Scalable Synthesis and Electrochemical Properties of Porous Si-CoSi2-C Composites as an Anode for Li-ion Batteries
Abstract
:1. Introduction
2. Experimental Procedure
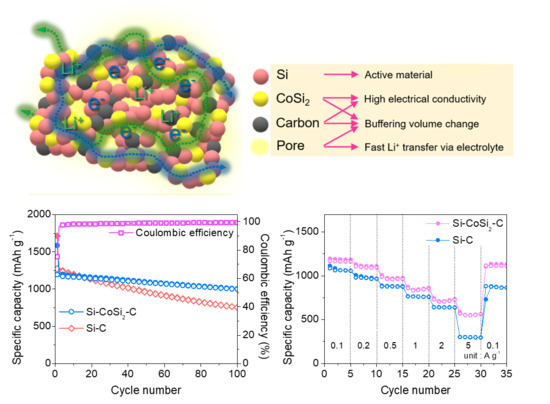
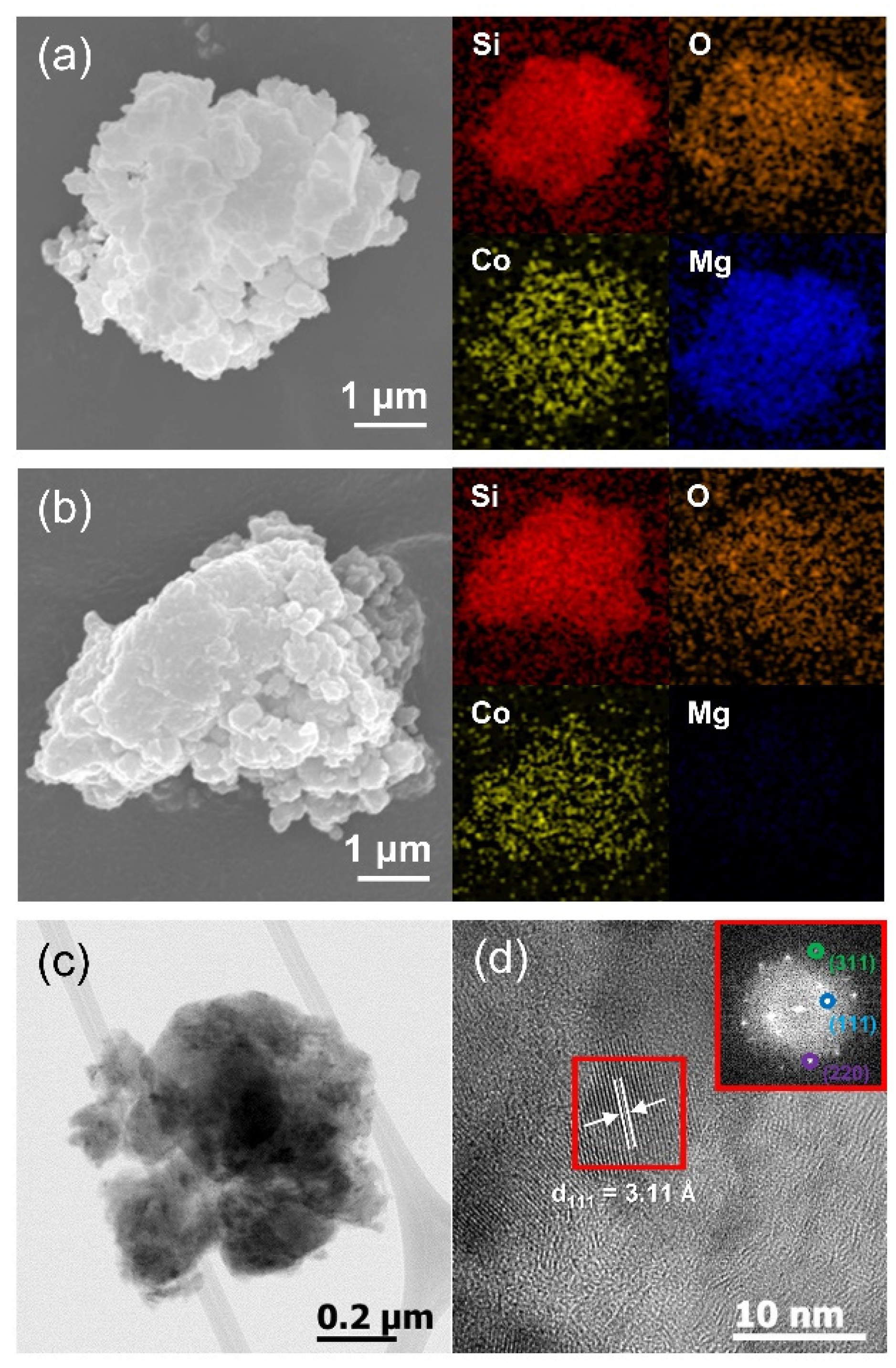
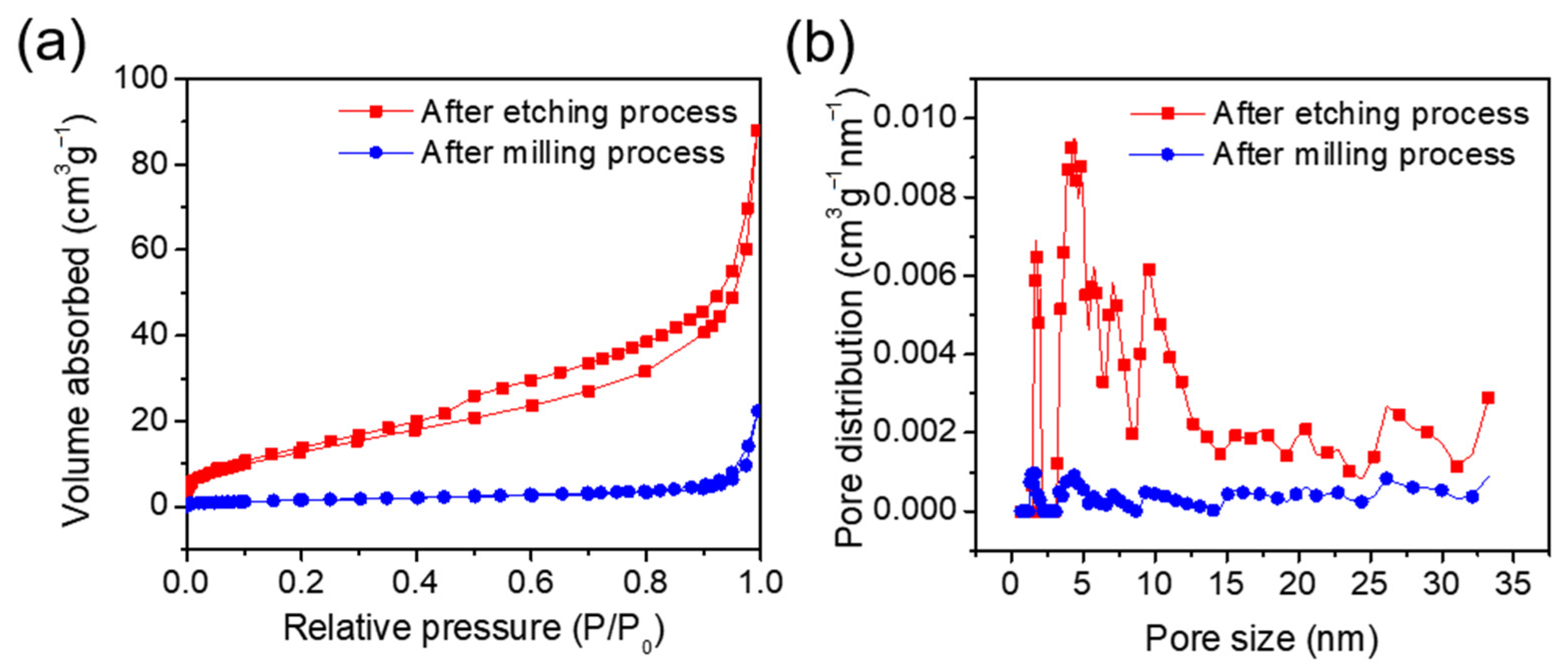
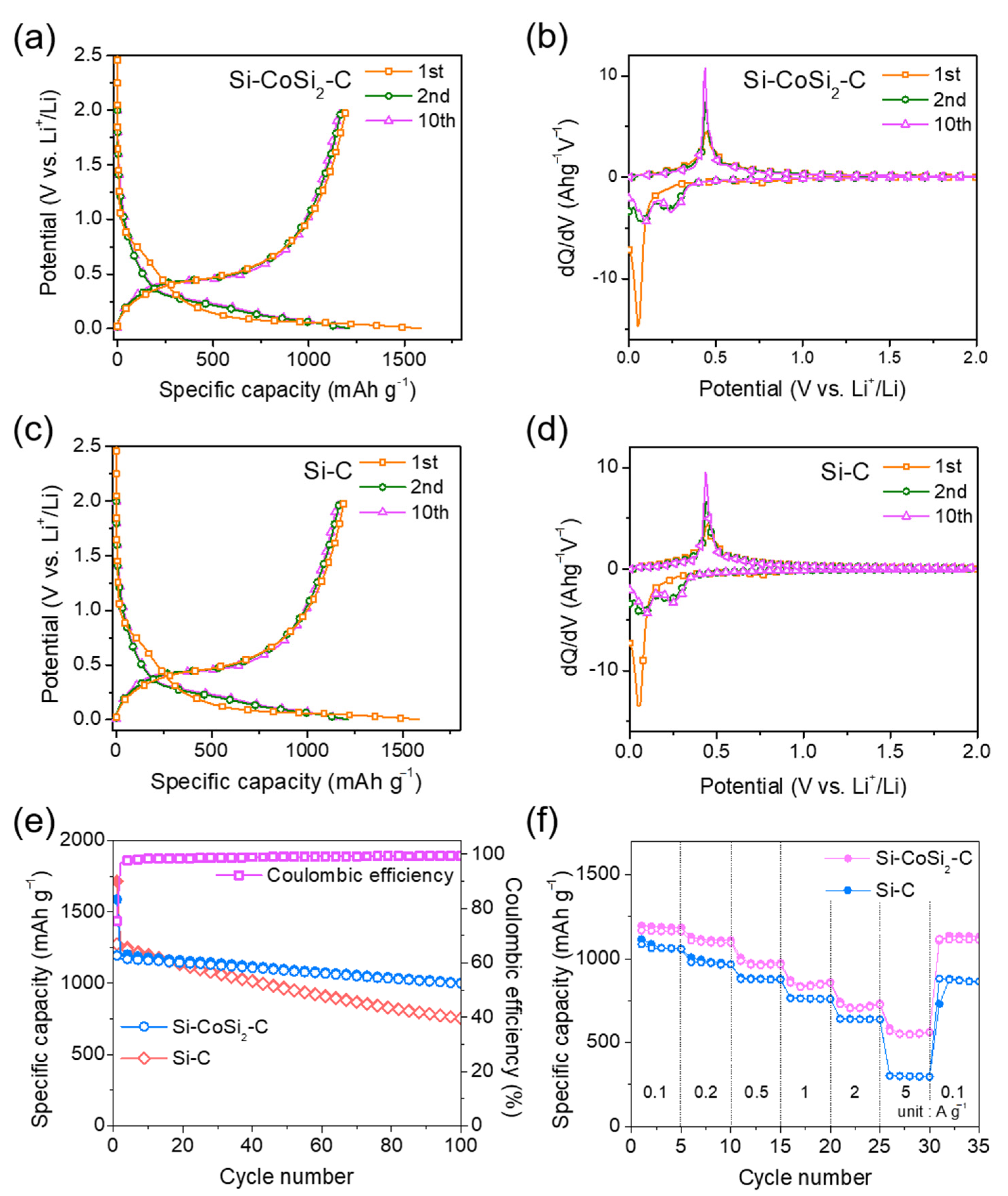
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novák, P. Insertion Electrode Materials for Rechargeable Lithium Batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef] [PubMed]
- Obrovac, M.N.; Chevrier, V.L. Alloy Negative Electrodes for Li-Ion Batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef]
- Yao, Y.; McDowell, M.T.; Ryu, I.; Wu, H.; Liu, N.; Hu, L.; Nix, W.D.; Cui, Y. Interconnected Silicon Hollow Nanospheres for Lithium-Ion Battery Anodes with Long Cycle Life. Nano Lett. 2011, 11, 2949–2954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, X.; Chen, L.; Yue, J.; Xu, H.; Yang, J.; Qian, Y. Novel mesoporous silicon nanorod as an anode material for lithium ion batteries. Electrochim. Acta 2014, 127, 252–258. [Google Scholar] [CrossRef]
- Wen, Z.; Lu, G.; Mao, S.; Kim, H.; Cui, S.; Yu, K.; Huang, X.; Hurley, P.T.; Mao, O.; Chen, J. Silicon nanotube anode for lithium-ion batteries. Electrochem. Commun. 2013, 29, 67–70. [Google Scholar] [CrossRef]
- Li, W.; Tang, Y.; Kang, W.; Zhang, Z.; Yang, X.; Zhu, Y.; Zhang, W.; Lee, C.-S. Core–Shell Si/C Nanospheres Embedded in Bubble Sheet-like Carbon Film with Enhanced Performance as Lithium Ion Battery Anodes. Small 2015, 11, 1345–1351. [Google Scholar] [CrossRef]
- Hu, Z.-G.; Tan, Z.-Y.; Sun, F.; Lin, Z.; Chen, J.; Tang, X.-y.; Luo, J.; Sun, L.; Zheng, R.-T.; Chen, Y.-C.; et al. The high cycling performance of ultra-thin Si nanowires fabricated by metal-assisted chemical etching method as lithium-ion batteries anode. J. Electroanal. Chem. 2020, 878, 114567. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.; Sohn, H.-J. Addition of Cu for carbon coated Si-based composites as anode materials for lithium-ion batteries. Electrochem. Commun. 2005, 7, 557–561. [Google Scholar] [CrossRef]
- Lin, L.; Ma, Y.; Xie, Q.; Wang, L.; Zhang, Q.; Peng, D.-L. Copper-Nanoparticle-Induced Porous Si/Cu Composite Films as an Anode for Lithium Ion Batteries. ACS Nano 2017, 11, 6893–6903. [Google Scholar] [CrossRef] [PubMed]
- Doh, C.-H.; Shin, H.-M.; Kim, D.-H.; Jeong, Y.-D.; Moon, S.-I.; Jin, B.-S.; Kim, H.-S.; Kim, K.-W.; Oh, D.-H.; Veluchamy, A. A new composite anode, Fe–Cu–Si/C for lithium ion battery. J. Alloys Compd. 2008, 461, 321–325. [Google Scholar] [CrossRef]
- Sugimoto, T.; Atsumi, Y.; Kono, M.; Kikuta, M.; Ishiko, E.; Yamagata, M.; Ishikawa, M. Application of bis(fluorosulfonyl)imide-based ionic liquid electrolyte to silicon–nickel–carbon composite anode for lithium-ion batteries. J. Power Sources 2010, 195, 6153–6156. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, J.; Cao, Y.; Yang, H.; Ai, X. Green Synthesis and Stable Li-Storage Performance of FeSi2/Si@C Nanocomposite for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 3753–3758. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, Y.; Huang, Q.-a.; Huang, X. Effects of thermal annealing on performance of silicon nitride anode for lithium-ion battery applications. J. Electroanal. Chem. 2019, 845, 119–125. [Google Scholar] [CrossRef]
- Kim, S.-O.; Manthiram, A. Low-cost carbon-coated Si-Cu3Si-Al2O3 nanocomposite anodes for high-performance lithium-ion batteries. J. Power Sources 2016, 332, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Kim, K.; Kim, H.-S.; Seo, H.; Lee, H.S.; Choi, H.; Kim, J.-H. Microstructure Design of Carbon-Coated Nb2O5–Si Composites as Reversible Li Storage Materials. Electron. Mater. Lett. 2020, 16, 376–384. [Google Scholar] [CrossRef]
- Zhou, N.; Wu, Y.; Li, Y.; Yang, J.; Zhou, Q.; Guo, Y.; Xia, M.; Zhou, Z. Interconnected structure Si@TiO2-B/CNTs composite anode applied for high-energy lithium-ion batteries. Appl. Surf. Sci. 2020, 500, 144026. [Google Scholar] [CrossRef]
- Park, E.; Kim, J.; Chung, D.J.; Park, M.-S.; Kim, H.; Kim, J.H. Si/SiOx-Conductive Polymer Core-Shell Nanospheres with an Improved Conducting Path Preservation for Lithium-Ion Battery. ChemSusChem 2016, 9, 2754–2758. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Merabet, H.; De Luna, Y.; Mohamed, K.; Bensalah, N. Fabrication of Si3N4@Si@Cu Thin Films by RF Sputtering as High Energy Anode Material for Li-Ion Batteries. Materials 2021, 14, 2824. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; Kim, K.-J.; Lee, S.-M. Preparation and Characterization of Core-Shell Structure Hard Carbon/Si-Carbon Composites with Multiple Shell Structures as Anode Materials for Lithium-Ion Batteries. Energies 2021, 14, 2104. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Zhai, T.; Li, H. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater. Chem. Front. 2018, 2, 437–455. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Huang, B.; Dai, Y. Achieving high energy density for lithium-ion battery anodes by Si/C nanostructure design. J. Mater. Chem. A 2019, 7, 2165–2171. [Google Scholar] [CrossRef]
- Wang, M.-S.; Song, W.-L.; Wang, J.; Fan, L.-Z. Highly uniform silicon nanoparticle/porous carbon nanofiber hybrids towards free-standing high-performance anodes for lithium-ion batteries. Carbon 2015, 82, 337–345. [Google Scholar] [CrossRef]
- Seo, H.; Kim, H.-S.; Kim, K.; Choi, H.; Kim, J.-H. Magnesium silicide-derived porous Sb-Si-C composite for stable lithium storage. J. Alloys Compd. 2019, 782, 525–532. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Wei, H.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Porosity- and Graphitization-Controlled Fabrication of Nanoporous Silicon@Carbon for Lithium Storage and Its Conjugation with MXene for Lithium-Metal Anode. Adv. Funct. Mater. 2020, 30, 1908721. [Google Scholar] [CrossRef]
- Shivaraju, G.C.; Sudakar, C.; Prakash, A.S. High-rate and long-cycle life performance of nano-porous nano-silicon derived from mesoporous MCM-41 as an anode for lithium-ion battery. Electrochim. Acta 2019, 294, 357–364. [Google Scholar] [CrossRef]
- Sohn, M.; Lee, D.G.; Park, H.-I.; Park, C.; Choi, J.-H.; Kim, H. Microstructure Controlled Porous Silicon Particles as a High Capacity Lithium Storage Material via Dual Step Pore Engineering. Adv. Funct. Mater. 2018, 28, 1800855. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, S.; Ying, H.; Yang, W.; Wang, J.; Han, W.-Q. New, Effective, and Low-Cost Dual-Functional Binder for Porous Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 14051–14058. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, S.; Ying, H.; Zhang, Z.; Han, W. Few-layered Ti3C2 MXene anchoring bimetallic selenide NiCo2Se4 nanoparticles for superior Sodium-ion batteries. Chem. Eng. J. 2021, 417, 129161. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, S.; Ying, H.; Yang, W.; Wang, J.; Guo, R.; Han, W. Fabrication of Fe nanocomplex pillared few-layered Ti3C2Tx MXene with enhanced rate performance for lithium-ion batteries. Nano Res. 2021, 14, 1218–1227. [Google Scholar] [CrossRef]
- Li, H.; Zhou, H. Enhancing the performances of Li-ion batteries by carbon-coating: Present and future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef]
- Yu, B.-C.; Hwa, Y.; Kim, J.-H.; Sohn, H.-J. Carbon coating for Si nanomaterials as high-capacity lithium battery electrodes. Electrochem. Commun. 2014, 46, 144–147. [Google Scholar] [CrossRef]
- Shen, X.; Tian, Z.; Fan, R.; Shao, L.; Zhang, D.; Cao, G.; Kou, L.; Bai, Y. Research progress on silicon/carbon composite anode materials for lithium-ion battery. J. Energy Chem. 2018, 27, 1067–1090. [Google Scholar] [CrossRef] [Green Version]
- García-Méndez, M.; Castillón, F.F.; Hirata, G.A.; Farías, M.H.; Beamson, G. XPS and HRTEM characterization of cobalt–nickel silicide thin films. Appl. Surf. Sci. 2000, 161, 61–73. [Google Scholar] [CrossRef]
- García-Méndez, M.; Galvan, D.H.; Posada-Amarillas, A.; Farías, M.H. Experimental and theoretical study of the electronic properties of CoSi2 and NiSi2. Appl. Surf. Sci. 2004, 230, 386–392. [Google Scholar] [CrossRef]
- Hu, C.-W.; Chang, T.-C.; Tu, C.-H.; Chen, Y.-D.; Lin, C.-C.; Chen, M.-C.; Lin, J.-Y.; Sze, S.M.; Tseng, T.-Y. Nitric Acid Oxidation of Si for the Tunneling Oxide Application on CoSi2 Nanocrystals Nonvolatile Memory. J. Electrochem. Soc. 2010, 157, H332. [Google Scholar] [CrossRef]
- Banik, S.; Kumar, P.P. Investigation of electronic structure of transition metal silicides MnSi1.75 and CoSi for enhanced thermoelectric properties. Solid State Commun. 2020, 307, 113807. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef]
- Nie, M.; Abraham, D.P.; Chen, Y.; Bose, A.; Lucht, B.L. Silicon Solid Electrolyte Interphase (SEI) of Lithium Ion Battery Characterized by Microscopy and Spectroscopy. J. Phys. Chem. C 2013, 117, 13403–13412. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93–A96. [Google Scholar] [CrossRef]
- Hatchard, T.D.; Dahn, J.R. In Situ XRD and Electrochemical Study of the Reaction of Lithium with Amorphous Silicon. J. Electrochem. Soc. 2004, 151, A838. [Google Scholar] [CrossRef]
- Kim, H.-S.; Cho, W.; Park, D.; Kim, K.; Jung, W.-S.; Choi, H.; Kim, J.-H. Zn-induced synthesis of porous SiOx materials as negative electrodes for Li secondary batteries. J. Alloys Compd. 2019, 803, 325–331. [Google Scholar] [CrossRef]
- Park, A.R.; Nam, M.G.; Kim, A.Y.; Kim, K.S.; Sher Shah, M.S.A.; Lee, J.Y.; Kim, W.-J.; Lee, J.K.; Yoo, P.J. Si/Co-CoSi2/reduced graphene oxide ternary nanocomposite anodes for Li-Ion batteries with enhanced capacity and cycling stability. J. Alloys Compd. 2017, 724, 1134–1142. [Google Scholar] [CrossRef]
- Kim, Y.; Woo, S.C.; Lee, C.S.; Park, J.S.; Seo, H.; Kim, J.-H.; Song, J.H. Electrochemical investigation on high-rate properties of graphene nanoplatelet-carbon nanotube hybrids for Li-ion capacitors. J. Electroanal. Chem. 2020, 863, 114060. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Hou, S.; Li, Y.; Lu, T.; Yao, Y.; Pan, L. Improved sodium-ion storage performance of Ti3C2Tx MXenes by sulfur doping. J. Mater. Chem. A 2018, 6, 1234–1243. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.; Yang, H.-R.; Yang, Y.; Kim, K.; Kim, S.H.; Lee, H.; Kim, J.-H. Scalable Synthesis and Electrochemical Properties of Porous Si-CoSi2-C Composites as an Anode for Li-ion Batteries. Materials 2021, 14, 5397. https://doi.org/10.3390/ma14185397
Seo H, Yang H-R, Yang Y, Kim K, Kim SH, Lee H, Kim J-H. Scalable Synthesis and Electrochemical Properties of Porous Si-CoSi2-C Composites as an Anode for Li-ion Batteries. Materials. 2021; 14(18):5397. https://doi.org/10.3390/ma14185397
Chicago/Turabian StyleSeo, Hyungeun, Hae-Ri Yang, Youngmo Yang, Kyungbae Kim, Sung Hyon Kim, Hyunseung Lee, and Jae-Hun Kim. 2021. "Scalable Synthesis and Electrochemical Properties of Porous Si-CoSi2-C Composites as an Anode for Li-ion Batteries" Materials 14, no. 18: 5397. https://doi.org/10.3390/ma14185397
APA StyleSeo, H., Yang, H.-R., Yang, Y., Kim, K., Kim, S. H., Lee, H., & Kim, J.-H. (2021). Scalable Synthesis and Electrochemical Properties of Porous Si-CoSi2-C Composites as an Anode for Li-ion Batteries. Materials, 14(18), 5397. https://doi.org/10.3390/ma14185397