High-Temperature Oxidation Behavior of AlTiNiCuCox High-Entropy Alloys
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Oxidation Behavior of AlTiNiCuCox Series High-Entropy Alloys at 800 °C
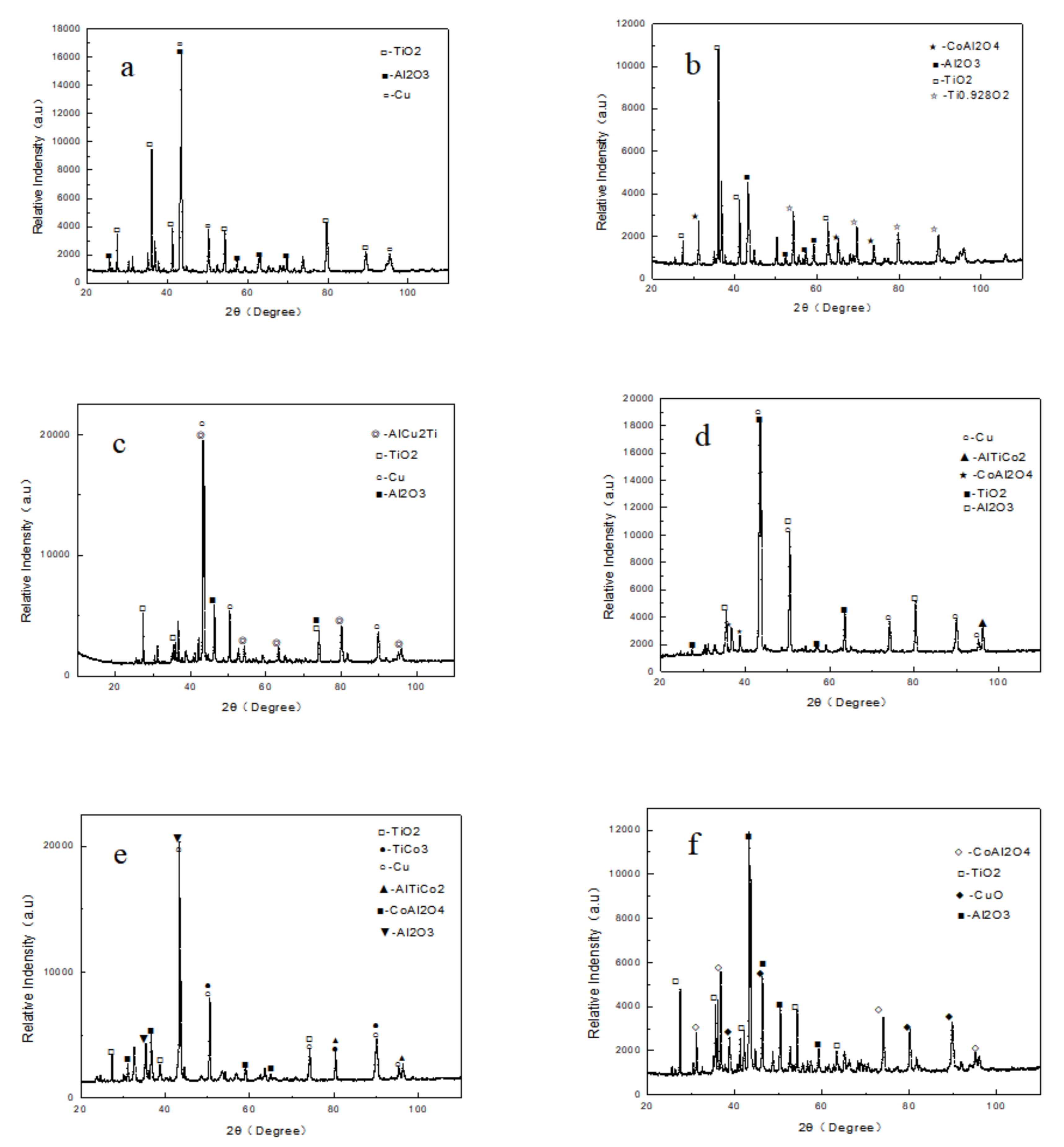
3.2. XRD Analysis of The Oxide Layer
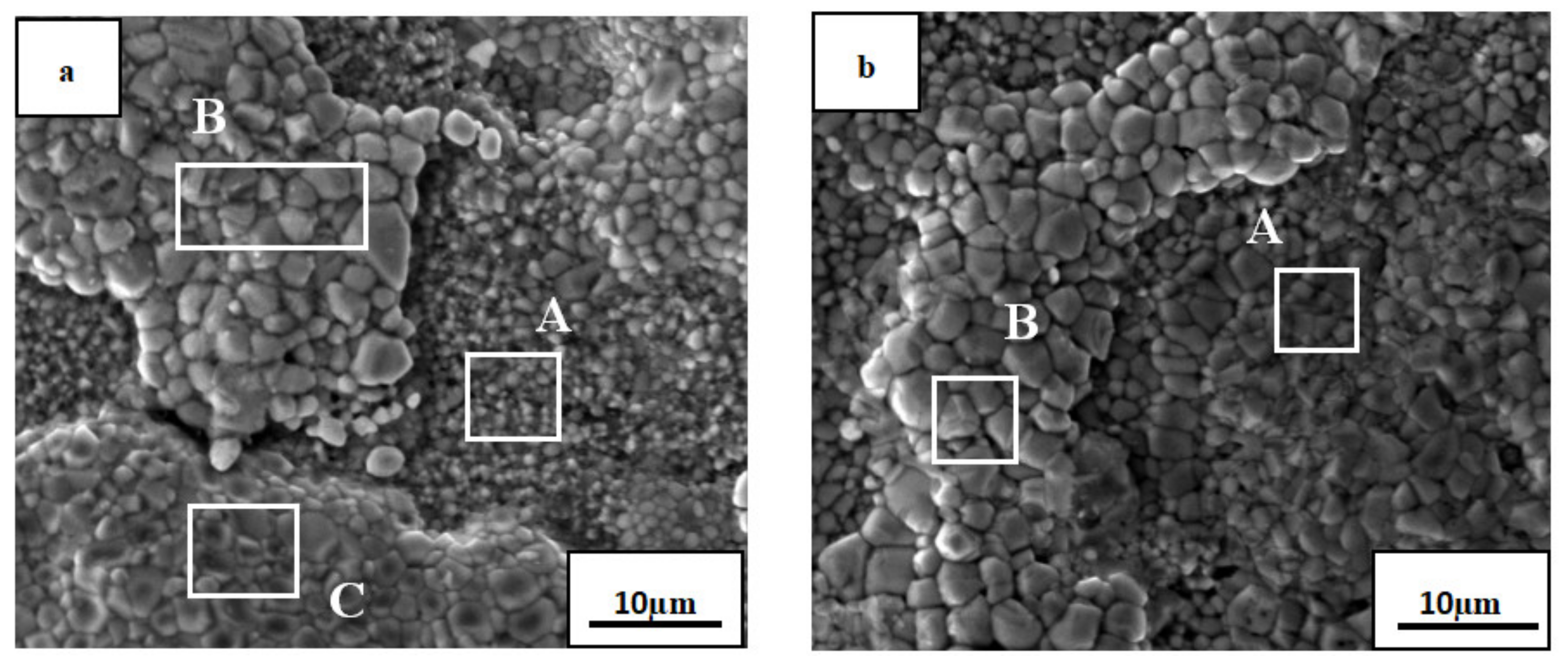
3.3. Surface Morphology of The Oxide Film
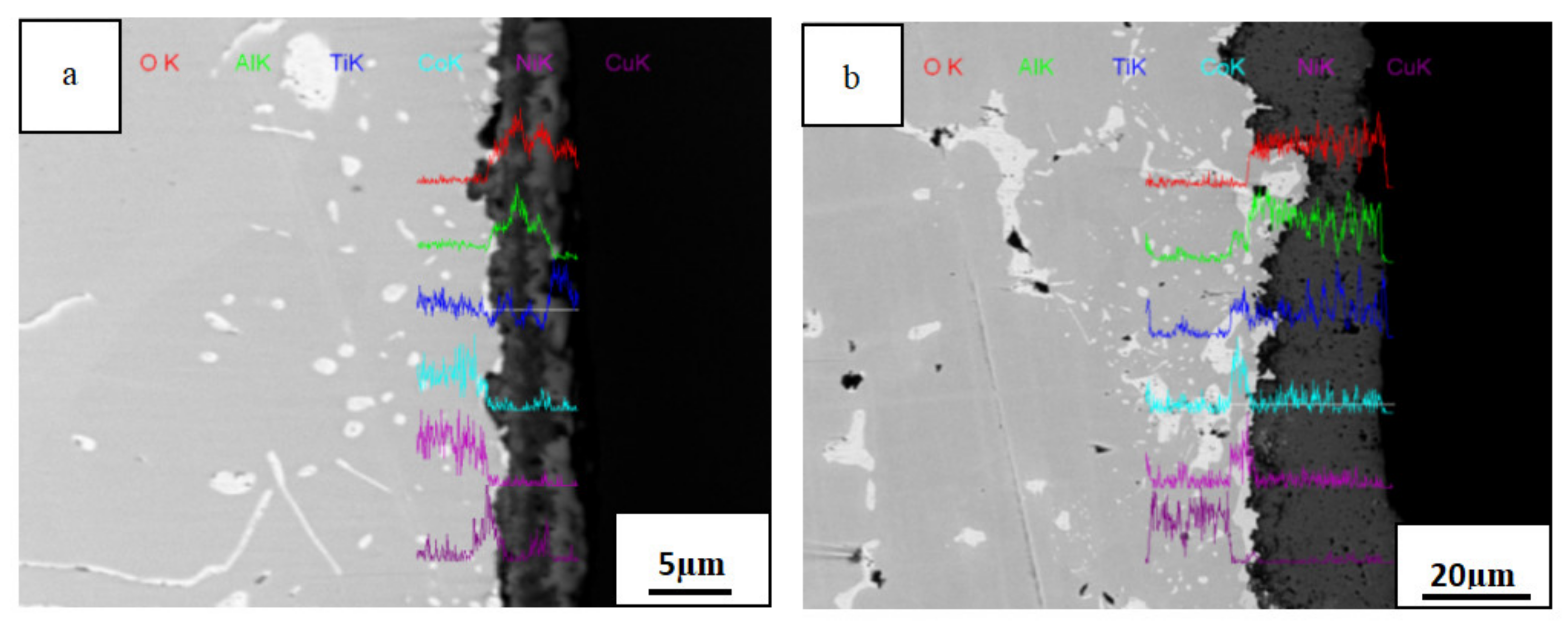
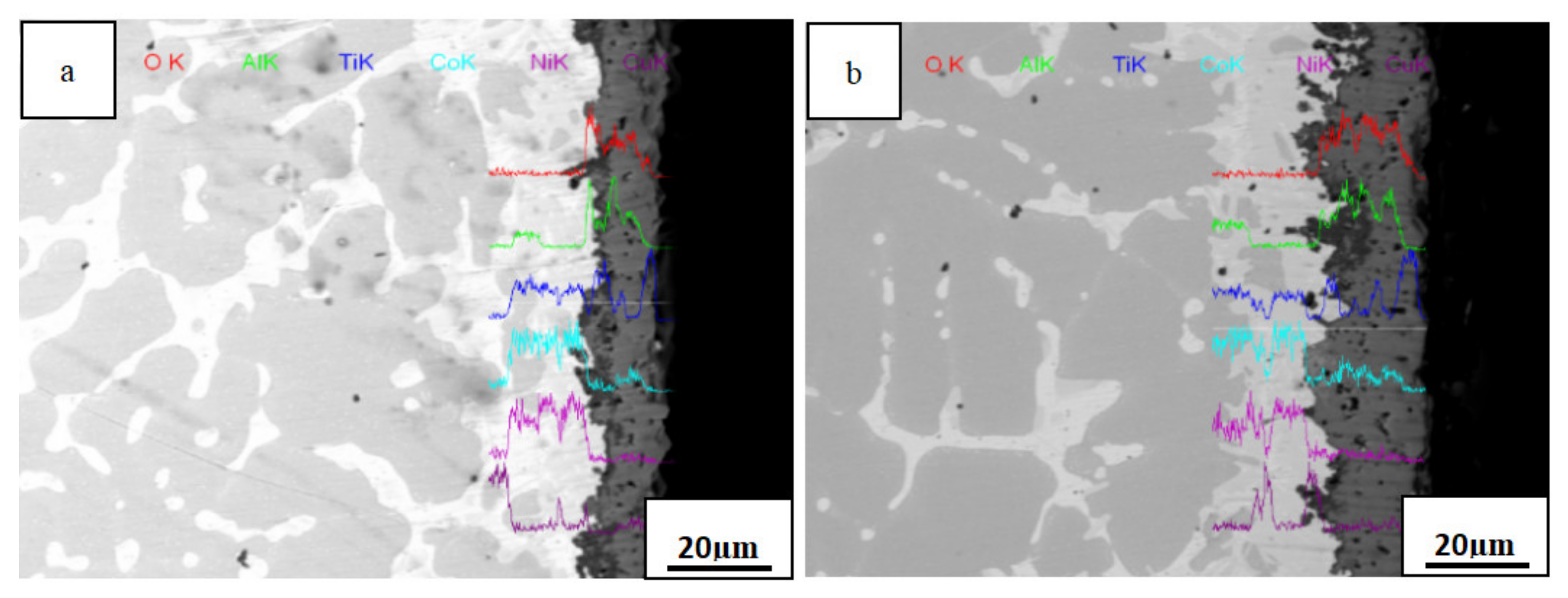
3.4. Cross-Sectional Morphology of The Oxide Film
3.5. Oxidation Mechanism
4. Conclusions
- 1.
- At 800 °C, AlTiNiCuCox (x = 0.5, 1.0, 1.5) series HEAs have certain high-temperature oxidation resistance and conform to the (n = 1) oxidation mode.The oxidation resistance of the AlTiNiCuCox series HEAs at high temperature is closely related to the Co content.
- 2.
- With increasing oxidation time, the overall oxide content in the alloys increases, and the high-temperature oxidation resistance becomes progressively worse. With increasing Co content, a transition layer gradually appears. Among the alloys, the oxide films of AlTiNiCuCo1.0 oxidized for 50 and 100 h were thinner; AlTiNiCuCo1.0 has higher oxidation resistance at high temperature compared to alloys with other amounts of Co that were tested.
- 3.
- In the process of high-temperature oxidation of AlTiNiCuCox series HEAs, the interface reaction initially dominates, and metal elements and oxygen in the alloy react to form ions as a result of direct contact; then, ions diffuse and are transported in the oxide film, and the diffusion process gradually becomes the dominant oxidation factor. Because the initial position and diffusion direction differ for each ion, the distribution of concentrations in the oxide layer–transition layer–matrix eventually differs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Chen, Y.; Duval, T.; Hung, U.; Yeh, J.; Shih, H. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Ren, M.; Yang, C.; Fu, H. Effects of Mn, Ti and V on the microstructure and properties of AlCrFeCoNiCu high entropy alloy. Mater. Sci. Eng. A 2008, 498, 482–486. [Google Scholar] [CrossRef]
- Yurchenko, N.; Stepanov, N.; Salishchev, G. Laves-phase formation criterion for high-entropy alloys. Mater. Sci. Technol. 2017, 33, 17–22. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Met. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Chen, T.-K.; Shun, T.; Yeh, J.; Wong, M. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188-189, 193–200. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, L.; He, W.; Liaw, P.K. Alloying and Processing Effects on the Aqueous Corrosion Behavior of High-Entropy Alloys. Entropy 2014, 16, 895–911. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W.; Shun, T.-T.; Chen, S.-K. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Sheng, H.; Gong, M.; Peng, L. Microstructural characterization and mechanical properties of an Al0.5CoCrFeCuNi high-entropy alloy in as-cast and heat-treated/quenched conditions. Mater. Sci. Eng. A 2013, 567, 14–20. [Google Scholar] [CrossRef]
- Garip, Y.; Ergin, N.; Ozdemir, O. Resistance sintering of CoCrFeNiAlx (x = 0.7, 0.85, 1) high entropy alloys: Microstructural char acterization, oxidation and corrosion properties. J. Alloy Compd. 2021, 877, 160180. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Chen, Y.; Liu, X.; Zhao, X.; Guo, F.; Xiao, P. Y-Hf co-doped AlCoCrFeNi high-entropy alloy coating with superior oxidation and spallation resistance at 1100 °C. Corros. Sci. 2021, 182, 109267. [Google Scholar] [CrossRef]
- Butler, T.M.; Alfano, J.P.; Martens, R.L.; Weaver, M.L. High-Temperature Oxidation Behavior of Al-Co-Cr-Ni-(Fe or Si) Multicomponent High-Entropy Alloys. JOM 2015, 67, 246–259. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Cheng, C.-Q.; Shang, J.-L.; Wang, R.; Li, P.; Zhao, J. Oxidation behavior of high-entropy alloys AlxCoCrFeNi (x = 0.15, 0.4) in supercritical water and comparison with HR3C steel. Trans. Nonferrous Met. Soc. China 2015, 25, 1341–1351. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Dimiduk, D.M.; Woodward, C.; Miracle, D.B. Oxidation behavior of a refractory NbCrMo0.5Ta0.5TiZr alloy. J. Mater. Sci. 2012, 47, 6522–6534. [Google Scholar] [CrossRef]
- Kofstad, P. High-temperature oxidation of titanium. J. Less Common Met. 1967, 12, 449–464. [Google Scholar] [CrossRef]
- Chen, H.; Kauffmann, A.; Gorr, B.; Schliephake, D.; Seemüller, C.; Wagner, J.; Christ, H.-J.; Heilmaier, M. Microstructure and mechanical properties at elevated temperatures of a new Al-containing refractory high-entropy alloy Nb-Mo-Cr-Ti-Al. J. Alloy Compd. 2016, 661, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Lu, X.; Yan, M.; Fu, Y. Evaluating stability, elastic and thermodynamic properties of AlTiNiCuCox (x = 0.5, 0.75, 1, 1.25, 1.5) high entropy alloys. Mater. Res. Express 2019, 6, 126581. [Google Scholar] [CrossRef]
- Krüger, J. Basic corrosion and oxidation: By John M. West. Ellis Horwood Ltd., Chichester, in association with Wiley, New York. Corros. Sci. 1982, 22, 159. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.; Pettit, F. High-Temperature Corrosion Resistance. JOM 1987, 39, 28–31. [Google Scholar] [CrossRef]
- Kubaschewski, O.; Hopkins, B. Oxidation mechanisms of niobium, tantalum, molybdenum and tungsten. J. Less Common Met. 1960, 2, 172–180. [Google Scholar] [CrossRef]

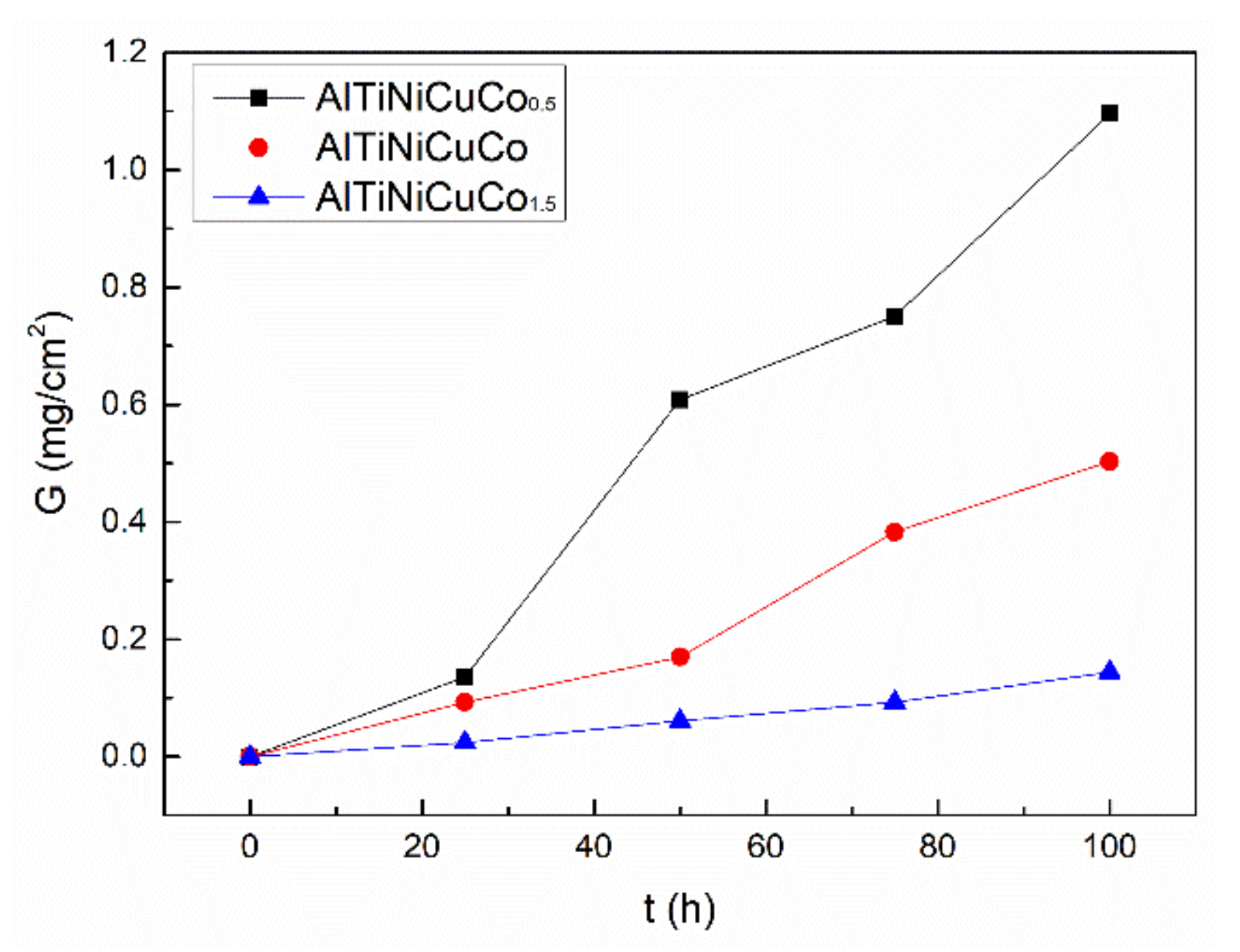



| Oxidation Time Co Content | 25 h | 50 h | 75 h | 100 h |
|---|---|---|---|---|
| x = 0.5 | 1.36 | 6.08 | 7.51 | 10.96 |
| x = 1.0 | 0.93 | 1.70 | 3.83 | 5.03 |
| x = 1.5 | 0.24 | 0.61 | 0.93 | 1.44 |
| Element | O | Al | Ti | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| wt% | 32.29 | 06.51 | 54.26 | 01.60 | 01.45 | 03.90 |
| at% | 57.58 | 06.88 | 32.31 | 00.77 | 00.70 | 01.75 |
| Element | O | Al | Ti | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| wt% | 30.46 | 03.90 | 58.12 | 02.18 | 01.44 | 03.90 |
| at% | 56.25 | 04.27 | 35.85 | 01.09 | 00.72 | 01.81 |
| Element | O | Al | Ti | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| wt% | 28.49 | 11.32 | 41.12 | 06.18 | 03.68 | 09.22 |
| at% | 52.82 | 12.44 | 25.47 | 03.11 | 01.86 | 04.30 |
| Position | O | Al | Ti | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| A | 46.34 | 30.93 | 08.83 | 02.41 | 02.09 | 09.40 |
| B | 56.41 | 03.00 | 36.72 | 01.04 | 00.84 | 01.99 |
| Position | O | Al | Ti | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| A | 43.22 | 23.10 | 07.58 | 13.78 | 07.31 | 05.02 |
| B | 36.17 | 00.40 | 00.85 | 04.59 | 04.54 | 53.44 |
| C | 51.66 | 02.90 | 13.32 | 20.48 | 03.50 | 08.15 |
| Position | O | Al | Ti | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| A | 51.24 | 03.59 | 19.56 | 12.50 | 07.58 | 05.54 |
| B | 36.09 | 00.63 | 00.90 | 02.33 | 03.00 | 57.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; He, Q.; Liu, G.; Zhang, Q.; Liu, G.; Huang, Z.; Zhu, X.; Fu, Y. High-Temperature Oxidation Behavior of AlTiNiCuCox High-Entropy Alloys. Materials 2021, 14, 5319. https://doi.org/10.3390/ma14185319
Wang J, He Q, Liu G, Zhang Q, Liu G, Huang Z, Zhu X, Fu Y. High-Temperature Oxidation Behavior of AlTiNiCuCox High-Entropy Alloys. Materials. 2021; 14(18):5319. https://doi.org/10.3390/ma14185319
Chicago/Turabian StyleWang, Junfeng, Qiaobai He, Guanqi Liu, Qi Zhang, Guotan Liu, Zhihao Huang, Xiaoshuo Zhu, and Yudong Fu. 2021. "High-Temperature Oxidation Behavior of AlTiNiCuCox High-Entropy Alloys" Materials 14, no. 18: 5319. https://doi.org/10.3390/ma14185319
APA StyleWang, J., He, Q., Liu, G., Zhang, Q., Liu, G., Huang, Z., Zhu, X., & Fu, Y. (2021). High-Temperature Oxidation Behavior of AlTiNiCuCox High-Entropy Alloys. Materials, 14(18), 5319. https://doi.org/10.3390/ma14185319






