Dendrimeric Structures in the Synthesis of Fine Chemicals
Abstract
:1. Introduction
2. Application of Dendrimers in the Science of Materials Field
3. Nano-Delivery Systems
4. Application of Dendrimers in Catalysis
5. Dendrimers Application in Organo-Catalysis
6. Dendrimers as Antiviral Agents
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Padilla De Jesús, O.L.; Ihre, H.R.; Gagne, L.; Fréchet, J.M.J.; Szoka, F.C. Polyester Dendritic Systems for Drug Delivery Applications: In Vitro and In Vivo Evaluation. Bioconjug. Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef]
- Zimmerman, S.C. Dendrimers in molecular recognition and self-assembly. Curr. Opin. Colloid Interface Sci. 1997, 2, 89–99. [Google Scholar] [CrossRef]
- Zeng, F.; Zimmerman, S.C. Dendrimers in Supramolecular Chemistry: From Molecular Recognition to Self-Assembly. Chem. Rev. 1997, 97, 1681–1712. [Google Scholar] [CrossRef]
- Hughes, G.A. Nanostructure-mediated drug delivery. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 22–30. [Google Scholar] [CrossRef]
- Kummu, M.; Ward, P.J.; de Moel, H.; Varis, O. Is physical water scarcity a new phenomenon? Global assessment of water shortage over the last two millennia. Environ. Res. Lett. 2010, 5, 34006. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.; Mahajan, G.; Phadke, S.; Adivarekar, R. V Application of polyamidoamine dendrimer in reactive dyeing of cotton. J. Text. Inst. 2018, 109, 823–831. [Google Scholar] [CrossRef]
- Li, J.; Wu, F.; Lin, L.; Guo, Y.; Liu, H.; Zhang, X. Flow fabrication of a highly efficient Pd/UiO-66-NH2 film capillary microreactor for 4-nitrophenol reduction. Chem. Eng. J. 2018, 333, 146–152. [Google Scholar] [CrossRef]
- Nabil, B.; Morshed, M.N.; Ahmida, E.A.; Nemeshwaree, B.; Christine, C.; Julien, V.; Olivier, T.; Abdelkrim, A. Development of new multifunctional filter based nonwovens for organics pollutants reduction and detoxification: High catalytic and antibacterial activities. Chem. Eng. J. 2019, 356, 702–716. [Google Scholar] [CrossRef]
- Chen, S.; Thota, S.; Wang, X.; Zhao, J. From solid to core shell to hollow Pt–Ag nanocrystals: Thermally controlled surface segregation to enhance catalytic activity and durability. J. Mater. Chem. A 2016, 4, 9038–9043. [Google Scholar] [CrossRef]
- Self-assembly, R.E.; Res, C.; Jiang, D.; Aida, T. Photoisomerization in dendrimers by harvesting of low-energy photons. Nature 1997, 388, 5–7. [Google Scholar]
- Laipniece, L.; Kampars, V. Synthesis and Thermal Properties of Azobenzene Core Polyester Dendrimers with Trityl Groups at the Periphery. Key Eng. Mater. 2018, 762, 171–175. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, P.; Wu, B.; Xing, Y.; Shi, K.; Fang, W.; Yu, H.; Wang, G. Photochromic Dendrimers for Photoswitched Solid-To-Liquid Transitions and Solar Thermal Fuels. ACS Appl. Mater. Interfaces 2020, 12, 50135–50142. [Google Scholar] [CrossRef]
- Han, G.D.; Park, S.S.; Liu, Y.; Zhitomirsky, D.; Cho, E.; Dincă, M.; Grossman, J.C. Photon energy storage materials with high energy densities based on diacetylene–azobenzene derivatives. J. Mater. Chem. A 2016, 4, 16157–16165. [Google Scholar] [CrossRef]
- Weis, P.; Wang, D.; Wu, S. Visible-Light-Responsive Azopolymers with Inhibited π–π Stacking Enable Fully Reversible Photopatterning. Macromolecules 2016, 49, 6368–6373. [Google Scholar] [CrossRef]
- Zhou, H.; Xue, C.; Weis, P.; Suzuki, Y.; Huang, S.; Koynov, K. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat. Chem. 2017, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Nemanashi, M. Dendrimers as alternative templates and pore-directing agents for the synthesis of micro- and mesoporous materials. J. Mater. Sci. 2018, 53, 12663–12678. [Google Scholar] [CrossRef]
- Dai, H.; Yang, J.; Ma, J.; Chen, F.; Fei, Z.; Zhong, M. A green process for the synthesis of controllable mesoporous silica materials. Microporous Mesoporous Mater. 2012, 147, 281–285. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Grinstaff, M.W. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv. Drug Deliv. Rev. 2008, 60, 1037–1055. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jain, K.; Mehra, N.K.; Jain, N.K. Dendrimers in anticancer drug delivery: Mechanism of interaction of drug and dendrimers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer nanodevices and gallic acid as novel strategies to fight chemoresistance in neuroblastoma cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Angelo, S.D.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.-L.; Lu, X.-Y. Solubilities of Gallic Acid and Its Esters in Water. J. Chem. Eng. Data 2007, 52, 37–39. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from oxidative stress. Drug Deliv. Transl. Res. 2019, 10, 259–270. [Google Scholar] [CrossRef]
- Lage, A.C.P.; Orlando Ladeira, L.; Mosqueira, L.; Magalhães Paniago, R.; Oliveira Castilho, R.; Amorim, J.M.; Pessoa, E.S.; Nuncira, J.; Faraco, A.A.G. Synthesis and characterization of gold nanorods using the natural products resveratrol, gallic acid, and a purified fraction of Stryphnodendron obovatum by seedless method. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100473. [Google Scholar] [CrossRef]
- Meschini, R.; D’Eliseo, D.; Filippi, S.; Bertini, L.; Bizzarri, B.M.; Botta, L.; Saladino, R.; Velotti, F. Tyrosinase-Treated Hydroxytyrosol-Enriched Olive Vegetation Waste with Increased Antioxidant Activity Promotes Autophagy and Inhibits the Inflammatory Response in Human THP-1 Monocytes. J. Agric. Food Chem. 2018, 66, 12274–12284. [Google Scholar] [CrossRef] [PubMed]
- Botta, G.; Bizzarri, B.M.; Garozzo, A.; Timpanaro, R.; Bisignano, B.; Amatore, D.; Palamara, A.T.; Nencioni, L.; Saladino, R. Carbon nanotubes supported tyrosinase in the synthesis of lipophilic hydroxytyrosol and dihydrocaffeoyl catechols with antiviral activity against DNA and RNA viruses. Bioorg. Med. Chem. 2015, 23, 5345–5351. [Google Scholar] [CrossRef]
- Mishra, M.K.; Kotta, K.; Hali, M.; Wykes, S.; Gerard, H.C.; Hudson, A.P.; Whittum-Hudson, J.A.; Kannan, R.M. PAMAM dendrimer-azithromycin conjugate nanodevices for the treatment of Chlamydia trachomatis infections. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 935–944. [Google Scholar] [CrossRef]
- Randomized, A.; Trial, C.; Schillinger, J.A.; Kissinger, P.; Calvet, H.; Whittington, W.L.H.; Ransom, R.A.Y.L.; Sternberg, M.R.; Berman, S.M.; Kent, C.K.; et al. Patient-Delivered Partner Treatment With Azithromycin to Prevent Repeated Chlamydia trachomatis Infection Among Women. Sex Transm Dis. 2003, 30, 49–56. [Google Scholar]
- Martin, D.H.; Mroczkowski, T.F.; Dalu, Z.A.; McCarty, J.; Jones, R.B.; Hopkins, S.J.; Johnson, R.B. A Controlled Trial of a Single Dose of Azithromycin for the Treatment of Chlamydial Urethritis and Cervicitis. N. Engl. J. Med. 1992, 327, 921–925. [Google Scholar] [CrossRef]
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int. J. Pharm. 2013, 441, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Cazenave, L.A.; Donehower, R.C. Taxol: A Novel Investigation^ Antimicrotubule Agent. J. Natl. Cancer Inst. 1990, 82, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Fellner, S.; Buschauer, A.; Fricker, G.; Fellner, S.; Bauer, B.; Miller, D.S.; Schaffrik, M.; Fankhänel, M.; Spruß, T.; Bernhardt, G.; et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo Find the latest version: Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Investig. 2002, 110, 1309–1318. [Google Scholar] [CrossRef]
- Najlah, M.; Freeman, S.; Attwood, D.; D’Emanuele, A. Synthesis and Assessment of First-Generation Polyamidoamine Dendrimer Prodrugs to Enhance the Cellular Permeability of P-gp Substrates. Bioconjug. Chem. 2007, 18, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Majoros, I.J.; Thomas, T.P.; Mehta, C.B.; Baker, J.R. Poly(amidoamine) Dendrimer-Based Multifunctional Engineered Nanodevice for Cancer Therapy. J. Med. Chem. 2005, 48, 5892–5899. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Mohammadnejad, J.; Yavari, K. Synthesis and evaluation of polyethylene glycol- and folic acid-conjugated polyamidoamine G4 dendrimer as nanocarrier. J. Drug Deliv. Sci. Technol. 2019, 50, 278–286. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Chen, S.; Yang, K.; Tuguntaev, R.G.; Mozhi, A.; Zhang, J.; Wang, P.C.; Liang, X.-J. Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 269–286. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; He, B.; Xu, Z.; Yin, M.; Yang, W.; Zhang, H.; Cao, J.; Shen, J. A functionalized fluorescent dendrimer as a pesticide nanocarrier: Application in pest control. Nanoscale 2015, 7, 445–449. [Google Scholar] [CrossRef]
- Maienfisch, P.; Angst, M.; Brandl, F.; Fischer, W.; Hofer, D.; Kayser, H.; Kobel, W.; Rindlisbacher, A.; Senn, R.; Steinemann, A. Chemistry and biology of thiamethoxam: A second generation neonicotinoid. Pest Manag. Sci. 2001, 57, 906–913. [Google Scholar] [CrossRef]
- Liang, Q.; Xing, P.; Huang, Z.; Dong, J.; Sharpless, K.B.; Li, X.; Jiang, B. Palladium-Catalyzed, Ligand-Free Suzuki Reaction in Water Using Aryl Fluorosulfates. Org. Lett. 2015, 17, 1942–1945. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Chung, D.W.; Rich, B. Sonogashira Couplings of Aryl Bromides: Room Temperature, Water Only, No Copper. Org. Lett. 2008, 10, 3793–3796. [Google Scholar] [CrossRef]
- Tukhani, M.; Panahi, F.; Khalafi-Nezhad, A. Supported Palladium on Magnetic Nanoparticles–Starch Substrate (Pd-MNPSS): Highly Efficient Magnetic Reusable Catalyst for C–C Coupling Reactions in Water. ACS Sustain. Chem. Eng. 2018, 6, 1456–1467. [Google Scholar] [CrossRef]
- Shaikh, T.M.; Hong, F. Palladium (II)—catalyzed Heck reaction of aryl halides and arylboronic acids with olefins under mild conditions. Belstein J. Org. Chem. 2013, 9, 1578–1588. [Google Scholar] [CrossRef] [Green Version]
- Papp, A.; Galbács, G.; Molnár, Á. Recyclable ligand-free mesoporous heterogeneous Pd catalysts for Heck coupling. Tetrahedron Lett. 2005, 46, 7725–7728. [Google Scholar] [CrossRef]
- Han, W.; Liu, N.; Liu, C.; Jin, Z.L. A ligand-free Heck reaction catalyzed by the in situ-generated palladium nanoparticles in PEG-400. Chin. Chem. Lett. 2010, 21, 1411–1414. [Google Scholar] [CrossRef]
- Niknam, E.; Moaddeli, A.; Khalafi-Nezhad, A. Palladium anchored on guanidine-terminated magnetic dendrimer (G3-Gu-Pd): An efficient nano-sized catalyst for phosphorous-free Mizoroki-Heck and copper-free Sonogashira couplings in water. J. Organomet. Chem. 2020, 923, 121369. [Google Scholar] [CrossRef]
- Alper, H.; Arya, P.; Bourque, S.C.; Jefferson, G.R.; Manzer, L.E. Heck reaction using palladium complexed to dendrimers on silica. Can. J. Chem. 2000, 78, 920–924. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Fanelli, A.; Piccinino, D.; De Angelis, M.; Dolfa, C.; Palamara, A.T.; Nencioni, L.; Zippilli, C.; Crucianelli, M.; Saladino, R. Synthesis of Stilbene and Chalcone Inhibitors of Influenza A Virus by SBA-15 Supported Hoveyda-Grubbs Metathesis. Catalysts 2019, 9, 983. [Google Scholar] [CrossRef] [Green Version]
- Karakhanov, E.; Maximov, A.; Kardasheva, Y.; Semernina, V.; Zolotukhina, A.; Ivanov, A.; Abbott, G.; Rosenberg, E.; Vinokurov, V. Pd Nanoparticles in Dendrimers Immobilized on Silica–Polyamine Composites as Catalysts for Selective Hydrogenation. ACS Appl. Mater. Interfaces 2014, 6, 8807–8816. [Google Scholar] [CrossRef]
- Hughes, M.A.; Nielsen, D.; Rosenberg, E.; Gobetto, R.; Viale, A.; Burton, S.D.; Ferel, J. Structural Investigations of Silica Polyamine Composites: Surface Coverage, Metal Ion Coordination, and Ligand Modification. Ind. Eng. Chem. Res. 2006, 45, 6538–6547. [Google Scholar] [CrossRef]
- Hughes, M.A.; Rosenberg, E. Characterization and Applications of Poly-Acetate Modified Silica Polyamine Composites. Sep. Sci. Technol. 2007, 42, 261–283. [Google Scholar] [CrossRef]
- Landarani-isfahani, A.; Mohammadpoor-baltork, I.; Mirkhani, V. Palladium nanoparticles immobilized on a nano- silica triazine dendritic polymer: A recyclable and sustainable nanoreactor for C–S cross-coupling. RSC Adv. 2020, 10, 21198–21205. [Google Scholar] [CrossRef]
- Lakshmi, K.; Rangasamy, R. Synthetic modification of silica coated magnetite cored PAMAM dendrimer to enrich branched Amine groups and peripheral carboxyl groups for environmental remediation. J. Mol. Struct. 2021, 1224, 129081. [Google Scholar] [CrossRef]
- Caminade, A.; Ouali, A.; Majoral, J.; Caminade, A. Chem Soc Rev Organocatalysis with dendrimers. Chem. Soc. Rev. 2012, 41, 4113–4125. [Google Scholar] [CrossRef]
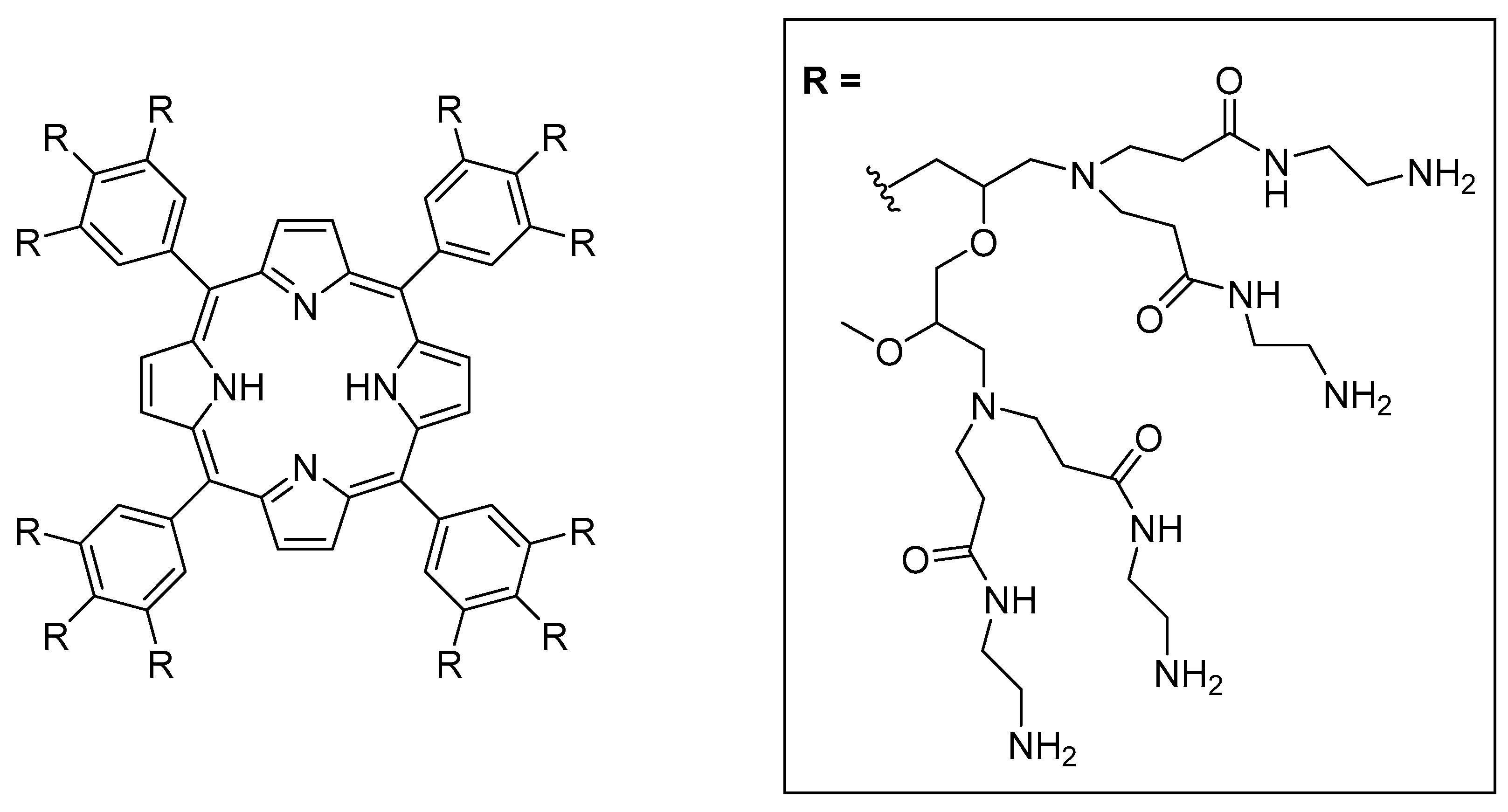
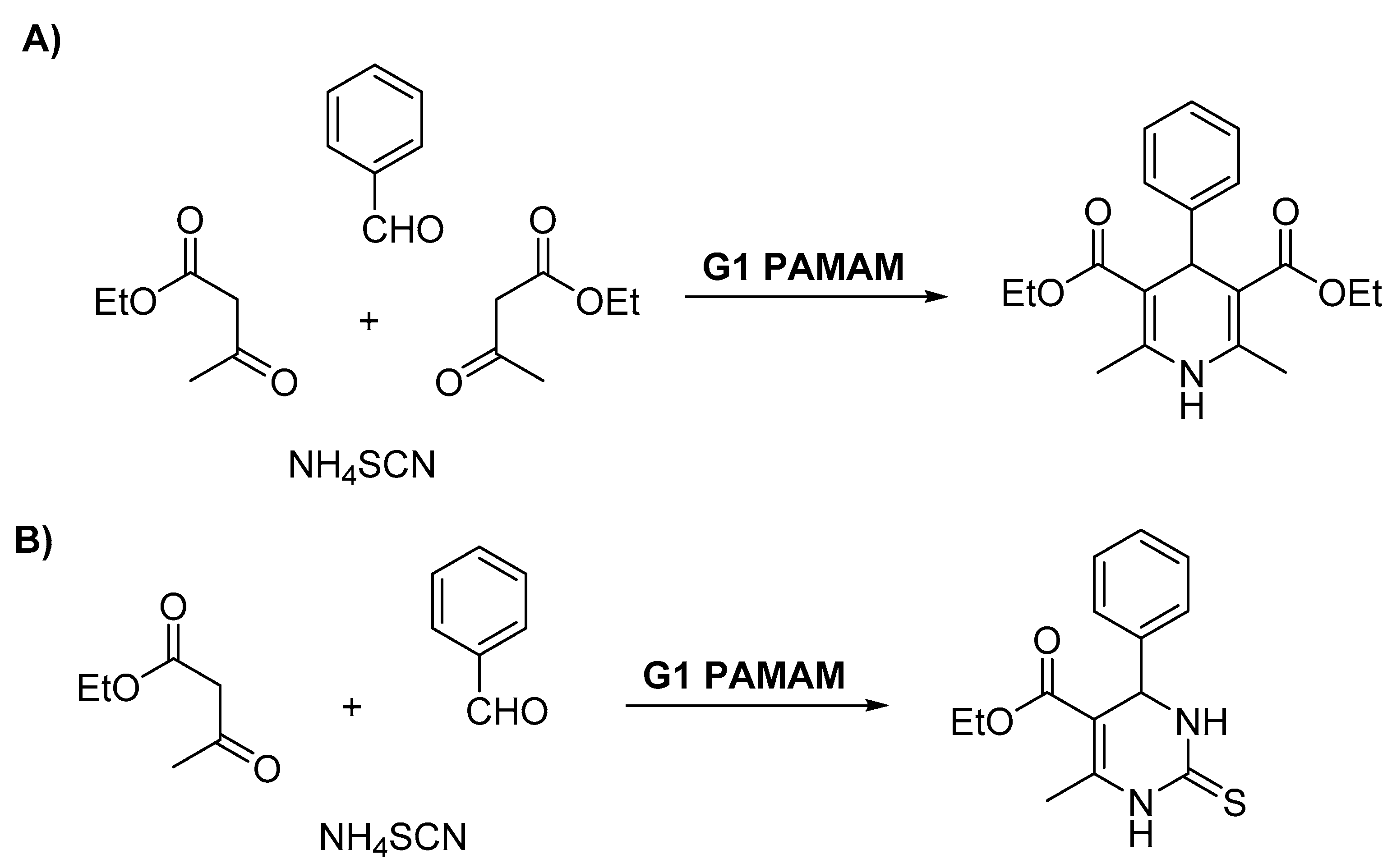
- Avudaiappan, G.; Unnikrishnan, V.; Sreekumar, K. Convenient Synthesis of Dihydropyridine and Dihydropyrimidinethione Derivatives Using a Porphyrin Cored G1 PAMAM Dendrimer as a Homogeneous Catalyst. Chem. Sel. 2020, 5, 506–514. [Google Scholar] [CrossRef]
- Sherlymole, P.B.; Anuf, R.; Krishna, A. Dendrimer with Interior Cavity as Catalytic Pockets for Substrate Molecules: Synthesis of Bisimidazoles and Molecular Docking Study. ChemistrySelect 2020, 5, 5055–5065. [Google Scholar] [CrossRef]
- Hiba, K.; Sreekumar, K. Multi- arm dendronized polymer as a unimolecular micelle: Synthesis, characterization and application as organocatalyst in the synthesis of N-unsubstituted 1,2,3-triazoles. React. Funct. Polym. 2021, 160, 104827. [Google Scholar] [CrossRef]
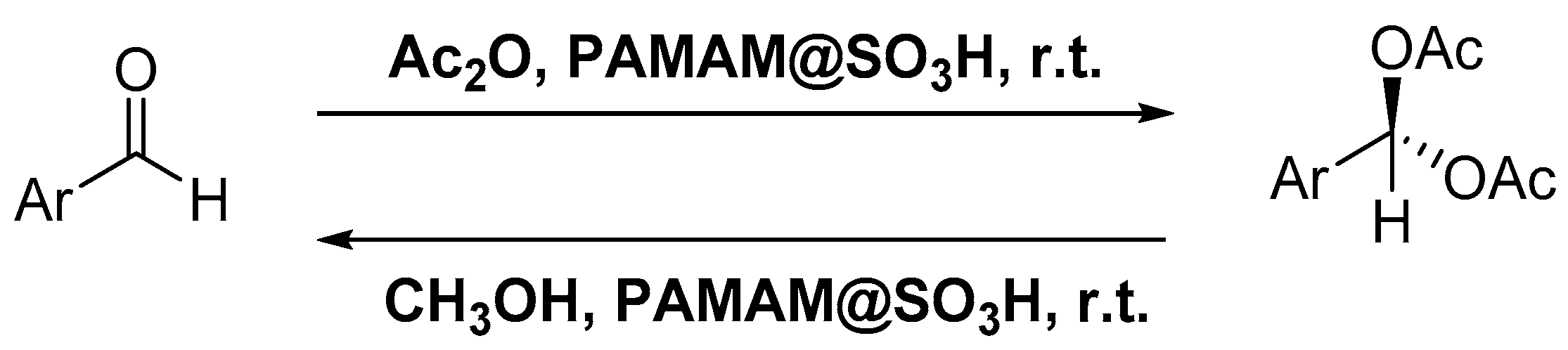
- Pourjavadi, A.; Hosseini, S.H. Functionalized Poly(Amidoamine) Dendrimer as a Strong Ionic Brønsted Acid Organocatalyst for Protection/Deprotection of Aldehydes. Phosphorus. Sulfur Silicon Relat. Elem. 2014, 189, 1794–1801. [Google Scholar] [CrossRef]
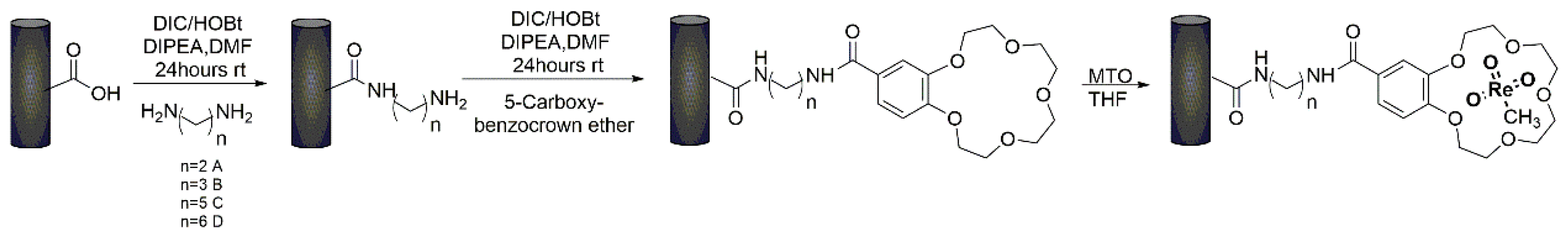
- Bizzarri, B.M.; Fanelli, A.; Botta, L.; Sadun, C.; Gontrani, L.; Ferella, F.; Crucianelli, M.; Saladino, R. Dendrimer crown-ether tethered multi-wall carbon nanotubes support methyltrioxorhenium in the selective oxidation of olefins to epoxides. RSC Adv. 2020, 10, 17185–17194. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Rotelli, L.; Botta, G.; Saladino, R. Current Advances in L-DOPA and DOPA-Peptidomimetics: Chemistry, Applications and Biological Activity. Curr. Med. Chem. 2015, 22, 4138–4165. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, B.M.; Pieri, C.; Botta, G.; Arabuli, L.; Mosesso, P.; Cinelli, S.; Schinoppi, A.; Saladino, R. Synthesis and antioxidant activity of DOPA peptidomimetics by a novel IBX mediated aromatic oxidative functionalization. RSC Adv. 2015, 5, 60354–60364. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Oka, H.; Onaga, T.; Koyama, T.; Guo, C.-T.; Suzuki, Y.; Esumi, Y.; Hatano, K.; Terunuma, D.; Matsuoka, K. Sialyl α(2→3) lactose clusters using carbosilane dendrimer core scaffolds as influenza hemagglutinin blockers. Bioorg. Med. Chem. Lett. 2008, 18, 4405–4408. [Google Scholar] [CrossRef]
- Rodríguez-Izquierdo, I.; Natalia, C.; García, F.; Muñoz-Fernandez, M.D.L. Ángeles G2-S16 sulfonate dendrimer as new therapy for treatment failure in HIV-1 entry inhibitors. Nanomedicine 2019, 14, 1095–1107. [Google Scholar] [CrossRef]
- Bourne, N.; Stanberry, L.R.; Kern, E.R.; Holan, G.; Matthews, B.; Bernstein, D.I. Dendrimers, a New Class of Candidate Topical Microbicides with Activity against Herpes Simplex Virus Infection. Antimicrob. Agents Chemother. 2000, 44, 2471–2474. [Google Scholar] [CrossRef] [Green Version]
- Razinkov, V.; Gazumyan, A.; Nikitenko, A.; Ellestad, G.; Krishnamurthy, G. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem. Biol. 2001, 8, 645–659. [Google Scholar] [CrossRef] [Green Version]
- Günther, S.C.; Maier, J.D.; Vetter, J.; Podvalnyy, N.; Khanzhin, N.; Hennet, T.; Stertz, S. Antiviral potential of 3′-sialyllactose- and 6′-sialyllactose-conjugated dendritic polymers against human and avian influenza viruses. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandeel, M.; Al-Taher, A.; Park, B.K.; Kwon, H.J.; Al-Nazawi, M. A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. J. Med. Virol. 2020, 92, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Rodríguez, I.R.; Sánchez, R.J.; Pavicic, C.; Muñoz, E.; Ángeles, M.; Fernández, M. Polyanionic carbosilane dendrimers as a new adjuvant in combination with latency reversal agents for HIV treatment. J. Nanobiotechnol. 2019, 17, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malkoch, M.; García-Gallego, S. CHAPTER 1: Introduction to Dendrimers and Other Dendritic Polymers. In Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures; Royal Society of Chemistry: London, UK, 2020; pp. 1–20. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, C.; Saito, K.; Kondo, E. Design of peptide-dendrimer conjugates with tumor homing and antitumor effects. Res. Chem. Intermed. 2018, 44, 4685–4695. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Ding, P.; Wang, M.; Guo, X.; Stuart, M.A.C.; Wang, J. Hierarchical polyion complex vesicles from PAMAM dendrimers. J. Colloid Interface Sci. 2021, 606, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Devadas, B.; Periasamy, A.P.; Bouzek, K. A review on poly(amidoamine) dendrimer encapsulated nanoparticles synthesis and usage in energy conversion and storage applications. Coord. Chem. Rev. 2021, 444, 214062. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Liviu Rus, L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Kulhari, H. Pharmaceutical Applications of Dendrimers, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–269. [Google Scholar] [CrossRef]
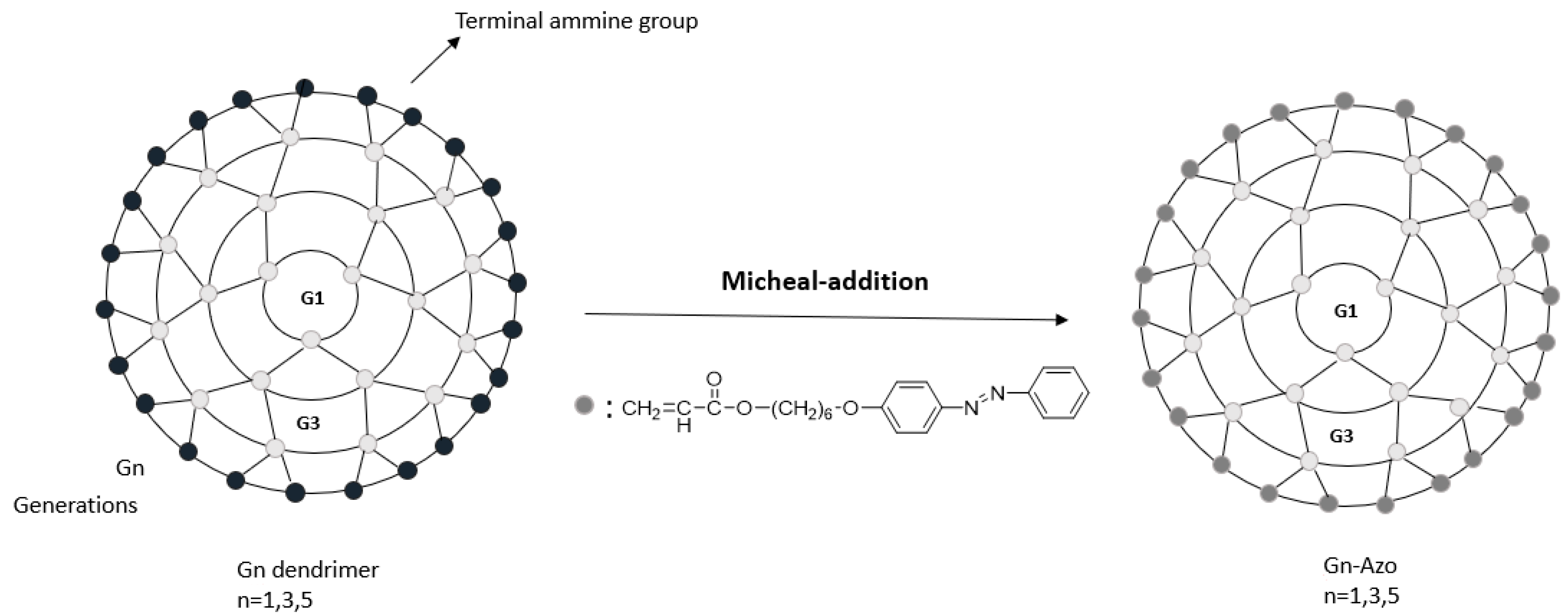
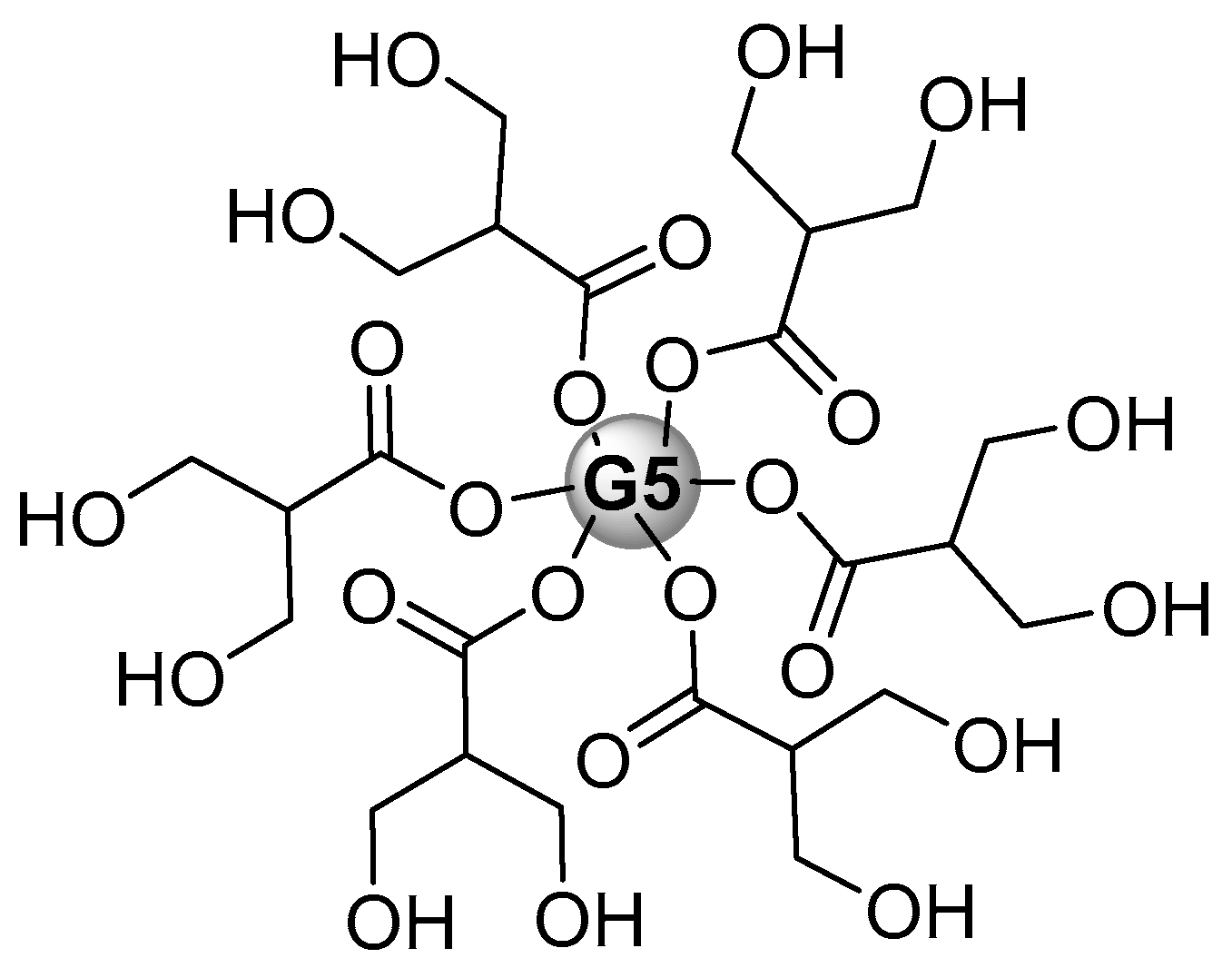
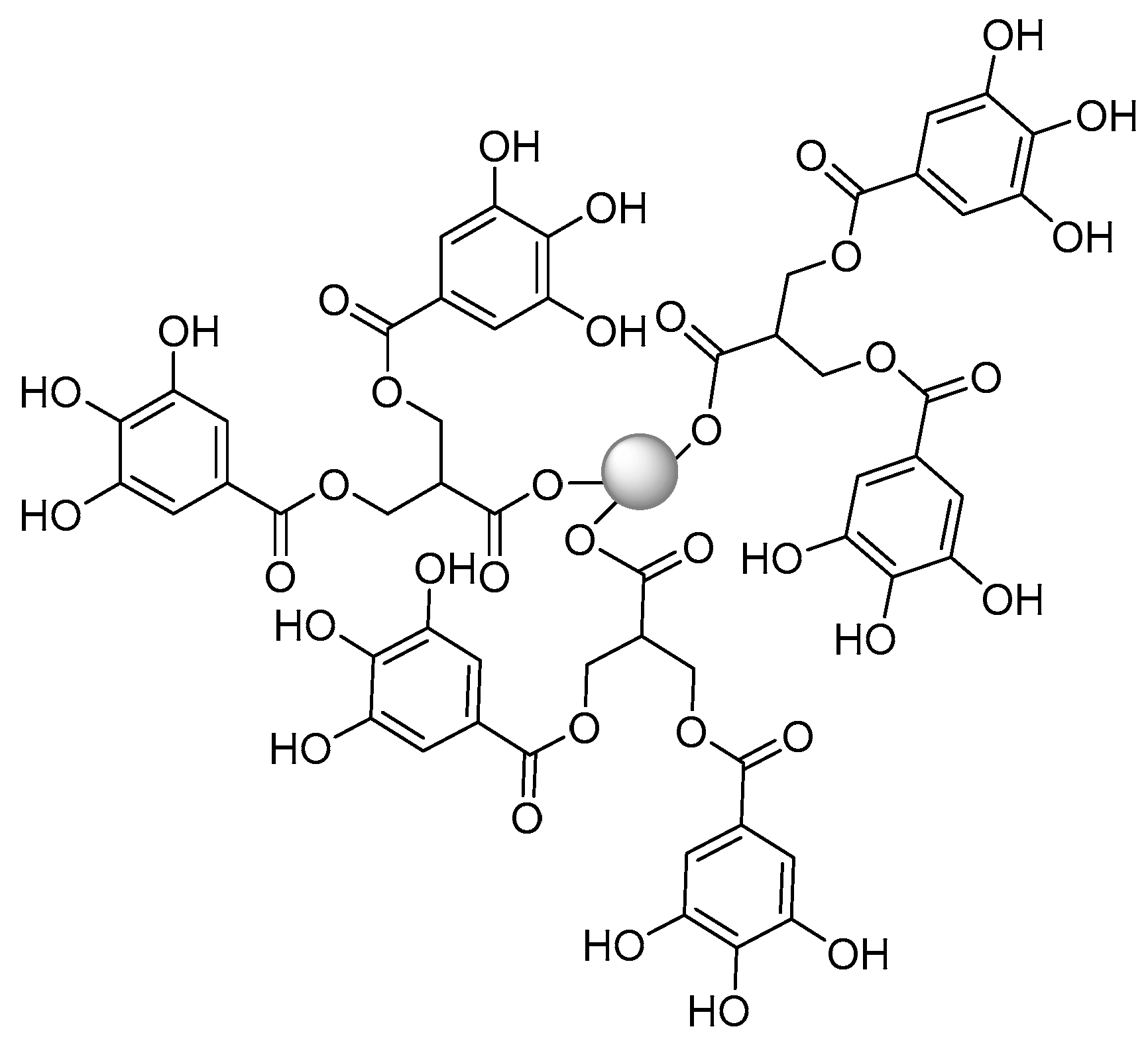

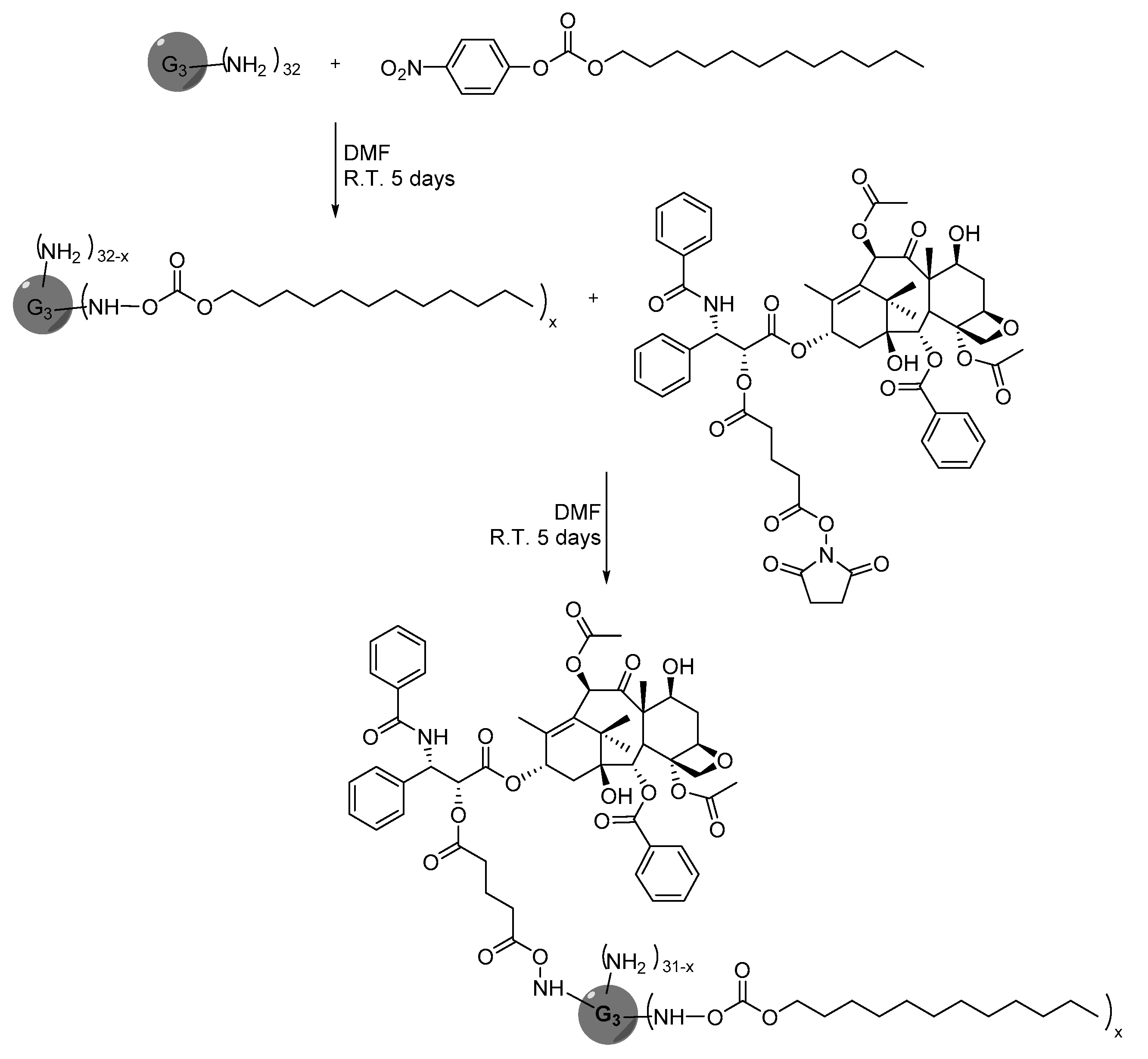









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzarri, B.M.; Fanelli, A.; Botta, L.; Zippilli, C.; Cesarini, S.; Saladino, R. Dendrimeric Structures in the Synthesis of Fine Chemicals. Materials 2021, 14, 5318. https://doi.org/10.3390/ma14185318
Bizzarri BM, Fanelli A, Botta L, Zippilli C, Cesarini S, Saladino R. Dendrimeric Structures in the Synthesis of Fine Chemicals. Materials. 2021; 14(18):5318. https://doi.org/10.3390/ma14185318
Chicago/Turabian StyleBizzarri, Bruno Mattia, Angelica Fanelli, Lorenzo Botta, Claudio Zippilli, Silvia Cesarini, and Raffaele Saladino. 2021. "Dendrimeric Structures in the Synthesis of Fine Chemicals" Materials 14, no. 18: 5318. https://doi.org/10.3390/ma14185318
APA StyleBizzarri, B. M., Fanelli, A., Botta, L., Zippilli, C., Cesarini, S., & Saladino, R. (2021). Dendrimeric Structures in the Synthesis of Fine Chemicals. Materials, 14(18), 5318. https://doi.org/10.3390/ma14185318







