Effects of a Natural Mordenite as Pozzolan Material in the Evolution of Mortar Settings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
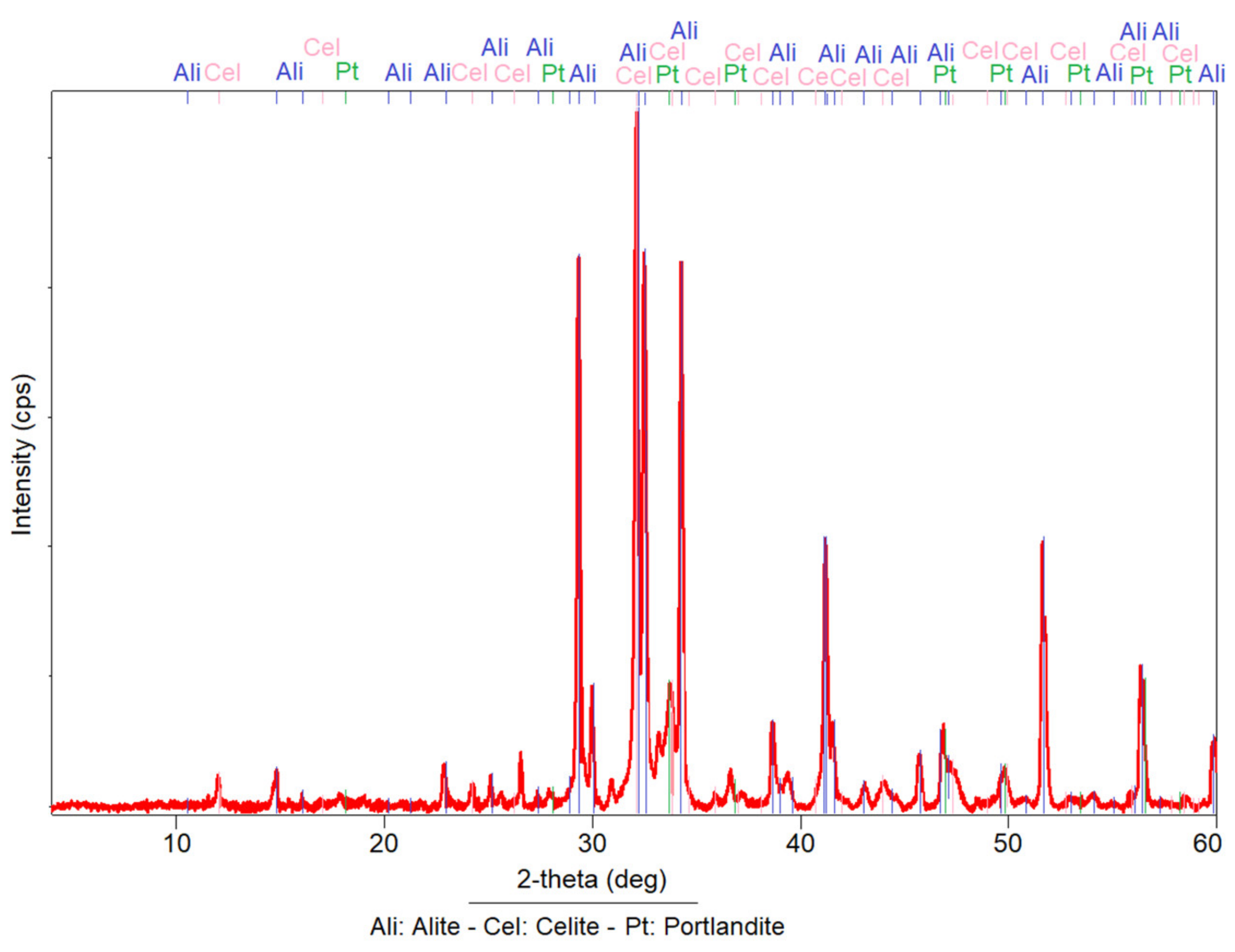
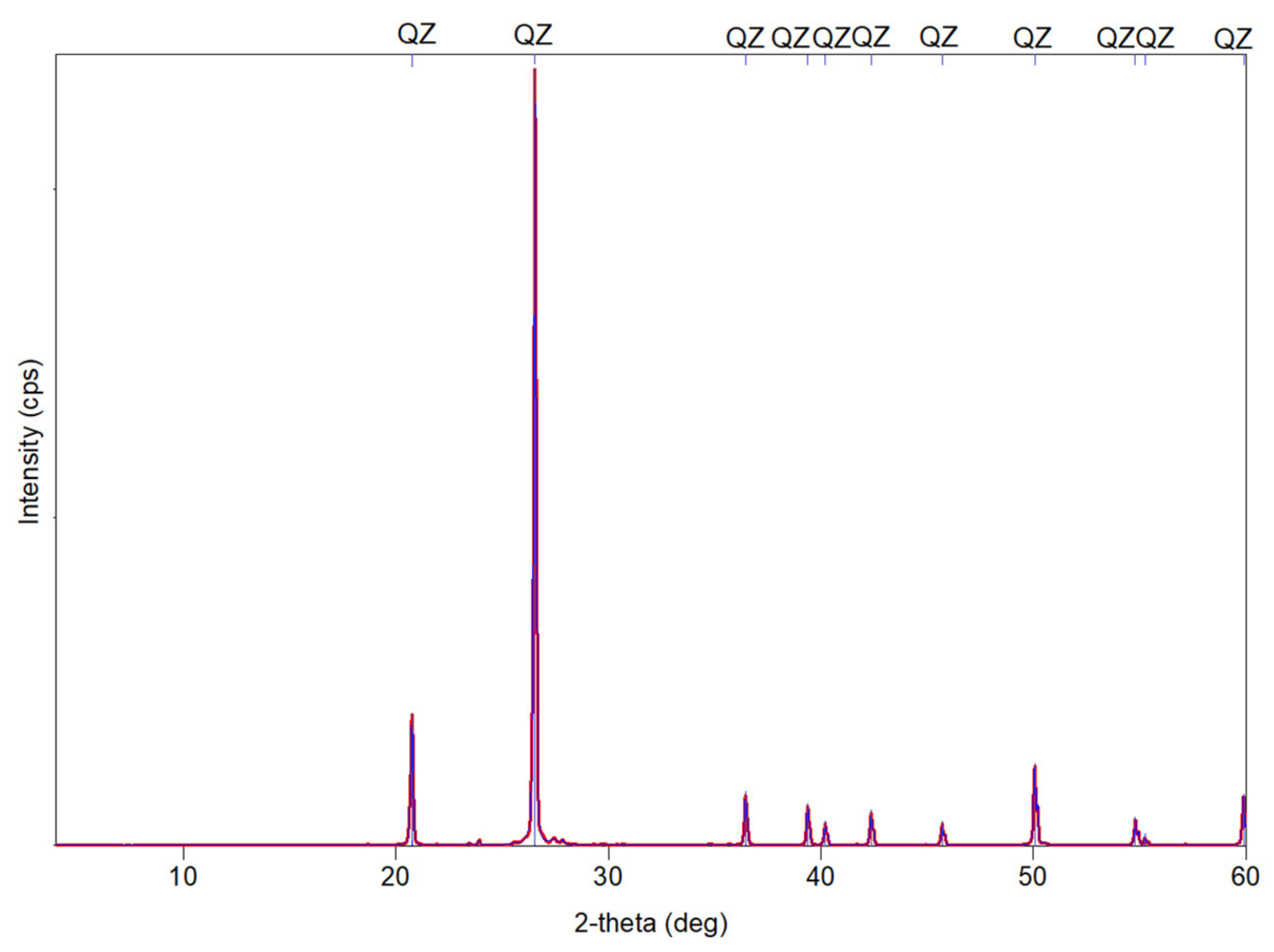
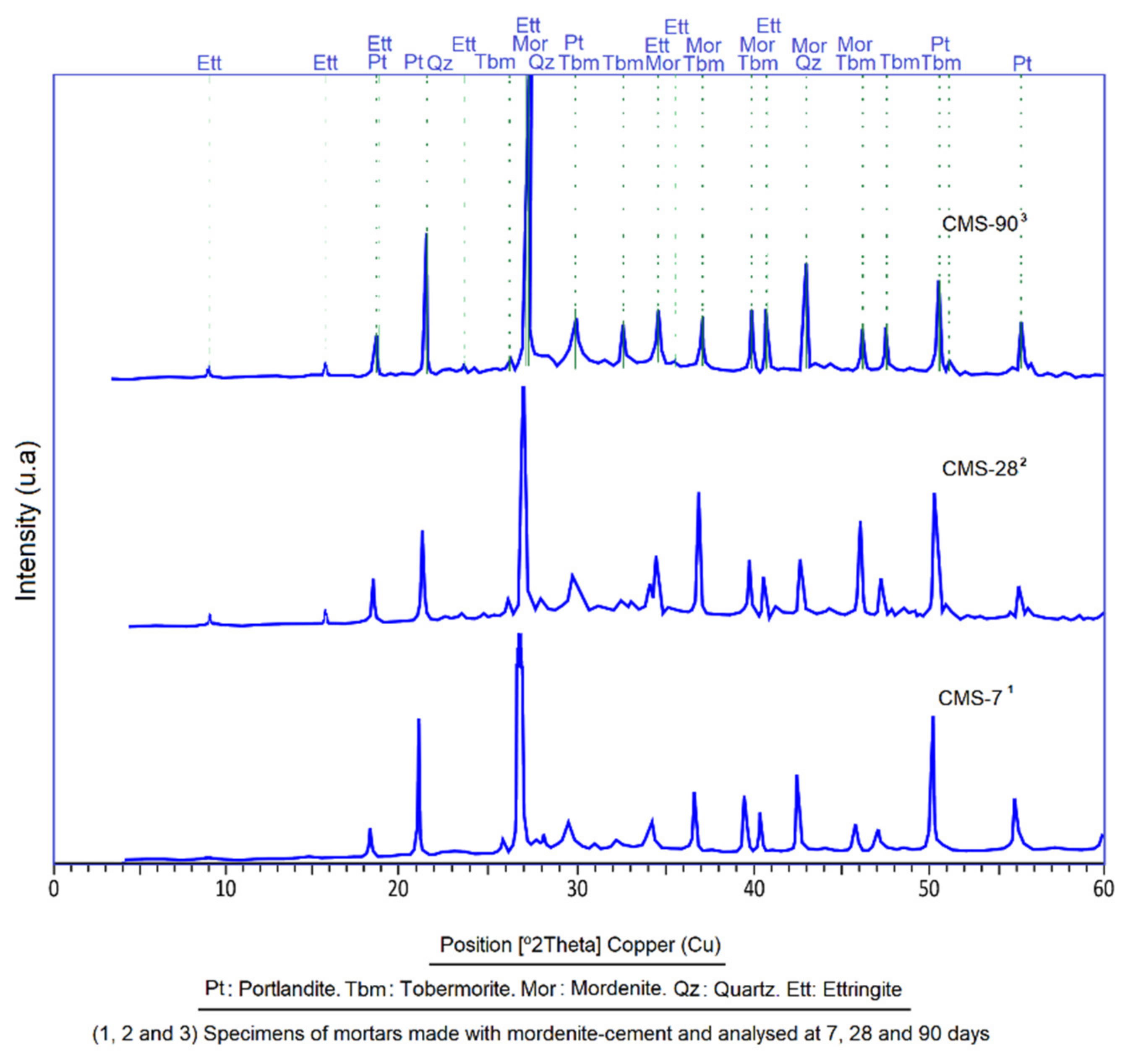
3.1. X-ray Diffraction (XRD)
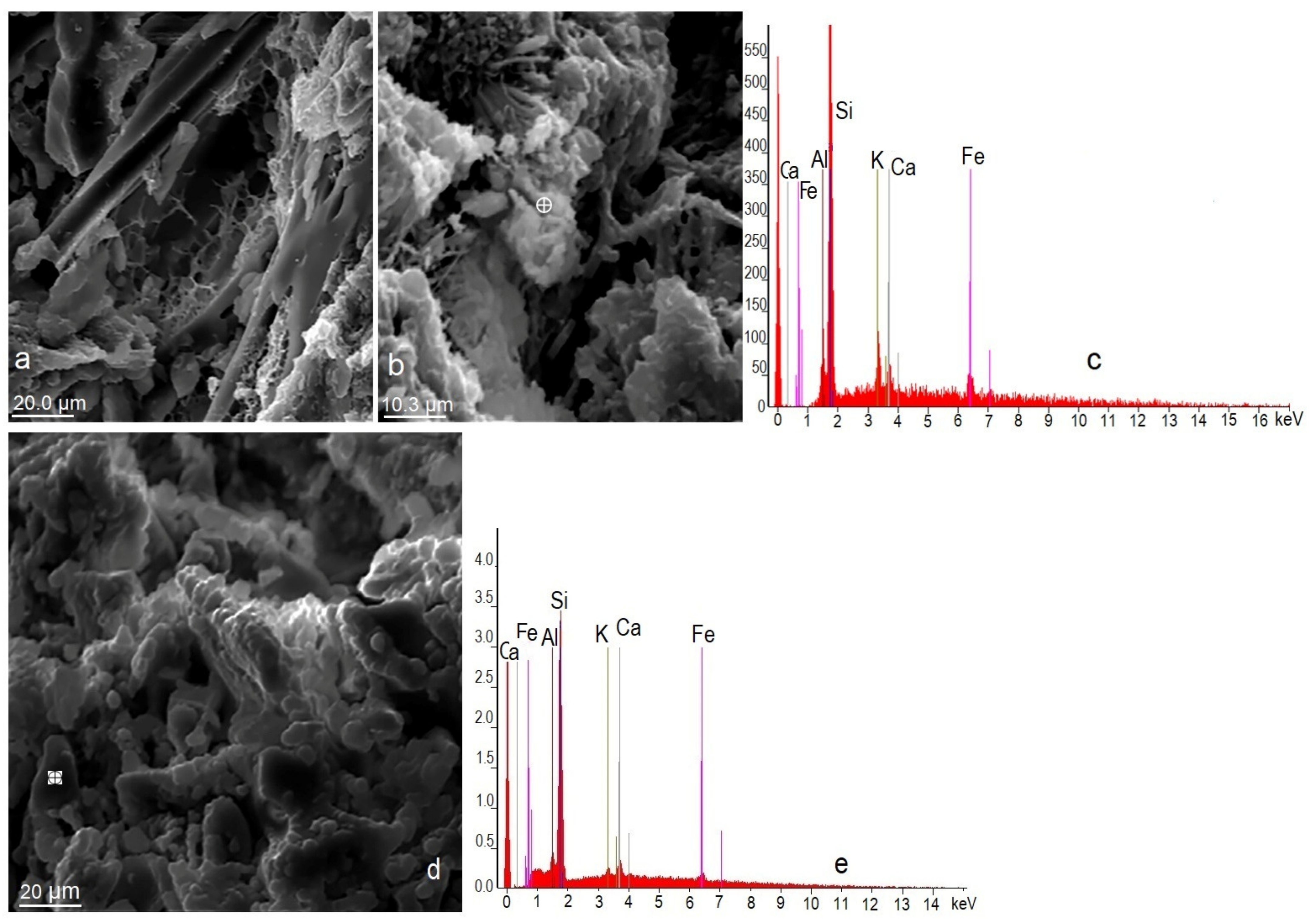
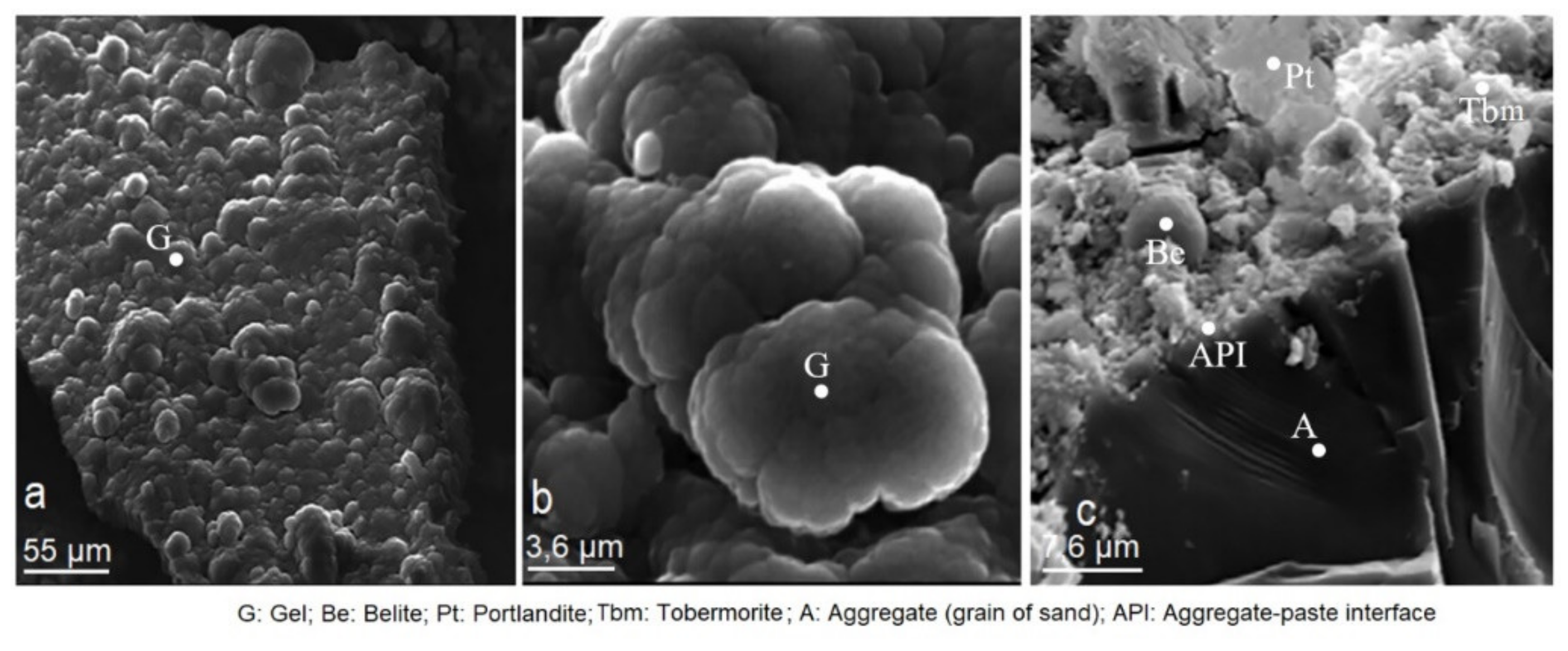
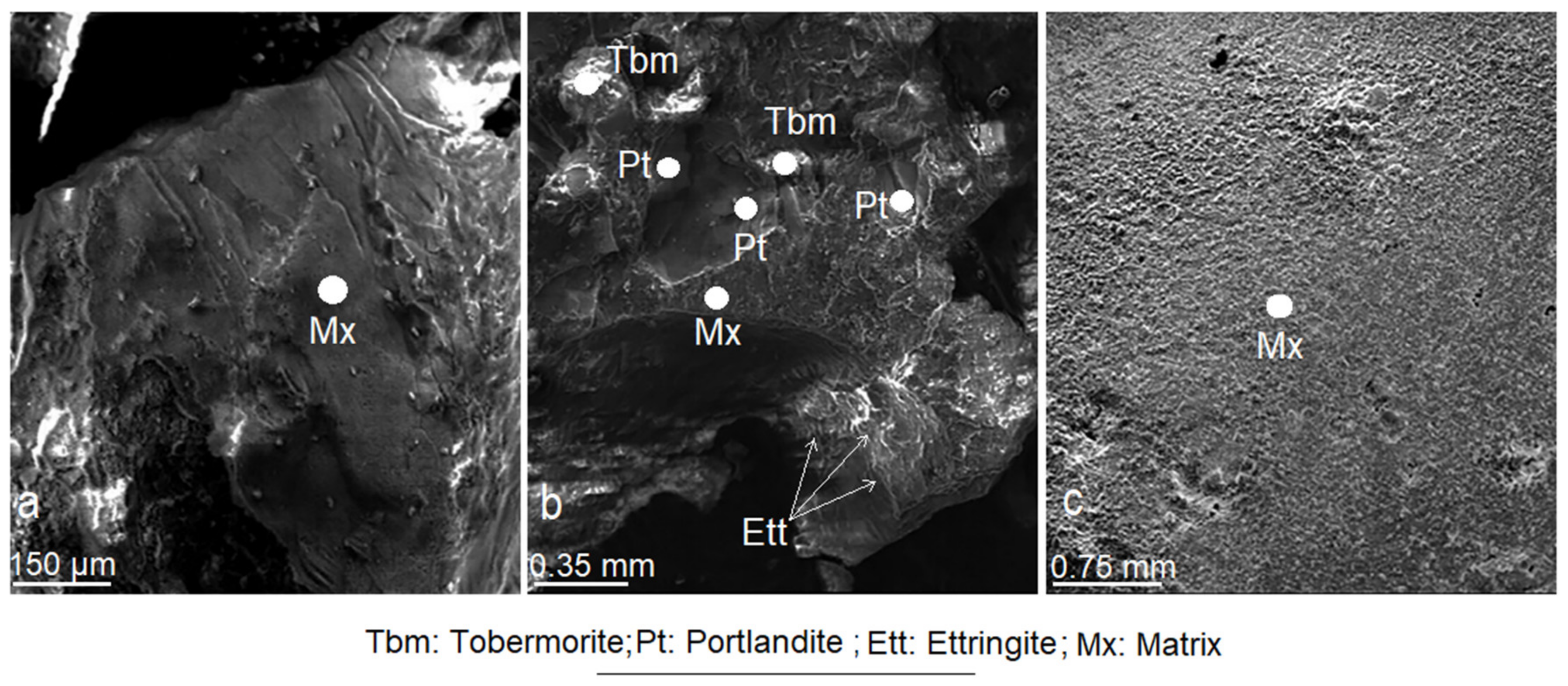
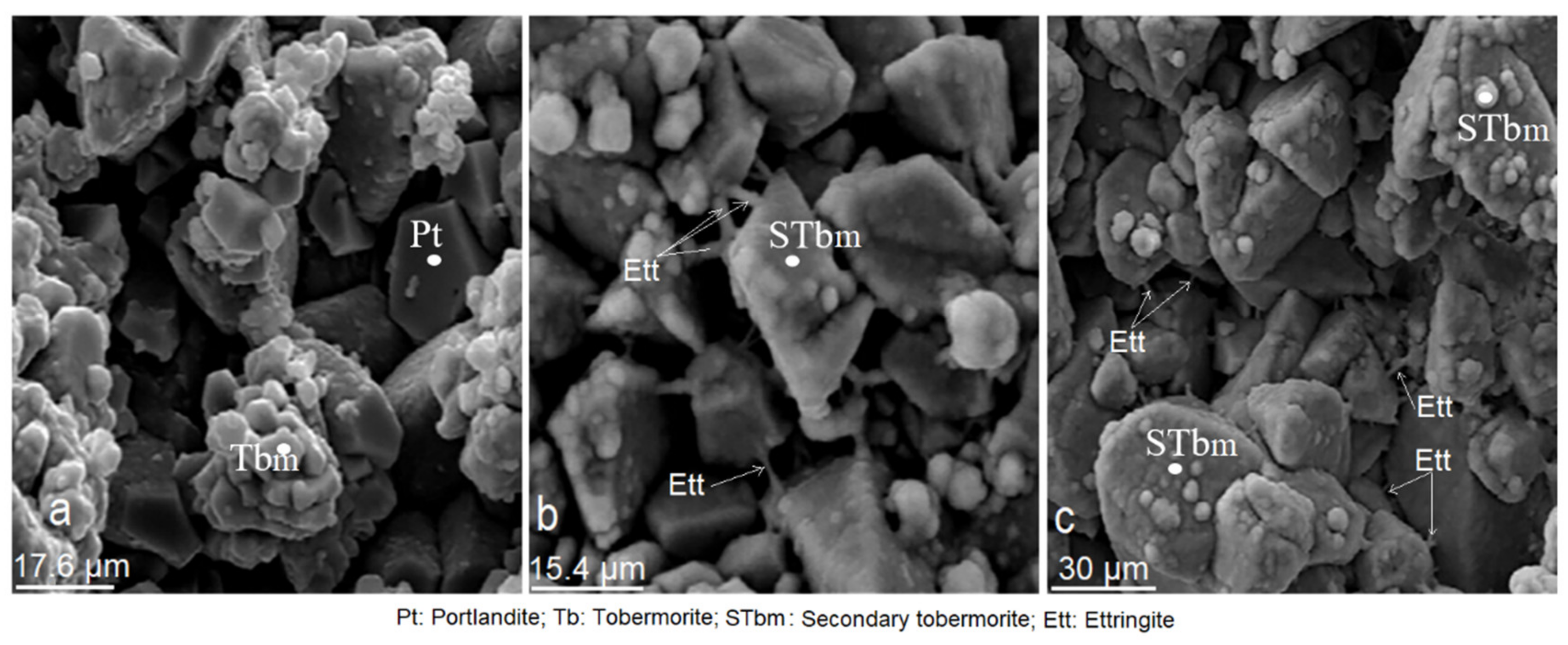
3.2. Scanning Electron Microscopy (SEM)
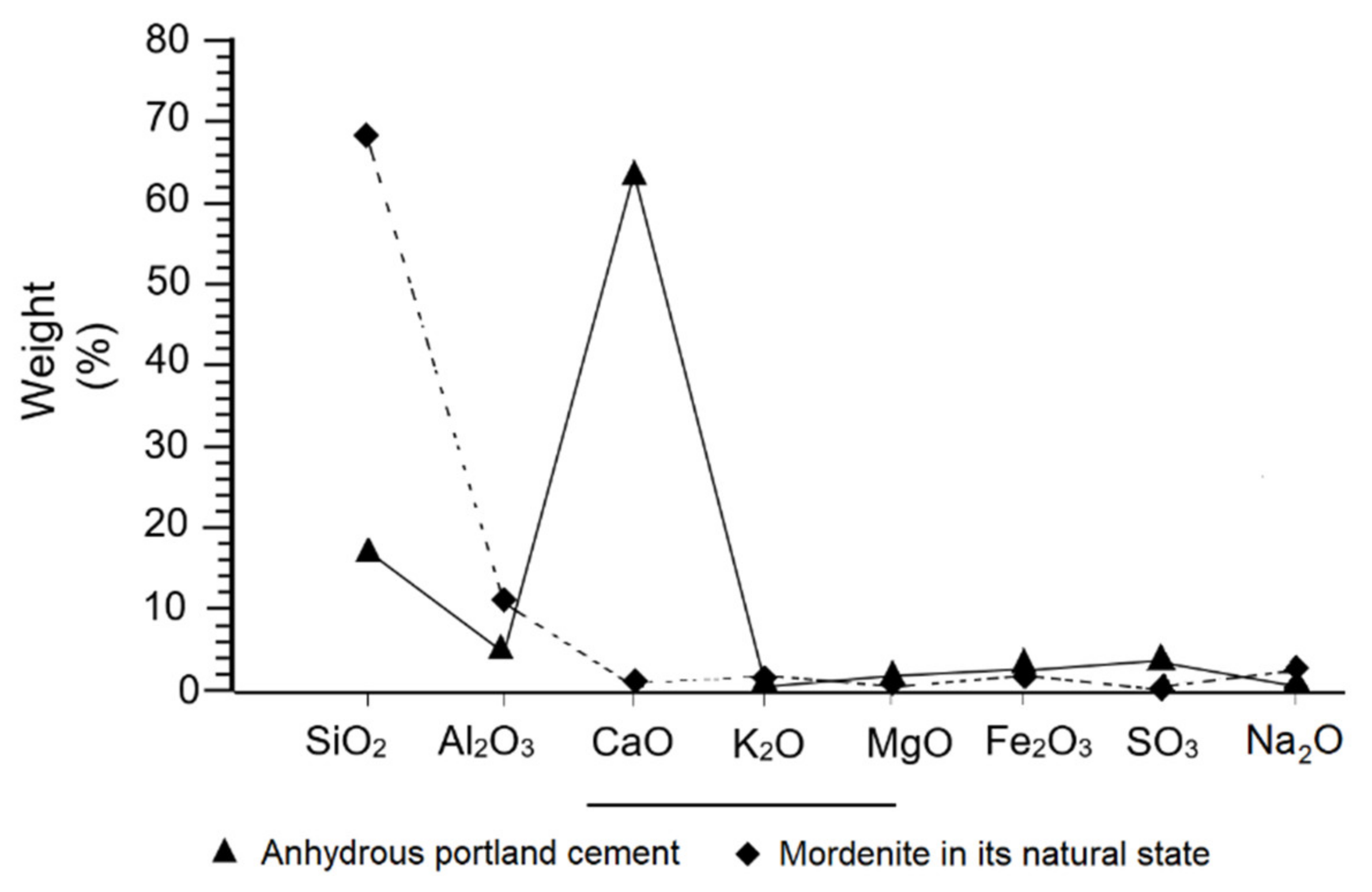
3.3. X-ray Fluorescence (XRF)
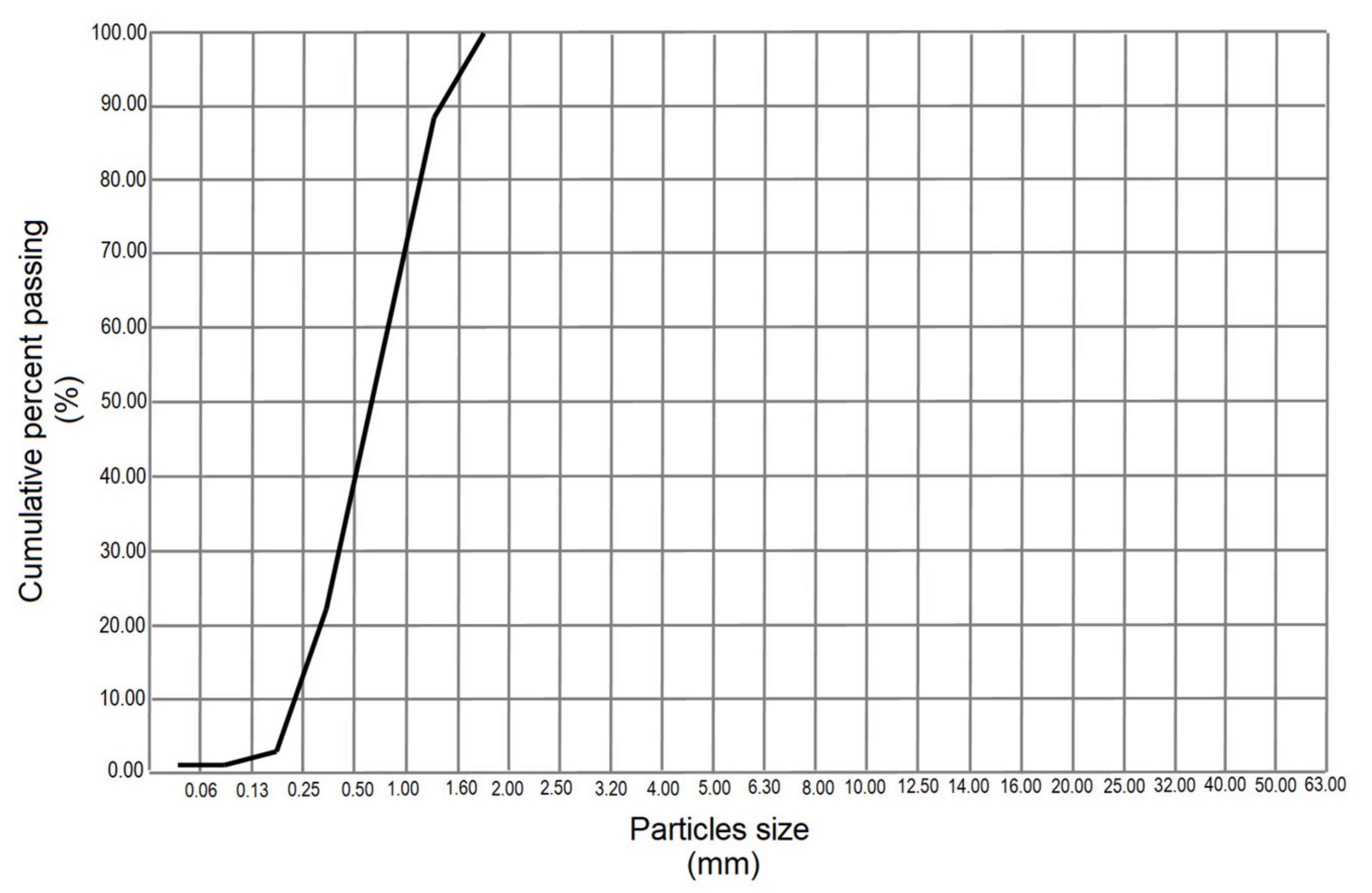
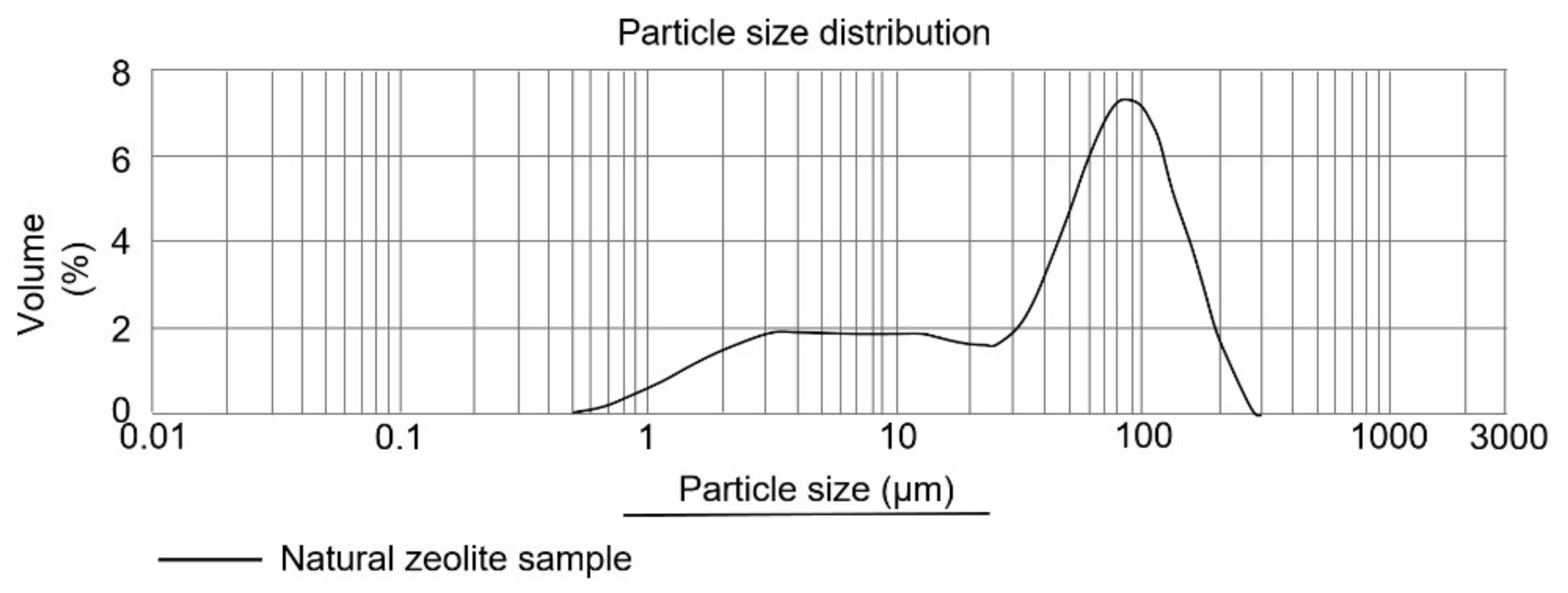
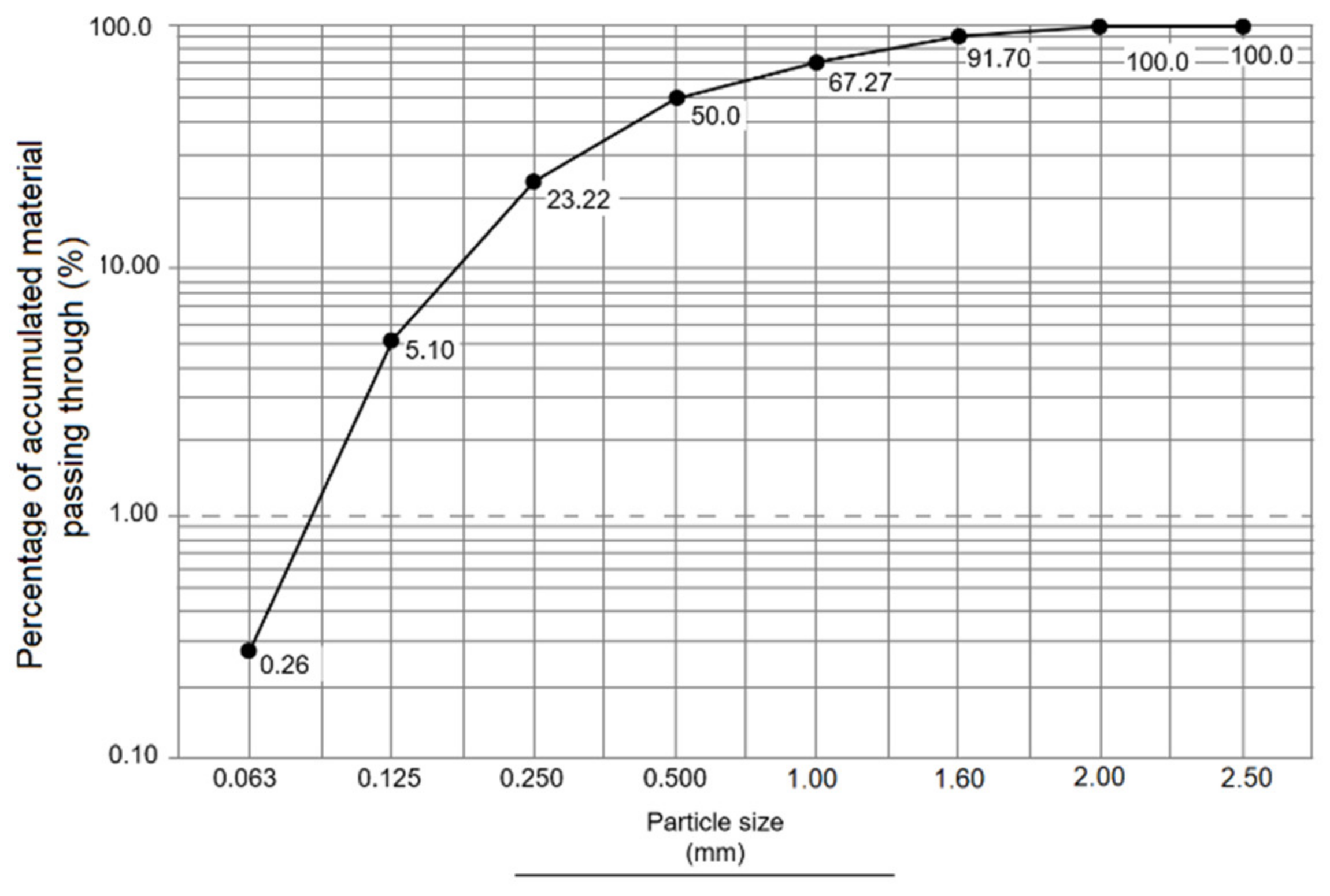
3.4. Granulometric Analysis
3.4.1. Mordenite Granulometric Analysis: Fine Particle Diameter and BET Surface
3.4.2. Granulometric Analysis of Standard Sand
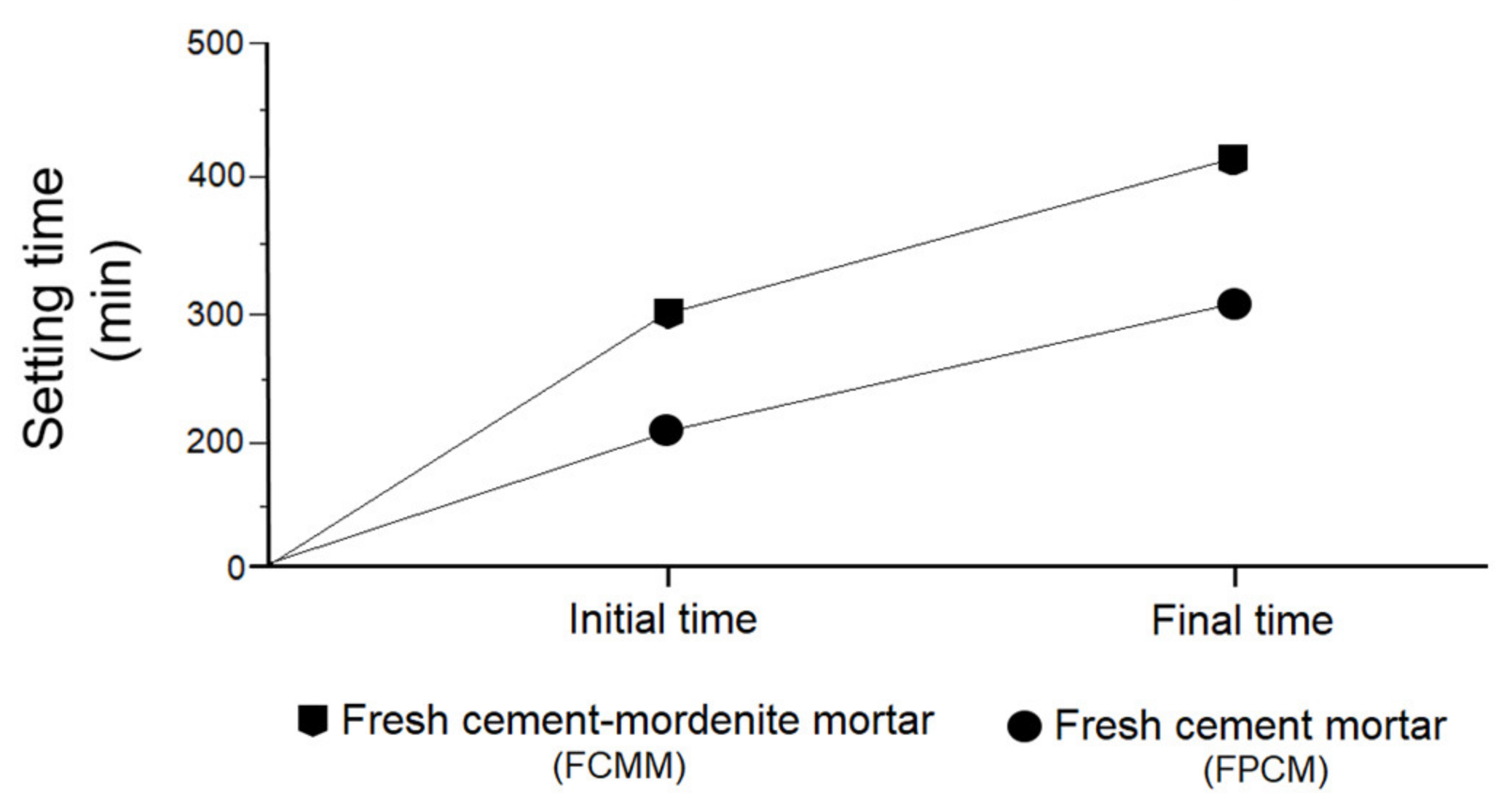
3.5. Effect of Mordenite on Initial and Final Setting Times of Fresh Mortar
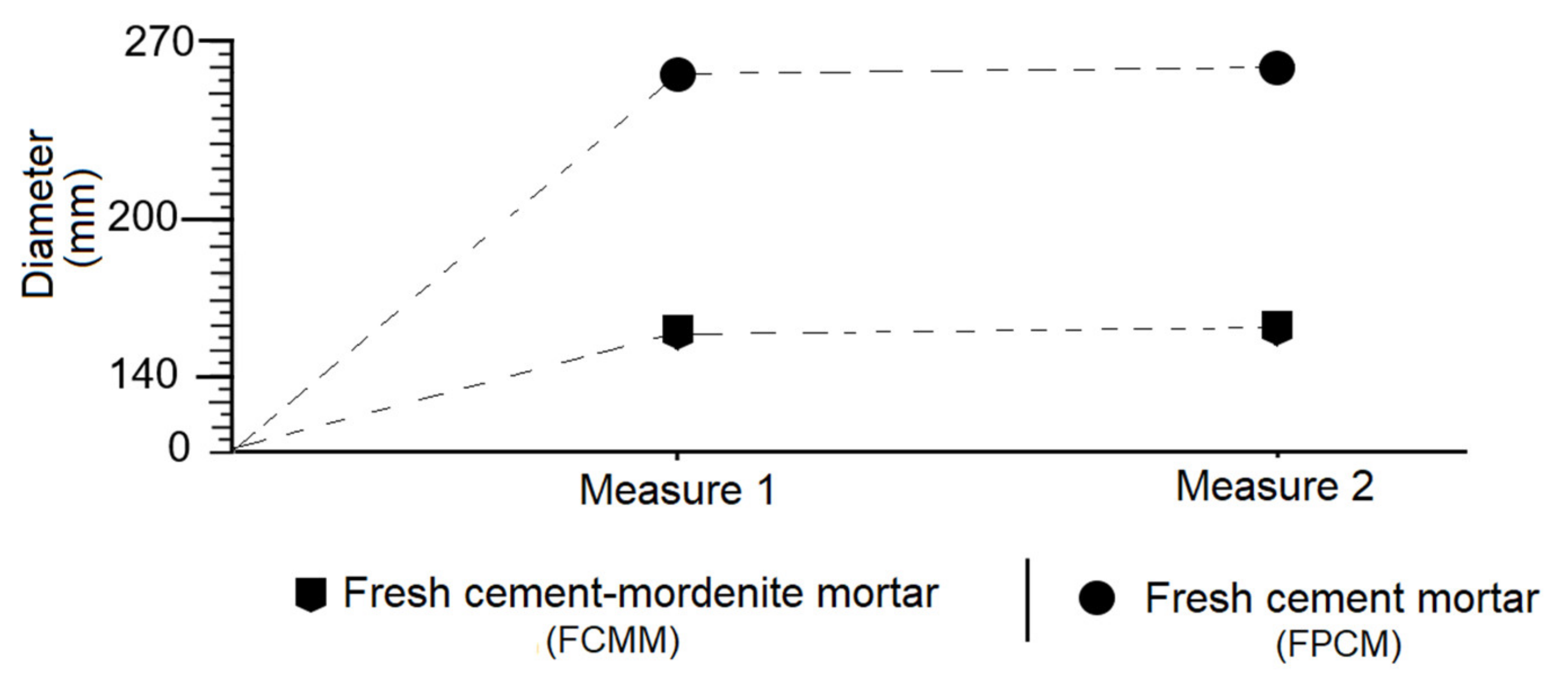
3.6. Effect of Mordenite on The Consistency of Fresh Mortar
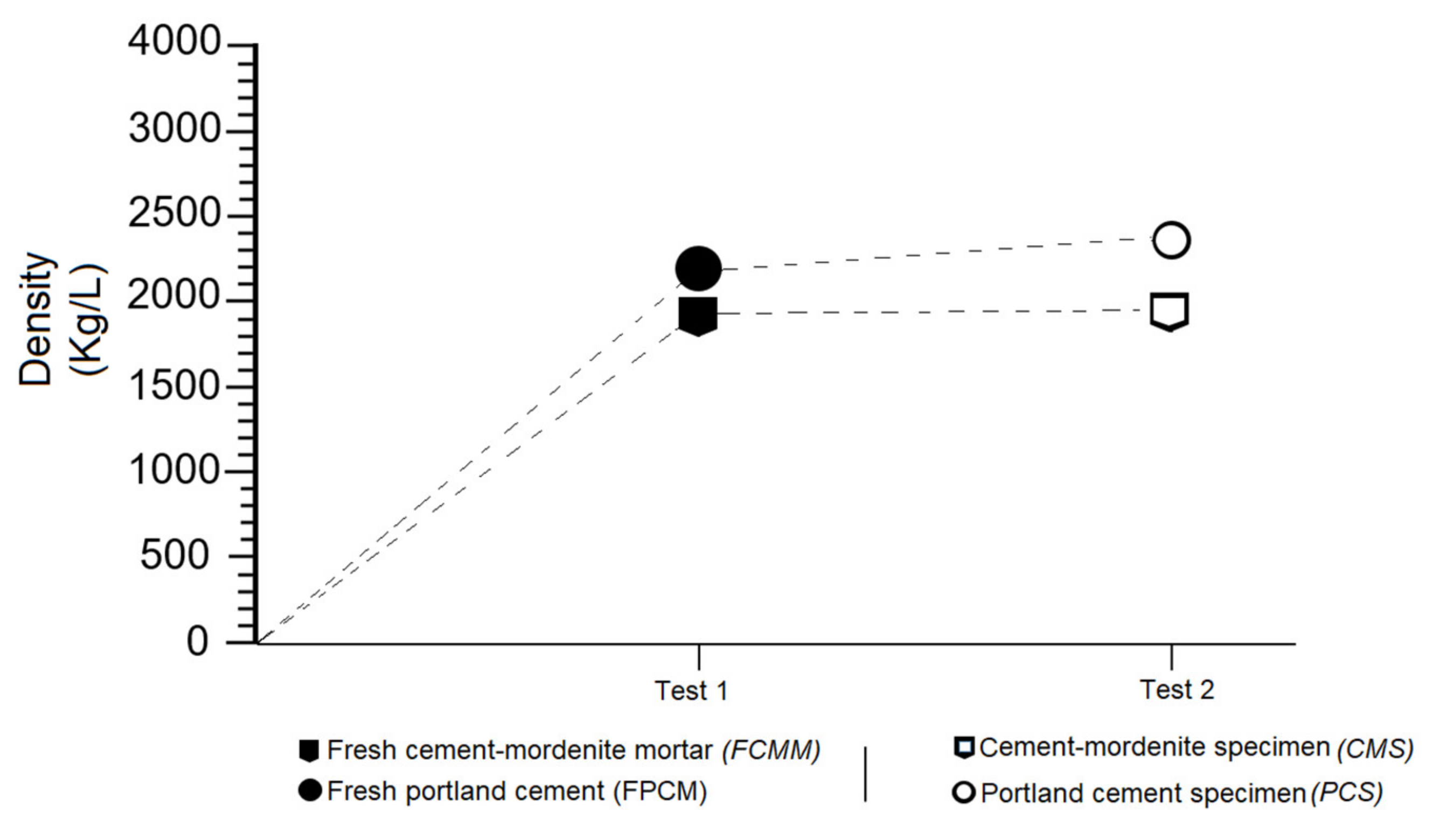
3.7. Effects of Mordenite on The Density of Fresh Mortar and Specimens
3.8. Effect of Mordenite on The Behaviour of Mechanical Strength of The Mortars
3.9. Effect of Mordenite on The Textural and Morphological Evolution of Minerals Formed during the Mortar Curing
3.10. Effects of Mordenite on The Growth of Minerals Formed during Mortar Curing
3.11. Effects of Mordenite in the Chemical Composition of Mortars
4. Conclusions
- The mordenite sample analysed in this research has a complex mineralogical composition where different mineral phases coexist. Mordenite is the main mineralogical phase and is accompanied by secondary phases such as smectite (montmorillonite), halloysite, illite, muscovite, biotite, plagioclase, quartz and amorphous phases.
- The chemical composition of the samples is calc-alkaline, where the major compounds SiO2, AI2O3, K2O and Na2O, stand out, as well as a high loss on ignition.
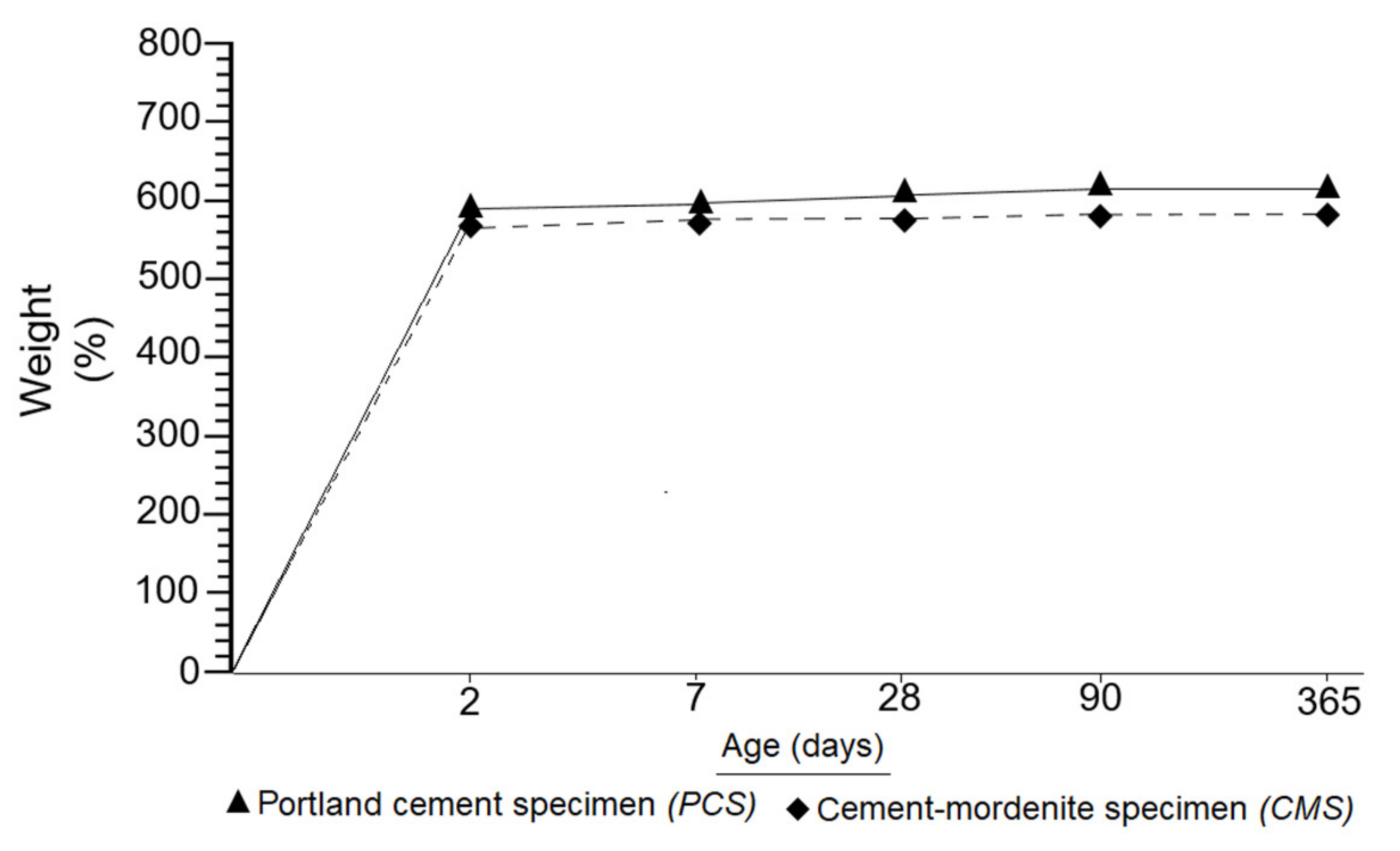
- Mortars made with partial substitution of cement with mordenite (CMM) showed the following properties regarding portland cement mortars (PCM): longer initial and final setting times, greater consistency, lower fluidity, lower density, lower weight and greater mechanical strength from 28 to 365 days.
- The study of CMS by DRX and SEM showed that the presence of mordenite slows down the hydration process, as seen in the longer initial and final setting times, which regulate the rapid hydration of C3A and allows slow-reacting silicates to be completely hydrated. As a result, a large amount of primary and late tobermorite has formed (28, 90 and 365 days), resulting in the formation of secondary tobermorite at the expense of portlandite. Mordenite´s action has made the ettringite formation a process that is not anomalous.
- According to what was analysed and discussed above, it is evident that the properties of mordenite, such as solid porous, cation exchange capacity (CEC), absorption-adsorption-desorption (ADD) properties, high contents of SiO2, Al2O3, K2O and Na2O, high LOI values, the presence of accompanying amorphous phases, as well as the long initial and final setting times and high mechanical strength deeply influence the positive evolution of the mortar setting, as was stated in the objectives and discussion of this work.
- In addition, the results achieved proved that mordenite behaves like a quality natural pozzolan, capable of partially replacing portland cement without affecting mechanical strength. On the other hand, the properties described justify their use in the manufacture of pozzolanic cements and mortars that are lighter, more resistant to overly aggressive natural environments, and more environmentally friendly, mainly in relation to low CO2 emissions.
- Finally, this work is new as it highlights the only deposit of zeolite of a mordenite type known in Spain.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazari, A.; Sanjayan, J. Compressive strength of functionally graded geopolymers: Role of position of layers. Constr. Build. Mater. 2015, 75, 31–34. [Google Scholar] [CrossRef]
- Gomathi, P.; Sivakumar, A. Accelerated curing effects on the mechanical performance of cold bonded and sintered fly ash aggregate concrete. Constr. Build. Mater. 2015, 77, 276–287. [Google Scholar] [CrossRef]
- Habert, G.; Choupay, N.; Montel, J.; Guillaume, D.; Escadeillas, G. Effects of the secondary minerals of the natural pozzolans on their pozzolanic activity. Cem. Concr. Res. 2008, 38, 963–975. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Pavlík, Z.; Jankovský, O.; Pokorný, J.; Tydlitát, V.; Svora, P.; Černý, R. Physical and chemical characterization of technogenic pozzolans for the application in blended cements. Constr. Build. Mater. 2018, 160, 106–116. [Google Scholar] [CrossRef]
- Frías, M.; Rodríguez, O.; Vegas, I.; Vigil, R. Properties of Calcined Clay Waste and its Influence on Blended Cement Behavior. J. Am. Ceram. Soc. 2008, 91, 1226–1230. [Google Scholar] [CrossRef]
- Küçükyıldırım, E.; Uzal, B. Characteristics of calcined natural zeolites for use in high-performance pozzolan blended cements. Constr. Build. Mater. 2014, 73, 229–234. [Google Scholar] [CrossRef]
- Tironia, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Assessment of pozzolanic activity of different calcined clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar] [CrossRef]
- Shi, C. An overview on the activation of reactivity of natural pozzolans. Can. J. Civ. Eng. 2001, 28, 778–786. [Google Scholar] [CrossRef]
- Quanlin, N.; Naiqian, F. Effect of modified zeolite on the expansion of alkaline silica reaction. Cem. Concr. Res. 2004, 35, 1784–1788. [Google Scholar] [CrossRef]
- Gong, K.; White, C. Impact of chemical variability of ground granulated blast-furnace slag on the phase formation in alkali-activated slag pastes. Cem. Concr. Res. 2016, 89, 310–319. [Google Scholar] [CrossRef] [Green Version]
- Coloma, J.R. Valorización de Residuos Orgánicos y Su Aprovechamiento en la Fabricación de Nuevos Hormigones. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2015; 755p. [Google Scholar]
- Frias, M.; Sánchez, M.I. Artificial pozzolans in eco-efficient concrete. In Eco-Efficient Concrete; Pacheco-Torgal, S.F., Jalali, J., Labrincha, V., John, M., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 105–122. [Google Scholar] [CrossRef]
- Feng, N.-Q.; Peng, G.-F. Applications of natural zeolite to construction and building materials in China. Constr. Build. Mater. 2005, 19, 579–584. [Google Scholar] [CrossRef]
- Ahmadi, B.; Shekarchizadeh, M. Use of natural zeolite as a supplementary cementitious material. Cem. Concr. Compos. 2010, 32, 134–141. [Google Scholar] [CrossRef]
- Bilim, C. Properties of cement mortars containing clinoptilolite as a supplementary cementitious material. Constr. Build. Mater. 2011, 25, 3175–3180. [Google Scholar] [CrossRef]
- Rosell-Lam, M.; Villar-Cociña, E.; Frías, M. Study on the pozzolanic properties of a natural Cuban zeolitic rock by conductometric method: Kinetic parameters. Constr. Build. Mater. 2011, 25, 644–650. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [Green Version]
- DeMuth, T.; Benco, L.; Hafner, J.; Toulhoat, H. Adsorption of water in mordenite? An ab initio study. Int. J. Quantum Chem. 2001, 84, 110–116. [Google Scholar] [CrossRef]
- Pavelić, K.; Hadžija, M. Medical applications of zeolites. In Handbook of Zeolite Science and Technology; Auerbach, S.M., Carrado, K.A., Dutta, P.K., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1141–1172. [Google Scholar] [CrossRef]
- Merida, A.; Kharchi, F. Pozzolan concrete durability on sulphate attack. Procedia Eng. 2015, 114, 832–837. [Google Scholar] [CrossRef] [Green Version]
- Małolepszy, J.; Grabowska, E. Sulphate attack resistance of cement with zeolite additive. Procedia Eng. 2015, 108, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Raggiotti, B.B.; Positieri, M.J.; Oshiro, A. Natural zeolite, a pozzolan for structural concrete. Procedia Struct. Integr. 2018, 11, 36–43. [Google Scholar] [CrossRef]
- Rodríguez-Camacho, R.; Uribe-Afif, R. Importance of using the natural pozzolans on concrete durability. Cem. Concr. Res. 2002, 32, 1851–1858. [Google Scholar] [CrossRef]
- Vejmelková, E.; Koňáková, D.; Kulovaná, T.; Keppert, M.; Žumár, J.; Rovnanikova, P.; Kersner, Z.; Sedlmajer, M.; Černý, R. Engineering properties of concrete containing natural zeolite as supplementary cementitious material: Strength, toughness, durability, and hygrothermal performance. Cem. Concr. Compos. 2015, 55, 259–267. [Google Scholar] [CrossRef]
- Moayyeri, N.; Oulapour, M.; Haghighi, A. Study of geotechnical properties of a gypsiferous soil treated with lime and silica fume. Geomech. Eng. 2019, 17, 195–206. [Google Scholar]
- Kumar, S.C.; Saha, P. Contribution of fly ash to the properties of mortar and concrete. Int. J. Earth Sci. Eng. 2011, 4, 1017–1023. [Google Scholar]
- Malhotra, V.M.; Kumar Mehta, P. Pozzolanic and cementitious materials. In Advances in Concrete Technology; Gordon and Breach Publishers: Amsterdam, The Netherlands, 1996; Volume 1, 191p, ISBN 2-88449-235-6. [Google Scholar]
- Marantos, I.; Christidis, G.E.; Ulmanu, M. Zeolite formation and deposit. In Handbook of Natural Zeolites; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 28–51. ISBN 978-1-60805-446-6. [Google Scholar] [CrossRef] [Green Version]
- Stocker, K.; Ellersdorfer, M.; Lehner, M.; Raith, J.G. Characterization and Utilization of Natural Zeolites in Technical Applications. Berg. Huettenmaenn. Monatsh. 2017, 162, 142–147. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.; Cecchin, D.; Costa, J.M.; Xavier, G.C.; de Fátima do Carmo, D.; Monteiro, S.N. Durability of coating mortars containing açaí fibers. Case Stud. Constr. Mater. 2020, 13, e00406. [Google Scholar] [CrossRef]
- Sedaghatdoost, A.; Behfarnia, K.; Moosaei, H.; Bayati, M.; Vaezi, M.S. Investigation on the mechanical properties and microstructure of eco-friendly mortar containing WGP at elevated temperature. Int. J. Concr. Struct. Mater. 2021, 15, 1. [Google Scholar] [CrossRef]
- Selvaranjan, K.; Gamage, J.; De Silva, G.; Navaratnam, S. Development of sustainable mortar using waste rice husk ash from rice mill plant: Physical and thermal properties. J. Build. Eng. 2021, 43, 102614. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, G.; Wu, D.; Fang, S.; Tang, Y. Investigation of using the ceramic polishing brick powder in engineered cementitious composites. J. Build. Eng. 2021, 43, 102489. [Google Scholar] [CrossRef]
- Shao, J.; Zhu, H.; Xue, G.; Yu, Y.; Borito, S.M.; Jiang, W. Mechanical and restrained shrinkage behaviors of cement mortar incorporating waste tire rubber particles and expansive agent. Constr. Build. Mater. 2021, 296, 123742. [Google Scholar] [CrossRef]
- Barreto, E.; Stafanato, K.; Marvila, M.; de Azevedo, A.; Ali, M.; Pereira, R.; Monteiro, S. Clay ceramic waste as pozzolan constituent in cement for structural concrete. Materials 2021, 14, 2917. [Google Scholar] [CrossRef]
- Kitsopoulos, K.P.; Dunham, A.C. Heulandite and mordenite-rich tuffs from Greece: A potential source for pozzolanic materials. Miner. Deposita 1996, 31, 576–583. [Google Scholar] [CrossRef]
- De La Villa, R.V.; Fernández, R.; García, R.; Villar-Cociña, E.; Frías, M. Pozzolanic activity and alkaline reactivity of a mordenite-rich tuff. Microporous Mesoporous Mater. 2009, 126, 125–132. [Google Scholar] [CrossRef]
- Mertens, G.; Snellings, R.; Van Balen, K.; Bicer-Simsir, B.; Verlooy, P.; Elsen, J. Pozzolanic reactions of common natural zeolites with lime and parameters affecting their reactivity. Cem. Concr. Res. 2009, 39, 233–240. [Google Scholar] [CrossRef]
- Cemento. Parte 1: Composición, Especificaciones y Criterios de Conformidad de los Cementos Comunes; Standard UNE-EN 197-1:2011; AENOR: Madrid, Spain, 2011.
- Methods of Testing Cement—Part 1: Determination of Strength, DIN EN 196-1:2016-11, German version EN 196-1:2016; European Committee for Standardization: Brussels, Belgium, 2016.
- Métodos de Ensayo de Cementos. Parte 3: Determinación del Tiempo de Fraguado y de la Estabilidad de Volumen; Standard UNE-EN 196-3:2005; AENOR: Madrid, Spain, 2005.
- Ensayos para Determinar las Propiedades Geométricas de los Áridos. Parte 1: Determinación de la Granulometría de las Partículas. Método del Tamizado; Standard UNE-EN 933-1:2012; AENOR: Madrid, Spain, 2012.
- Ensayos para Determinar las Propiedades Geométricas de los Áridos. Parte 10: Evaluación de los Finos. Granulometría de los Fillers (Tamizado en Corriente de Aire); Standard UNE-EN 933-10:2010; AENOR: Madrid, Spain, 2010.
- Ensayos para Determinar las Propiedades Mecánicas y Físicas de los Áridos. Parte 6: Determinación de la Densidad de Partículas y la Absorción de Agua; Standard UNE-EN 1097-6:2000; AENOR: Madrid, Spain, 2000.
- Métodos de Ensayo de Cementos. Parte 1: Determinación de Resistencias; Standard UNE-EN 196-1:2018; AENOR: Madrid, Spain, 2018.
- Métodos de Ensayo para Morteros de Albañilería. Parte 3: Determinación de la Consistencia del Mortero Fresco (por la Mesa de Sacudidas); Standard UNE-EN 1015-3:2000; AENOR: Madrid, Spain, 2000.
- Métodos de Ensayo de los Morteros para Albañilería. Parte 6: Determinación de la Densidad Aparente del Mortero Fresco; Standard UNE-EN 1015-6:1999/A1:2007; AENOR: Madrid, Spain, 2007.
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2007; 485p, ISBN 9780444530677762. [Google Scholar]
- Stamatakis, M.G.; Regueiro, M.; Calvo José, P.; Fragoulis, D.; Stamatakis, G. A study of zeolitic tuffs associated with bentonite deposits from Almeria, Spain and Kimolos Island, Greece and their industrial potential as pozzolanas in the cement industry. Hell. J. Geosci. 2010, 45, 283–292. [Google Scholar]
- Talero, R. ¿Puede el concepto “capacidad de cambio” de las arcillas, ser íntegramente aplicable a las puzolanas? Mater. Constr. 2004, 54, 17–36. [Google Scholar] [CrossRef] [Green Version]
- Pusch, R. Chapter 6: Mechanical properties of clays and clay minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; pp. 247–260. [Google Scholar] [CrossRef]
- Vivaldi, J.L.M. The bentonites of Cabo de Gata (Southeast Spain) and of Guelaya volcanic province (North Morocco). Clays Clay Miner. 1962, 11, 327–357. [Google Scholar] [CrossRef]
- Costafreda, J.L. Geología, Caracterización y Aplicaciones de las Rocas Zeolitizadas del Complejo Volcánico de Cabo de Ga-ta-Almería. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2008; 515p. [Google Scholar]
- Kitsopoulos, K.P. The Genesis of a mordenite deposit by hydrothermal alteration of pyroclastics on Polyegos Island, Greece. Clays Clay Miner. 1997, 45, 632–648. [Google Scholar] [CrossRef]
- IZA Commission on Natural Zeolites. Available online: http://www.iza-online.org/natural/Datasheets/Mordenite/mordenite.htm (accessed on 9 July 2021).
- Moradian, M.; Hu, Q.; Aboustait, M.; Ley, M.T.; Hanan, J.C.; Xiao, X.; Rose, V.; Winarski, R.; Scherer, G. Multi-scale observations of structure and chemical composition changes of Portland cement systems during hydration. Constr. Build. Mater. 2019, 212, 486–499. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.Z.; Rudert, V.; Trettin, R. Effect of fineness and particle size distribution of granulated blast-furnace slag on the hydraulic reactivity in cement systems. Adv. Cem. Res. 2005, 17, 161–167. [Google Scholar] [CrossRef]
- Payra, P.; Dutta, P. Zeolite: A primer. In Handbook of Zeolite Science and Technology, 1st ed.; Auerbach, S.M., Carrado, K.A., Dutta, P.K., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–24. [Google Scholar]
- Cortes, D.; Kim, H.-K.; Palomino, A.; Santamarina, J. Rheological and mechanical properties of mortars prepared with natural and manufactured sands. Cem. Concr. Res. 2008, 38, 1142–1147. [Google Scholar] [CrossRef]
- Kocak, Y.; Tascı, E.; Kaya, U. The effect of using natural zeolite on the properties and hydration characteristics of blended cements. Constr. Build. Mater. 2013, 47, 720–727. [Google Scholar] [CrossRef]
- Uzal, B.; Turanlı, L. Blended cements containing high volume of natural zeolites: Properties, hydration and paste microstructure. Cem. Concr. Compos. 2012, 34, 101–109. [Google Scholar] [CrossRef]
- Karakurt, C.; Kurama, H.; Topçu, I.B. Utilization of natural zeolite in aerated concrete production. Cem. Concr. Compos. 2010, 32, 1–8. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Siddique, R.; Klimek, B.; Franus, M. The use of zeolite, lightweight aggregate and boiler slag in restoration renders. Constr. Build. Mater. 2017, 142, 162–174. [Google Scholar] [CrossRef]
- Pekgöz, M.; Tekin, I. Microstructural investigation and strength properties of structural lightweight concrete produced with zeolitic tuff aggregate. J. Build. Eng. 2021, 43, 102863. [Google Scholar] [CrossRef]
- Sai Teja, G.; Prasad Ravella, D.; Chandra Sekhara Rao, P.V. Studies on self-curing self-compacting concretes containing zeolite admixture. Mater. Today Proc. 2021, 43, 2355–2360. [Google Scholar] [CrossRef]
- Vijayan, D.S.; Arvindan, S.; Parthiban, D.; Saravanan, B.; Kalpana, M. Natural aggregates used for light weight concrete—A review. IOP Conf. Series: Mater. Sci. Eng. 2020, 993, 012042. [Google Scholar] [CrossRef]
- Janotka, I.; Krajči, L.; Dzivák, M. Properties and utilization of zeolite-blended Portland cements. Clays Clay Miner. 2003, 51, 616–624. [Google Scholar] [CrossRef]
- Houston, J.R.; Maxwell, R.S.; Carroll, S.A. Transformation of meta-stable calcium silicate hydrates to tobermorite: Reaction kinetics and molecular structure from XRD and NMR spectroscopy. Geochem. Trans. 2009, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Kapeluszna, E.; Kotwica, L.; Malata, G.; Murzyn, P.; Nocuń-Wczelik, W. The effect of highly reactive pozzolanic material on the early hydration of alite—C3A—gypsum synthetic cement systems. Constr. Build. Mater. 2020, 251, 118879. [Google Scholar] [CrossRef]
- Borštnar, M.; Daneu, N.; Dolenec, S. Phase development and hydration kinetics of belite-calcium sulfoaluminate cements at different curing temperatures. Ceram. Int. 2020, 46, 29421–29428. [Google Scholar] [CrossRef]




| Specimen Sample | Composition | Proportion (g) | Test Age (Days) |
|---|---|---|---|
| CMS-7 * CMS-28 CMS-90 CMS-365 | Cement Mordenite Standard sand Distilled water | Cement (375) Mordenite (125) Distilled water (225) Standard sand (1350) | 7/28/90/365 |
| PCS-7 ** PCS-28 PCS-90 PCS-365 | Cement Standard sand Distilled water | Cement (450) Distilled water (225) Standard sand (1350) | 7/28/90/365 |
| Sample | SiO2 | Al2O3 | CaO | Na20 | K2O | MgO | Fe2O3 | SO3 | LOI | Si/Al |
|---|---|---|---|---|---|---|---|---|---|---|
| Mordenite | 68.30 | 11.95 | 1.15 | 2.89 | 1.38 | 1.29 | 1.56 | 0.03 | 12.53 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costafreda, J.L.; Martín, D.A.; Presa, L.; Parra, J.L. Effects of a Natural Mordenite as Pozzolan Material in the Evolution of Mortar Settings. Materials 2021, 14, 5343. https://doi.org/10.3390/ma14185343
Costafreda JL, Martín DA, Presa L, Parra JL. Effects of a Natural Mordenite as Pozzolan Material in the Evolution of Mortar Settings. Materials. 2021; 14(18):5343. https://doi.org/10.3390/ma14185343
Chicago/Turabian StyleCostafreda, Jorge L., Domingo A. Martín, Leticia Presa, and José Luis Parra. 2021. "Effects of a Natural Mordenite as Pozzolan Material in the Evolution of Mortar Settings" Materials 14, no. 18: 5343. https://doi.org/10.3390/ma14185343
APA StyleCostafreda, J. L., Martín, D. A., Presa, L., & Parra, J. L. (2021). Effects of a Natural Mordenite as Pozzolan Material in the Evolution of Mortar Settings. Materials, 14(18), 5343. https://doi.org/10.3390/ma14185343







