Improving the Room-Temperature Ferromagnetism in ZnO and Low-Doped ZnO:Ag Films Using GLAD Sputtering
Abstract
1. Introduction
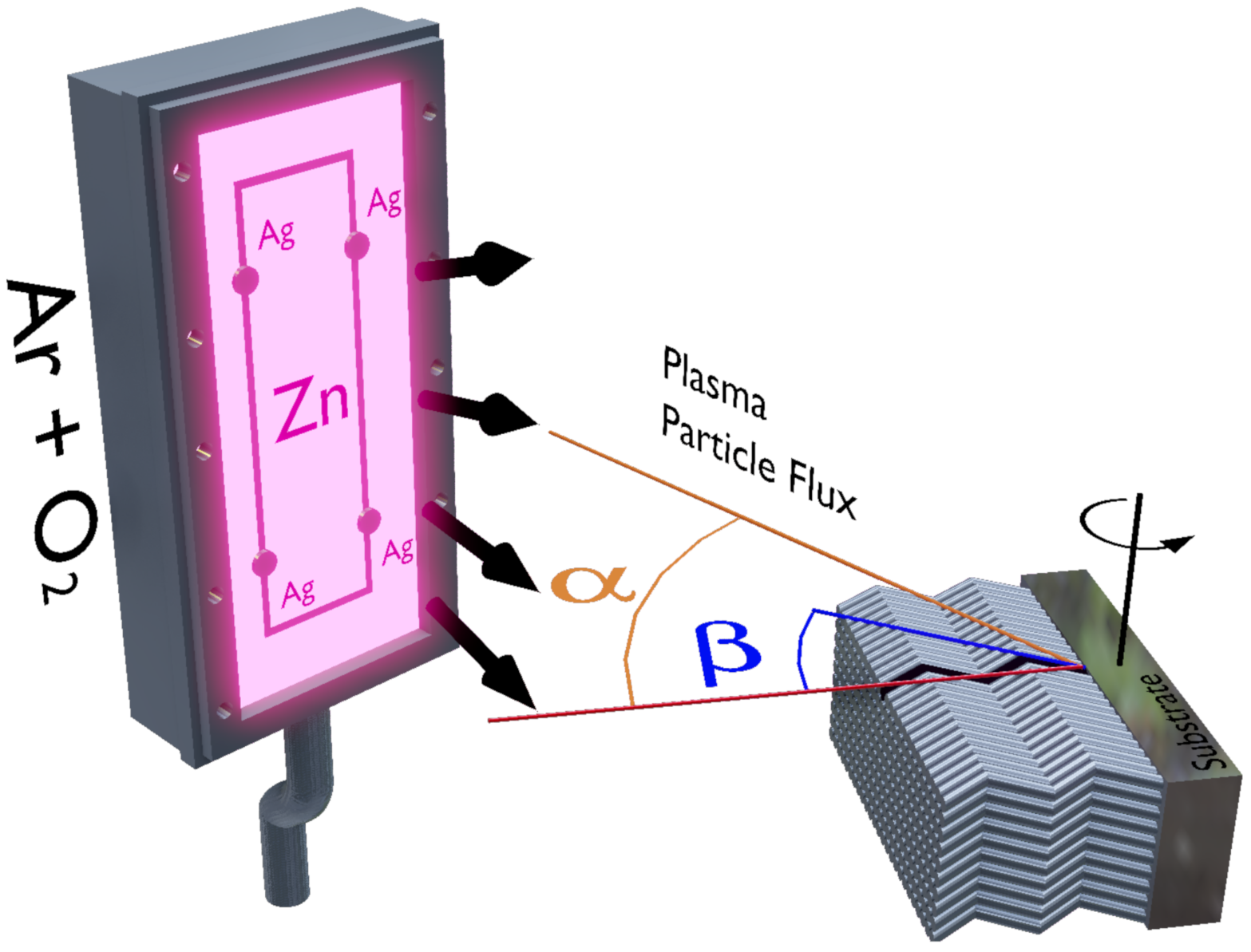
2. Experiment
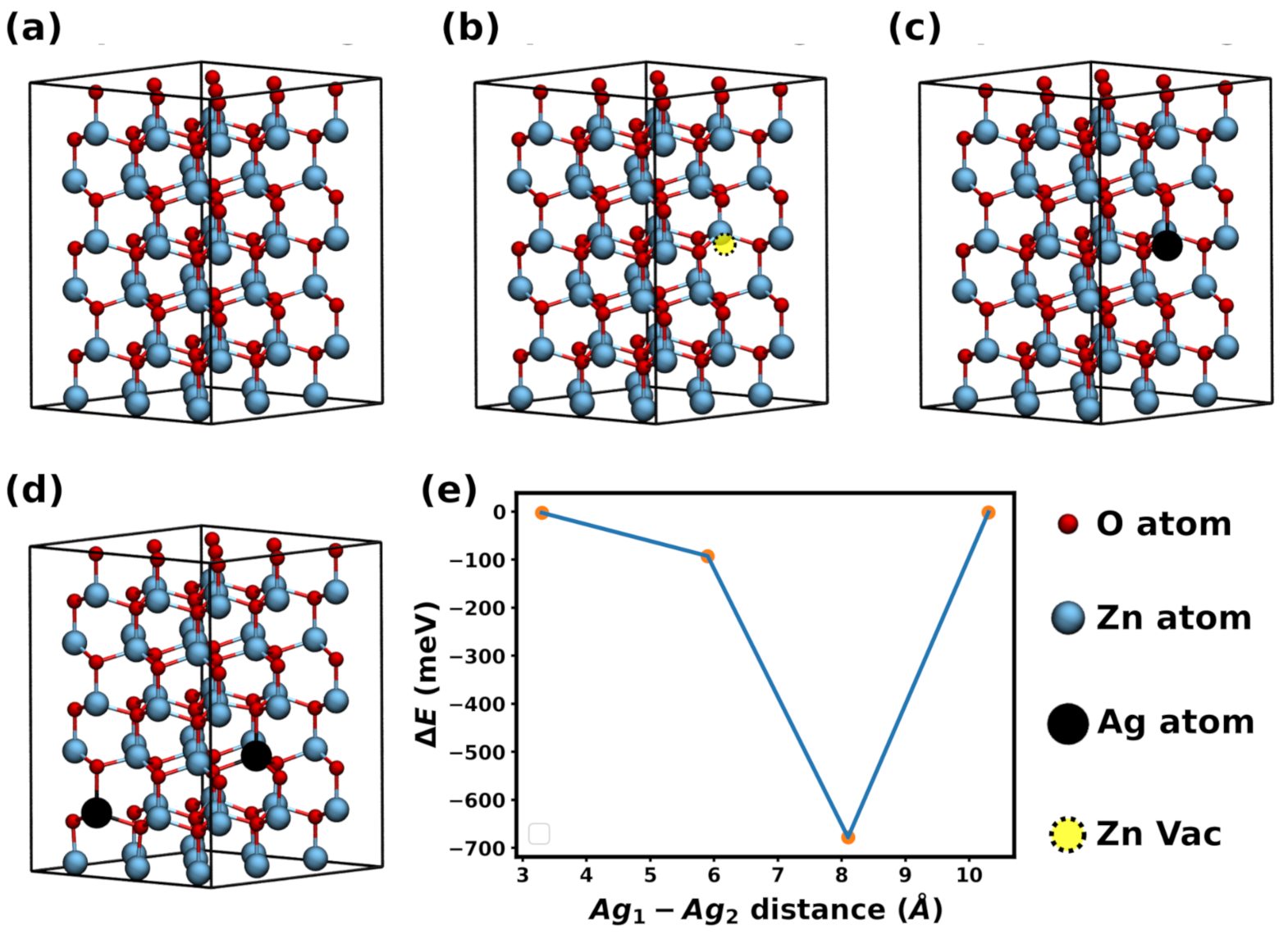
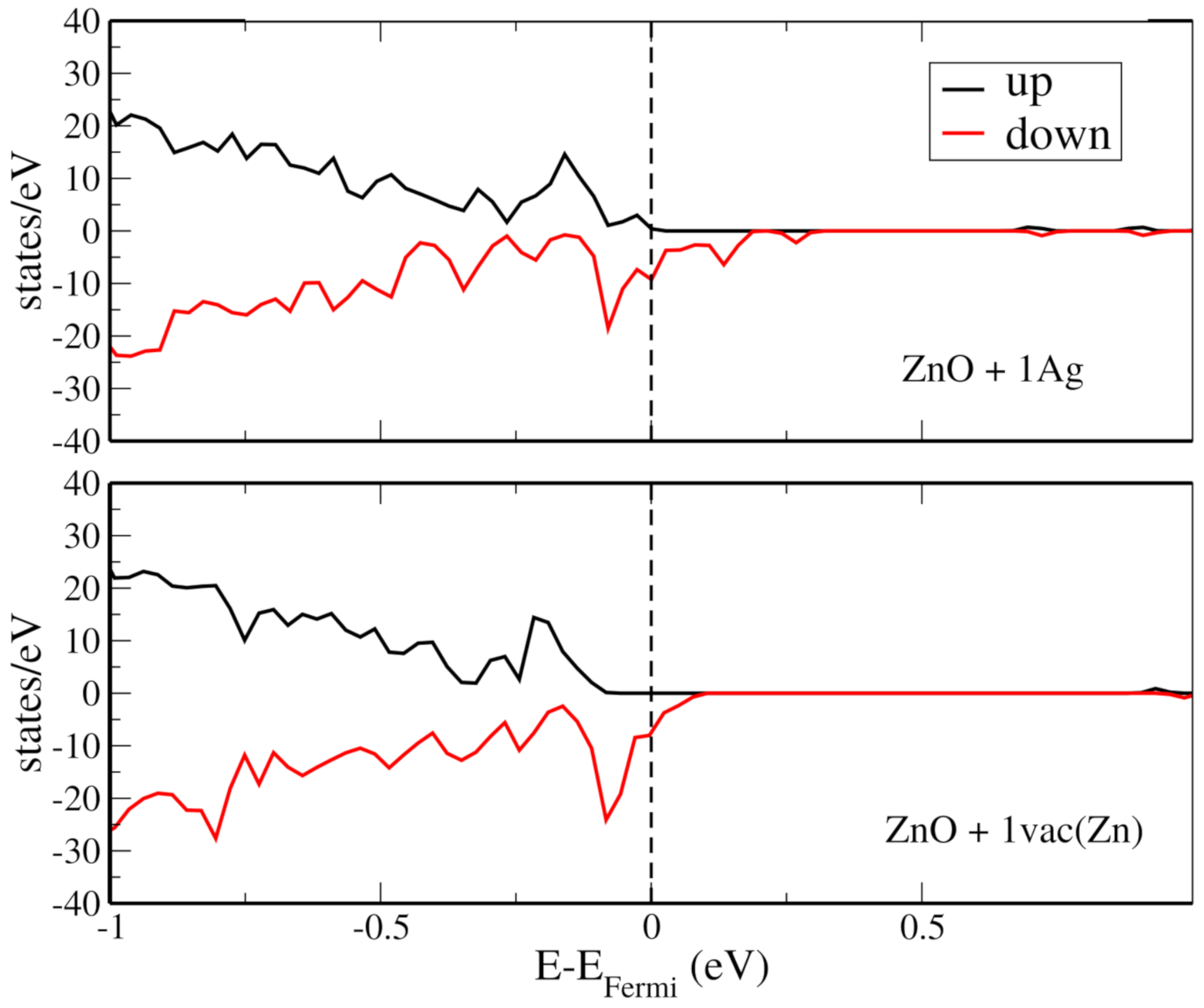
3. Computational Simulation
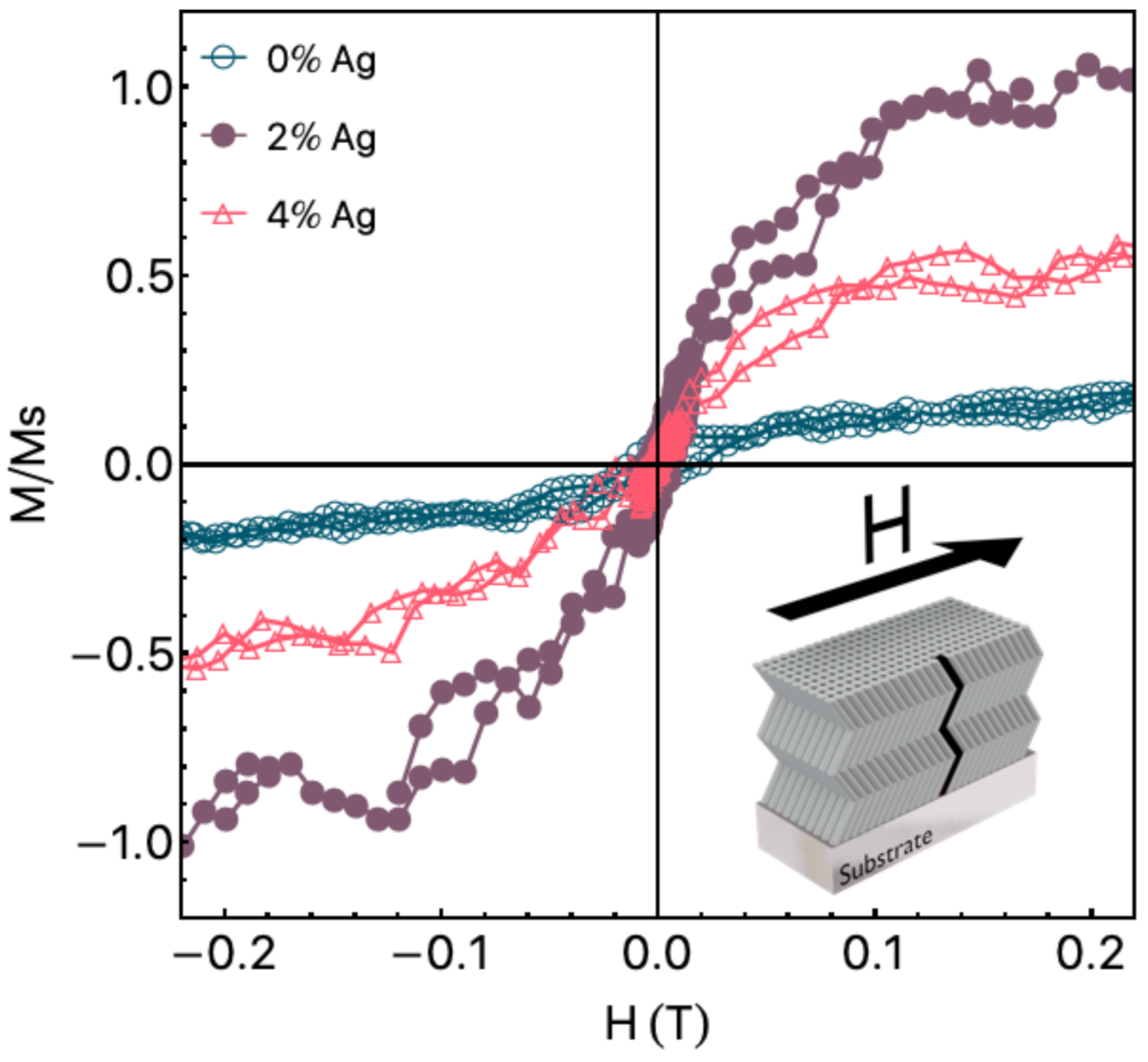
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, J.; Choo, E.S.G.; Tang, X.; Sheng, Y.; Ding, J.; Xue, J. Synthesis of ZnO/Pt nanoflowers and their photocatalytic applications. Nanotechnology 2010, 21, 185606. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Martin, N.; Lanceros-Méndez, S.; Vaz, F. Tuning electrical resistivity anisotropy of ZnO thin films for resistive sensor applications. Thin Solid Film. 2018, 654, 93–99. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hu, A.; Zhang, T.; Xue, X.J.; Wen, J.Z.; Duley, W.W. Subwavelength plasmonic waveguides based on ZnO nanowires and nanotubes: A theoretical study of thermo-optical properties. Appl. Phys. Lett. 2010, 96, 043109. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, T.S.; Lubguban, J.A.; White, H.W.; Kim, B.J.; Park, Y.S.; Youn, C.J. Next generation of oxide photonic devices: ZnO-based ultraviolet light emitting diodes. Appl. Phys. Lett. 2006, 88, 241108. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Usman, A.; Nazli, H.; Sagheer, R.; Riaz, S.; Naseem, S. Dielectric and magnetic properties of dilute magnetic semiconductors Ag-doped ZnO thin films. Appl. Phys. A 2020, 126, 559. [Google Scholar] [CrossRef]
- He, M.; Tian, Y.F.; Springer, D.; Putra, I.A.; Xing, G.Z.; Chia, E.E.M.; Cheong, S.A.; Wu, T. Polaronic transport and magnetism in Ag-doped ZnO. Appl. Phys. Lett. 2011, 99, 222511. [Google Scholar] [CrossRef]
- Volnianska, O.; Boguslawski, P.; Kaczkowski, J.; Jakubas, P.; Jezierski, A.; Kaminska, E. Theory of doping properties of Ag acceptors in ZnO. Phys. Rev. B 2009, 80, 245212. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z. ZnO thin films produced by magnetron sputtering. Ceram. Int. 2004, 30, 1155–1159. [Google Scholar] [CrossRef]
- Ahmed Khan, Z.; Rai, A.; Roy Barman, S.; Ghosh, S. Green luminescence and room temperature ferromagnetism in Cu doped ZnO. Appl. Phys. Lett. 2013, 102, 022105. [Google Scholar] [CrossRef]
- Ahmed Khan, Z.; Ghosh, S. Robust room temperature ferromagnetism in Cu doped ZnO thin films. Appl. Phys. Lett. 2011, 99, 042504. [Google Scholar] [CrossRef]
- Venkatesh, P.S.; Purushothaman, V.; Muthu, S.E.; Arumugam, S.; Ramakrishnan, V.; Jeganathan, K.; Ramamurthi, K. Role of point defects on the enhancement of room temperature ferromagnetism in ZnO nanorods. CrystEngComm 2012, 14, 4713. [Google Scholar] [CrossRef]
- Hosseini, S.; Sarsari, I.A.; Kameli, P.; Salamati, H. Effect of Ag doping on structural, optical, and photocatalytic properties of ZnO nanoparticles. J. Alloy. Compd. 2015, 640, 408–415. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Venkatesan, M.; Fitzgerald, C.B. Donor impurity band exchange in dilute ferromagnetic oxides. Nat. Mater. 2005, 4, 173–179. [Google Scholar] [CrossRef]
- Herng, T.S.; Qi, D.C.; Berlijn, T.; Yi, J.B.; Yang, K.S.; Dai, Y.; Feng, Y.P.; Santoso, I.; Sánchez-Hanke, C.; Gao, X.Y.; et al. Room-Temperature Ferromagnetism of Cu-Doped ZnO Films Probed by Soft X-Ray Magnetic Circular Dichroism. Phys. Rev. Lett. 2010, 105, 207201. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Singh, B.; Khan, Z.A.; Vijaya, A.R.; Tarafder, K.; Ghosh, S. Origin of ferromagnetism in Cu-doped ZnO. Sci. Rep. 2019, 9, 2461. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Vijaya, A.R.; Khan, Z.A.; Tarafder, K.; Kumar, A.; Wadhwa, M.K.; Singh, B.; Ghosh, S. Ferromagnetism from non-magnetic ions: Ag-doped ZnO. Sci. Rep. 2019, 9, 20039. [Google Scholar] [CrossRef]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.; Lin, H.; Wang, G. Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: Experimental and DFT studies. Ceram. Int. 2020, 46, 1494–1502. [Google Scholar] [CrossRef]
- Gherab, K.; Al-Douri, Y.; Hashim, U.; Ameri, M.; Bouhemadou, A.; Batoo, K.M.; Adil, S.F.; Khan, M.; Raslan, E.H. Fabrication and characterizations of Al nanoparticles doped ZnO nanostructures-based integrated electrochemical biosensor. J. Mater. Res. Technol. 2020, 9, 857–867. [Google Scholar] [CrossRef]
- Yang, R.; Yan, X.; Li, Y.; Zhang, X.; Chen, J. Nitrogen-Doped Porous Carbon-ZnO Nanopolyhedra Derived from ZIF-8: New Materials for Photoelectrochemical Biosensors. ACS Appl. Mater. Interfaces 2017, 9, 42482–42491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Weng, Z.; Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Biomedical Applications of Functionalized ZnO Nanomaterials: From Biosensors to Bioimaging. Adv. Mater. Interfaces 2016, 3, 1500494. [Google Scholar] [CrossRef]
- Muthuchamy, N.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. High-performance glucose biosensor based on green synthesized zinc oxide nanoparticle embedded nitrogen-doped carbon sheet. J. Electroanal. Chem. 2018, 816, 195–204. [Google Scholar] [CrossRef]
- Tak, M.; Gupta, V.; Tomar, M. An electrochemical DNA biosensor based on Ni doped ZnO thin film for meningitis detection. J. Electroanal. Chem. 2017, 792, 8–14. [Google Scholar] [CrossRef]
- Guan, J.G.; Miao, Y.Q.; Zhang, Q.J. Impedimetric biosensors. J. Biosci. Bioeng. 2004, 97, 219–226. [Google Scholar] [CrossRef]
- Kraus, L. GMI modeling and material optimization. Sens. Actuators A Phys. 2003, 106, 187–194. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.; Levit, V. Magnetic Dynabeads® detection by sensitive element based on giant magnetoimpedance. Biosens. Bioelectron. 2005, 20, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Taschuk, M.T.; Hawkeye, M.M.; Brett, M.J. Glancing Angle Deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; William Andrew Publishing: Norwich, NY, USA, 2010. [Google Scholar]
- Ferreira, A.; Borges, J.; Lopes, C.; Martin, N.; Lanceros-Mendez, S.; Vaz, F. Piezoresistive response of nano-architectured TixCuy thin films for sensor applications. Sens. Actuators A Phys. 2016, 247, 105–114. [Google Scholar] [CrossRef][Green Version]
- Ferreira, A.; Lopes, C.; Martin, N.; Lanceros-Méndez, S.; Vaz, F. Nanostructured functional Ti-Ag electrodes for large deformation sensor applications. Sens. Actuators A Phys. 2014, 220, 204–212. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chiang, C.Y.; Chen, Y.C.; Lin, Y.C.; Ono, T.; Tsai, Y.C. Study and fabrication of a flexible Zr-based metallic glass thin film strain gauge. Jpn. J. Appl. Phys. 2020, 59, SIIG10. [Google Scholar] [CrossRef]
- Santos, J.G.S.; Correa, M.A.; Ferreira, A.; Carvalho, B.R.; da Silva, R.B.; Bohn, F.; Lanceiros-Méndez, S.; Vaz, F. Magnetic Response Dependence of ZnO Based Thin Films on Ag Doping and Processing Architecture. Materials 2020, 13, 2907. [Google Scholar] [CrossRef]
- Viswanatha, R.; Naveh, D.; Chelikowsky, J.R.; Kronik, L.; Sarma, D.D. Magnetic Properties of Fe/Cu Codoped ZnO Nanocrystals. J. Phys. Chem. Lett. 2012, 3, 2009–2014. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, H.; Cheng, X. Electronic and magnetic properties of all 3 d transition-metal-doped ZnO monolayers. Int. J. Quantum Chem. 2013, 113, 2243–2250. [Google Scholar] [CrossRef]
- Wen, J.Q.; Zhang, J.M.; Qiu, Z.G.; Yang, X.; Li, Z.Q. The investigation of Ce doped ZnO crystal: The electronic, optical and magnetic properties. Phys. B Condens. Matter 2018, 534, 44–50. [Google Scholar] [CrossRef]
- Traversa, E.; Bearzotti, A. A novel humidity-detection mechanism for ZnO dense pellets. Sens. Actuators B. Chem. 1995, 23, 181–186. [Google Scholar] [CrossRef]
- Karimov, K.S.; Cheong, K.Y.; Saleem, M.; Murtaza, I.; Farooq, M.; Mohd Noor, A.F. Ag/PEPC/NiPc/ZnO/Ag thin film capacitive and resistive humidity sensors. J. Semicond. 2010, 31, 0540021–0540026. [Google Scholar] [CrossRef]
- Souza, R.P.A.; Motta, F.V.; Nascimento, J.H.O.; Bomio, M.R.D.; Borges, F.M.M.; Correa, M.A.; Longo, E.; Li, M.S.; Bohn, F.; Paskocimas, C.A. Effect of Ag clusters doping on the photoluminescence, photocatalysis and magnetic properties of ZnO nanorods prepared by facile microwave-assisted hydrothermal synthesis. J. Mater. Sci. Mater. Electron. 2017, 28, 11059–11069. [Google Scholar] [CrossRef]
- Ferreira, A.; Correa, M.A.; Lanceros-Méndez, S.; Bohn, F.; Vaz, F. Modulation of the magnetoimpedance effect of ZnO:Ag/NiFe heterostructures by thermal annealing. J. Mater. Sci. 2020, 55, 5961–5968. [Google Scholar] [CrossRef]
- Nakhodkin, N.G.; Shaldervan, A.I. Effect of vapour incidence angles on profile and properties of condensed films. Thin Solid Film. 1972, 10, 109–122. [Google Scholar] [CrossRef]
- Robbie, K.; Shafai, C.; Brett, M.J. Thin films with nanometer-scale pillar microstructure. J. Mater. Res. 1999, 14, 3158–3163. [Google Scholar] [CrossRef]
- Tait, R.N.; Smy, T.; Brett, M.J. Modelling and characterization of columnar growth in evaporated films. Thin Solid Film. 1993, 226, 196–201. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter 2002, 14, 2745. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Troullier, N.; Martins, J.L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, L.; Bylander, D.M. Efficacious Form for Model Pseudopotentials. Phys. Rev. Lett. 1982, 48, 1425–1428. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Domingues, R.P.; Rodrigues, M.S.; Lopes, C.; Pedrosa, P.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Thin films composed of metal nanoparticles (Au, Ag, Cu) dispersed in AlN: The influence of composition and thermal annealing on the structure and plasmonic response. Thin Solid Film. 2019, 676, 12–25. [Google Scholar] [CrossRef]
- Etiemble, A.; Lopes, C.; Nkou Bouala, G.I.; Borges, J.; Malchère, A.; Langlois, C.; Vaz, F.; Steyer, P. Fracture resistance of Ti-Ag thin films deposited on polymeric substrates for biosignal acquisition applications. Surf. Coat. Technol. 2019, 358, 646–653. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Chen, G.; Kawazoe, Y.; Jena, P. Vacancy-induced magnetism in ZnO thin films and nanowires. Phys. Rev. B 2008, 77, 205411. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa, M.A.; Ferreira, A.; Tromer, R.M.; Machado, L.D.; Gamino, M.; França Junior, S.A.N.; Bohn, F.; Vaz, F. Improving the Room-Temperature Ferromagnetism in ZnO and Low-Doped ZnO:Ag Films Using GLAD Sputtering. Materials 2021, 14, 5337. https://doi.org/10.3390/ma14185337
Correa MA, Ferreira A, Tromer RM, Machado LD, Gamino M, França Junior SAN, Bohn F, Vaz F. Improving the Room-Temperature Ferromagnetism in ZnO and Low-Doped ZnO:Ag Films Using GLAD Sputtering. Materials. 2021; 14(18):5337. https://doi.org/10.3390/ma14185337
Chicago/Turabian StyleCorrea, Marcio A., Armando Ferreira, Raphael M. Tromer, Leonardo D. Machado, Matheus Gamino, Sergio A. N. França Junior, Felipe Bohn, and Filipe Vaz. 2021. "Improving the Room-Temperature Ferromagnetism in ZnO and Low-Doped ZnO:Ag Films Using GLAD Sputtering" Materials 14, no. 18: 5337. https://doi.org/10.3390/ma14185337
APA StyleCorrea, M. A., Ferreira, A., Tromer, R. M., Machado, L. D., Gamino, M., França Junior, S. A. N., Bohn, F., & Vaz, F. (2021). Improving the Room-Temperature Ferromagnetism in ZnO and Low-Doped ZnO:Ag Films Using GLAD Sputtering. Materials, 14(18), 5337. https://doi.org/10.3390/ma14185337








