Silver Nanoparticles Effects on In Vitro Germination, Growth, and Biochemical Activity of Tomato, Radish, and Kale Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Culture Medium
2.2. In Vitro Physical Growing Conditions
2.3. Characteristics of Nanoparticles
2.4. Plant Material—Tested Genotypes and In Vitro Culture Initiation
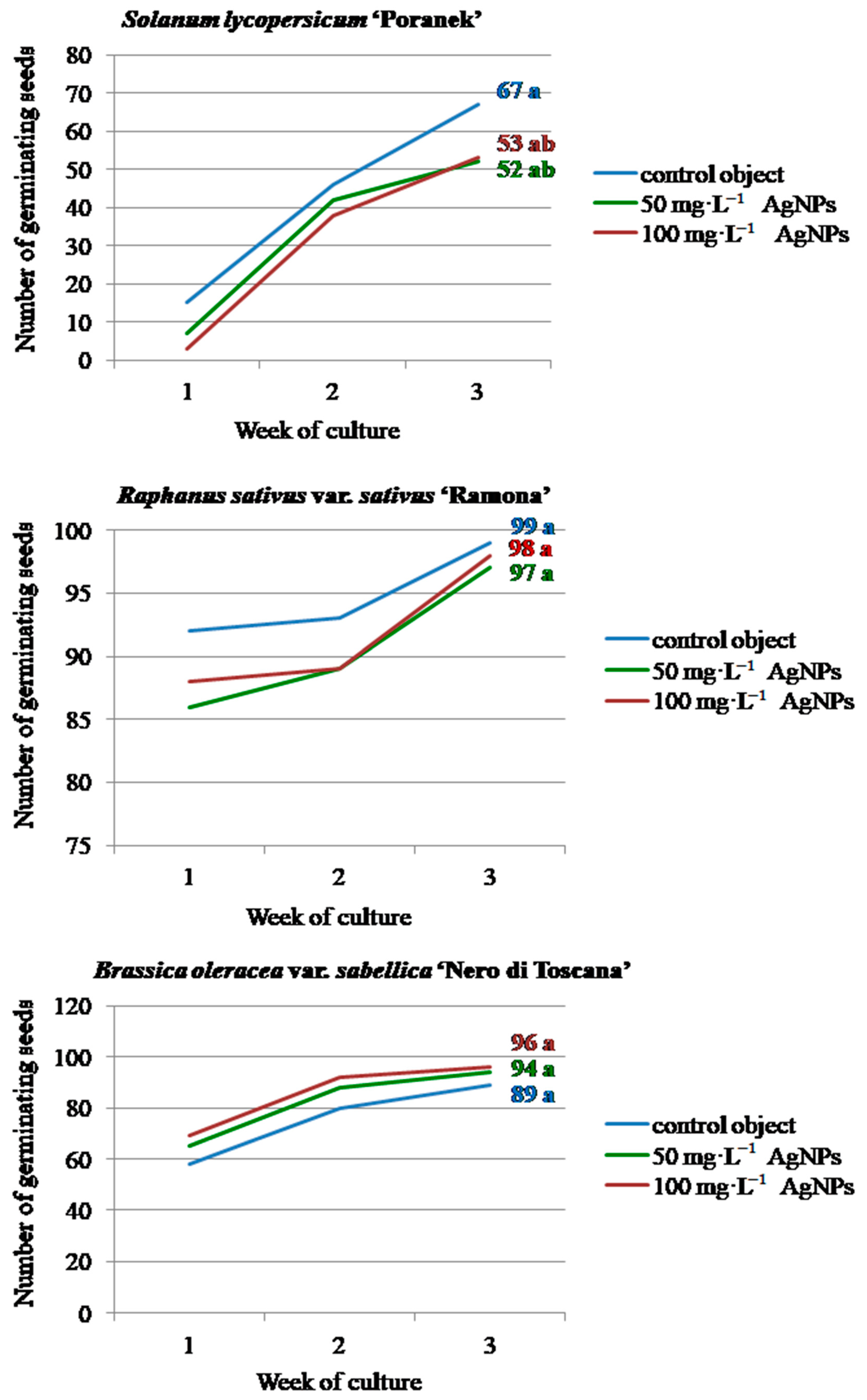
2.5. Plant Material—Observations of In Vitro Cultures and Biometric Evaluation
2.6. Plant Material—Biochemical Array
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatami, M. Stimulatory and inhibitory effects of nanoparticulates on seed germination and seedling vigor indices. In Nanoscience and Plant-Soil Systems; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 357–385. [Google Scholar] [CrossRef]
- Fayez, K.A.; El-Deeb, B.A.; Mostafa, N.Y. Toxicity of biosynthetic silver nanoparticles on the growth, cell ultrastructure and physiological activities of barley plant. Acta Physiol. Plant. 2017, 39, 155. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Mahendran, D.; Geetha, N.; Venkatachalam, P. 2019 Role of silver nitrate and silver nanoparticles in tissue culture medium and enhanced the plant growth and development. In In Vitro Plant Breeding towards Novel Agronomic Traits; Kumar, M., Muthusamy, A., Kumar, V., Bhalla-Sarin, N., Eds.; Springer Nature: Singapore, 2019; pp. 59–74. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Timoteo, C.; Paiva, R.; dos Reis, M.V.; Claro, P.I.C.; Ferraz, L.M.; Marconcini, J.M.; de Oliviera, J.E. In vitro growth of Physalis peruviana L. affected by silver nanoparticles. 3 Biotech 2019, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Raza, M.A.; Rizwan, M.; Tariq, R.; Ali, S.; Huang, L. Silver nanoparticles improved plant growth and reduced the sodium and chlorine accumulation in pearl millet: A life cycle study. ESPR 2021, 28, 13712–13724. [Google Scholar] [CrossRef]
- Pacheco, I.; Buzea, C. Nanoparticle interactions with plants. In Nanoscience and Plant-Soil Systems; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 323–355. [Google Scholar] [CrossRef]
- Álvarez, S.P.; Tapia, M.A.M.; Vega, M.E.G.; Ardisana, E.F.H.; Medina, A.J.C.; Zamora, G.L.F.; Bustamante, D.V. Nanotechnology and Plant Tissue Culture. In Plant Nanobionics; Prasad, R., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 333–370. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A. Gold nanoparticles affect the cryopreservation efficiency of In Vitro-derived shoot tips of bleeding heart. PCTOC 2021, 146, 297–311. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Miler, N. Silver and gold nanoparticles impact on in vitro adventitious organogenesis in chrysanthemum, gerbera and Cape Primrose. Sci. Hortic. 2019, 257, 108766. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Kulus, D. Silver nanoparticles induce genetic, biochemical, and phenotype variation in chrysanthemum. PCTOC 2020, 143, 331–344. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Gurunathan, S.; Chung, I.-M. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanopartciles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 2015, 252, 1031–1046. [Google Scholar] [CrossRef]
- Homaee, M.B.; Ehsanpour, A.A. Silver nanoparticles and silver ions: Oxidative stress responses and toxicity in potato (Solanum tuberosum L.) grown in vitro. Hortic. Environ. Biotechnol. 2016, 57, 544–553. [Google Scholar] [CrossRef]
- Yasur, J.; Rani, P.U. Environmental effects of nanosilver: Impact on castor seed germination, seedling growth, and plant physiology. Environ. Sci. Pollut. Res. 2013, 20, 8636–8648. [Google Scholar] [CrossRef]
- Han, C.; Yang, P. Studies on the molecular mechanism of seed germination. Proteomics 2015, 15, 1671–1679. [Google Scholar] [CrossRef]
- Szőllősi, R.; Molnár, Á.; Kondar, S.; Kolbert, Z. Dual effect of nanomaterials on germination and seedling growth: Stimulation vs. phytotoxcity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef]
- Shukla, P.; Chaurasia, P.; Yonis, K.; Qadri, O.S.; Faridi, S.A.; Srivastava, G. Nanotechnology in sustainable agriculture: Studies from seed priming to post-harvest management. Nanotechnol. Environ. Eng. 2019, 4, 11. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Wojnarowicz, J. Zinc oxide and zinc oxide nanoparticles impact on in vitro germination and seedling growth in Allium cepa L. Materials 2020, 13, 2784. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Z.; Rui, Y.; Ren, J.; Hou, T.; Wu, S.; Rui, M.; Jiang, F.; Liu, L. Effect of different nanoparticles on seed germination and seedling growth in rice. In Proceedings of the 2nd Annual International Conference on Advanced Material Engineering (AME 2016), Wuhan, China, 15–17 April 2016; Bhatnagar, A.K., Dahai, R., Pandey, K.M., Haeri, H., Andrievskiy, R.A., Ziara, M., Feng, G., Eds.; Advances in Engineering Research. Atlantis Press: Dordrecht, The Netherlands, 2016; Volume 85, pp. 166–173. [Google Scholar]
- Parveen, A.; Rao, S. Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum. J. Clust. Sci. 2015, 26, 693–701. [Google Scholar] [CrossRef]
- Bayramzadeh, V.; Ghadiri, M.; Davoodi, M.H. Effects of silver nanoparticle exposure on germination and early growth of Pinus sylvestris and Alnus subcordata. Sains Malays 2019, 48, 937–944. [Google Scholar] [CrossRef]
- Almutairi, Z.M.; Alharbi, A. Effect of silver nanoparticles on seed germination of crop plants. J. Adv. Agric. 2015, 4, 283–288. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Domeradzka-Gajda, K.; Nocuń, M.; Roszak, J.; Janasik, B.; Quarles, C.D., Jr.; Wąsowicz, W.; Grobelny, J.; Tomaszewska, E.; Celichowski, G.; Ranoszek-Soliwoda, K.; et al. A study on the in vitro percutaneous absorption of silver nanoparticles in combination with aluminum chloride, methyl paraben or di-n-butyl phthalate. Toxicol. Lett. 2017, 272, 38–48. [Google Scholar] [CrossRef]
- Pudlarz, A.; Czechowska, E.; Ranoszek-Soliwoda, K.; Tomaszewska, E.; Celichowski, G.; Grobelny, J.; Szemraj, J. Immobilization of recombinant human catalase on gold and silver nanoparticles. Appl. Biochem. Biotechnol. 2018, 185, 717–735. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. In Methods of Biochemical Analysis; Glick, D., Ed.; Willey: New York, NY, USA, 1954; pp. 357–425. [Google Scholar] [CrossRef]
- Nowogórska, A.; Patykowski, J. Selected reactive oxygen species and antioxidany enzymes in common bean after Pseudomonas syringae pv. phaseolicola and Botrytis cinerea infection. Acta Physiol. Plant. 2015, 37, 1725. [Google Scholar] [CrossRef] [Green Version]
- Mehrian, S.K.; Heidari, R.; Rahmani, F.; Najafi, S. Effect of chemical synthesis silver nanoparticles on germianation indices and seedling growth in seven varietes of Lycopersicon esculentum Mill (tomato) plants. J. Clust. Sci. 2016, 27, 327–340. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Pardha Saradhi, P.; Khanna, P.K.; Arora, S. Silver nanoparticles-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, B.R.; Singh, A.; Keswani, C.; Naqvi, A.H.; Singh, H.B. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS ONE 2014, 9, e97881. [Google Scholar] [CrossRef] [PubMed]
- Bernard, F.; Moghadam, N.N.; Mirzahani, F. The effect of colloidal silver nanoparticles on the level of lignification and hyperhydricity syndrome in Thymus deanensis vitro shoots: A possibile involvement of bonded polyamines. In Vitro Cell Dev Biol–Plant 2015, 51, 546–553. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parveen, A.; Mazhari, B.B.Z.; Rao, S. Impact of bio-nanogold on seed germination and seedling growth in Pennisetum glaucum. Enzyme Microb. Technol. 2016, 95, 107–111. [Google Scholar] [CrossRef]
- Mahakham, W.; Theerakulpisut, P.; Maensiri, S.; Phumying, S.; Sarmah, A.K. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles agent for promoting maize seed germination. Sci. Total Environ. 2016, 573, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009, 117, 143–148. [Google Scholar] [CrossRef]
- Dimpka, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Garcia-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Barriga-Castro, E.D.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Nino-Medina, G. Zinc oxide nanoparticles boost phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef] [Green Version]
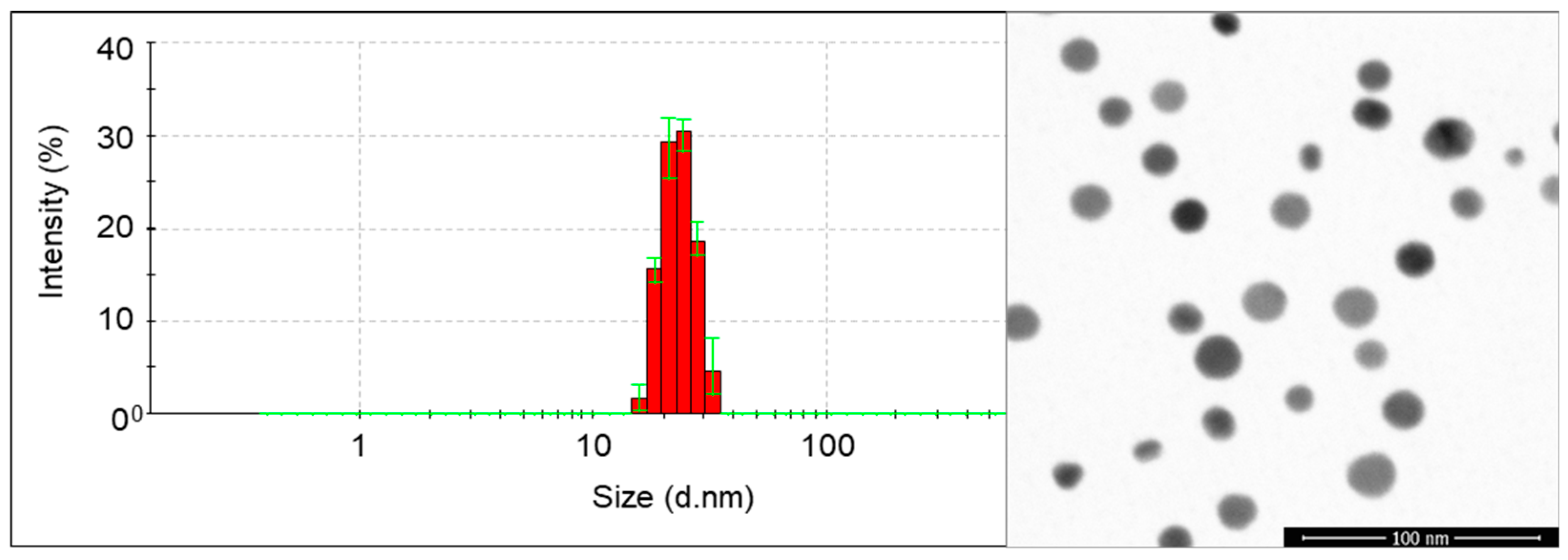

| Concentration of AgNPs | Shoot Length (cm) | Shoot Fresh Weight (mg) | Shoot Dry Weight (mg) | Root Length (cm) | Root Fresh Weight (mg) | Root Dry Weight (mg) |
|---|---|---|---|---|---|---|
| Solanum lycopersicum ‘Poranek’ | ||||||
| control object | 7.01 ± 3.14 a | 211.94 ± 161.39 a | 10.49 ± 2.18 a | 7.93 ± 6.48 a | 48.69 ± 56.94 a | 3.84 ± 1.33 a |
| 50 mg·L−1 | 5.47 ± 1.21 ab | 143.52 ± 39.08 ab | 7.94 ± 3.46 ab | 6.62 ± 2.35 a | 25.28 ± 7.79 ab | 2.64 ± 1.59 a |
| 100 mg·L−1 | 3.64 ± 0.89 b | 63.34 ± 46.15 b | 3.31 ± 2.10 b | 5.80 ± 2.54 a | 10.34 ± 7.30 b | 1.45 ± 0.59 a |
| Raphanus sativus var. sativus ‘Ramona’ | ||||||
| control object | 11.69 ± 2.30 a | 377.05 ± 109.06 b | 23.59 ± 2.33 b | 8.87 ± 5.62 b | 53.47 ± 27.29 a | 3.63 ± 0.89 a |
| 50 mg·L−1 | 9.97 ± 3.65 b | 496.03 ± 249.87 a | 29.32 ± 3.97 a | 10.79± 5.75ab | 34.93 ± 24.81 b | 3.82 ± 1.08 a |
| 100 mg·L−1 | 9.98 ± 2.96 b | 515.06 ± 206.82 a | 30.14 ± 3.40 a | 12.07 ± 3.72 a | 40.65 ± 24.20 b | 3.65 ± 0.71 a |
| Brassica oleracea var. sabellica ‘Nero di Toscana’ | ||||||
| control object | 9.26 ± 2.69 b | 130.45 ± 41.20 b | 10.30 ± 0.84 b | 8.49 ± 1.86 ab | 15.13 ± 7.43 ab | 2.00 ± 0.33 b |
| 50 mg·L−1 | 11.36 ± 2.53 a | 196.07 ± 107.55 ab | 15.26 ± 3.12 a | 9.89 ± 2.80 a | 9.22 ± 8.50 b | 2.19 ± 0.43 b |
| 100 mg·L−1 | 9.78 ± 3.26 b | 245.39 ± 126.41 a | 17.98 ± 0.66 a | 8.32 ± 2.77 b | 19.64 ± 14.66 a | 2.97 ± 0.48a 1 |
| Concentration of AgNPs | Chlorophyll a Content (mg·g−1 FW) | Chlorophyll b Content (mg·g−1 FW) | Chlorophyll a/b Ratio | Chlorophylls (a + b) Content (mg·g−1 FW) | Carotenoids Content (mg·g−1 FW) | Chlorophylls/ Carotenoids Ratio | Anthocyanins Content (mg·g−1 FW) | Phenolic Compounds Content (mg·g−1 FW) | Total Protein Content (mg·g−1 FW) | SOD Activity (U) | GPOX Activity (U) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solanum lycopersicum ‘Poranek’ | |||||||||||
| control object | 1.30 ± 0.25 a | 0.54 ± 0.13 a | 2.45 ± 0.14 b | 1.84 ± 0.38 a | 0.36 ± 0.07 a | 5.05 ± 0.10 a | 0.17 ± 0.03 b | 1.50 ± 0.09 a | 26.64 ± 0.68 a | 2.48 ± 0.75 a | 5.54 ± 0.66 b |
| 50 mg·L−1 | 0.77 ± 0.10 b | 0.23 ± 0.04 b | 3.43 ± 0.25 a | 1.00 ± 0.14 b | 0.23 ± 0.02 b | 4.42 ± 0.20 b | 0.19 ± 0.02 ab | 1.52 ± 0.11 a | 26.25 ± 1.57 a | 2.20 ± 1.06 a | 6.79 ± 2.16 b |
| 100 mg·L−1 | 0.83 ± 0.17 b | 0.28 ± 0.09 b | 3.05 ± 0.51 a | 1.12 ± 0.26 b | 0.24 ± 0.05 b | 4.69 ± 0.36 ab | 0.23 ± 0.03 a | 1.57 ± 0.16 a | 26.99 ± 1.16 a | 4.11 ± 0.79 a | 10.85 ± 0.78 a |
| Raphanus sativus var. sativus ‘Ramona’ | |||||||||||
| control object | 1.10 ± 0.05 ab | 0.38 ± 0.02 b | 2.90 ± 0.26 a | 1.48 ± 0.05 ab | 0.27 ± 0.02 ab | 5.60 ± 0.34 b | 0.30 ± 0.05 a | 1.65 ± 0.08 ab | 13.77 ± 0.25 a | 7.18 ± 2.24 a | 5.16 ± 1.03 a |
| 50 mg·L−1 | 1.25 ± 0.20 a | 0.50 ± 0.10 a | 2.52 ± 0.17 b | 1.75 ± 0.30 a | 0.30 ± 0.05 a | 5.92 ± 0.17 ab | 0.29 ± 0.06 ab | 1.82 ± 0.10 a | 13.41 ± 0.31 a | 6.56 ± 1.71 a | 4.12 ± 0.74 a |
| 100 mg·L−1 | 1.02 ± 0.04 b | 0.39 ± 0.02 b | 2.61 ± 0.09 b | 1.41 ± 0.06 b | 0.23 ± 0.01 b | 6.18 ± 0.13 a | 0.22 ± 0.05 b | 1.51 ± 0.19 b | 12.16 ± 0.51 b | 8.36 ± 0.98 a | 2.18± 0.94b |
| Brassica oleracea var. sabellica ‘Nero di Toscana’ | |||||||||||
| control object | 1.76 ± 0.11 a | 0.70 ± 0.04 a | 2.52 ± 0.05 a | 2.45 ± 0.14 a | 0.48 ± 0.03 a | 5.19 ± 0.10 a | 0.13 ± 0.03 a | 1.05 ± 0.39 a | 13.21 ± 0.64 a | 2.63 ± 0.11 a | 1.28 ± 0.51 b |
| 50 mg·L−1 | 1.41 ± 0.10 b | 0.58 ± 0.06 b | 2.47 ± 0.27 a | 1.99 ± 0.14 b | 0.36 ± 0.03 b | 5.60 ± 0.48 a | 0.09 ± 0.02 c | 1.02 ± 0.04 a | 13.35 ± 0.13 a | 2.64 ± 0.13 a | 3.09 ± 1.87 ab |
| 100 mg·L−1 | 1.54 ± 0.17 b | 0.65 ± 0.09 b | 2.37 ± 0.18 a | 2.19 ± 0.25 ab | 0.40 ± 0.05 b | 5.49 ± 0.27 a | 0.11 ± 0.03 b | 1.08 ± 0.01 a | 13.23 ± 0.20 a | 2.52 ± 0.18 a | 4.11 ± 1.13 a 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tymoszuk, A. Silver Nanoparticles Effects on In Vitro Germination, Growth, and Biochemical Activity of Tomato, Radish, and Kale Seedlings. Materials 2021, 14, 5340. https://doi.org/10.3390/ma14185340
Tymoszuk A. Silver Nanoparticles Effects on In Vitro Germination, Growth, and Biochemical Activity of Tomato, Radish, and Kale Seedlings. Materials. 2021; 14(18):5340. https://doi.org/10.3390/ma14185340
Chicago/Turabian StyleTymoszuk, Alicja. 2021. "Silver Nanoparticles Effects on In Vitro Germination, Growth, and Biochemical Activity of Tomato, Radish, and Kale Seedlings" Materials 14, no. 18: 5340. https://doi.org/10.3390/ma14185340
APA StyleTymoszuk, A. (2021). Silver Nanoparticles Effects on In Vitro Germination, Growth, and Biochemical Activity of Tomato, Radish, and Kale Seedlings. Materials, 14(18), 5340. https://doi.org/10.3390/ma14185340






