Assessment of Long Lived Isotopes in Alkali-Silica Resistant Concrete Designed for Nuclear Installations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Neutron-Activation Analysis
2.3. Expansion Testing
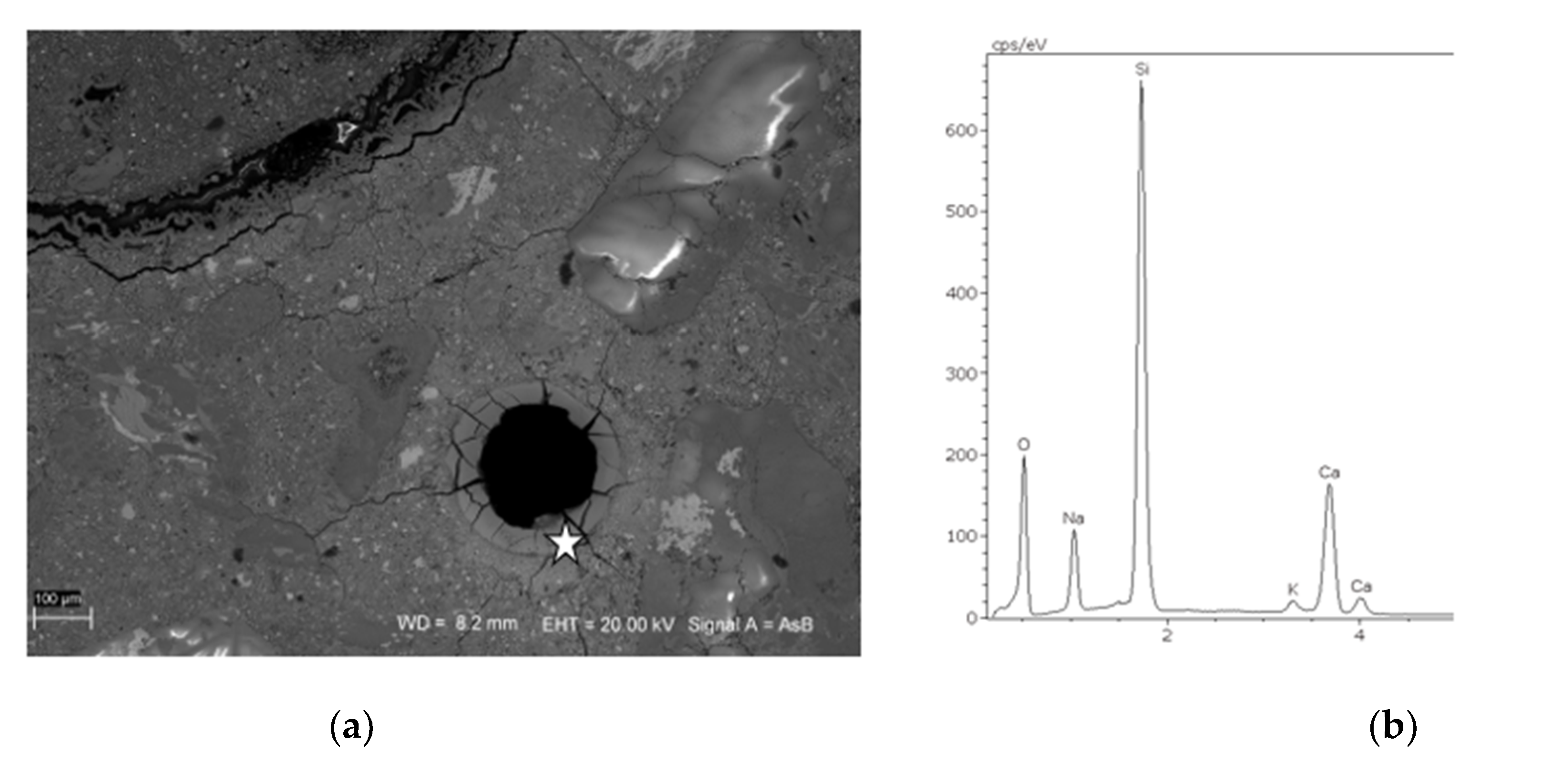
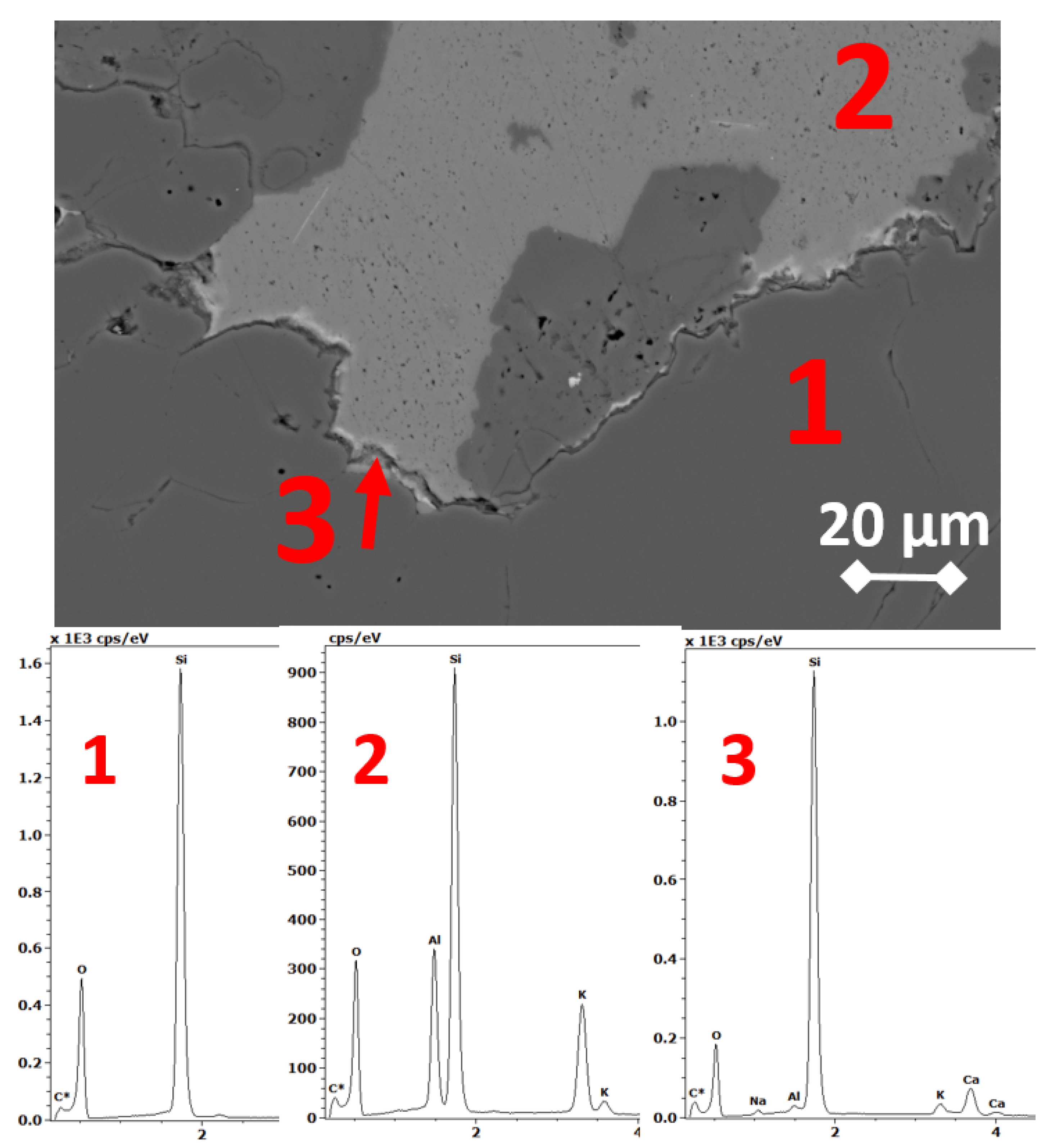
2.4. Scanning Electron Microscopy and Energy-Dispersive Spectrometry (SEM-EDS)
3. Results
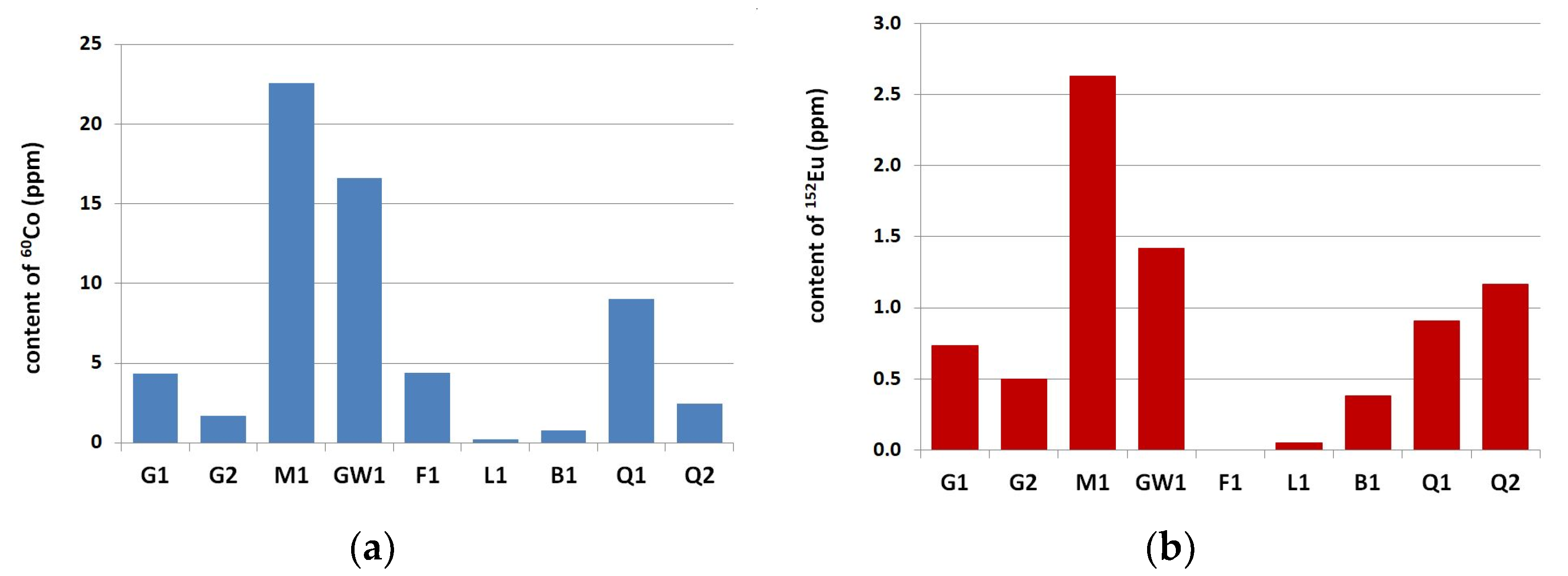
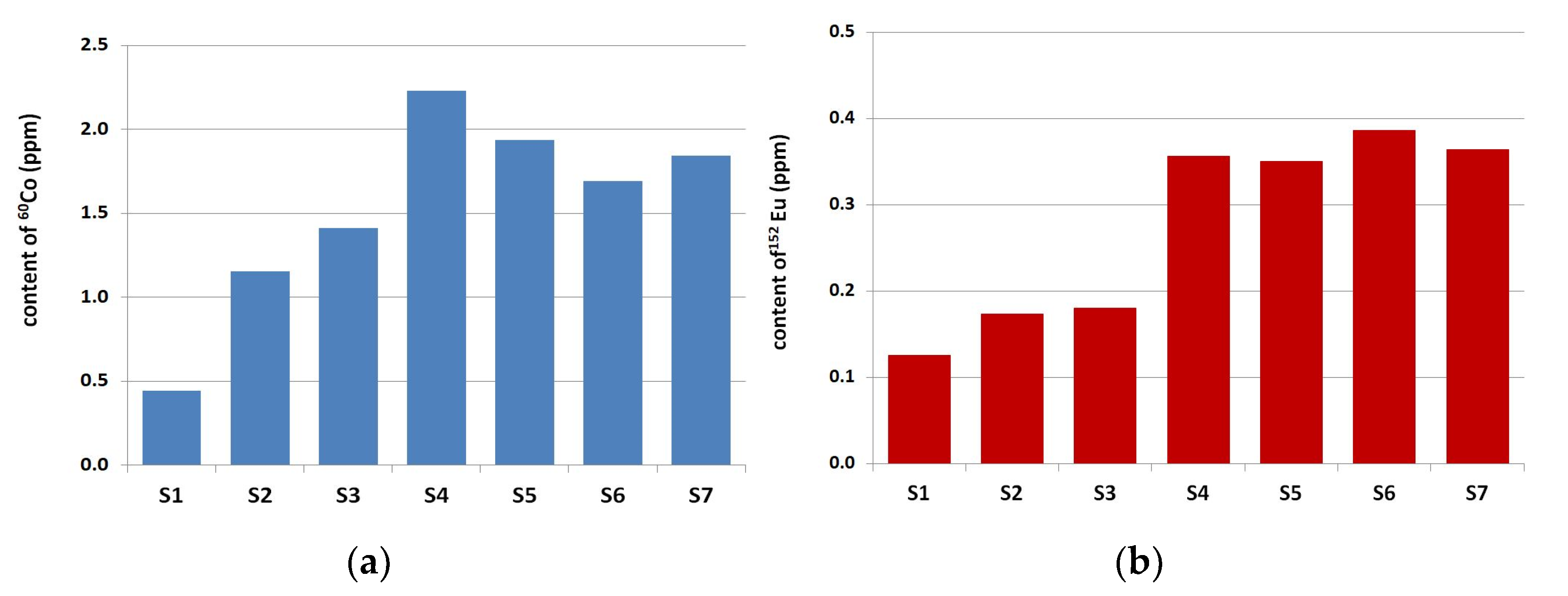
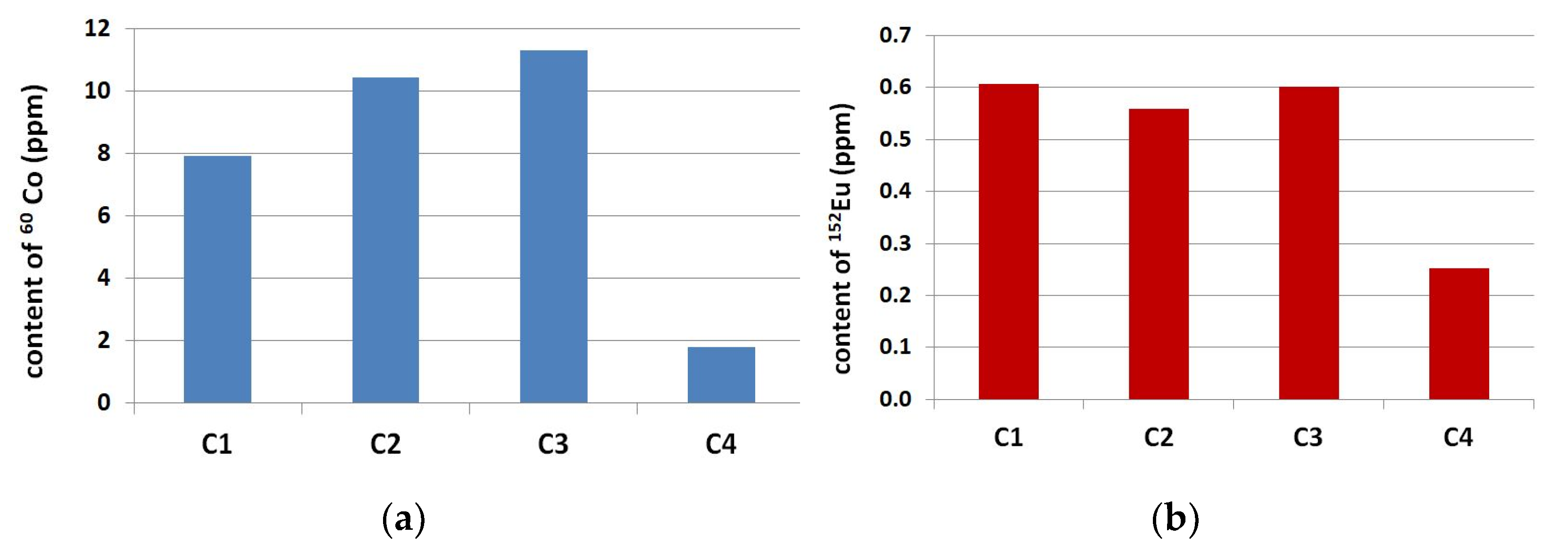
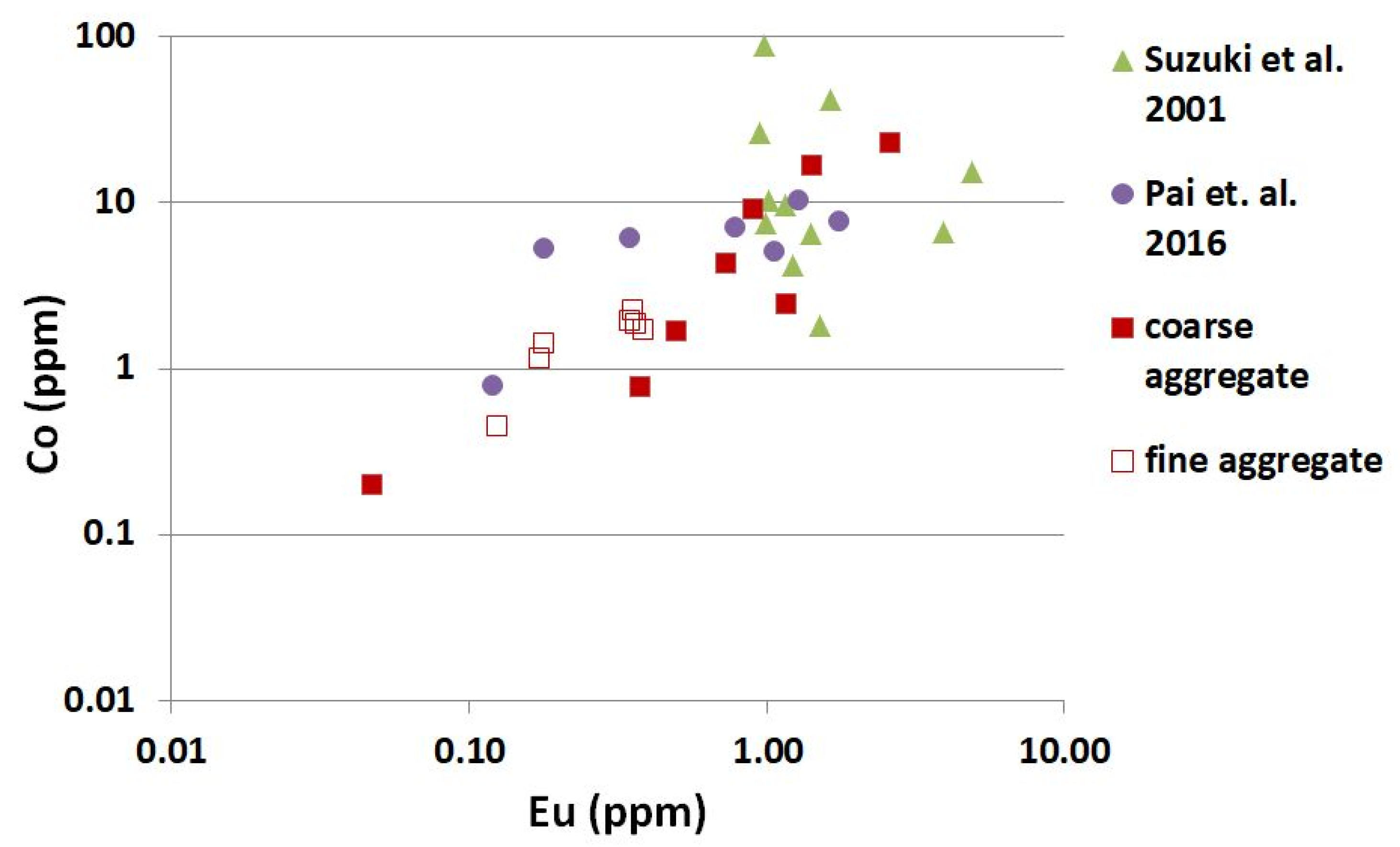
3.1. Neutron-Activation Analysis
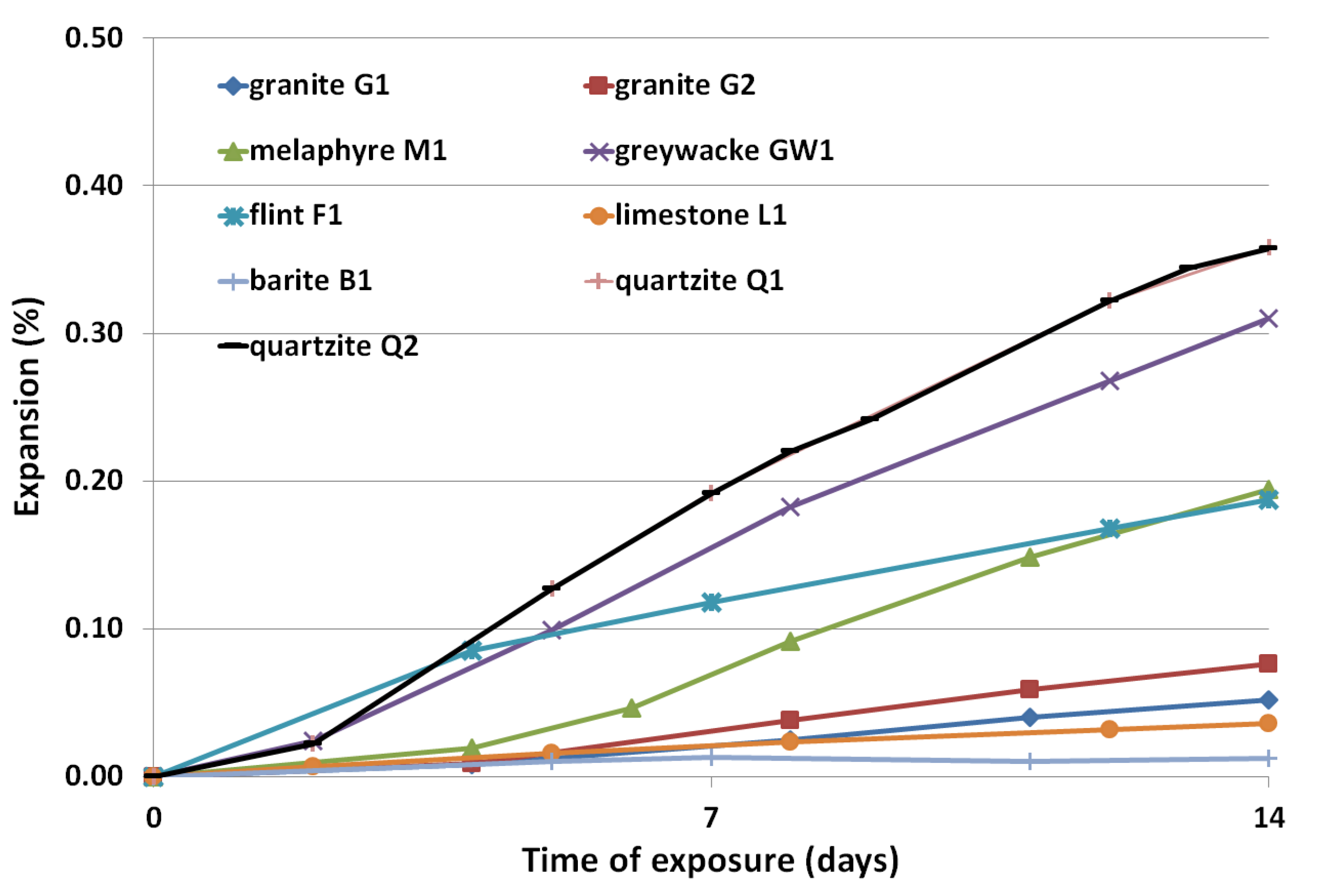
3.2. Expansion Results
3.3. SEM Microstructure Analysis
4. Discussion
5. Conclusions
- The concentration of 60Co and 152Eu activated by neutron radiation in natural fine aggregate was lower than in natural coarse aggregate. The average content of 60Co and 152Eu in natural siliceous fine aggregate amounted to 1.71 ± 0.38 ppm and 0.30 ± 0.09 ppm, respectively. It was 8.73 ± 7.98 ppm and 1.05 ± 0.83 ppm in natural coarse aggregate.
- The influence of the sand origin on the 60Co and 152Eu content was clearly visible in the analyzed natural fine aggregate. Lower concentrations of Cobalt and Europium were present in the river sands, compared to fossil fine aggregate.
- The lowest values of 60Co and 152Eu concentration were found in limestone, both in fine aggregate, at 0.44 ppm and 0.13 ppm, and in coarse aggregate, at 0.20 ppm and 0.05 ppm, respectively.
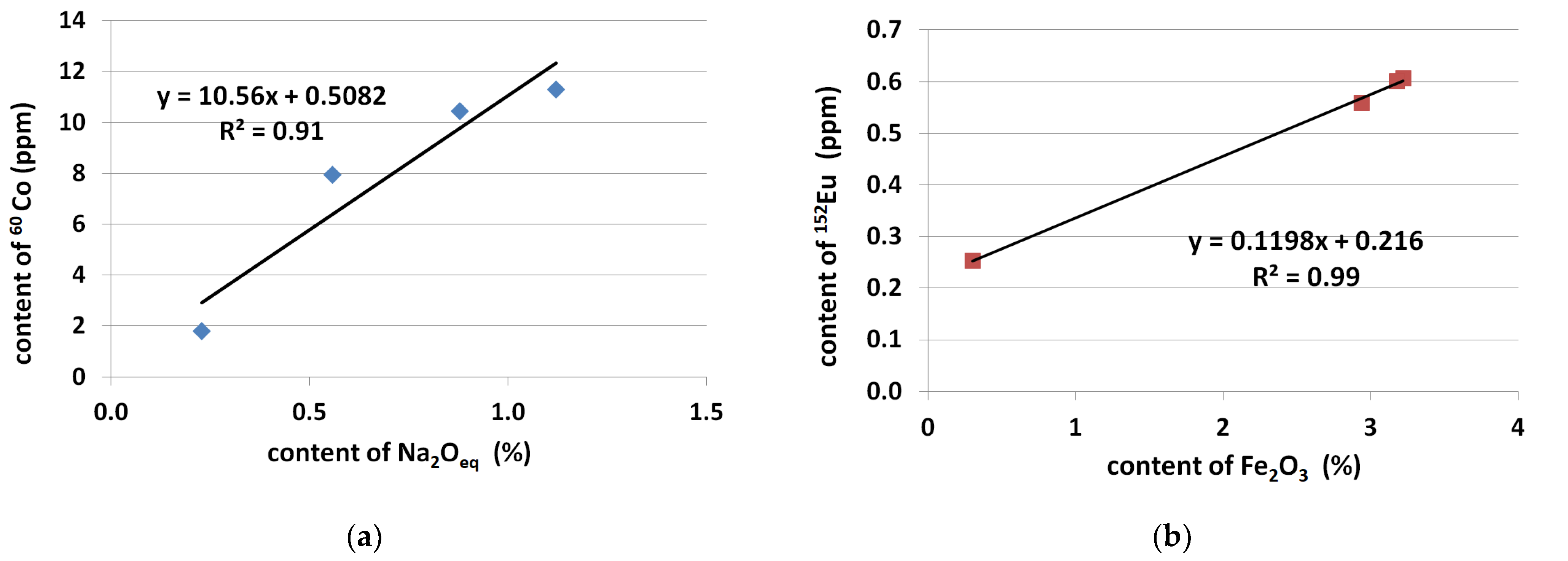
- For the considered range of Portland cements CEM I 42.5 R and 52.5 R the content of 60Co was proportional to the content of Fe2O3 and the content of 152Eu was proportional to the total content of alkalis.
- Quartzite and greywacke aggregates were found to be highly reactive in the alkaline environment of Portland cement concrete.
- Due to the high potential of alkali-silica reaction, it is not recommended to use quartzite as a coarse aggregate, as well as siliceous river sand as a fine aggregate for shielding concrete, despite the low contents of 60Co and 152Eu. At the same time, it is suggested to use the cement with the lowest alkali content, both due to the possibility of alkali-silica reaction of the aggregate in concrete, as well as a lower content of 60Co.
- Limestone with low content of siliceous minerals is good for preventing the alkalisilica reaction and the formation of 60Co and 152Eu radioisotopes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IAEA. Clearance Levels for Radionuclides in Solid Materials: Application of the Exemption Principles, Interim Report for Comment; IAEA TECDOC-855: Vienna, Austria, 1996. [Google Scholar]
- Deckert, A.; Thierfeldt, S.; Kugeler, E.; Neuhaus, I. Definition of Clearance Levels for the Release of Radioactively Contaminated Buildings and Building Rubble, Final Report Contract C1/ETU/970040; European Commission, Radiation Protection 114; BrenkSystemplanung: Aachen, Germany, 1999. [Google Scholar]
- Kimura, K.-I.; Hasegawa, A.; Hayashi, K.; Uematsu, M.; Ogata, T.; Tanosaki, T.; Yoshino, R.; Sato, M.; Saito, M.; Kinno, M. Development of Low-Activation Design Method for Reduction of Radioactive Waste Below Clearance Level. In Proceedings of the International Conference on Nuclear Engineering 2008, Orlando, FL, USA, 11–15 May 2008. [Google Scholar]
- AC09617981. The Safety Case and Safety Assessment for the Disposal of Radioactive Waste, SSG-23; IAEA: Vienna, Austria, 2012. [Google Scholar]
- Suzuki, A.; Iida, T.; Moriizumi, J.; Sakuma, Y.; Takada, J.; Yamasaki, K.; Yoshimoto, T. Trace Elements with Large Activation Cross Section in Concrete Materials in Japan. J. Nucl. Sci. Technol. 2001, 38, 542–550. [Google Scholar] [CrossRef][Green Version]
- Gauld, I.C.; Ryman, J.C. Nuclide Importance to Criticality Safety, Decay Heating, and Source Terms Related to Transport and Interim Storage of High-Burnup LWR Fuel; Oak Ridge National Lab.(ORNL): Oak Ridge, TN, USA, 2000. [Google Scholar] [CrossRef][Green Version]
- Reches, Y. A multi-scale review of the effects of gamma radiation on concrete. Results Mater. 2019, 2, 100039. [Google Scholar] [CrossRef]
- IAEA. Ageing Management of Concrete Structures in Nuclear Power Plants, Vienna, Nucl. Energy Ser. NP-T-3.5:1–372; IAEA: Vienna, Austria, 2016. [Google Scholar]
- Kurtis, K.; Xi, Y.; Glinicki, M.A.; Provis, J.; Gianni, E.; Fu, T. Can we design concrete to survive nuclear environments? Concr. Int. 2017, 39, 29–35. [Google Scholar]
- Bamonte, P.; Gambrova, P.G. Properties of concrete required in nuclear power plants. In Infrastructure Systems for Nuclear Energy; Hsu, T.T.C., Wu, C.-L., Lin, J.-L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Saouma, V.E.; Hariri-Ardebili, M.A. A proposed aging management program for alkali silica reactions in a nuclear power plant. Nucl. Eng. Des. 2014, 277, 248–264. [Google Scholar] [CrossRef]
- Duchesne, J.; Bérubé, M.-A. Long-term effectiveness of supplementary cementing materials against alkali–silica reaction. Cem. Concr. Res. 2001, 31, 1057–1063. [Google Scholar] [CrossRef]
- Saha, A.K.; Khan, M.; Sarker, P.; Shaikh, F.; Pramanik, A. The ASR mechanism of reactive aggregates in concrete and its mitigation by fly ash: A critical review. Constr. Build. Mater. 2018, 171, 743–758. [Google Scholar] [CrossRef]
- Kawabata, Y.; Yamada, K. The mechanism of limited inhibition by fly ash on expansion due to alkali–silica reaction at the pessimum proportion. Cem. Concr. Res. 2017, 92, 1–15. [Google Scholar] [CrossRef]
- Menéndez, E.; Sanjuán, M.; García-Roves, R.; Argiz, C.; Recino, H. Durability of Blended Cements Made with Reactive Aggregates. Materials 2021, 14, 2948. [Google Scholar] [CrossRef]
- Olagüe, C.; Castro, P.; Lopez, W. Reacción álcali-sílice en áridos para pavimentos de hormigón en el Estado de Chihuahua, México. Mater. Construcción 2002, 52, 19–31. [Google Scholar] [CrossRef]
- Gautam, B.P.; Panesar, D.K.; Sheikh, S.A.; Vecchio, F.J.; Orbovic, N. Alkali aggregate reaction in nuclear concrete structures: Part 2: Concrete materials aspects. In Proceedings of the 23rd SMiRT Conference 2015, Manchester, UK, 10–14 August 2015. [Google Scholar]
- Beaver, J.; Jiang, L.; Sherman, M. Inspection and monitoring of ASR-affected structures at Seabrook Station, NH. In Proceedings of the SMiRT Conference 2013, San Francisco, CA, USA, 18–23 August 2013. [Google Scholar]
- Khan, A.A.; Gray, M.; Shenton, B.; Panesar, D.; Elgohary, M. Assessment, repair and monitoring of the Gentilly-1 concrete containment structure. In Proceedings of the 16th SMiRT Conference 2001, Washington, DC, USA, 12–17 August 2001. [Google Scholar]
- Ferche, A.C.; Gautam, B.; Habibi, F.; Panesar, D.K.; Sheikh, S.A.; Vecchio, F.J.; Orbovic, N. Material, structural and modelling aspects of alkali aggregate reaction in concrete. Nucl. Eng. Des. 2019, 351, 87–93. [Google Scholar] [CrossRef]
- PN-EN 1097-6. Tests for Mechanical and Physical Properties of Aggregates. Determination of Particle Density and Water Absorption; Polish Committee for Standardization: Warsaw, Poland, 2013. [Google Scholar]
- PN-EN 197-1:2012, Cement. Composition, Specifications and Conformity Criteria for Common Cements; Polish Committee for Standardization: Warsaw, Poland, 2012. [Google Scholar]
- PN-EN 196-2:2013-11. Method of Testing Cement—Part 2: Chemical Analysis of Cement; Polish Committee for Standardization: Warsaw, Poland, 2011. [Google Scholar]
- PN-EN 196-6: 2019-01. Methods of Testing Cement. Determination of Fineness; Polish Committee for Standardization: Warsaw, Poland, 2019. [Google Scholar]
- PN-EN 196-3:2016-12. Methods of Testing Cement. Determination of Setting Times and Soundness; Polish Committee for Standardization: Warsaw, Poland, 2016. [Google Scholar]
- De Corte, F.; Simonits, A. Recommended nuclear data for use in the k0 standardization of neutron activation analysis. At. Data Nucl. Data Tables 2003, 85, 47–67. [Google Scholar] [CrossRef]
- Szentmiklósi, L.; Párkányi, D.; Sziklai-László, I. Upgrade of the Budapest neutron activation analysis laboratory. J. Radioanal. Nucl. Chem. 2016, 309, 91–99. [Google Scholar] [CrossRef]
- Szentmiklósi, L.; Maróti, B.; Párkányi, D.; Harsányi, I.; Révay, Z. High-energy detector calibration data for k 0-neutron activation analysis. J. Radioanal. Nucl. Chem. 2017, 315, 743–750. [Google Scholar] [CrossRef]
- Simonits, A.; Östör, J.; Kálvin, S.; Fazekas, B. HyperLab: A new concept in gamma-ray spectrum analysis. J. Radioanal. Nucl. Chem. 2003, 257, 589–595. [Google Scholar] [CrossRef]
- De Corte, F.; Van Sluijs, R.; Simonits, A.; Kučera, J.; Smodiš, B.; Byrne, A.R.; De Wispelaere, A.; Bossus, D.; Frána, J.; Horák, Z.; et al. Installation and calibration of Kayzero-assisted NAA in three Central European countries via a Copernicus project. Appl. Radiat. Isot. 2001, 55, 347–354. [Google Scholar] [CrossRef]
- Garbacik, A.; Glinicki, M.A.; Jóźwiak-Niedźwiedzka, D.; Adamski, G.; Gibas, K. Technical Guidelines for the Classification of Domestic Aggregates and Prevention of the Alkali-Aggregate Reaction in Concrete Used in Road Pavements and Road Engineering Facilities, The Institute of Ceramics and Building Materials, Institute of Fundamental Technological Research Polish Academy of Sciences. 2019; (In Polish). Available online: https://www.gddkia.gov.pl/pl/1118/dokumenty-techniczne (accessed on 15 June 2021).
- ASTM C 1293. Standard Test Method for Determination of Length Change of Concrete Due to Alkali-Silica Reaction; ASTM Int.: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Nixon, P.J.; Sims, I. RILEM Recommended Test Method: AAR-3—Detection of Potential Alkali-Reactivity—38 °C Test Method for Aggregate Combinations Using Concrete Prisms. In RILEM Recommendations for the Prevention of Damage by Alkali-Aggregate Reactions in New Concrete Structures; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Kinno, M.; Kimura, K.-I.; Nakamura, T. Raw Materials for Low-Activation Concrete Neutron Shields. J. Nucl. Sci. Technol. 2002, 39, 1275–1280. [Google Scholar] [CrossRef]
- Pai, B.H.V.; Shanbhag, A.A.; Prabhath Ravi, K.; Thakare, S.V.; Jagadeesan, K.C.; Krishnamoorthy, A.; Sarkar, P.K.; Nandy, M. Estimation of trace element concentration and neutron induced radioactivity in rock samples of different geological compositions for neutron shielding. Indian J. Pure Appl. Phys. 2016, 54, 7–14. [Google Scholar]
- Sachlová, Š.; Kuchaová, A.; Pøikryl, R.; Pertold, Z.; Nekvasilová, Z. Factors affecting ASR potential of quartzite from a single quarry (Bohemian Massif, Czech Republic). In Proceedings of the Conference: 12th SGA Biennial Meeting 2013, Upsala, Sweden, 12–15 August 2013. [Google Scholar]
- Jensen, V.; Sujjavanich, S. Alkali silica reaction in concrete foundations in Thailand. In Proceedings of the 15th International Conference on Alkali-Aggregates Reaction 2016, Sao-Paulo, Brazil, 3–7 July 2016. [Google Scholar]
- Jóźwiak-Niedźwiedzka, D.; Gibas, K.; Brandt, A.M.; Glinicki, M.A.; Dąbrowski, M.; Denis, P. Mineral Composition of Heavy Aggregates for Nuclear Shielding Concrete in Relation to Alkali-silica Reaction. Procedia Eng. 2015, 108, 162–169. [Google Scholar] [CrossRef]
- Jóźwiak-Niedźwiedzka, D.; Glinicki, M.A.; Gibas, K.; Baran, T. Alkali-Silica Reactivity of High Density Aggregates for Radiation Shielding Concrete. Materials 2018, 11, 2284. [Google Scholar] [CrossRef] [PubMed]
- Latifee, E.R. State-of-the-Art Report on Alkali Silica Reactivity Mitigation Effectiveness Using Different Types of Fly Ashes. J. Mater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Baran, T.; Glinicki, M.A.; Jóźwiak-Niedźwiedzka, D. The properties of special cements for shielding constructions in nuclear power plants. Cement Wapno Beton 2016, 21, 207–216. [Google Scholar]
- ASTM C1778. Standard Guide for Reducing the Risk of Deleterious Alkali-Aggregate Reaction in Concrete; ASTM Int.: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Ichikawa, T.; Koizumi, H. Possibility of Radiation-Induced Degradation of Concrete by Alkali-Silica Reaction of Aggregates. J. Nucl. Sci. Technol. 2002, 39, 880–884. [Google Scholar] [CrossRef]
- Field, K.; Remec, I.; Le Pape, Y. Radiation effects in concrete for nuclear power plants—Part I: Quantification of radiation exposure and radiation effects. Nucl. Eng. Des. 2015, 282, 126–143. [Google Scholar] [CrossRef]
- Le Pape, Y.; Alsaid, M.H.F.; Giorla, A.B. Rock-Forming Minerals Radiation-Induced Volumetric Expansion—Revisiting Literature Data. J. Adv. Concr. Technol. 2018, 16, 191–209. [Google Scholar] [CrossRef]
- Maruyama, I.; Kontani, O.; Takizawa, M.; Sawada, S.; Ishikawao, S.; Yasukouchi, J.; Sato, O.; Etoh, J.; Igari, T. Development of Soundness Assessment Procedure for Concrete Members Affected by Neutron and Gamma-Ray Irradiation. J. Adv. Concr. Technol. 2017, 15, 440–523. [Google Scholar] [CrossRef]



| Aggregate | Designation | Density, g/cm3 [21] |
|---|---|---|
| quartzite | Q1 | 2.62 |
| Q2 | 2.60 | |
| granite | G1 | 2.63 |
| G2 | 2.64 | |
| flint | F1 | 2.65 |
| melaphyre | M1 | 2.70 |
| greywacke | GW1 | 2.70 |
| limestone | L1 | 2.71 |
| baryte | B1 | 4.20 |
| Constituent | C1 | C2 | C3 | C4 |
|---|---|---|---|---|
| CEM I 42.5R | CEM I 52.5R | CEM I 42.5R | CEM I 52.5R | |
| SiO2 | 19.03 | 19.42 | 19.43 | 24.40 |
| Al2O3 | 4.84 | 5.45 | 4.84 | 2.11 |
| Fe2O3 | 3.22 | 2.94 | 3.18 | 0.30 |
| CaO | 63.64 | 64.1 | 61.81 | 68.40 |
| MgO | 1.15 | 1.75 | 2.56 | 0.66 |
| SO3 | 2.97 | 3.5 | 3.93 | 2.09 |
| Na2O | 0.21 | 0.24 | 0.41 | 0.17 |
| K2O | 0.53 | 0.97 | 1.08 | 0.09 |
| LOI | 3.34 | 2.50 | 2.67 | 1.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak-Niedźwiedzka, D.; Gméling, K.; Antolik, A.; Dziedzic, K.; Glinicki, M.A. Assessment of Long Lived Isotopes in Alkali-Silica Resistant Concrete Designed for Nuclear Installations. Materials 2021, 14, 4595. https://doi.org/10.3390/ma14164595
Jóźwiak-Niedźwiedzka D, Gméling K, Antolik A, Dziedzic K, Glinicki MA. Assessment of Long Lived Isotopes in Alkali-Silica Resistant Concrete Designed for Nuclear Installations. Materials. 2021; 14(16):4595. https://doi.org/10.3390/ma14164595
Chicago/Turabian StyleJóźwiak-Niedźwiedzka, Daria, Katalin Gméling, Aneta Antolik, Kinga Dziedzic, and Michał A. Glinicki. 2021. "Assessment of Long Lived Isotopes in Alkali-Silica Resistant Concrete Designed for Nuclear Installations" Materials 14, no. 16: 4595. https://doi.org/10.3390/ma14164595
APA StyleJóźwiak-Niedźwiedzka, D., Gméling, K., Antolik, A., Dziedzic, K., & Glinicki, M. A. (2021). Assessment of Long Lived Isotopes in Alkali-Silica Resistant Concrete Designed for Nuclear Installations. Materials, 14(16), 4595. https://doi.org/10.3390/ma14164595






