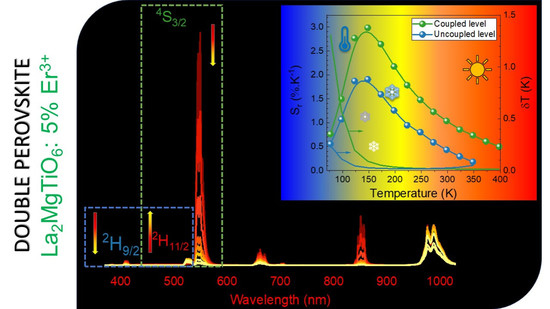
Exploration of the Temperature Sensing Ability of La2MgTiO6:Er3+ Double Perovskites Using Thermally Coupled and Uncoupled Energy Levels
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Characterization
3. Results and Discussion
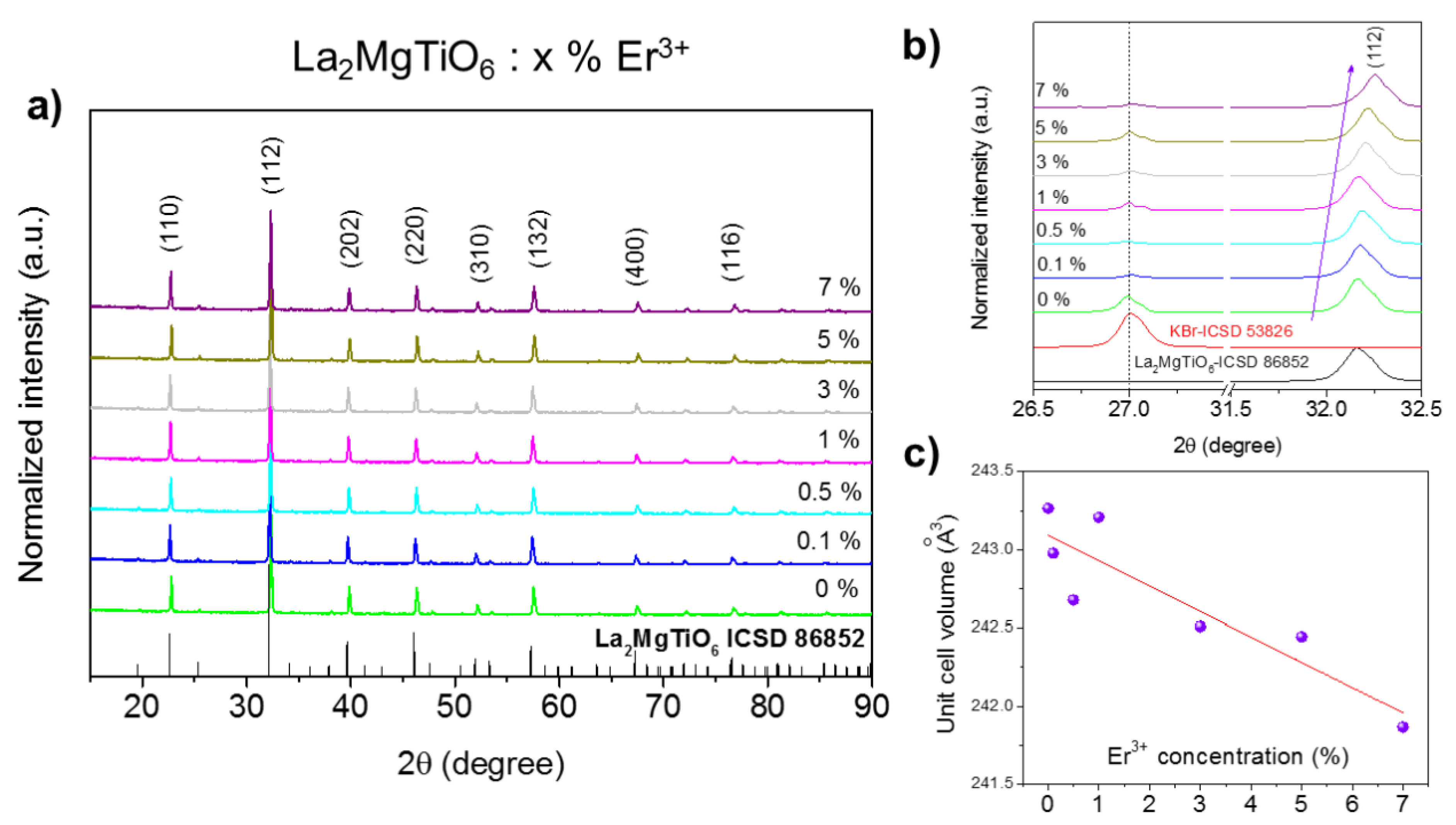
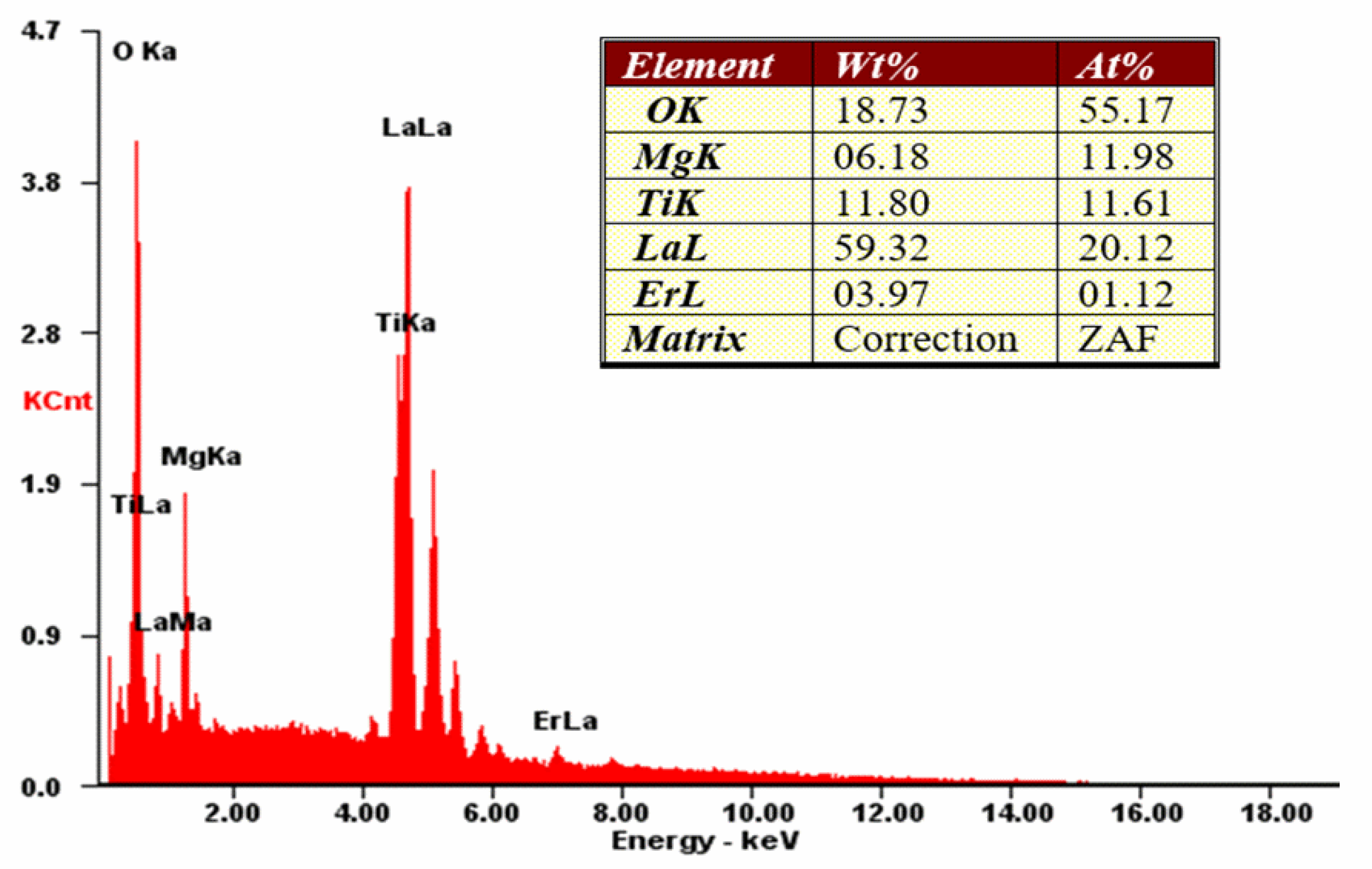
3.1. Structural and Morphological Characterization
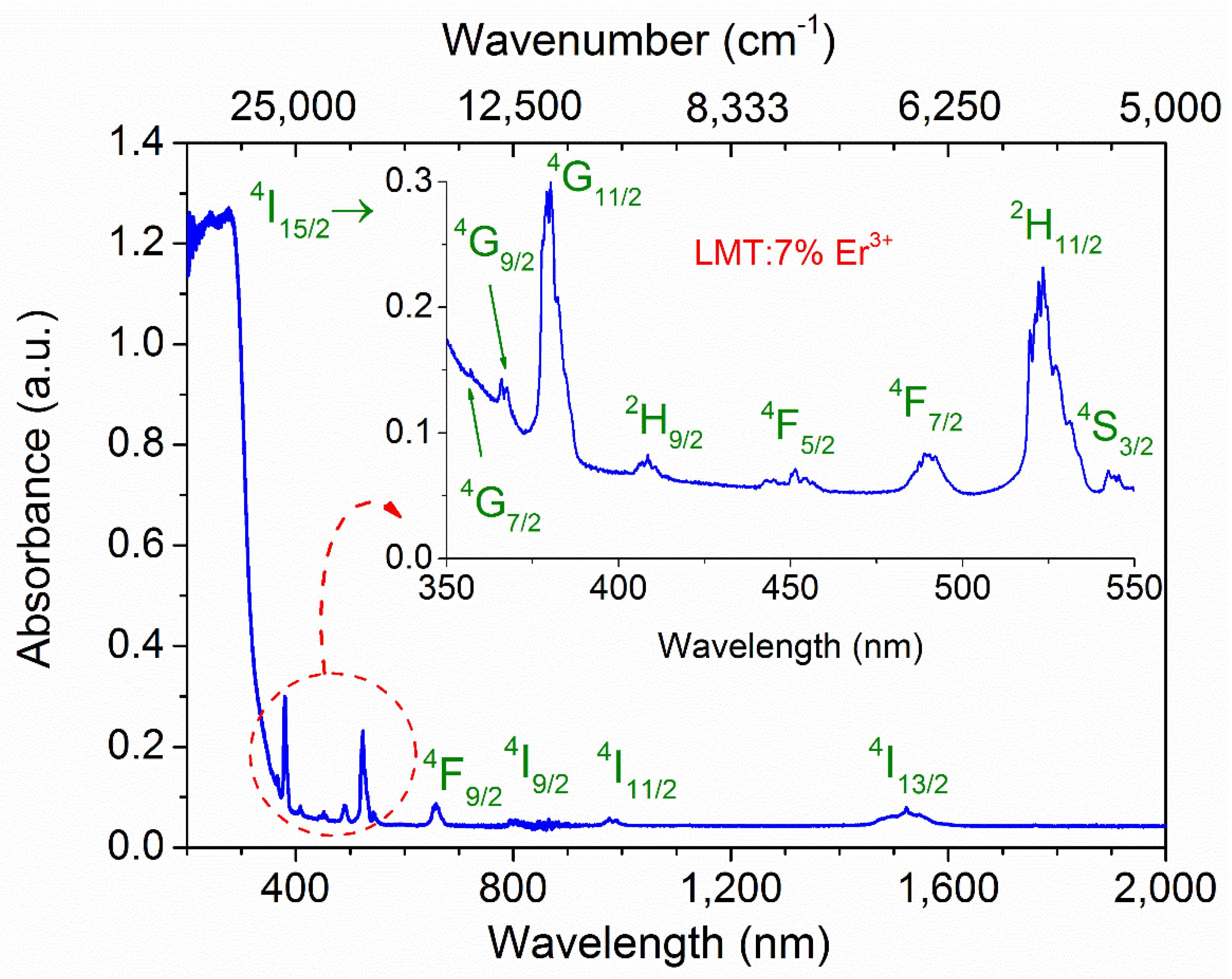
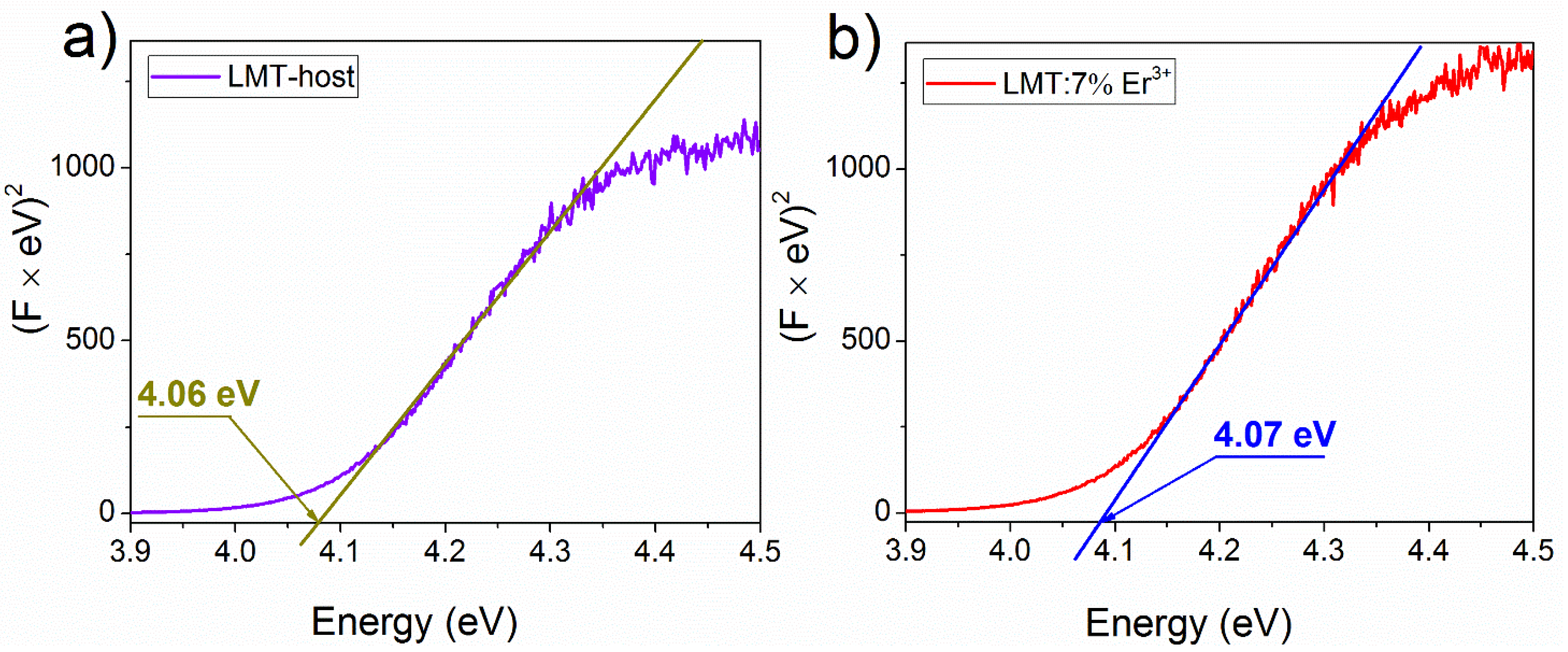
3.2. Absorption Spectrum
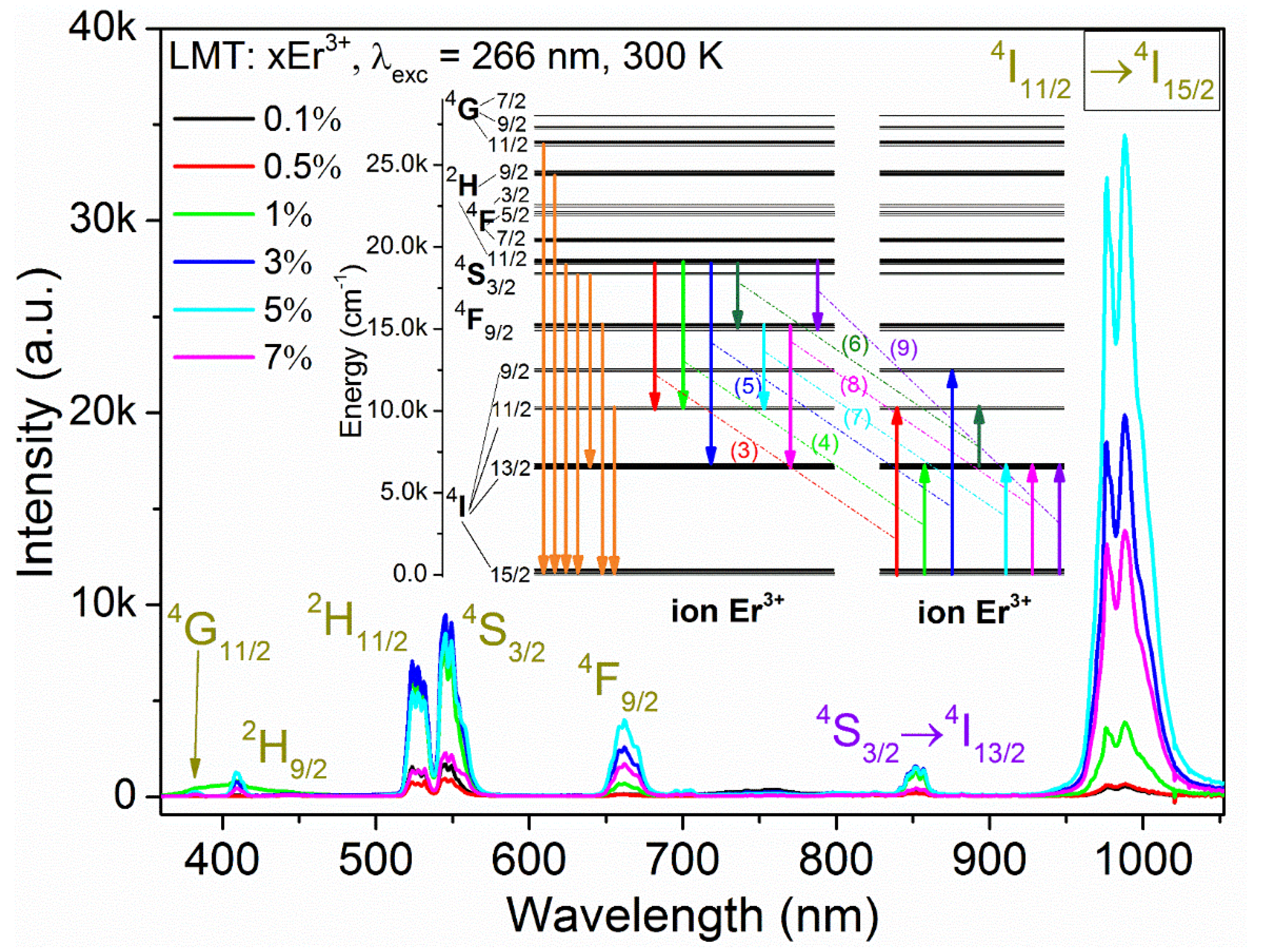
3.3. Luminescence Studies
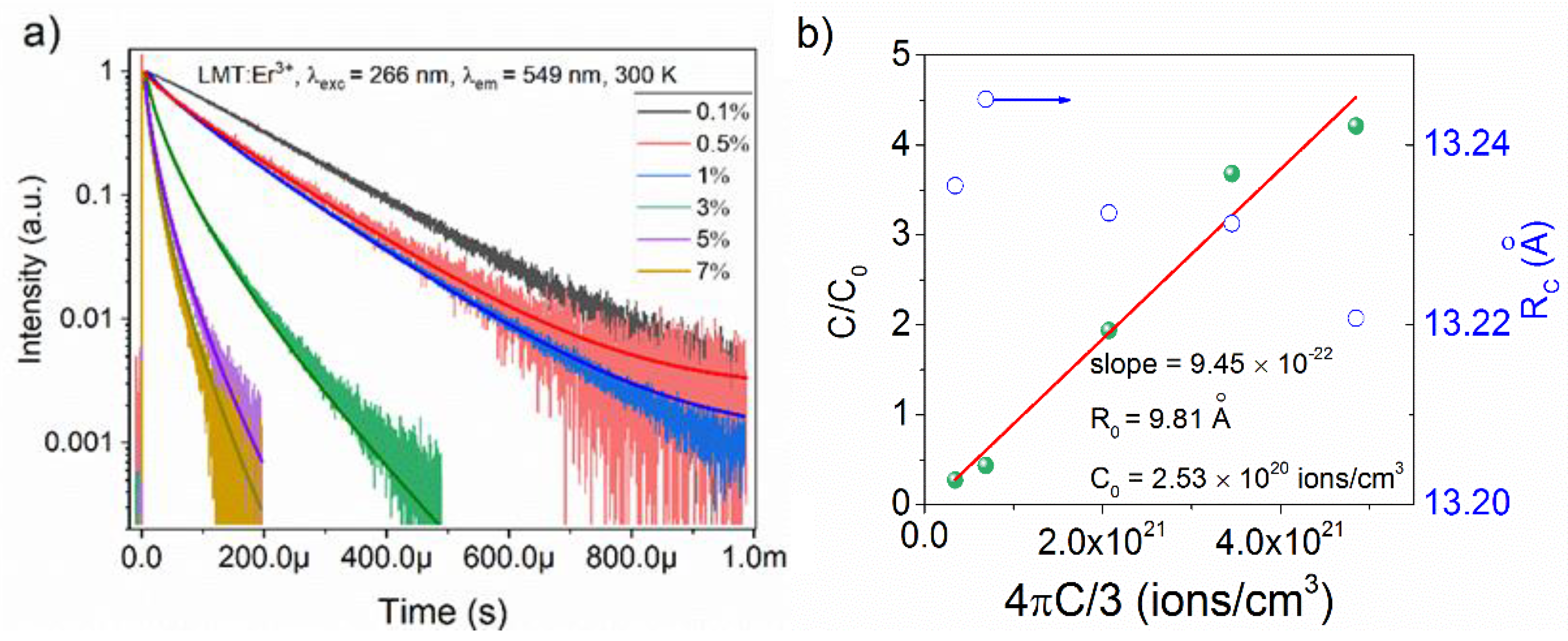
3.4. Luminescent Decay Profiles
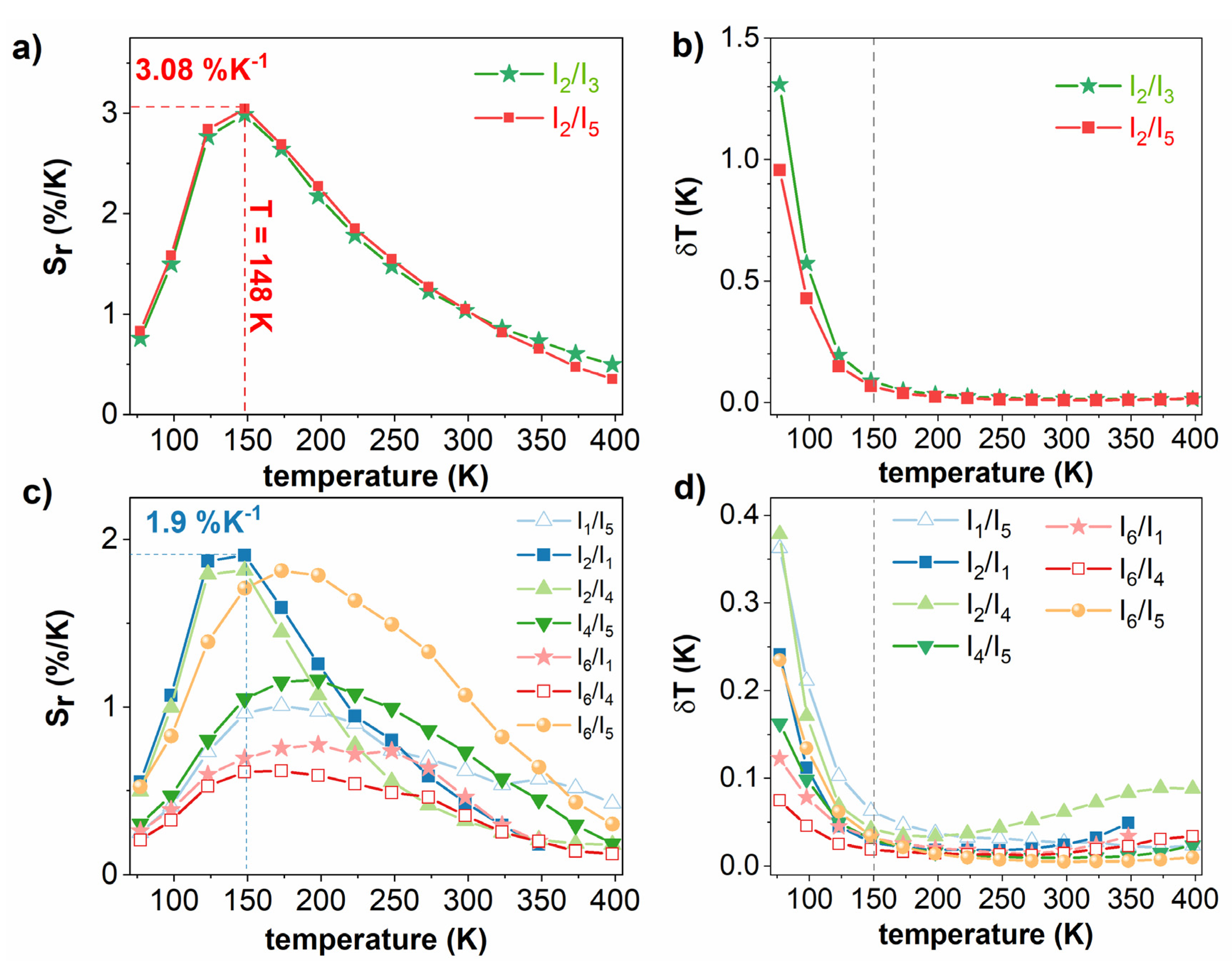
3.5. Temperature-Sensing Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefańska, D.; Bondzior, B.; Vu, T.H.Q.; Grodzicki, M.; Dereń, P.J. Temperature sensitivity modulation through changing the vanadium concentration in a La2MgTiO6:V5+,Cr3+ double perovskite optical thermometer. Dalton Trans. 2021, 50, 9851–9857. [Google Scholar] [CrossRef]
- Vu, T.; Bondzior, B.; Stefańska, D.; Dereń, P. Influence of temperature on near-infrared luminescence, energy transfer mechanism and the temperature sensing ability of La2MgTiO6:Nd3+ double perovskites. Sensors Actuators A Phys. 2021, 317, 112453. [Google Scholar] [CrossRef]
- Bondzior, B.; Stefańska, D.; Vũ, T.; Miniajluk-Gaweł, N.; Dereń, P. Red luminescence with controlled rise time in La2MgTiO6:Eu3+. J. Alloys Compd. 2021, 852, 157074. [Google Scholar] [CrossRef]
- Antic-Fidancev, E.; Deren, P.; Krupa, J.-C. Energy levels and crystal field calculations of Er3+ in LaAlO3. J. Alloys Compd. 2004, 380, 376–379. [Google Scholar] [CrossRef]
- Dereń, P.; Mahiou, R.; Pazik, R.; Lemanski, K.; Strek, W.; Boutinaud, P. Upconversion emission in CaTiO3:Er3+ nanocrystals. J. Lumin. 2008, 128, 797–799. [Google Scholar] [CrossRef]
- Deren, P.; Mahiou, R. Spectroscopic characterisation of LaAlO3 crystal doped with Er3+ ions. Opt. Mater. 2007, 29, 766–772. [Google Scholar] [CrossRef]
- Su, B.; Xie, H.; Tan, Y.; Zhao, Y.; Yang, Q.; Zhang, S. Luminescent properties, energy transfer, and thermal stability of double perovskites La2MgTiO6:Sm3+, Eu3+. J. Lumin. 2018, 204, 457–463. [Google Scholar] [CrossRef]
- Li, W.; Chen, T.; Xia, W.; Yang, X.; Xiao, S. Near-infrared emission of Yb3+ sensitized by Mn4+ in La2MgTiO6. J. Lumin. 2018, 194, 547–550. [Google Scholar] [CrossRef]
- Srivastava, A.; Comanzo, H.; Brik, M. Luminescence of Bi3+ in the double perovskite, La2MgTiO6. Opt. Mater. 2018, 75, 809–813. [Google Scholar] [CrossRef]
- Yin, X.; Yao, J.; Wang, Y.; Zhao, C.; Huang, F. Novel red phosphor of double perovskite compound La2MgTiO6:x Eu3+. J. Lumin. 2012, 132, 1701–1704. [Google Scholar] [CrossRef]
- Huang, J.; Qin, M.; Yu, J.; Ma, A.; Yu, X.; Liu, J.; Zheng, Z.; Wang, X. La2MgTiO6:Eu2+/TiO2-based composite for methyl orange (MO) decomposition. Appl. Phys. A 2019, 125, 862. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Brik, M.G.; Comanzo, H.A.; Beers, W.W.; Cohen, W.E.; Pocock, T. Spectroscopy of Mn4+ in Double Perovskites, La2LiSbO6 and La2LiSbO6: Deep Red Photon Generators for Agriculture LEDs. ECS J. Solid State Sci. Technol. 2018, 7, R3158–R3162. [Google Scholar] [CrossRef]
- Li, L.; Qin, F.; Zhou, Y.; Miao, J.; Zhang, Z. Origin of the giant thermal enhancement of the Er3+ ion’s 4I9/2-4I15/2 photoluminescence. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117862. [Google Scholar] [CrossRef]
- Gai, S.; Zhu, H.; Gao, P.; Zhou, C.; Kong, Z.; Molokeev, M.S.; Qi, Z.; Zhou, Z.; Xia, M. Structure analysis, tuning photoluminescence and enhancing thermal stability on Mn4+-doped La2-xYxMgTiO6 red phosphor for agricultural lighting. Ceram. Int. 2020, 46, 20173–20182. [Google Scholar] [CrossRef]
- Takeda, Y.; Kato, H.; Kobayashi, M.; Kobayashi, H.; Kakihana, M. Photoluminescence Properties of Mn4+-activated Perovskite-type Titanates, La2MTiO6:Mn4+(M = Mg and Zn). Chem. Lett. 2015, 44, 1541–1543. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Su, B.; Tan, Y.; Zhao, Y.; Chai, S. Effect of Gd3+, Bi3+, or Sm3+ on luminescent properties of La2−x MgTiO6:x Eu3+ phosphors. Luminescence 2018, 33, 1450–1455. [Google Scholar] [CrossRef]
- Shi, R.; Lin, L.-T.; Dorenbos, P.; Liang, H. Development of a potential optical thermometric material through photoluminescence of Pr3+ in La2MgTiO6. J. Mater. Chem. C 2017, 5, 10737–10745. [Google Scholar] [CrossRef]
- Brandão-Silva, A.C.; Gomes, M.A.; Novais, S.M.V.; Macedo, Z.S.; Avila, J.F.M.; Rodrigues, J.J.; Alencar, M.A.R.C. Size influence on temperature sensing of erbium-doped yttrium oxide nanocrystals exploiting thermally coupled and uncoupled levels’ pairs. J. Alloys Compd. 2018, 731, 478–488. [Google Scholar] [CrossRef]
- Rijckaert, H.; Kaczmarek, A.M. Ho3+–Yb3+ doped NaGdF4 nanothermometers emitting in BW-I and BW-II. Insight into the particle growth intermediate steps. Chem. Commun. 2020, 56, 14365–14368. [Google Scholar] [CrossRef]
- Stefańska, D.; Dereń, P.J. High Efficiency Emission of Eu2+ Located in Channel and Mg-Site of Mg2Al4Si5O18 Cordierite and Its Potential as a Bi-Functional Phosphor toward Optical Thermometer and White LED Application. Adv. Opt. Mater. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Stefańska, D.; Stefanski, M.; Dereń, P.J. Unusual emission generated from Ca2Mg0.5AlSi1.5O7:Eu2+ and its potential for UV-LEDs and non-contact optical thermometry. J. Alloys Compd. 2021, 863, 158770. [Google Scholar] [CrossRef]
- Trojan-Piegza, J.; Brites, C.D.S.; Ramalho, J.F.C.B.; Wang, Z.; Zhou, G.; Wang, S.; Carlos, L.D.; Zych, E. La0.4Gd1.6Zr2O7:0.1%Pr transparent sintered ceramic—A wide-range luminescence thermometer. J. Mater. Chem. C 2020, 8, 7005–7011. [Google Scholar] [CrossRef]
- Sójka, M.; Brites, C.D.S.; Carlos, L.D.; Zych, E. Exploiting bandgap engineering to finely control dual-mode Lu2(Ge,Si)O5:Pr3+ luminescence thermometers. J. Mater. Chem. C 2020, 8, 10086–10097. [Google Scholar] [CrossRef]
- Brites, C.; Millán, A.; Carlos, L. Lanthanides in Luminescent Thermometry. Metals 2016, 339–427. [Google Scholar] [CrossRef]
- Hua, Y.; Yu, J.S. Strong Green Emission of Erbium(III)-Activated La2MgTiO6 Phosphors for Solid-State Lighting and Optical Temperature Sensors. ACS Sustain. Chem. Eng. 2021, 9, 5105–5115. [Google Scholar] [CrossRef]
- Wade, S.; Collins, S.F.; Baxter, G. Fluorescence intensity ratio technique for optical fiber point temperature sensing. J. Appl. Phys. 2003, 94, 4743–4756. [Google Scholar] [CrossRef]
- Ćirić, A.; Aleksić, J.; Barudžija, T.; Antić, Ž.; Đorđević, V.; Medić, M.; Periša, J.B.; Zeković, I.; Mitrić, M.; Dramićanin, M.D. Comparison of Three Ratiometric Temperature Readings from the Er3+ Upconversion Emission. Nanomaterials 2020, 10, 627. [Google Scholar] [CrossRef] [Green Version]
- Rakov, N.; Maciel, G.S. Exploring the 4I13/2 → 4I15/2 radiative transition from Er3+ in Y2O3 for temperature sensing. J. Lumin. 2018, 199, 293–297. [Google Scholar] [CrossRef]
- Ran, W.; Noh, H.M.; Park, S.H.; Lee, B.R.; Kim, J.H.; Jeong, J.H.; Shi, J.; Liu, G. Simultaneous bifunctional application of solid-state lighting and ratiometric optical thermometer based on double perovskite LiLaMgWO6:Er3+ thermochromic phosphors. RSC Adv. 2019, 9, 7189–7195. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ölsä, J.; Säilynoja, E.; Lamminmäki, R.-J.; Deren, P.; Strek, W.; Porcher, P. Crystal field energy level scheme of Er3+ in GdOCl Parametric analysis. J. Chem. Soc. Faraday Trans. 1997, 93, 2241–2246. [Google Scholar] [CrossRef]
- Stręk, W.; Dereń, P.; Maruszewski, K.; Pawlik, E.; Wojcik, W.; Malashkevich, G.; Gaishun, V.; Strȩk, W. Spectroscopic properties of erbium doped silica glasses obtained by sol-gel method. J. Alloys Compd. 1998, 275-277, 420–423. [Google Scholar] [CrossRef]
- Macalik, L.; Dereń, P.; Hanuza, J.; Stręk, W.; Demidovich, A.; Kuzmin, A. Effect of random distribution and molecular interactions on optical properties of Er3+ dopant in KY(WO4)2 and Ho3+ in KYb(WO4)2. J. Mol. Struct. 1998, 450, 179–192. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Levin, I.; Vanderah, T.A.; Amos, T.G.; Maslar, J.E. Structural Behavior and Raman Spectra of Perovskite-Like Solid Solutions (1 − x)LaMg0.5Ti 0.5O3−xLa2/3TiO3. Chem. Mater. 2005, 17, 3273–3280. [Google Scholar] [CrossRef]
- Inokuti, M.; Hirayama, F. Influence of Energy Transfer by the Exchange Mechanism on Donor Luminescence. J. Chem. Phys. 1965, 43, 1978–1989. [Google Scholar] [CrossRef]
- Puchalska, M.; Watras, A. A clear effect of charge compensation through Na+ co-doping on luminescent properties of new CaGa4O7:Nd3+. J. Alloys Compd. 2016, 688, 253–260. [Google Scholar] [CrossRef]
- Puchalska, M.; Watras, A. A clear effect of charge compensation through Na+ co-doping on the luminescence spectra and decay kinetics of Nd3+-doped CaAl4O7. J. Solid State Chem. 2016, 238, 259–266. [Google Scholar] [CrossRef]
- Rudnicka, D.; Dereń, P. Preliminary spectroscopic properties of K4SrSi3O9 doped with Eu3+. Opt. Mater. 2013, 35, 2531–2534. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, B.; Zhong, H.; Sun, J.; Cheng, L.; Li, X.; Zhang, J.; Tian, Y.; Lu, W.; Wan, J.; et al. Optical Transition, Excitation State Absorption, and Energy Transfer Study of Er3+, Nd3+ Single-Doped, and Er3+/Nd3+ Codoped Tellurite Glasses for Mid-Infrared Laser Applications. J. Am. Ceram. Soc. 2011, 94, 1766–1772. [Google Scholar] [CrossRef]
- Llanos, J.; Espinoza, D.; Castillo, R. Energy transfer in single phase Eu3+-doped Y2WO6 phosphors. RSC Adv. 2017, 7, 14974–14980. [Google Scholar] [CrossRef] [Green Version]
- Bondzior, B.; Stefanska, D.; Kubiak, A.; Dereń, P. Spectroscopic properties of K4SrSi3O9 doped with Sm3+. J. Lumin. 2015, 173, 38–43. [Google Scholar] [CrossRef]
- Bin Im, W.; Fellows, N.N.; Denbaars, S.; Seshadri, R.; Kim, Y.-I. LaSr2AlO5, a Versatile Host Compound for Ce3+-Based Yellow Phosphors: Structural Tuning of Optical Properties and Use in Solid-State White Lighting. Chem. Mater. 2009, 21, 2957–2966. [Google Scholar] [CrossRef]
- Dong, B.; Wang, X.J.; Li, C.R.; Liu, D.P. Er3+-Y3+-Codoped Al2O3 for High-Temperature Sensing. IEEE Photon-Technol. Lett. 2008, 20, 117–119. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Shen, J.; Wang, X.; Yan, X. Controlling optical temperature behaviors of Er3+ doped Sr2CaWO6 through doping and changing excitation powers. Opt. Mater. Express 2018, 8, 1926–1939. [Google Scholar] [CrossRef]
- Kumar, K.U.; Santos, W.Q.; Silva, W.F.; Jacinto, C. Two photon thermal sensing in Er3+/Yb3+ Co-doped nanocrystalline NaNbO3. J. Nanosci. Nanotechnol. 2013, 13, 6841–6845. [Google Scholar] [CrossRef] [PubMed]
- Ran, W.; Noh, H.M.; Park, S.H.; Lee, B.R.; Kim, J.H.; Jeong, J.H.; Shi, J. Er3+-Activated NaLaMgWO6 double perovskite phosphors and their bifunctional application in solid-state lighting and non-contact optical thermometry. Dalton Trans. 2019, 48, 4405–4412. [Google Scholar] [CrossRef]
- Šević, D.; Rabasovic, M.; Križan, J.; Savić-Šević, S.; Marinkovic, B.P.; Nikolic, M. Effects of temperature on luminescent properties of Gd2O3:Er, Yb nanophosphor. Opt. Quantum Electron. 2020, 52, 1–9. [Google Scholar] [CrossRef]
- León-Luis, S.F.; Monteseguro, V.; Rodriguez-Mendoza, U.; Rathaiah, M.; Venkatramu, V.; Lozano-Gorrin, A.; Valiente, R.; Munoz, A.; Lavin, V. Optical nanothermometer based on the calibration of the Stokes and upconverted green emissions of Er3+ ions in Y3Ga5O12 nano-garnets. RSC Adv. 2014, 4, 57691–57701. [Google Scholar] [CrossRef] [Green Version]


| Material | λexc (nm) | Temperature Range (K) | Sm (%·K−1) | Tm (K) | δTm (K) | References |
|---|---|---|---|---|---|---|
| Al2O3:Er3+, Y3+ | 978 | 295–973 | 0.35 | 450 | 0.3 | [45] |
| Sr2CaWO6:Er3+, La3+ | 980 | 250–2000 | 0.5 | 439 | - | [46] |
| NaNbO3:Er3+ | 976 | 293–353 | 0.51 | 355 | - | [47] |
| NaLaMgWO6:Er3+ | 378 | 303–483 | 1.04 | 303 | - | [48] |
| Gd2O3:Er3+, Yb3+ | 980 | 300–550 | 1.18 | 300 | - | [49] |
| Y2O3:Er3+ Coupled levels | 379 | 296–500 | 1.54 | 483 | - | [18] |
| Uncoupled levels | 379 | 303–434 | 0.87 | 434 | - | [18] |
| LiLaMgWO6:Er3+ | 378 | 303–483 | 2.24 | 483 | - | [29] |
| Y3Ga5O12:Er3+ | 488 | 300-850 | 0.64 | 547 | - | [50] |
| La2MgTiO6:Er3+ | 380 | 303–483 | 1.107 | 303 | - | [25] |
| (solid-state) | ||||||
| La2MgTiO6:Er3+ | 266 | 77–398 | 2.98 | 148 | 0.09 | This work |
| (coprecipitation) | ||||||
| Coupled levels | ||||||
| Uncoupled levels | 266 | 77–398 | 1.9 | 148 | 0.027 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, T.H.Q.; Bondzior, B.; Stefańska, D.; Dereń, P.J. Exploration of the Temperature Sensing Ability of La2MgTiO6:Er3+ Double Perovskites Using Thermally Coupled and Uncoupled Energy Levels. Materials 2021, 14, 5557. https://doi.org/10.3390/ma14195557
Vu THQ, Bondzior B, Stefańska D, Dereń PJ. Exploration of the Temperature Sensing Ability of La2MgTiO6:Er3+ Double Perovskites Using Thermally Coupled and Uncoupled Energy Levels. Materials. 2021; 14(19):5557. https://doi.org/10.3390/ma14195557
Chicago/Turabian StyleVu, Thi Hong Quan, Bartosz Bondzior, Dagmara Stefańska, and Przemysław J. Dereń. 2021. "Exploration of the Temperature Sensing Ability of La2MgTiO6:Er3+ Double Perovskites Using Thermally Coupled and Uncoupled Energy Levels" Materials 14, no. 19: 5557. https://doi.org/10.3390/ma14195557
APA StyleVu, T. H. Q., Bondzior, B., Stefańska, D., & Dereń, P. J. (2021). Exploration of the Temperature Sensing Ability of La2MgTiO6:Er3+ Double Perovskites Using Thermally Coupled and Uncoupled Energy Levels. Materials, 14(19), 5557. https://doi.org/10.3390/ma14195557