Hydrothermal Co-Processing of Coal Fly Ash Cenospheres and Soluble Sr(II) as Environmentally Sustainable Approach to Sr-90 Immobilization in a Mineral-like Form
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Hydrothermal Procedures
2.2.1. Hydrothermal Crystallization
2.2.2. Hydrothermal Sorption
2.3. Characterization Techniques
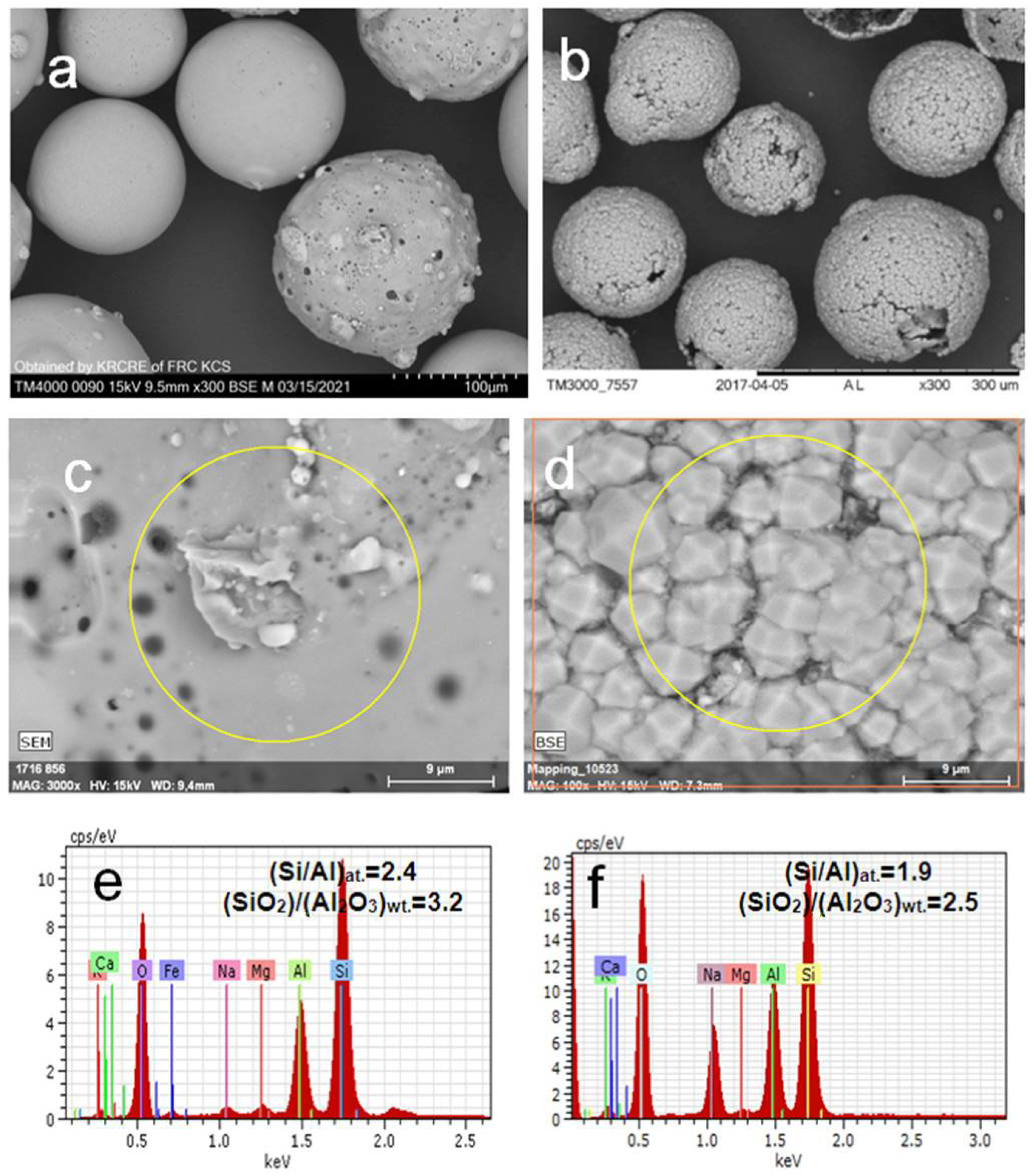
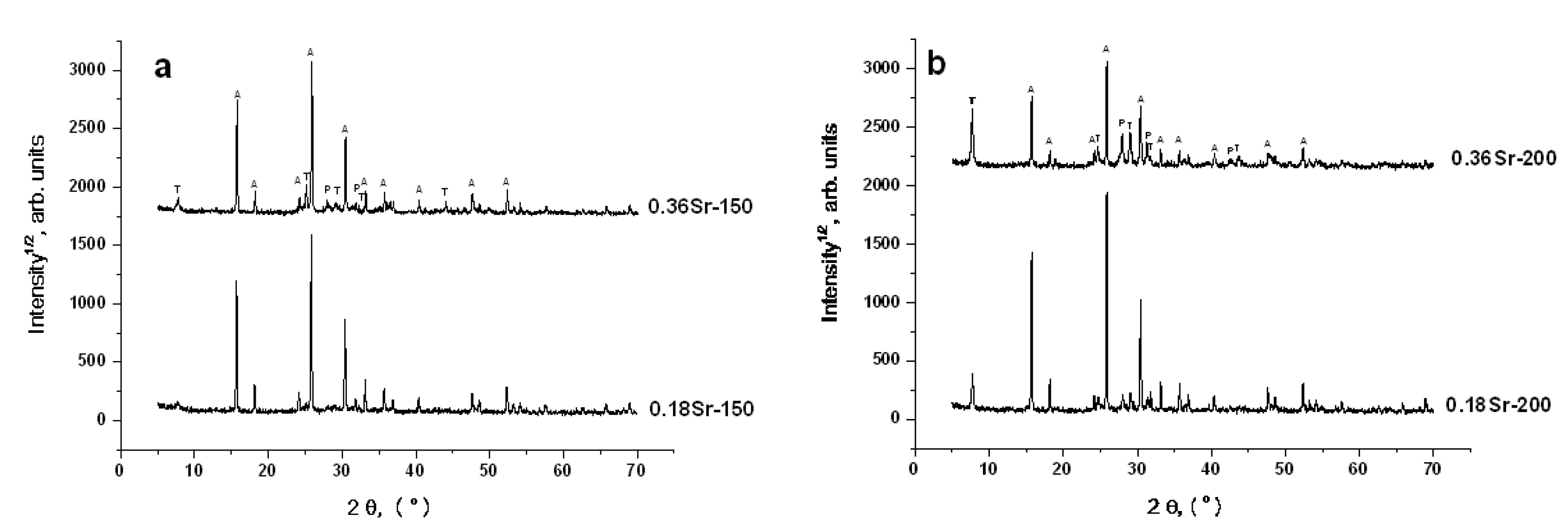
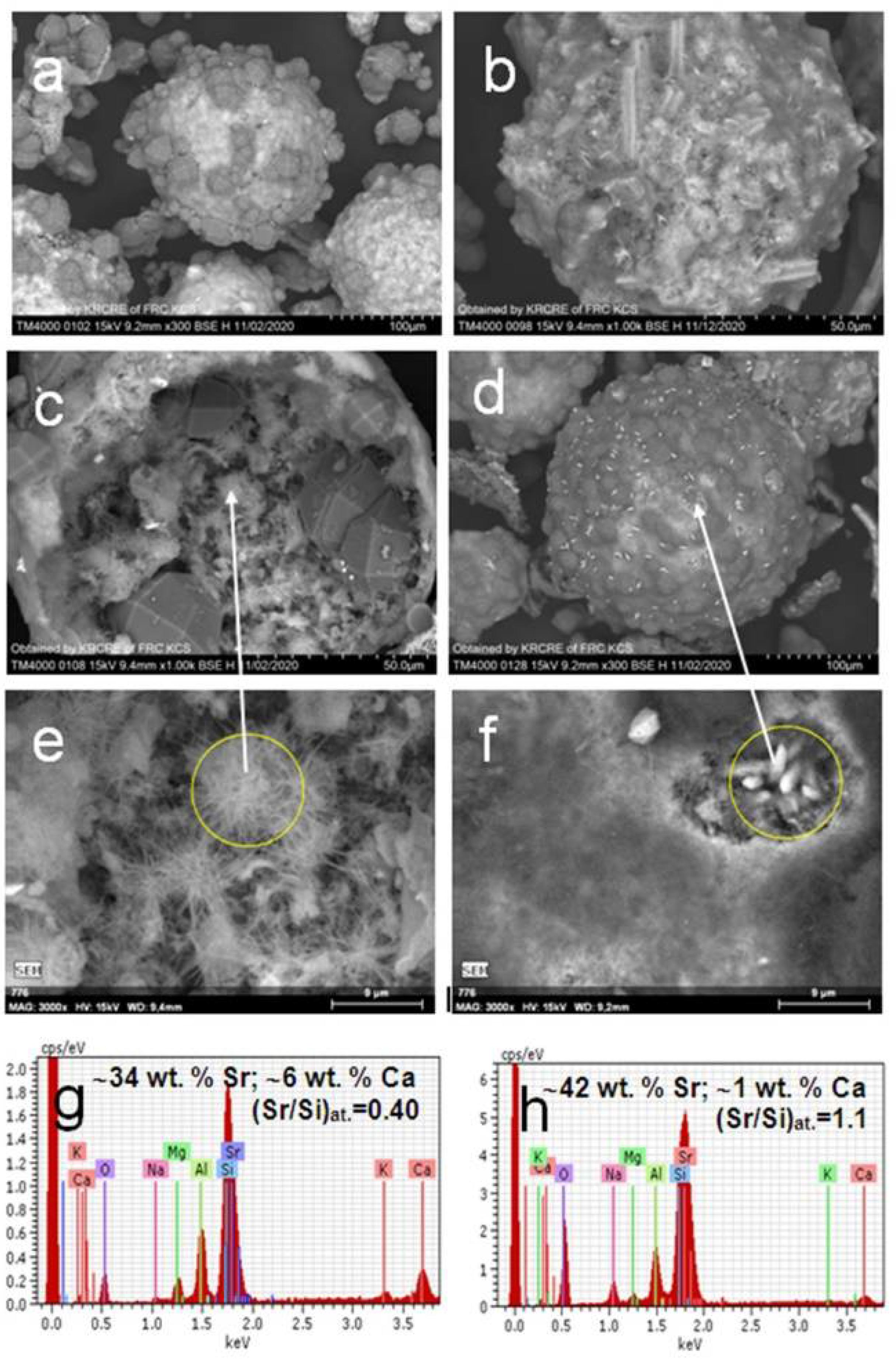
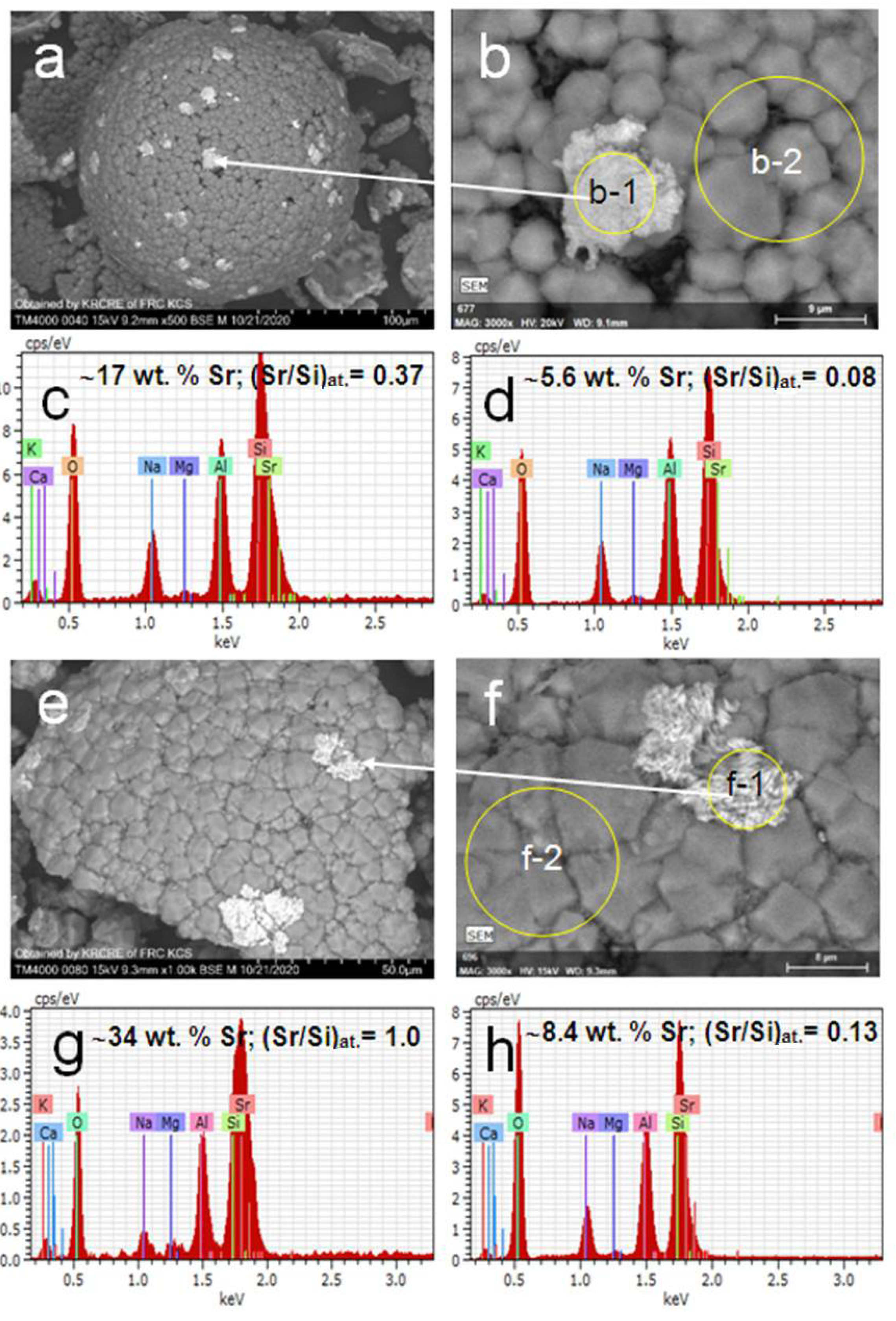
3. Results and Discussion
3.1. Hydrothermal Crystallization
3.2. Hydrothermal Sorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ye, J.; Zubair, M.; Wang, S.; Cai, Y.; Zhang, P. Power production waste. Water Environ. 2019, 91, 1091–1096. [Google Scholar] [CrossRef]
- Harris, D.; Heidrich, C.; Feuerborn, J. Global Aspects on Coal Combustion Products. Available online: https://www.coaltrans.com/insights/article/global-aspects-on-coal-combustion-products (accessed on 5 August 2021).
- Gollakota, A.R.K.; Volli, V.; Shu, C.-M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Jantzen, C.M.; Lee, W.E.; Ojovan, M.I. Radioactive waste (RAW) conditioning, immobilization, and encapsulation processes and technologies: Overview and advances. In Radioactive Waste Management and Contaminated Site Clean-Up. Processes, Technologies and International Experience, 1st ed.; Lee, W.E., Ojovan, M.I., Jantzen, C.M., Eds.; Woodhead Published Limited: Oxford, UK; Woodhead Published Limited: Cambridge, UK; Woodhead Published Limited: Philadelphia, PA, USA; Woodhead Published Limited: New Delhi, India, 2013; Chapter 6; pp. 171–272. [Google Scholar]
- Grutzeck, M.W.; Siemer, D.D. Zeolites synthesized from class F fly ash and sodium aluminate slurry. J. Am. Ceram. Soc. 1997, 80, 2449–2453. [Google Scholar] [CrossRef]
- Tian, Q.; Sasaki, K. Application of fly ash-based materials for stabilization/solidification of cesium and strontium. Environ. Sci. Pollut. Res. Int. 2019, 26, 23542–23554. [Google Scholar] [CrossRef]
- Ogata, F.; Kobayashi, Y.; Uematsu, Y.; Nakamura, T.; Kawasaki, N. Zeolite produced from fly ash by thermal treatment in alkaline solution and its capability to adsorb Cs(I) and Sr(II) in aqueous solution. Yakugaku Zasshi 2020, 140, 729–737. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; Rakhimov, R.Z.; Rakhimova, N.L.; Ojovan, M.I. Cementitious Materials for Nuclear Waste Immobilization, 1st ed.; John Wiley & Son: Chichester, UK, 2015; pp. 67–68. [Google Scholar]
- Cozzi, A.D.; Bannochie, C.J.; Burket, P.R.; Crawford, C.L.; Jantzen, C.M. Immobilization of radioactive waste in fly ash based geopolymers. In Proceedings of the 2011 World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9−12 May 2011; Available online: http://www.flyash.info/2011/190-Cozzi-2011.pdf (accessed on 5 August 2021).
- Liu, X.; Ding, Y.; Lu, X. Immobilization of simulated radionuclide 90Sr by fly ash-slag-metakaolin-based geopolymer. Nucl. Technol. 2017, 198, 64–69. [Google Scholar] [CrossRef]
- Jha, B.; Singh, D.N. A review on synthesis, characterization and industrial application of fly ash zeolites. J. Mater. Educ. 2011, 33, 65–132. [Google Scholar]
- Mimura, H.; Yokota, K.; Akiba, K.; Onodera, Y. Alkali hydrothermal synthesis of zeolites from coal fly ash and their uptake properties of cesium ion. J. Nucl. Sci. Technol. 2001, 38, 766–772. [Google Scholar] [CrossRef][Green Version]
- Mishra, T.; Tiwari, S.K. Studies on sorption properties of zeolite derived from Indian fly ash. J. Hazard. Mater. 2006, B137, 299–303. [Google Scholar] [CrossRef]
- Goni, S.; Bustos, A.M.G.; Lorenzo, M.P. Efficiency of fly ash belite cement and zeolite matrices for immobilizing cesium. J. Hazard. Mater. 2006, 137, 1608–1617. [Google Scholar] [CrossRef]
- Donald, I.W. Waste Immobilization in Glass and Ceramic Based Hosts: Radioactive, Toxic and Hazardous Wastes, 1st ed.; John Wiley & Son: Chichester, UK, 2010; pp. 221–240. [Google Scholar]
- Orlova, A.I.; Ojovan, M.I. Ceramic mineral waste-forms for nuclear waste immobilization. Materials 2019, 12, 2638. [Google Scholar] [CrossRef]
- Gatta, G.D.; Rinaldi, R.; McIntyre, G.J.; Nenert, G.; Bellatreccia, F.; Guastoni, A.; Ventura, G.D. On the crystal structure and crystal chemistry of pollucite, (Cs,Na)16Al16Si32O96·nH2O: A natural microporous material of interest in nuclear technology. Am. Miner. 2009, 94, 1560–1568. [Google Scholar] [CrossRef]
- Blissett, R.S.; Rowson, N.A. A review of the multi-component utilisation of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Kondratenko, E.V.; Salanov, A.N.; Bajukov, O.A.; Talyshev, A.A.; Maksimov, N.G.; Nizov, V.A.; Anshits, A.G. Novel microdesign of oxidation catalysts. Part 1. Glass crystal microspheres as new catalysts for the oxidative conversion of methane. Catal. Today 1998, 42, 267–272. [Google Scholar] [CrossRef]
- Koopman, M.; Chawla, K.K.; Ricci, W.; Carlisle, K.; Gladsyz, G.M.; Lalor, M.; Jones, M.L.; Kerr, K.; George, M.P.; Gouadec, G.; et al. Titania-coated glass microballoons and cenospheres for environmental applications. J. Mater. Sci. 2009, 44, 1435–1441. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.; Li, F.; Cheng, X.; Chen, T. Investigation into electrical conductivity and electromagnetic interference shielding effectiveness of silicone rubber filled with Ag-coated cenosphere particles. Polym. Test. 2010, 29, 609–612. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R.; Diaz-Somoano, M.; Martinez-Tarazona, M.R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 2. Characterization of ceramic cenosphere and salt concentrates. Fuel 2004, 83, 585–603. [Google Scholar] [CrossRef]
- Fenelonov, V.B.; Mel’gunov, S.; Parmon, V.N. The properties of cenospheres and the mechanism of their formation during high-temperature coal combustion at thermal power plants. Powder Part. J. 2010, 28, 189–208. [Google Scholar] [CrossRef]
- Hirajima, T.; Petrus, H.T.B.M.; Oosako, Y.; Nonaka, M.; Sasaki, K.; Ando, T. Recovery of cenospheres from coal fly ash using a dry separation process: Separation estimation and potential application. Int. J. Miner. Process. 2010, 95, 18–24. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 27, 1–12. [Google Scholar] [CrossRef]
- Danish, A.; Mosaberpanah, M.A. Formation mechanism and applications of cenospheres: A review. J. Mater. Sci. 2020, 55, 4539–4557. [Google Scholar] [CrossRef]
- Anshits, N.N.; Mikhailova, O.A.; Salanov, A.N.; Anshits, A.G. Chemical composition and structure of the shell of fly ash non-perforated cenospheres produced from the combustion of the Kuznetsk coal (Russia). Fuel 2010, 89, 1849–1862. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Mikhaylova, O.A.; Anshits, A.G. Composition and morphology of fly ash cenospheres produced from the combustion of Kuznetsk coal. Energy Fuels 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Vasilieva, N.G.; Mikhaylova, O.A.; Rogovenko, E.S.; Zhizhaev, A.M.; Anshits, A.G. Characterization of fly ash cenospheres produced from the combustion of Ekibastuz coal. Energy Fuels 2015, 29, 5390–5403. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Rogovenko, E.S.; Solovyov, L.A.; Anshits, A.G. Gas permeation properties of hollow glass-crystalline microspheres. RSC Adv. 2014, 20, 9997–10000. [Google Scholar] [CrossRef]
- Vereshchagina, T.A.; Vereshchagin, S.N.; Shishkina, N.N.; Mikhaylova, O.A.; Solovyov, L.A.; Anshits, A.G. One-step fabrication of hollow aluminosilicate microspheres with a composite zeolite/glass crystalline shell. Microporous Mesoporous Mater. 2013, 169, 207–211. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, A.G.; Bobko, A.A.; Khramtsov, V.V.; Salanov, A.N.; Kirilyuk, I.A.; Grigor’ev, I.A. Perforated cenosphere-supported pH-sensitive spin probes. Rus. Chem. Bull. 2008, 57, 493–498. [Google Scholar] [CrossRef]
- Vereshchagina, T.A.; Kutikhina, E.A.; Fomenko, E.V.; Solovyov, L.A.; Vereshchagin, S.N.; Anshits, A.G. ZrMo2O7(OH)2(H2O)2 coated microsphere glass supports derived from coal fly ash cenospheres as a novel sorbent for radionuclide trapping. J. Environ. Chem. Eng. 2019, 7, 102887. [Google Scholar] [CrossRef]
- Vereshchagina, T.A.; Anshits, N.N.; Sharonova, O.M.; Vasil’eva, N.G.; Vereshchagin, S.N.; Shishkina, N.N.; Fomenko, E.V.; Anshits, A.G. Polyfunctional microspherical materials for long-term burial of liquid radioactive wastes. Glass Phys. Chem. 2008, 34, 547–558. [Google Scholar] [CrossRef]
- Vasilieva, N.G.; Vereshchagina, T.A. Solidification of Cs-137-bearing radioactive waste in cenosphere-based mineral-like hosts for long-term disposal in granithoids. SFU J. Chem. 2015, 3, 346–358. [Google Scholar] [CrossRef]
- Vereshchagina, T.A.; Vereshchagin, S.N.; Shishkina, N.N.; Vasilieva, N.G.; Solovyov, L.A.; Anshits, A.G. Microsphere zeolite materials derived from coal fly ash cenospheres as precursors to mineral-like aluminosilicate hosts for 135,137Cs and 90Sr. J. Nucl. Mater. 2013, 437, 11–18. [Google Scholar] [CrossRef]
- Vereshchagina, T.A.; Kutikhina, E.A.; Chernykh, Y.Y.; Solovyov, L.A.; Zhizhaev, A.M.; Vereshchagin, S.N.; Anshits, A.G. One-step immobilization of cesium and strontium from alkaline solutions via a facile hydrothermal route. J. Nucl. Mater. 2018, 510, 243–255. [Google Scholar] [CrossRef]
- Vereshchagina, T.A.; Kutikhina, E.A.; Chernykh, Y.Y.; Solovyov, L.A.; Zhizhaev, A.M.; Vereshchagin, S.N.; Fomenko, E.V. Cenosphere-sourced hydrothermal synthesis of pollucite-analcime solid solutions as a low-temperature method to immobilize 137Cs in a mineral-like form. J. Nucl. Mater. 2020, 532, 152073. [Google Scholar] [CrossRef]
- Cements and Materials for Cement Production. Chemical Analysis Methods; State Standard (GOST) No. 5382-91; IPK Izdatel’stvo standartov: Moscow, Russia, 1991.
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Solovyov, L.A. Full-profile refinement by derivative difference minimization. J. Appl. Crystallogr. 2004, 37, 743–749. [Google Scholar] [CrossRef]
- Vereshchagina, T.A.; Kutikhina, E.A.; Solovyov, L.A.; Vereshchagin, S.N.; Mazurova, E.V.; Chernykh, Y.Y.; Anshits, A.G. Synthesis and structure of analcime and analcime-zirconia composite derived from coal fly ash cenospheres. Microporous Mesoporous Mater. 2018, 258, 228–235. [Google Scholar] [CrossRef]
- Wise, W.S. Handbook of Natural Zeolites; Colella, C., Ed.; International Zeolite Association, Natural Zeolites Commission; A. de Frede Edotore: Napoli, Italy, 2013; 334p. [Google Scholar]
- Parsons, I. Feldspars. In Encyclopedia of Geology, 2nd ed.; Alderton, D., Elias, S.A., Eds.; Academic Press: London, UK, 2021; pp. 271–286. [Google Scholar]
- McConnell, J.D.C. The hydrated calcium silicates riversideite, tobermorite, and plombierite. Miner. Mag. 1954, 30, 293–305. [Google Scholar] [CrossRef]
- Komarneni, S.; White, W.B. Hydrothermal reactions of strontium and transuranic simulator elements with clay minerals, zeolites, and shales. Clays Clay Miner. 1983, 31, 113–121. [Google Scholar] [CrossRef]
- Ames, L.L. Cation exchange properties of wairakite and analcime. Am. Miner. 1966, 51, 903–909. [Google Scholar]
- Redkin, A.F.; Hemley, J.J. Experimental Cs and Sr sorption on analcime in rock-buffered systems at 250−300 °C and Psat and the thermodynamic evaluation of mineral solubilities and phase relations. Eur. J. Miner. 2000, 12, 999–1014. [Google Scholar] [CrossRef]

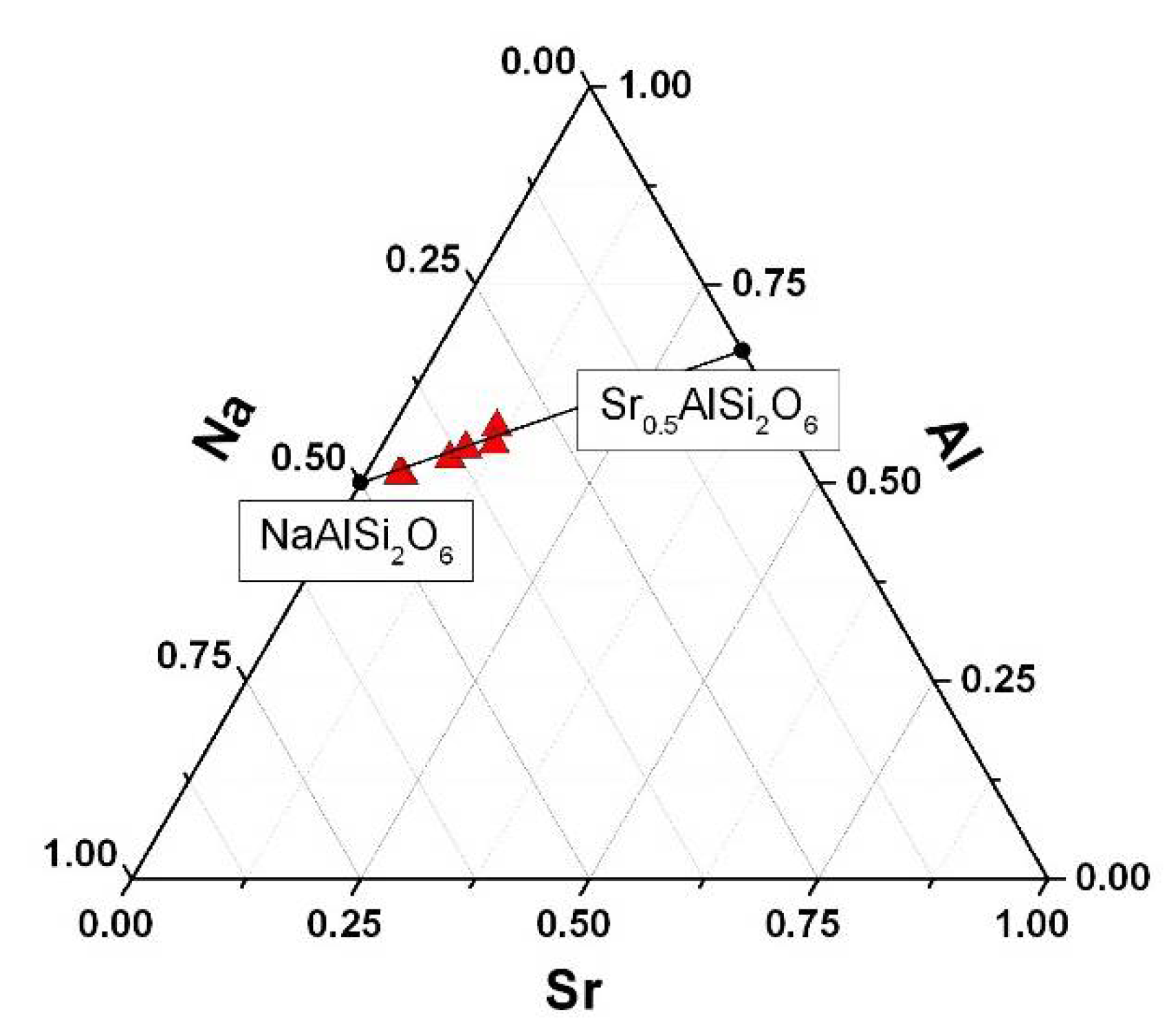

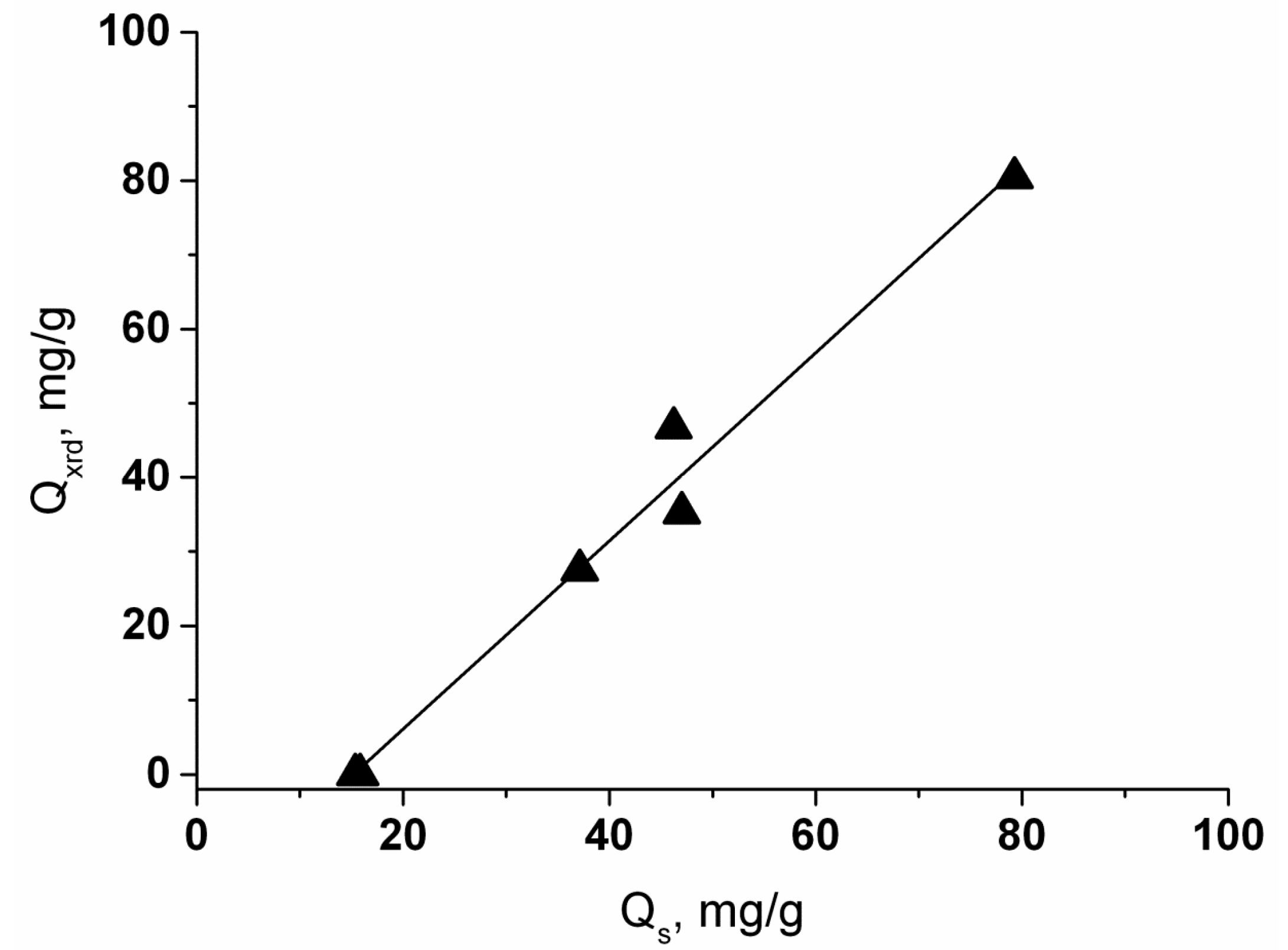
| System | Sample | T, °C | E, % |
|---|---|---|---|
| 5.5SiO2/1.0Al2O3/1.0SrO/3.65Na2O/270H2O (Sr/Si)at. = 0.18 | 0.18Sr-150 | 150 | 99.99 |
| 0.18Sr-180 | 180 | 99.99 | |
| 0.18Sr-200 | 200 | 99.99 | |
| 5.5SiO2/1.0Al2O3/2.0SrO/3.65Na2O/270H2O (Sr/Si)at. = 0.36 | 0.36Sr-150 | 150 | >99.99 |
| 0.36Sr-180 | 180 | >99.99 | |
| 0.36Sr-200 | 200 | 99.99 |
| Sample | Composition | Lattice Parameter, Å | Sr2+ Sorption, mg/g | E, % | Δm 3, % (40–150 °C) | Tm, 4 °C |
|---|---|---|---|---|---|---|
| ANA | NaAlSi2O6·H2O 1 | 13.7332 (1) | - | - | 7.77 (0.65) | 302 |
| 1000Sr/ANA-25 | NaAlSi2O6·H2O 1 | 13.7337 (8) | 15.9 | 22.7 | 7.89 (0.84) | 299 |
| 500Sr/ANA-25 | NaAlSi2O6·H2O 1 | 13.7339 (8) | 15.4 | 31.7 | 7.80 (0.79) | 300 |
| 1000Sr/ANA-150 | Na0.82Sr0.09AlSi2O6·H2O 2 | 13.7288 (6) | 47.0 | 47.7 | 7.81 (0.68) | 295 |
| 500Sr/ANA-150 | Na0.86Sr0.07AlSi2O6·H2O 2 | 13.7298 (5) | 37.1 | 72.7 | 7.74 (0.67) | 303 |
| 1000Sr/ANA-200 | Na0.58Sr0.21AlSi2O6·H2O 2 | 13.7168 (9) | 79.3 | 80.0 | 7.77 (0.55) | 291 |
| 500Sr/ANA-200 | Na0.76Sr0.12AlSi2O6·H2O 2 | 13.7241 (8) | 46.2 | 91.7 | 7.78 (0.54) | 305 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereshchagina, T.; Kutikhina, E.; Solovyov, L.; Vereshchagin, S.; Mazurova, E.; Anshits, A. Hydrothermal Co-Processing of Coal Fly Ash Cenospheres and Soluble Sr(II) as Environmentally Sustainable Approach to Sr-90 Immobilization in a Mineral-like Form. Materials 2021, 14, 5586. https://doi.org/10.3390/ma14195586
Vereshchagina T, Kutikhina E, Solovyov L, Vereshchagin S, Mazurova E, Anshits A. Hydrothermal Co-Processing of Coal Fly Ash Cenospheres and Soluble Sr(II) as Environmentally Sustainable Approach to Sr-90 Immobilization in a Mineral-like Form. Materials. 2021; 14(19):5586. https://doi.org/10.3390/ma14195586
Chicago/Turabian StyleVereshchagina, Tatiana, Ekaterina Kutikhina, Leonid Solovyov, Sergei Vereshchagin, Elena Mazurova, and Alexander Anshits. 2021. "Hydrothermal Co-Processing of Coal Fly Ash Cenospheres and Soluble Sr(II) as Environmentally Sustainable Approach to Sr-90 Immobilization in a Mineral-like Form" Materials 14, no. 19: 5586. https://doi.org/10.3390/ma14195586
APA StyleVereshchagina, T., Kutikhina, E., Solovyov, L., Vereshchagin, S., Mazurova, E., & Anshits, A. (2021). Hydrothermal Co-Processing of Coal Fly Ash Cenospheres and Soluble Sr(II) as Environmentally Sustainable Approach to Sr-90 Immobilization in a Mineral-like Form. Materials, 14(19), 5586. https://doi.org/10.3390/ma14195586







