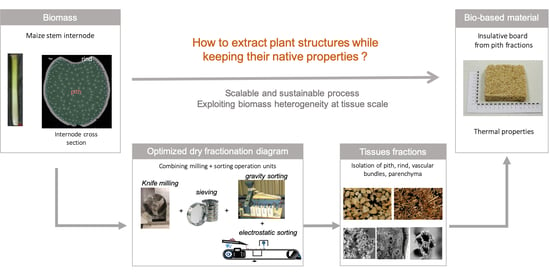
Preserving the Cellular Tissue Structure of Maize Pith Though Dry Fractionation Processes: A Key Point to Use as Insulating Agro-Materials
Abstract
Highlights
- Pith and rind were isolated from maize stem after knife milling and density sorting
- Vascular bundles were sorted using electrostatic separation
- Shear stress during grinding preserves the cellular structure of maize pith in the particles
- Insulating boards made with maize pith exhibit thermal conductivities similar to conventional insulating materials
- Pith particles isolated by dry fractionation processes keep their thermal insulative properties
1. Introduction
2. Material and Methods
2.1. Materials
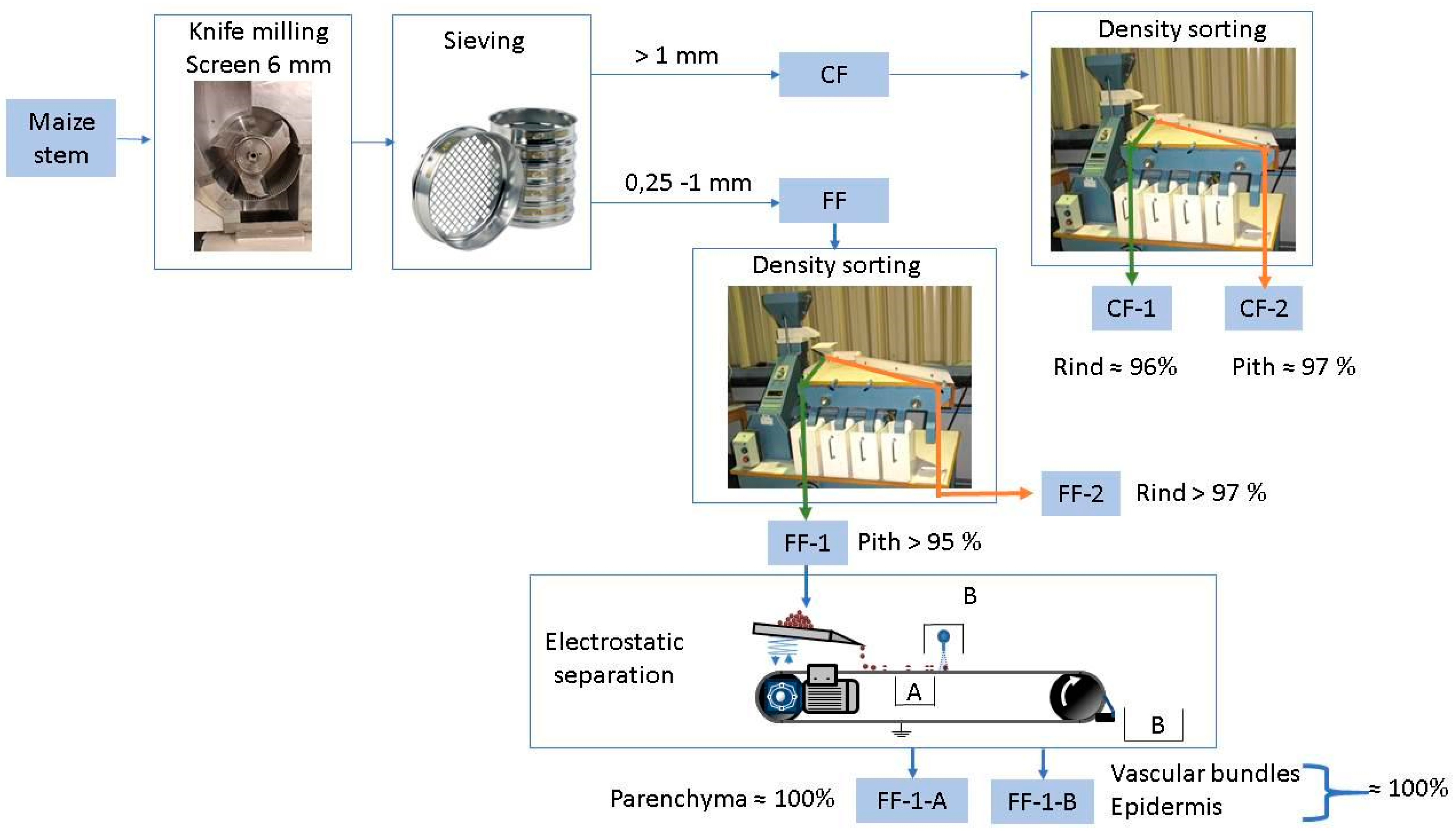
2.2. Dry Fractionation Processes
2.3. Particle Characterization
2.4. Preparation of Insulating Thermal Boards and Thermal Characterization
3. Results and Discussion
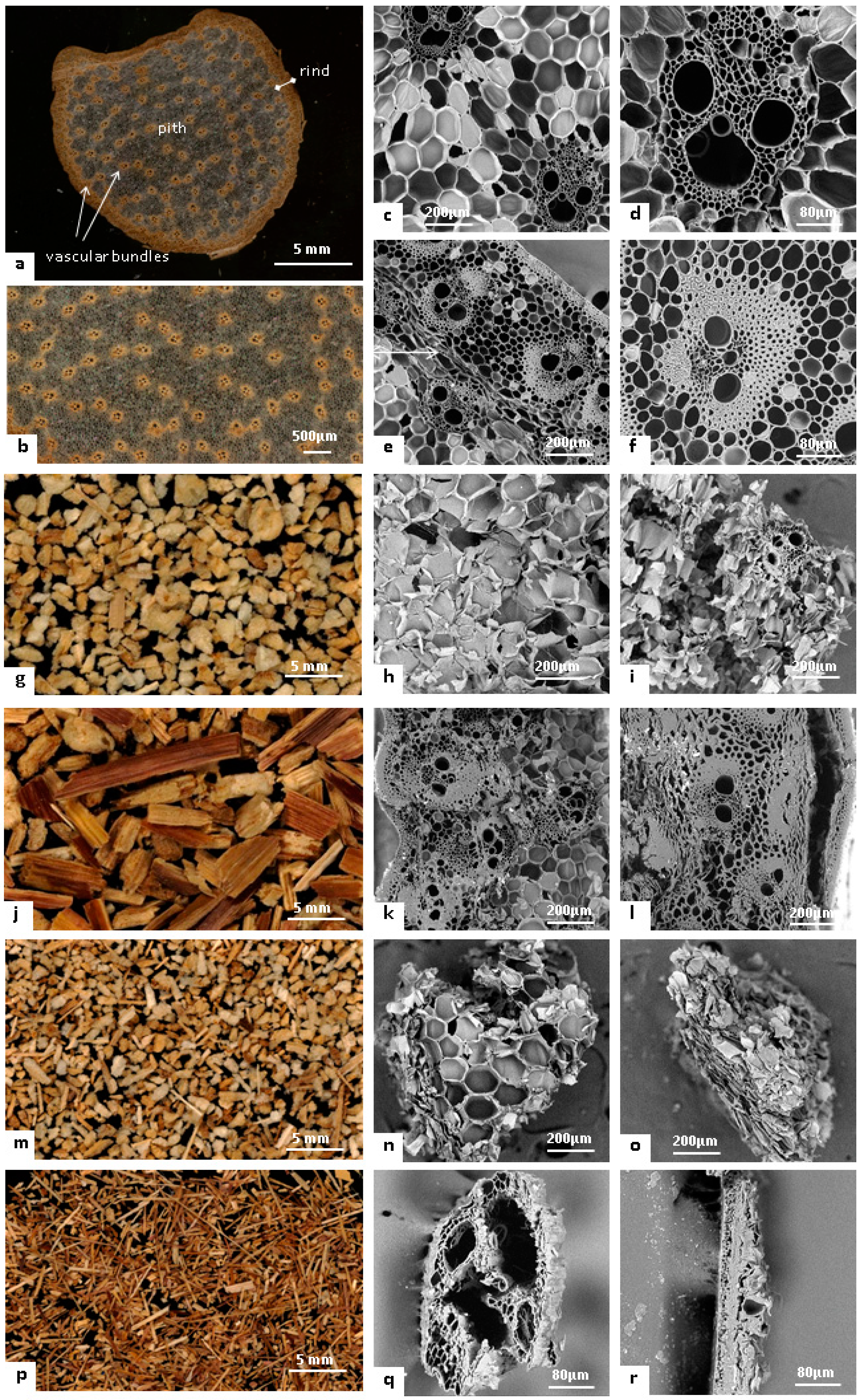
3.1. Pith and Rind Isolation from Maize Stem
3.2. Isolation of Vascular Bundles
3.3. Insulation Materials from Pith Tissue: Impact of Isolation Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature (See Figure 1)
| CF | Coarse fraction obtained from knife milling (6 mm sieving mesh) and sieving (>1 mm) |
| CF_1 | Dense fraction obtained from gravity sorting of the coarse fraction (CF) |
| CF_2 | Light fraction obtained from the gravity sorting of the coarse fraction (CF) |
| DF_Coarse | Dry fractionated coarse pith fraction obtained after gravity sorting of the >2 mm sieved fraction from knife milling (10 mm sieving mesh). |
| DF_Medium | Dry fractionated medium pith fraction obtained after gravity sorting of the 1–2 mm sieved fraction from knife milling (10 mm sieving mesh) |
| FF | Fine fraction obtained from knife milling (6 mm sieving mesh) and sieving (0.25–1 mm) |
| FF-1 | Dense fraction obtained from gravity sorting of the fine fraction (FF) |
| FF-2 | Light fraction obtained from the gravity sorting of the fine fraction (FF) |
| FF-1-A | Fraction deviated by the ionic cloud during the electrostatic sorting of the fine fraction 1 (FF-1) |
| FF-1-B | Fraction not deviated by the ionic cloud during the electrostatic sorting of the fine fraction 1 (FF-1) |
| Manual_Pith | Manually extracted fraction, by hand dissection of the stem |
| SEM | Scanning Electron Microscopy |
References
- Stafford-Smith, M.; Griggs, D.; Gaffney, O.; Ullah, F.; Reyers, B.; Kanie, N.; Stigson, B.; Shrivastava, P.; Leach, M.; O’Connell, D. Integration: The key to implementing the Sustainable Development Goals. Sustain. Sci. 2017, 12, 911–919. [Google Scholar] [CrossRef]
- Gerssen-Gondelach, S.; Saygin, D.; Wicke, B.; Patel, M.; Faaij, A. Competing uses of biomass: Assessment and comparison of the performance of bio-based heat, power, fuels and materials. Renew. Sustain. Energy Rev. 2014, 40, 964–998. [Google Scholar] [CrossRef]
- Madurwar, M.V.; Ralegaonkar, R.V.; Mandavgane, S. Application of agro-waste for sustainable construction materials: A review. Constr. Build. Mater. 2013, 38, 872–878. [Google Scholar] [CrossRef]
- Toor, M.; Kumar, S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef] [PubMed]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Pavel, C.; Blagoeva, D. Competitive Landscape of the EU’s Insulation Materials Industry for Energy-Efficient Buildings; JRC Techical Report; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/building-insulation-materials-market-510.html (accessed on 25 August 2021).
- Muscat, A.; de Olde, E.M.; de Boer, I.; Ripoll-Bosch, R. The battle for biomass: A systematic review of food-feed-fuel competition. Glob. Food Secur. 2020, 25, 100330. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.; Kovacic, Z.; de Boer, I.; Ripoll-Bosch, R. Food, energy or biomaterials? Policy coherence across agro-food and bioeconomy policy domains in the EU. Environ. Sci. Policy 2021, 123, 21–30. [Google Scholar] [CrossRef]
- Mayer-Laigle, C.; Blanc, N.; Rajaonarivony, R.K.; Rouau, X. Comminution of Dry Lignocellulosic Biomass, a Review: Part, I. From Fundamental Mechanisms to Milling Behaviour. Bioeng. 2018, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Rouau, X. New dry technology of environmentally friendly biomass refinery: Glucose yield and energy efficiency. Biotechnol. Biofuels 2014, 7, 138. [Google Scholar] [CrossRef]
- Palumbo, M.; Avellaneda, J.; Lacasta, A. Availability of crop by-products in Spain: New raw materials for natural thermal insulation. Resour. Conserv. Recycl. 2015, 99, 1–6. [Google Scholar] [CrossRef]
- Esau, K. Anatomy of Seed Plants; John Willey & Sons: New York, New York, USA, 1977; p. 550. [Google Scholar]
- Mati-Baouche, N.; De Baynast, H.; Lebert, A.; Sun, S.; Lopez-Mingo, C.J.S.; Leclaire, P.; Michaud, P. Mechanical, thermal and acoustical characterizations of an insulating bio-based composite made from sunflower stalks particles and chitosan. Ind. Crop. Prod. 2014, 58, 244–250. [Google Scholar] [CrossRef]
- Pinto, J.; Cruz, D.; Paiva, A.; Pereira, S.; Tavares, P.; Fernandes, L.S.G.; Varum, H. Characterization of corn cob as a possible raw building material. Constr. Build. Mater. 2012, 34, 28–33. [Google Scholar] [CrossRef]
- Pinto, J.; Paiva, A.; Varum, H.; Costa, A.; Cruz, D.; Pereira, S. Corn’s cob as a potential ecological thermal insulation material. Ener. Build. 2011, 43, 1985–1990. [Google Scholar] [CrossRef]
- Viel, M.; Collet, F.; Lanos, C. Development and characterization of thermal insulation materials from renewable resources. Constr. Build. Mater. 2019, 214, 685–697. [Google Scholar] [CrossRef]
- Czajkowski, Ł.; Wojcieszak, D.; Olek, W.; Przybył, J. Thermal properties of fractions of corn stover. Constr. Build. Mater. 2019, 210, 709–712. [Google Scholar] [CrossRef]
- Etuk, S.; Akpabio, L.; Akpan, I. Comparative study of thermal transport in Zea mays straw and Zea mays heartwood (cork) boards. Therm. Sci. 2010, 14, 31–38. [Google Scholar] [CrossRef]
- Palumbo, M. Contribution to the Development of New Bio-Based Termal Insulation Materials Made from Vegetal Pith and Natural Binders. Universitat Politècnica de Catalunya, 2015. Available online: http://tdx.cat/handle/10803/314580 (accessed on 8 September 2021).
- Palumbo, M.; Lacasta, A.M.; Giraldo, M.P.; Haurie, L.; Correal, E. Bio-based insulation materials and their hygrothermal per-formance in a building envelope system (ETICS), Ener. Build. 2018, 174, 147–155. [Google Scholar]
- Anderson, W.F.; Akin, D.E. Structural and chemical properties of grass lignocelluloses related to conversion for biofuels. J. Ind. Microbiol. Biotechnol. 2008, 35, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xia, Q.; Zha, B.; Sun, J.; Xu, B.; Chen, Z. Triboelectric separation of wheat bran tissues: Influence of tribo-material, water content, and particle size. J. Food Process. Eng. 2019, 43, e13346. [Google Scholar] [CrossRef]
- Jin, S.; Chen, H. Fractionation of fibrous fraction from steam-exploded rice straw. Process. Biochem. 2007, 42, 188–192. [Google Scholar] [CrossRef]
- Papatheofanous, M.; Billa, E.; Koullas, D.; Monties, B.; Koukios, E. Optimizing multisteps mechanical-chemical fractionation of wheat straw components. Ind. Crop. Prod. 1998, 7, 249–256. [Google Scholar] [CrossRef]
- Motte, J.-C.; Delenne, J.-Y.; Barron, C.; Dubreucq, E.; Mayer-Laigle, C. Elastic properties of packing of granulated cork: Effect of particle size. Ind. Crop. Prod. 2017, 99, 126–134. [Google Scholar] [CrossRef]
- Mayer-Laigle, C.; Bourmaud, A.; Shah, D.; Follain, N.; Beaugrand, J. Unravelling the consequences of ultra-fine milling on physical and chemical characteristics of flax fibres. Powder Technol. 2020, 360, 129–140. [Google Scholar] [CrossRef]
- Mayer-Laigle, C.; Barakat, A. Electrostatic Separation as an Entry into Environmentally Eco-Friendly Dry Biorefining of Plant Materials. J. Biosens. Bioelectron. 2017, 8, 08. [Google Scholar] [CrossRef]
- Chen, H. Integrated industrial lignocellulose biorefinery chains. In Lignocellulose Biorefinery Engineering; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 219–245. [Google Scholar]
- Wilson, J.R.; Mertens, D.R.; Hatfield, R.D. Isolates of cell-types from sorghum stems—Digestion, cell-wall and anatomical characteristics. J. Sci. Food Agric. 1993, 63, 407–417. [Google Scholar] [CrossRef]
- Legland, D.; El-Hage, F.; Méchin, V.; Reymond, M. Histological quantification of maize stem sections from FASGA-stained images. Plant Methods 2017, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; De Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review. Bioresour. Technol. 2013, 134, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Trivelato, P.; Mayer-Laigle, C.; Barakat, A.; Fulcrand, H.; Aouf, C. Douglas bark dry fractionation for polyphenols isolation: From forestry waste to added value products. Ind. Crop. Prod. 2016, 86, 12–15. [Google Scholar] [CrossRef]
- Nuez, L.; Beaugrand, J.; Shah, D.; Mayer-Laigle, C.; Bourmaud, A.; D’Arras, P.; Baley, C. The potential of flax shives as reinforcements for injection moulded polypropylene composites. Ind. Crop. Prod. 2020, 148, 112324. [Google Scholar] [CrossRef]
- Barros-Rios, J.; Santiago, R.; Malvar, R.A.; Jung, H.-J.G. Chemical composition and cell wall polysaccharide degradability of pith and rind tissues from mature maize internodes. Anim. Feed Sci. Technol. 2012, 172, 226–236. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, H.; Zhang, Y.; Yu, L. Cell morphology and chemical characteristics of corn stover fractions. Ind. Crop. Prod. 2012, 37, 130–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Legay, S.; Barrière, Y.; Méchin, V.; Legland, D. Color Quantification of Stained Maize Stem Section Describes Lignin Spatial Distribution within the Whole Stem. J. Agric. Food Chem. 2013, 61, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
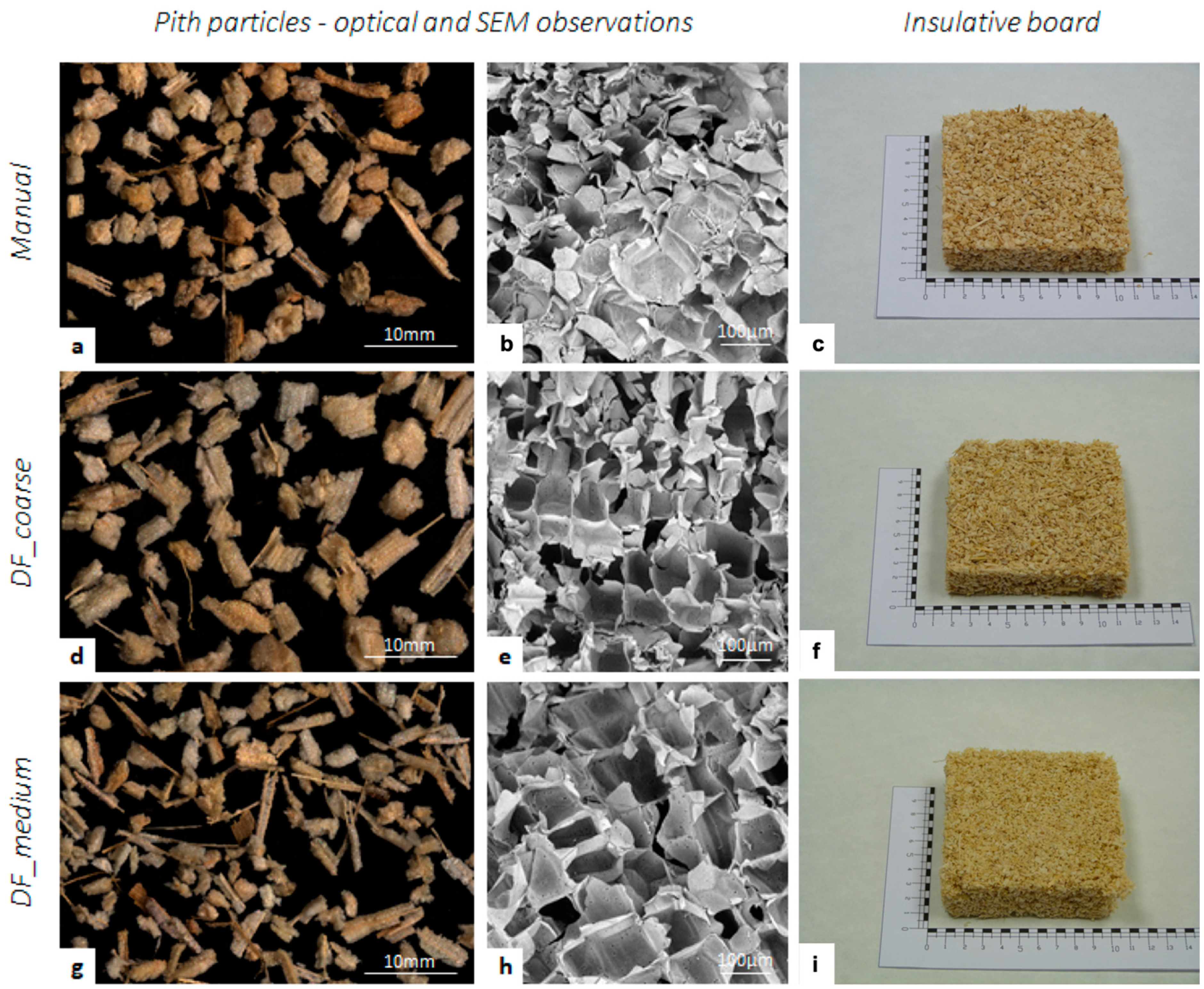
| Manual_Pith | DF_Coarse | DF_Medium | |
|---|---|---|---|
| Density (kg·m−3) | 37 | 33 | 23 |
| Thermal conductivity λ (Wm·K−1) | 0.039 | 0.037 | 0.035 |
| Volumetric heat capacity α (J m−3·K−1) | 0.953 × 10−6 | 0.916 × 10−6 | 1.115 × 10−6 |
| c·ρ (m2·s−1) | 0.040 × 106 | 0.040 × 106 | 0.031 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer-Laigle, C.; Haurie Ibarra, L.; Breysse, A.; Palumbo, M.; Mabille, F.; Lacasta Palacio, A.M.; Barron, C. Preserving the Cellular Tissue Structure of Maize Pith Though Dry Fractionation Processes: A Key Point to Use as Insulating Agro-Materials. Materials 2021, 14, 5350. https://doi.org/10.3390/ma14185350
Mayer-Laigle C, Haurie Ibarra L, Breysse A, Palumbo M, Mabille F, Lacasta Palacio AM, Barron C. Preserving the Cellular Tissue Structure of Maize Pith Though Dry Fractionation Processes: A Key Point to Use as Insulating Agro-Materials. Materials. 2021; 14(18):5350. https://doi.org/10.3390/ma14185350
Chicago/Turabian StyleMayer-Laigle, Claire, Laia Haurie Ibarra, Amélie Breysse, Marina Palumbo, Frédéric Mabille, Ana Maria Lacasta Palacio, and Cécile Barron. 2021. "Preserving the Cellular Tissue Structure of Maize Pith Though Dry Fractionation Processes: A Key Point to Use as Insulating Agro-Materials" Materials 14, no. 18: 5350. https://doi.org/10.3390/ma14185350
APA StyleMayer-Laigle, C., Haurie Ibarra, L., Breysse, A., Palumbo, M., Mabille, F., Lacasta Palacio, A. M., & Barron, C. (2021). Preserving the Cellular Tissue Structure of Maize Pith Though Dry Fractionation Processes: A Key Point to Use as Insulating Agro-Materials. Materials, 14(18), 5350. https://doi.org/10.3390/ma14185350