Recent Advances in the Fabrication and Environmental Science Applications of Cellulose Nanofibril-Based Functional Materials
Abstract
:1. Introduction
2. Fabrication of CNF-Based 1D, 2D, and 3D Functional Materials
2.1. CNF-Based 1D Nanomaterials
2.2. CNF-Based 2D Films/Membranes
2.3. CNF Hydrogels
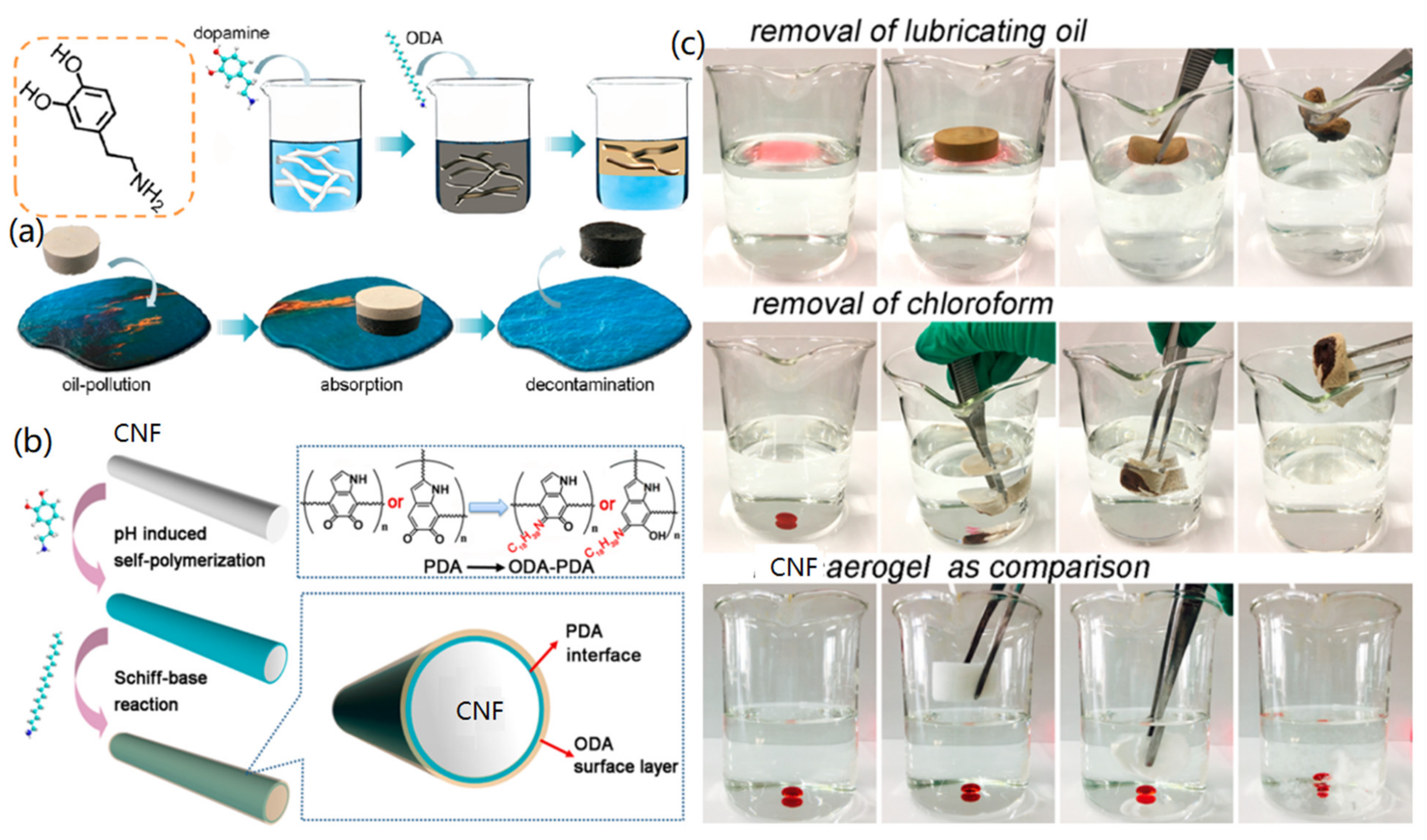
2.4. CNF Aerogels
3. Environmental Science Applications
3.1. Removal of Heavy Metal Ions
3.2. Removal of Anions
3.3. Removal of Organic Dyes
3.4. Oil Removal
3.5. Removal of Bio-Contents
4. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Qi, P.F.; Liu, X.M.; Wei, G. Synthesis of Three-Dimensional Graphene-Based Hybrid Materials for Water Purification: A Review. Nanomaterials 2019, 9, 1123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.J.; Cui, J.X.; Lu, T.; Tang, G.S.; Wu, S.T.; Ma, W.J.; Huang, C.B. Robust, functionalized reduced graphene-based nanofibrous membrane for contaminated water purification. Chem. Eng. J. 2021, 404, 126347. [Google Scholar] [CrossRef]
- Xie, X.Q.; Chen, C.; Zhang, N.; Tang, Z.R.; Jiang, J.J.; Xu, Y.J. Microstructure and surface control of MXene films for water purification. Nat. Sustain. 2019, 2, 856–862. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.Y.; Graham, N.; Yu, W.Z.; Sun, K.N. Two-dimensional MXene incorporated graphene oxide composite membrane with enhanced water purification performance. J. Membr. Sci. 2020, 593, 117431. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mezzenga, R. Protein nanofibrils for next generation sustainable water purification. Nat. Commun. 2021, 12, 3248. [Google Scholar] [CrossRef] [PubMed]
- Bethke, K.; Palantoken, S.; Andrei, V.; Ross, M.; Raghuwanshi, V.S.; Kettemann, F.; Greis, K.; Ingber, T.T.K.; Stuckrath, J.B.; Valiyaveettil, S.; et al. Functionalized Cellulose for Water Purification, Antimicrobial Applications, and Sensors. Adv. Funct. Mater. 2018, 28, 1800409. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trcek, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Dichiara, A. Hybridization between cellulose nanofibrils and faceted silver nanoparticles used with surface enhanced Raman scattering for trace dye detection. Int. J. Biol. Macromol. 2020, 143, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Yuan, Z.Y.; Wang, A.; Wang, C.P.; Qu, J.L.; Chen, C.; Wei, B.; Kapu, N.S.; Wen, Y.B. Cellulose nanofibril-polymer hybrids for protecting drilling fluid at high salinity and high temperature. Carbohyd. Polym. 2020, 229, 115465. [Google Scholar] [CrossRef]
- Yang, J.; Xu, F.; Han, C.R. Metal ion mediated cellulose nanofibrils transient network in covalently cross-linked hydrogels: Mechanistic insight into morphology and dynamics. Biomacromolecules 2017, 18, 1019–1028. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, Y.S.; Liu, Y.; Lai, W.C.; Huang, F.; Ou, A.P.; Qin, R.; Liu, X.Y.; Wang, X. Ester crosslinking enhanced hydrophilic cellulose nanofibrils aerogel. ACS Sustain. Chem. Eng. 2018, 6, 11979–11988. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Onelli, O.D.; Jacucci, G.; Lovikka, V.; Rojas, O.J.; Ikkala, O.; Vignolini, S. Anomalous-diffusion-assisted brightness in white cellulose nanofibril membranes. Adv. Mater. 2018, 30, 1704050. [Google Scholar] [CrossRef]
- Hong, H.J.; Lim, J.S.; Hwang, J.Y.; Kim, M.; Jeong, H.S.; Park, M.S. Carboxymethlyated cellulose nanofibrils(CMCNFs) embedded in polyurethane foam as a modular adsorbent of heavy metal ions. Carbohyd. Polym. 2018, 195, 136–142. [Google Scholar] [CrossRef]
- Qin, F.M.; Fang, Z.Q.; Zhou, J.; Sun, C.; Chen, K.H.; Ding, Z.X.; Li, G.H.; Qiu, X.Q. Efficient removal of Cu2+ in water by carboxymethylated cellulose nanofibrils: Performance and mechanism. Biomacromolecules 2019, 20, 4466–4475. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Tapia-Orozco, N.; Ibarra-Cabrera, R.; Tecante, A.; Gimeno, M.; Parra, R.; Garcia-Arrazola, R. Removal strategies for endocrine disrupting chemicals using cellulose-based materials as adsorbents: A review. J. Environ. Chem. Eng. 2016, 4, 3122–3142. [Google Scholar] [CrossRef]
- Du, H.S.; Liu, W.M.; Zhang, M.L.; Si, C.L.; Zhang, X.Y.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohyd. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Chen, Y.M.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.H.; Duan, G.G.; Jiang, S.H.; Zhang, K. Recent progress on nanocellulose aerogels: Preparation, modification, composite fabrication, applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Misenan, M.S.M.; Janudin, N.; Shah, N.A.A.; Kasim, N.; Yusoff, W.Y.W.; Noor, S.A.M.; Jamal, S.H.; et al. Nanocellulose: A bioadsorbent for chemical contaminant remediation. RSC Adv. 2021, 11, 7347–7368. [Google Scholar] [CrossRef]
- Tshikovhi, A.; Mishra, S.B.; Mishra, A.K. Nanocellulose-based composites for the removal of contaminants from wastewater. Int. J. Biol. Macromol. 2020, 152, 616–632. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Haider, M.K.; Ullah, A.; Sarwar, M.N.; Saito, Y.; Sun, L.; Park, S.; Kim, I.S. Lignin-mediated in-situ synthesis of CuO nanoparticles on cellulose nanofibers: A potential wound dressing material. Int. J. Biol. Macromol. 2021, 173, 315–326. [Google Scholar] [CrossRef]
- Wahab, J.A.; Kim, I.S.; Ni, Q.Q. A comparative study on synthesis of AgNPs on cellulose nanofibers by thermal treatment and DMF for antibacterial activities. Mater. Sci. Eng. C 2019, 98, 1179–1195. [Google Scholar] [CrossRef]
- Habibi, S.; Jamshidi, M. Synthesis of TiO2 nanoparticles coated on cellulose nanofibers with different morphologies: Effect of the template and sol-gel parameters. Mater. Sci. Semicon. Proc. 2020, 109, 104927. [Google Scholar] [CrossRef]
- Gopiraman, M.; Saravanamoorthy, S.; Baskar, R.; Ilangovan, A.; Ill-Min, C. Green synthesis of Ag@Au bimetallic regenerated cellulose nanofibers for catalytic applications. New J. Chem. 2019, 43, 17090–17103. [Google Scholar] [CrossRef]
- Phan, D.N.; Dorjjugder, N.; Khan, M.Q.; Saito, Y.; Taguchi, G.; Lee, H.; Mukai, Y.; Kim, I.S. Synthesis and attachment of silver and copper nanoparticles on cellulose nanofibers and comparative antibacterial study. Cellulose 2019, 26, 6629–6640. [Google Scholar] [CrossRef]
- Yu, Z.C.; Hu, C.S.; Guan, L.T.; Zhang, W.W.; Gu, J. Green synthesis of cellulose nanofibrils decorated with Ag nanoparticles and their application in colorimetric detection of L-cysteine. ACS Sustain. Chem. Eng. 2020, 8, 12713–12721. [Google Scholar] [CrossRef]
- Bandi, R.; Alle, M.; Park, C.W.; Han, S.Y.; Kwon, G.J.; Kim, N.H.; Kim, J.C.; Lee, S.H. Cellulose nanofibrils/carbon dots composite nanopapers for the smartphone-based colorimetric detection of hydrogen peroxide and glucose. Sens. Actuators B 2021, 330. [Google Scholar] [CrossRef]
- Chen, M.W.; Liu, T.; Zhang, X.B.; Zhang, R.Q.; Tang, S.; Yuan, Y.H.; Xie, Z.J.; Liu, Y.J.; Wang, H.; Fedorovich, K.V.; et al. Photoinduced enhancement of uranium extraction from seawater by MOF/Black phosphorus quantum dots heterojunction anchored on cellulose nanofiber aerogel. Adv. Funct. Mater. 2021, 31, 2100106. [Google Scholar] [CrossRef]
- Guo, S.H.; Zhang, P.C.; Feng, Y.; Wang, Z.G.; Li, X.D.; Yao, J.F. Rational design of interlaced Co9S8/carbon composites from ZIF-67/cellulose nanofibers for enhanced lithium storage. J. Alloy. Compd. 2020, 818, 152911. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Chen, G.X.; Wang, X.J.; Zhou, S.H.; Yu, J.; Feng, X.; Li, L.W.; Chen, P.; Qi, H.S. Eco-friendly bioinspired interface design for high-performance cellulose nanofibril/carbon nanotube nanocomposites. ACS Appl. Mater. Interfaces 2020, 12, 55527–55535. [Google Scholar] [CrossRef] [PubMed]
- Visanko, M.; Liimatainen, H.; Sirvio, J.A.; Haapala, A.; Sliz, R.; Niinimaki, J.; Hormi, O. Porous thin film barrier layers from 2,3-dicarboxylic acid cellulose nanofibrils for membrane structures. Carbohyd. Polym. 2014, 102, 584–589. [Google Scholar] [CrossRef]
- Zhang, X.F.; Feng, Y.; Wang, Z.G.; Jia, M.M.; Yao, J.F. Fabrication of cellulose nanofibrils/UiO-66-NH2 composite membrane for CO2/N-2 separation. J. Membrane Sci. 2018, 568, 10–16. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, Y.B.; Liu, Z.X.; Zhang, B.L.; Zhang, Q.Y. Ultrathin, biomimetic multifunctional leaf-like silver nanowires/Ti(3)C(2)Tx MXene/cellulose nanofibrils nanocomposite film for high-performance electromagnetic interference shielding and thermal management. J. Alloy. Compd. 2021, 860, 158151. [Google Scholar] [CrossRef]
- Xu, W.L.; Xin, B.J.; Yang, X. Carbonization of electrospun polyacrylonitrile (PAN)/cellulose nanofibril (CNF) hybrid membranes and its mechanism. Cellulose 2020, 27, 3789–3804. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Si, J.H.; Cui, Z.X.; Ye, J.H.; Wang, X.F.; Wang, Q.T.; Peng, K.P.; Chen, W.Z.; Chen, S.C. Biomimetic composite scaffolds based on surface modification of polydopamine on electrospun poly(lactic acid)/cellulose nanofibrils. Carbohyd. Polym. 2017, 174, 750–759. [Google Scholar] [CrossRef]
- Naeem, M.A.; Alfred, M.; Lv, P.F.; Zhou, H.M.; Wei, Q.F. Three-dimensional bacterial cellulose-electrospun membrane hybrid structures fabricated through in-situ self-assembly. Cellulose 2018, 25, 6823–6830. [Google Scholar] [CrossRef]
- Zhou, X.X.; Wang, Y.; Gong, C.C.; Liu, B.; Wei, G. Production, structural design, functional control, and broad applications of carbon nanofiber-based nanomaterials: A comprehensive review. Chem. Eng. J. 2020, 402, 126189. [Google Scholar] [CrossRef]
- Zhou, X.X.; Liu, B.; Chen, Y.; Guo, L.; Wei, G. Carbon nanofiber-based three-dimensional nanomaterials for energy and environmental applications. Mater. Adv. 2020, 1, 2163–2181. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Narwade, V.N.; Bogle, K.A.; Kokol, V. TEMPO-oxidized cellulose nanofibrils–graphene oxide composite films with improved dye adsorption properties. Polym. Bull. 2020, 77, 6175–6189. [Google Scholar] [CrossRef]
- Sim, K.; Youn, H.J. Preparation of porous sheets with high mechanical strength by the addition of cellulose nanofibrils. Cellulose 2016, 23, 1383–1392. [Google Scholar] [CrossRef]
- Gorgieva, S.; Vogrincic, R.; Kokol, V. The effect of membrane structure prepared from carboxymethyl cellulose and cellulose nanofibrils for cationic dye removal. J. Polym. Environ. 2019, 27, 318–332. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, W.S.; Gong, C.C.; Liu, B.; Li, Y.D.; Wang, L.C.; Su, Z.Q.; Wei, G. Recent advances in the fabrication, functionalization, and bioapplications of peptide hydrogels. Soft Matter 2020, 16, 10029–10045. [Google Scholar] [CrossRef]
- Lu, J.S.; Han, X.; Dai, L.; Li, C.Y.; Wang, J.F.; Zhong, Y.D.; Yu, F.X.; Si, C.L. Conductive cellulose nanofibrils-reinforced hydrogels with synergetic strength, toughness, self-adhesion, flexibility and adjustable strain responsiveness. Carbohyd. Polym. 2020, 250, 117010. [Google Scholar] [CrossRef]
- Lu, P.; Liu, R.; Liu, X.; Wu, M. Preparation of self-supporting bagasse cellulose nanofibrils hydrogels induced by Zinc ions. Nanomaterials 2018, 8, 800. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Qu, J.L.; Wang, A.; Wang, C.P.; Chen, B.; Wang, Z.G.; Wu, B.B.; Wei, B.; Wen, Y.B.; Yuan, Z.Y. Hydrogels prepared from cellulose nanofibrils via ferric ion-mediated crosslinking reaction for protecting drilling fluid. Carbohyd. Polym. 2019, 212, 67–74. [Google Scholar] [CrossRef]
- Aarstad, O.; Heggset, E.B.; Pedersen, I.S.; Bjornoy, S.H.; Syverud, K.; Strand, B.L. Mechanical properties of composite hydrogels of alginate and cellulose nanofibrils. Polymers 2017, 9, 378. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.S.; Zhu, W.Y.; Dai, L.; Si, C.L.; Ni, Y.H. Fabrication of thermo- and pH-sensitive cellulose nanofibrils-reinforced hydrogel with biomass nanoparticles. Carbohyd. Polym. 2019, 215, 289–295. [Google Scholar] [CrossRef]
- Sun, X.H.; Tyagi, P.; Agate, S.; McCord, M.G.; Lucia, L.A.; Pal, L. Highly tunable bioadhesion and optics of 3D printable PNIPAm/cellulose nanofibrils hydrogels. Carbohyd. Polym. 2020, 234, 115898. [Google Scholar] [CrossRef]
- Baniasadi, H.; Ajdary, R.; Trifol, J.; Rojas, O.J.; Seppala, J. Direct ink writing of aloe vera/cellulose nanofibrils bio-hydrogels. Carbohyd. Polym. 2021, 266. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Zeng, J.S.; Hu, F.G.; Cheng, Z.; Wang, B.; Chen, K.F. Isolation and rheological characterization of cellulose nanofibrils (CNFs) produced by microfluidic homogenization, ball-milling, grinding and refining. Cellulose 2021, 28, 3389–3408. [Google Scholar] [CrossRef]
- Chen, N.S.; Zhu, J.Y.; Tong, Z.H. Fabrication of microfibrillated cellulose gel from waste pulp sludge via mild maceration combined with mechanical shearing. Cellulose 2016, 23, 2573–2583. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Xie, W.; Deng, H. Extraction, isolation and characterization of nanocrystalline cellulose from industrial kelp (Laminaria japonica) waste. Carbohyd. Polym. 2017, 173, 353–359. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing-thawing. J. Mater. Chem. A 2014, 2, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Mandin, S.; Moreau, S.; Talantikite, M.; Novales, B.; Maigret, J.E.; Cathala, B.; Moreau, C. Cellulose nanofibrils/xyloglucan bio-based aerogels with shape recovery. Gels 2021, 7, 5. [Google Scholar] [CrossRef]
- Olsson, R.T.; Samir, M.A.S.A.; Salazar-Alvarez, G.; Belova, L.; Strom, V.; Berglund, L.A.; Ikkala, O.; Nogues, J.; Gedde, U.W. Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat. Nanotechnol. 2010, 5, 584–588. [Google Scholar] [CrossRef]
- Li, Y.Z.; Grishkewich, N.; Liu, L.L.; Wang, C.; Tam, K.C.; Liu, S.Q.; Mao, Z.P.; Sui, X.F. Construction of functional cellulose aerogels via atmospheric drying chemically cross-linked and solvent exchanged cellulose nanofibrils. Chem. Eng. J. 2019, 366, 531–538. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kaskela, A.; Rojas, O.J.; Kauppinen, E.I.; Ikkala, O. Ambient-dried cellulose nanofibril aerogel membranes with high tensile strength and their use for aerosol collection and templates for transparent, flexible devices. Adv. Funct. Mater. 2015, 25, 6618–6626. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.Q.; Lu, C.H.; He, X.; Zhang, X.F.; Zhang, W.; Zhang, X.M. Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Francisco, E.; Bonilla-Cruz, J.; Marquez-Lamas, U.; Suarez-Jacobo, A.; Longoria-Rodriguez, F.; Rivera-Haro, J.; Russell, P.; Ali, Z.; Yin, C.Y.; Lara-Ceniceros, T.E. Entangled cellulose nanofibrils/nanosheets derived from native mexican agave for lead(II) ion removal. Cellulose 2020, 27, 8785–8798. [Google Scholar] [CrossRef]
- Vadakkekara, G.J.; Thomas, S.; Nair, C.P.R. Maleic acid modified cellulose for scavenging lead from water. Int. J. Biol. Macromol. 2019, 129, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Maatar, W.; Boufi, S. Poly(methacylic acid-co-maleic acid) grafted nanofibrillated cellulose as a reusable novel heavy metal ions adsorbent. Carbohyd. Polym. 2015, 126, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Keplinger, T.; Wang, X.Q.; Burgert, I. Nanofibrillated cellulose composites and wood derived scaffolds for functional materials. J. Mater. Chem. A 2019, 7, 2981–2992. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.C.; You, Y.Z.; Yang, X.; Jiang, X.; Lu, Q.L.; Wang, T.; Huang, B.; Lu, B.L. Microwave-assisted facile synthesis of TEMPO-oxidized cellulose beads with high adsorption capacity for organic dyes. Cellulose 2017, 24, 5025–5040. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.L.; Shi, C.; Yu, H.Q.; Sheng, G.P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef]
- Kose, K.; Mavlan, M.; Nuruddin, M.; Youngblood, J.P. TEMPO-oxidized cellulose nanofiber based polymeric adsorbent for use in iron removal. Cellulose 2020, 27, 4623–4635. [Google Scholar] [CrossRef]
- Yang, Z.H.; Ren, L.L.; Jin, L.F.; Huang, L.; He, Y.J.; Tang, J.W.; Yang, W.C.; Wang, H.Y. In-situ functionalization of poly(m-phenylenediamine) nanoparticles on bacterial cellulose for chromium removal. Chem. Eng. J. 2018, 344, 441–452. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Wang, R.; Wang, Y.; Zhang, X. A novel cellulose hydrogel coating with nanoscale Fe(0) for Cr(VI) adsorption and reduction. Sci. Total. Environ. 2020, 726, 138625. [Google Scholar] [CrossRef]
- Venalainen, S.H.; Hartikainen, H. Resource-efficient purification of acidic multi-metal process water by means of anionic nanofibrillated cellulose. J. Clean. Prod. 2018, 185, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Anirudhan, T.S.; Rauf, T.A.; Rejeena, S.R. Removal and recovery of phosphate ions from aqueous solutions by amine functionalized epichlorohydrin-grafted cellulose. Desalination 2012, 285, 277–284. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Senan, P. Adsorption of phosphate ions from water using a novel cellulose-based adsorbent. Chem. Ecol. 2011, 27, 147–164. [Google Scholar] [CrossRef]
- Cui, G.R.; Liu, M.; Chen, Y.; Zhang, W.; Zhao, J.Q. Synthesis of a ferric hydroxide-coated cellulose nanofiber hybrid for effective removal of phosphate from wastewater. Carbohyd. Polym. 2016, 154, 40–47. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Chen, K.L.; Zhou, J.L.; Liu, Q.X. Removal of heavy metal and sulfate ions by cellulose derivative-based biosorbents. Cellulose 2018, 25, 2531–2545. [Google Scholar] [CrossRef]
- Muqeet, M.; Malik, H.; Mahar, R.B.; Ahmed, F.; Khatri, Z.; Carlson, K. Cationization of cellulose nanofibers for the removal of sulfate ions from aqueous solutions. Ind. Eng. Chem. Res. 2017, 56, 14078–14088. [Google Scholar] [CrossRef]
- Yu, X.L.; Tong, S.R.; Ge, M.F.; Zuo, J.C. Removal of fluoride from drinking water by cellulose@hydroxyapatite nanocomposites. Carbohyd. Polym. 2013, 92, 269–275. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.J.; Wang, H.C.; Sedgwick, A.C.; Gardiner, J.E.; Bull, S.D.; Xiao, H.N.; James, T.D. Dual-function cellulose composites for fluorescence detection and removal of fluoride. Dyes Pigments 2018, 149, 669–675. [Google Scholar] [CrossRef]
- Li, D.P.; Ning, X.A.; Yuan, Y.Q.; Hong, Y.X.; Zhang, J.P. Ion-exchange polymers modified bacterial cellulose electrodes for the selective removal of nitrite ions from tail water of dyeing wastewater. J. Environ. Sci. 2020, 91, 62–72. [Google Scholar] [CrossRef]
- Purington, E.; Bousfield, D.; Gramlich, W.M. Fluorescent dye adsorption in aqueous suspension to produce tagged cellulose nanofibers for visualization on paper. Cellulose 2019, 26, 5117–5131. [Google Scholar] [CrossRef]
- Stefelova, J.; Slovak, V.; Siqueira, G.; Olsson, R.T.; Tingaut, P.; Zimmermann, T.; Sehaqui, H. Drying and pyrolysis of cellulose nanofibers from wood, bacteria, and algae for char application in oil absorption and dye adsorption. ACS Sustain. Chem. Eng. 2017, 5, 2679–2692. [Google Scholar] [CrossRef]
- Quinlan, P.J.; Tanvir, A.; Tam, K.C. Application of the central composite design to study the flocculation of an anionic azo dye using quaternized cellulose nanofibrils. Carbohyd. Polym. 2015, 133, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Maatar, W.; Boufi, S. Microporous cationic nanofibrillar cellulose aerogel as promising adsorbent of acid dyes. Cellulose 2017, 24, 1001–1015. [Google Scholar] [CrossRef]
- Sehaqui, H.; de Larraya, U.P.; Tingaut, P.; Zimmermann, T. Humic acid adsorption onto cationic cellulose nanofibers for bioinspired removal of copper(II) and a positively charged dye. Soft Matter 2015, 11, 5294–5300. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.J.; Li, X.X.; Li, J.Y.; Wang, L.W.; Jin, W.J.; Liu, J.; Pei, Y.; Tang, K.Y. Efficient removal of anionic dye (Congo red) by dialdehyde microfibrillated cellulose/chitosan composite film with significantly improved stability in dye solution. Int. J. Biol. Macromol. 2018, 107, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, A.; Sabzi, M.; Azimi, H. 3D porous bioadsorbents based on chitosan/alginate/cellulose nanofibers as efficient and recyclable adsorbents of anionic dye. Carbohyd. Polym. 2021, 265, 118075. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Huang, Y.J. Bacterial cellulose nanofibers decorated with phthalocyanine: Preparation, characterization and dye removal performance. Mater. Lett. 2015, 142, 235–237. [Google Scholar] [CrossRef]
- Chen, S.L.; Huang, X.J.; Xu, Z.K. Decoration of phthalocyanine on multiwalled carbon nanotubes/cellulose nanofibers nanocomposite for decoloration of dye wastewater. Compos. Sci. Technol. 2014, 101, 11–16. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhang, X.F.; He, X.; Zhang, W.; Zhang, X.X.; Lu, C.H. In situ synthesis of MnO2 coated cellulose nanofibers hybrid for effective removal of methylene blue. Carbohyd. Polym. 2014, 110, 302–308. [Google Scholar] [CrossRef]
- Sun, B.F.; Yuan, Y.N.; Li, H.L.; Li, X.Y.; Zhang, C.H.; Guo, F.; Liu, X.H.; Wang, K.A.; Zhao, X.S. Waste-cellulose-derived porous carbon adsorbents for methyl orange removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Hasanpour, M.; Motahari, S.; Jing, D.; Hatami, M. Investigation of operation parameters on the removal efficiency of methyl orange pollutant by cellulose/zinc oxide hybrid aerogel. Chemosphere 2021, 284, 131320. [Google Scholar] [CrossRef] [PubMed]
- Vala, R.M.K.; Tichagwa, L.; Dikio, E.D. Evaluation of N-terminated siloxanes grafted onto lignocellulose as adsorbent for the removal of phenol red from water. Int. J. Environ. Sci. Technol. 2015, 12, 2723–2730. [Google Scholar] [CrossRef] [Green Version]
- Narwade, V.N.; Khairnar, R.S.; Kokol, V. In-situ synthesised hydroxyapatite-loaded films based on cellulose nanofibrils for phenol removal from wastewater. Cellulose 2017, 24, 4911–4925. [Google Scholar] [CrossRef] [Green Version]
- Hoang, A.T.; Nizetic, S.; Duong, X.Q.; Rowinski, L.; Nguyen, X.P. Advanced super-hydrophobic polymer-based porous absorbents for the treatment of oil-polluted water. Chemosphere 2021, 277, 130274. [Google Scholar] [CrossRef]
- Liu, H.Z.; Geng, B.Y.; Chen, Y.F.; Wang, H.Y. Review on the aerogel-type oil sorbents derived from nanocellulose. ACS Sustain. Chem. Eng. 2017, 5, 49–66. [Google Scholar] [CrossRef]
- Fu, Q.L.; Ansari, F.; Zhou, Q.; Berglund, L.A. Wood nanotechnology for strong, mesoporous, and hydrophobic biocomposites for selective separation of oil/water mixtures. ACS Nano 2018, 12, 2222–2230. [Google Scholar] [CrossRef]
- Kollarigowda, R.H.; Abraham, S.; Montemagno, C.D. Antifouling cellulose hybrid biomembrane for effective oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 29812–29819. [Google Scholar] [CrossRef]
- Zhu, W.X.; Huang, W.; Zhou, W.H.; Qiu, Z.; Wang, Z.; Li, H.J.; Wang, Y.G.; Li, J.; Xie, Y.J. Sustainable and antibacterial sandwich-like Ag-Pulp/CNF composite paper for oil/water separation. Carbohyd. Polym. 2020, 245, 116587. [Google Scholar] [CrossRef]
- Mulyadi, A.; Zhang, Z.; Deng, Y.L. Fluorine-Free Oil Absorbents Made from Cellulose Nanofibril Aerogels. ACS Appl. Mater. Interfaces 2016, 8, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, O.; Suopajarvi, T.; Osterberg, M.; Liimatainen, H. Hydrophobic, superabsorbing aerogels from choline chloride-based deep eutectic solvent pretreated and silylated cellulose nanofibrils for selective oil removal. ACS Appl. Mater. Interfaces 2017, 9, 25029–25037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.N.; Xiao, S.L.; Gan, W.T.; Liu, Q.; Amer, H.; Rosenau, T.; Li, J.; Lu, Y. Mussel adhesive-inspired design of superhydrophobic nanofibrillated cellulose aerogels for oil/water separation. ACS Sustain. Chem. Eng. 2018, 6, 9047–9055. [Google Scholar] [CrossRef]
- Sun, F.F.; Liu, W.; Dong, Z.X.; Deng, Y.L. Underwater superoleophobicity cellulose nanofibril aerogel through regioselective sulfonation for oil/water separation. Chem. Eng. J. 2017, 330, 774–782. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.F.; Xu, P.; Chen, B.B. Super-elastic and highly hydrophobic/superoleophilic sodium alginate/cellulose aerogel for oil/water separation. Cellulose 2018, 25, 3533–3544. [Google Scholar] [CrossRef]
- Tabuchi, M.; Baba, Y. Design for DNA separation medium using bacterial cellulose fibrils. Anal Chem 2005, 77, 7090–7093. [Google Scholar] [CrossRef]
- Demirci, S.; Celebioglu, A.; Uyar, T. Surface modification of electrospun cellulose acetate nanofibers via RAFT polymerization for DNA adsorption. Carbohyd. Polym. 2014, 113, 200–207. [Google Scholar] [CrossRef]
- Rajesh, S.; Crandall, C.; Schneiderman, S.; Menkhaus, T.J. Cellulose-graft-polyethyleneamidoamine anion-exchange nanofiber membranes for simultaneous protein adsorption and virus filtration. ACS Appl. Nano Mater. 2018, 1, 3321–3330. [Google Scholar] [CrossRef]
- Hassan, M.; Abou-Zeid, R.; Hassan, E.; Berglund, L.; Aitomaki, Y.; Oksman, K. Membranes based on cellulose nanofibers and activated carbon for removal of escherichia coli bacteria from water. Polymers 2017, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Mi, X.; Albukhari, S.M.; Heldt, C.L.; Heiden, P.A. Virus and chlorine adsorption onto guanidine modified cellulose nanofibers using covalent and hydrogen bonding. Carbohyd. Res. 2020, 498, 108153. [Google Scholar] [CrossRef]
- Huang, W.J.; Wang, Y.X.; Chen, C.; Law, J.L.M.; Houghton, M.; Chen, L.Y. Fabrication of flexibleself-standing all-cellulose nanofibrous composite membranes for virus removal. Carbohyd. Polym. 2016, 143, 9–17. [Google Scholar] [CrossRef]
- Gustafsson, S.; Mihranyan, A. Strategies for tailoring the pore-size distribution of virus retention filter papers. ACS Appl. Mater. Interfaces 2016, 8, 13759–13767. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Tao, J.H.; Ma, C.X.; Yang, J.; Gu, T.T.; Liu, J.C. Carboxylated cellulose nanofiber/montmorillonite nanocomposite for the removal of levofloxacin hydrochloride antibiotic from aqueous solutions. RSC Adv. 2020, 10, 42038–42053. [Google Scholar] [CrossRef]
- Yao, Q.F.; Fan, B.T.; Xiong, Y.; Jin, C.D.; Sun, Q.F.; Sheng, C.M. 3D assembly based on 2D structure of cellulose nanofibril/graphene oxide hybrid aerogel for adsorptive removal of antibiotics in water. Sci. Rep. 2017, 7, 45914. [Google Scholar] [CrossRef] [Green Version]
- Khatri, V.; Halasz, K.; Trandafilovic, L.V.; Dimitrijevic-Brankovic, S.; Mohanty, P.; Djokovic, V.; Csoka, L. ZnO-modified cellulose fiber sheets for antibody immobilization. Carbohyd. Polym. 2014, 109, 139–147. [Google Scholar] [CrossRef]
- Zeng, Z.H.; Wu, T.T.; Han, D.X.; Ren, Q.; Siqueira, G.; Nystrom, G. Ultralight, flexible, and biomimetic nanocellulose/silver nanowire aerogels for electromagnetic interference shielding. ACS Nano 2020, 14, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
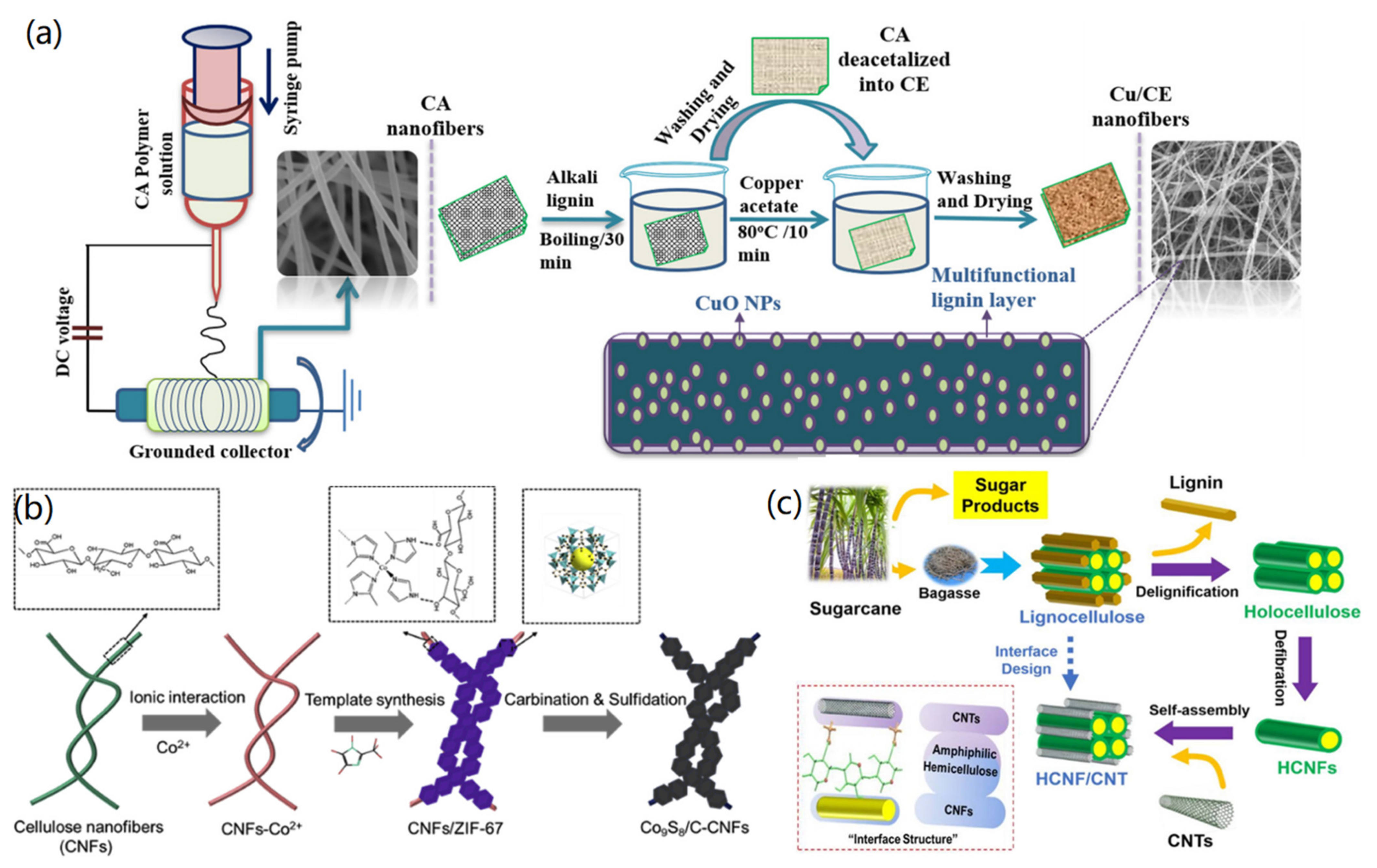







| Dimension | Materials | Synthesis Method | Properties and Applications | Ref. |
|---|---|---|---|---|
| 1D CNF composites | CuO/CNF | Electrospinning, templated synthesis | Antioxidant activity, antibacterial activity | [25] |
| Ag/CNF | Thermal treatment and DMF reduction | Antibacterial activity | [26] | |
| TiO2/CNF | Sol-gel synthesis | Photodegradation of methylene blue | [27] | |
| Ag@Au/CNF | Ionic binding and green synthesis | Catalytic activity, nitrophenol reduction and aza-Michael reactions | [28] | |
| Cu/CNF and Ag/CNF | Ionic binding and chemical reduction | Antibacterial study | [29] | |
| Ag/CNF | Green synthesis | Colorimetric sensors | [30] | |
| Carbon dot/CNF | Microwave synthesis | Colorimetric biosensors | [31] | |
| BP@/CNF-MOF | In situ solvothermal synthesis | Photocatalytic reduction of U(VI) | [32] | |
| ZIF-67/CNF | Ionic interaction, templated synthesis | Catalytic activity, energy storage | [33] | |
| HCNF/CNT | Self-assembly and interface interactions | High strength, high relative humidity, good electrical conductivity | [34] | |
| 2D films/membranes | Carboxylic CNFs | Vacuum filtration | High rejection efficiencies (74–80%) | [35] |
| CNF/MOF hybrids | Vacuum filtration | CO2 and N2 separation | [36] | |
| Ag NWs-Ti3C2Tx/CNFs | Vacuum filtration | Electromagnetic interference shielding and thermal management | [37] | |
| PAN/CNF | Electrospinning | High breaking strength and conductivity | [38] | |
| PLA/CNF | Electrospinning | Enhanced bioactivity, cell culture | [39] | |
| Polymer/CNF | Electrospinning and self-assembly | Interlocking multiple membrane layers | [40] | |
| 3D hydrogels | CNFs/PAA | Fe3+-mediated gelation | High stability, flexibility, conductivity, wearable devices | [47] |
| TEMPO-CNFs | Zn2+-mediated gelation | Colorimetric indicator of food spoilage | [48] | |
| P(AMPS-DMA)-CNF | Fe3+-mediated gelation | Salt tolerance and thermal stability | [49] | |
| CNF/alginate | Electrostatic interaction | Tissue engineering and regenerative medicine | [50] | |
| AL-PEG-CNFs | Self-assembling and -COOH/NH2 interaction | Thermo- and pH-responsive | [51] | |
| PNIPAm/CNF | 3D printing | Temperature-sensitive antibacterial properties | [52] | |
| Aloe vera/CNF | Ink printing | Porosity higher than 80% and a high-water uptake capacity of up to 46 g/g | [53] | |
| 3D aerogels | TEMPO-CNFs | Freeze-thawing | Super water absorption and shape memory | [58] |
| CNF/xyloglucan | Freeze-casting | Water absorption and shape memory | [59] | |
| Magnetic CNFs | Freeze-casting | Dry, lightweight, porous, flexible | [60] | |
| CNF-Si-PEI | Atmospheric drying | Very high specific surface area, and good flexibility | [61] | |
| CNFs | Freeze-thawing and ambient drying | Highly tensile, transparent, flexible | [62] | |
| PEI/CNFs | Freeze-drying | Drug delivery | [63] | |
| BP@CNF-MOF | Freeze-drying, solvothermal synthesis, drying | Photocatalytic reduction of U(IV) | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Guo, L.; Wei, G. Recent Advances in the Fabrication and Environmental Science Applications of Cellulose Nanofibril-Based Functional Materials. Materials 2021, 14, 5390. https://doi.org/10.3390/ma14185390
Zhang L, Guo L, Wei G. Recent Advances in the Fabrication and Environmental Science Applications of Cellulose Nanofibril-Based Functional Materials. Materials. 2021; 14(18):5390. https://doi.org/10.3390/ma14185390
Chicago/Turabian StyleZhang, Lianming, Lei Guo, and Gang Wei. 2021. "Recent Advances in the Fabrication and Environmental Science Applications of Cellulose Nanofibril-Based Functional Materials" Materials 14, no. 18: 5390. https://doi.org/10.3390/ma14185390
APA StyleZhang, L., Guo, L., & Wei, G. (2021). Recent Advances in the Fabrication and Environmental Science Applications of Cellulose Nanofibril-Based Functional Materials. Materials, 14(18), 5390. https://doi.org/10.3390/ma14185390







