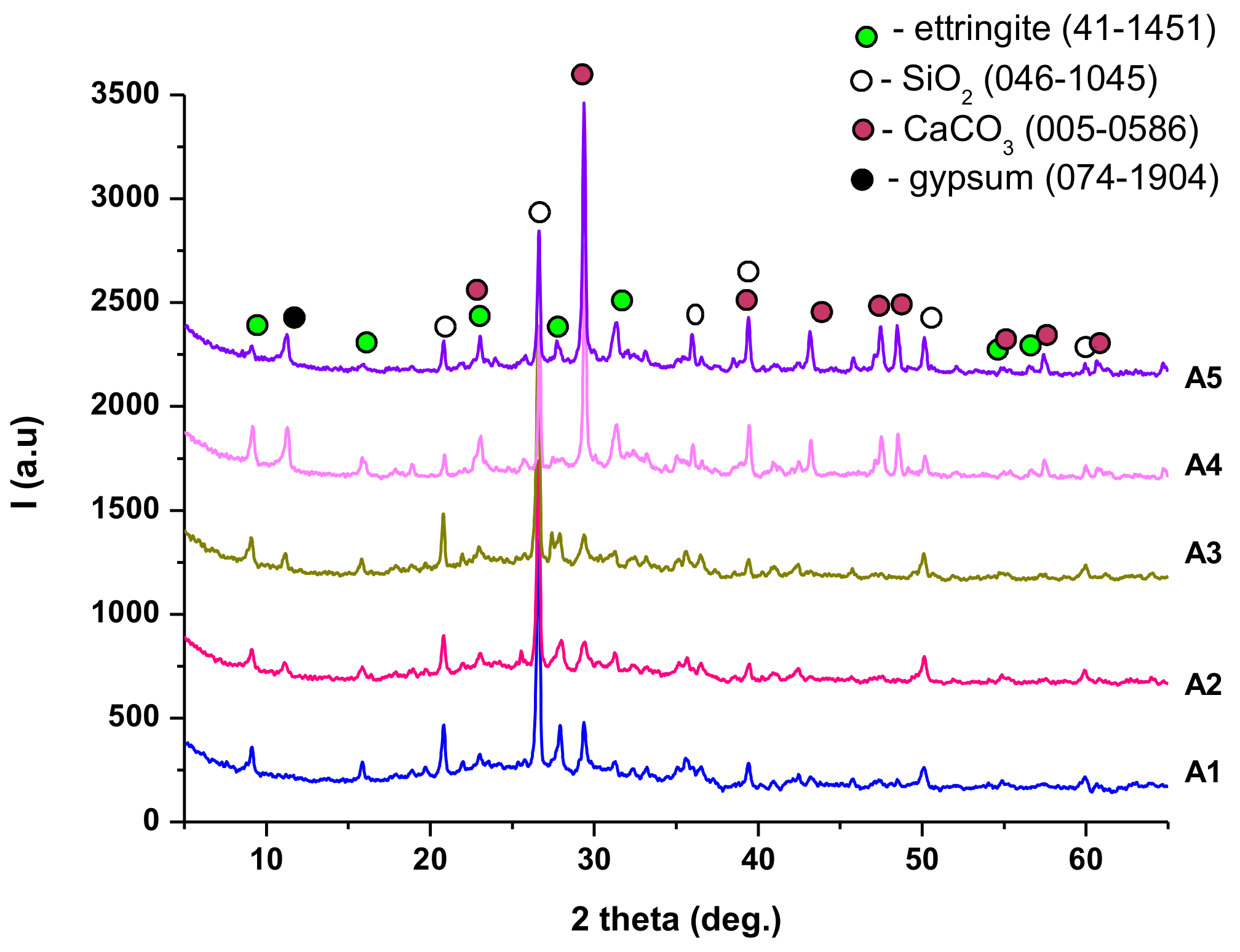

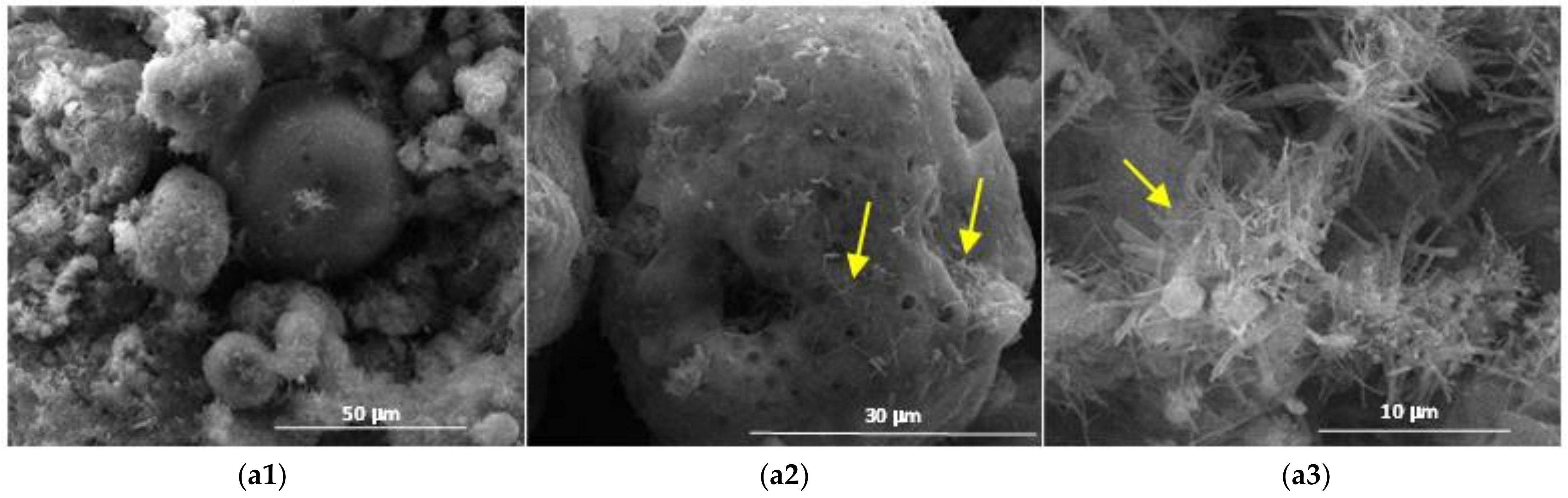


| Symbol Cementitious Systems | Fly Ash | Bottom Ash | INERCEM E | INERCEM A | Wastewater * |
|---|---|---|---|---|---|
| wt.% | |||||
| A1 | 51 | 0 | 4 | 0 | 45 |
| A2 | 47 | 0 | 6 | 2 | 45 |
| A3 | 47 | 0 | 4 | 4 | 45 |
| A4 | 0 | 52 | 6 | 2 | 40 |
| A5 | 0 | 47 | 4 | 4 | 45 |
| INERCEM | Oxide Composition (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | |
| A | 19.85 | 6.5 | 2.75 | 64.38 | 1.4 | 0.54 | 1.07 | 3.49 |
| E | 24.58 | 8.75 | 4.35 | 52.03 | 1.78 | 0.53 | 1.25 | 2.19 |
| INERCEM | Mineralogical Composition (wt.%) | |||||||
| C3S | C2S | Tricalcium aluminate | C4AF | Gypsum | Calcite | |||
| cC3A | oC3A | C3A | ||||||
| A | 67.25 | 8.14 | 4.25 | 3.46 | 8.97 | 1.47 | 0 | 2.97 |
| E | 33.8 | 5.96 | 3.05 | 1.53 | 4.58 | 5.08 | 1.16 | 7.54 |
| Material/Element | H2O | Cl | As | Cr | Cd | Ni | Cu | Zn | Ba | Hg | Mo | Pb | Sb | Se | V | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | mg/kg | |||||||||||||||
| Fly ash | 0.5 | 0 | 27 | 73 | 0 | 67 | 55 | 77 | 274 | 0 | 0 | 22 | 0 | 0 | 199 | 305 |
| Bottom ash | 5.0 | 0.1 | 0 | 126 | 0 | 105 | 35 | 1151 | 821 | 1 | 15 | 27 | 23 | 0 | 16 | 205 |
| Material/Element | H2O | Cl | As | Cr | Cd | Ni | Cu | Zn | Ba | Hg | Mo | Pb | Sb | Se | V | Mn |
| wt.% | mg/dm3 | |||||||||||||||
| Max. conc. allowed by specific legislation [25] | - | 0.5 | 0.1 | 1 | 0.2 | 0.5 | 0.1 | 0.5 | - | 0.05 | 0.1 | 0.2 | - | 0.1 | - | 1 |
| Wastewater | 97.4 | 0.3 | 0.0 | 4.1 | 0 | 2.3 | 2.7 | 4.7 | 12 | 0 | 0 | 0 | 0 | 0 | 2.1 | 4.2 |
| Material | Oxide composition (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L.O.I * | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | P2O5 | ZnO | |
| Bottom ash | 8.8 | 34.6 | 3.7 | 10.0 | 19.1 | 1.6 | 7.3 | 1.1 | 1.7 | 2.2 | 1.3 |
| Fly ash | 3.4 | 52.9 | 22.1 | 8.5 | 7.4 | 2.5 | 0.5 | 1.5 | 0.7 | 0.18 | 0.02 |
| Element | As | Ba | Cd | Cr | Hg | Mo | Sb | Se | Zn | Cu | Ni | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maxim. allowed (mg/kg) [24] | 25 | 300 | 5 | 70 | 2 | 30 | 5 | 7 | 200 | 100 | 40 | 50 |
| A1 | 0 | 5.4 | 0 | 1.15 | 0.2 | 0.25 | 1.35 | 1.2 | 0 | 2.1 | 0 | 0.2 |
| A2 | 0 | 5.5 | 0.1 | 0.95 | 0.15 | 0.1 | 1.7 | 1.2 | 0.5 | 2.1 | 0 | 0.1 |
| A3 | 0 | 5.9 | 0.15 | 1.3 | 0.2 | 0.2 | 1.6 | 1.2 | 1.25 | 2.1 | 0 | 0.2 |
| A4 | 0 | 5.85 | 0 | 1.55 | 0.2 | 0.2 | 1.65 | 1.3 | 0.5 | 1.35 | 0 | 0.3 |
| A5 | 0 | 6.25 | 0.2 | 1.5 | 0.2 | 0.25 | 1.45 | 1.3 | 0.65 | 1.3 | 0 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oproiu, C.-L.; Voicu, G.; Bădănoiu, A.; Nicoară, A.-I. The Solidification/Stabilization of Wastewater (From a Landfill Leachate) in Specially Designed Binders Based on Coal Ash. Materials 2021, 14, 5610. https://doi.org/10.3390/ma14195610
Oproiu C-L, Voicu G, Bădănoiu A, Nicoară A-I. The Solidification/Stabilization of Wastewater (From a Landfill Leachate) in Specially Designed Binders Based on Coal Ash. Materials. 2021; 14(19):5610. https://doi.org/10.3390/ma14195610
Chicago/Turabian StyleOproiu, Carmen-Lidia, Georgeta Voicu, Alina Bădănoiu, and Adrian-Ionuţ Nicoară. 2021. "The Solidification/Stabilization of Wastewater (From a Landfill Leachate) in Specially Designed Binders Based on Coal Ash" Materials 14, no. 19: 5610. https://doi.org/10.3390/ma14195610
APA StyleOproiu, C.-L., Voicu, G., Bădănoiu, A., & Nicoară, A.-I. (2021). The Solidification/Stabilization of Wastewater (From a Landfill Leachate) in Specially Designed Binders Based on Coal Ash. Materials, 14(19), 5610. https://doi.org/10.3390/ma14195610







