Nanocrystalline Cellulose from Microcrystalline Cellulose of Date Palm Fibers as a Promising Candidate for Bio-Nanocomposites: Isolation and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation Method of NCC
2.3. Characterization Methods
2.3.1. Yield Determination
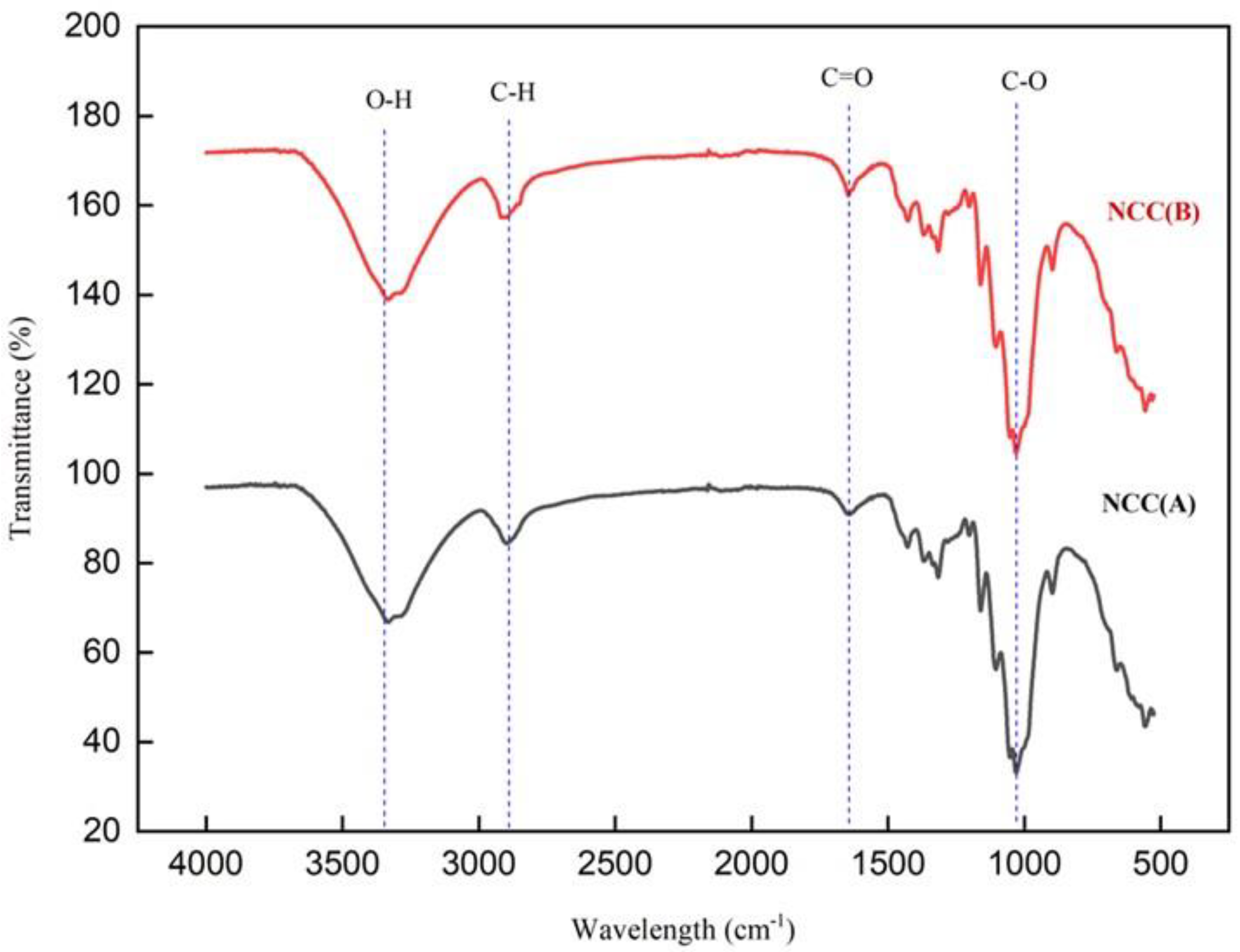
2.3.2. FTIR Examination
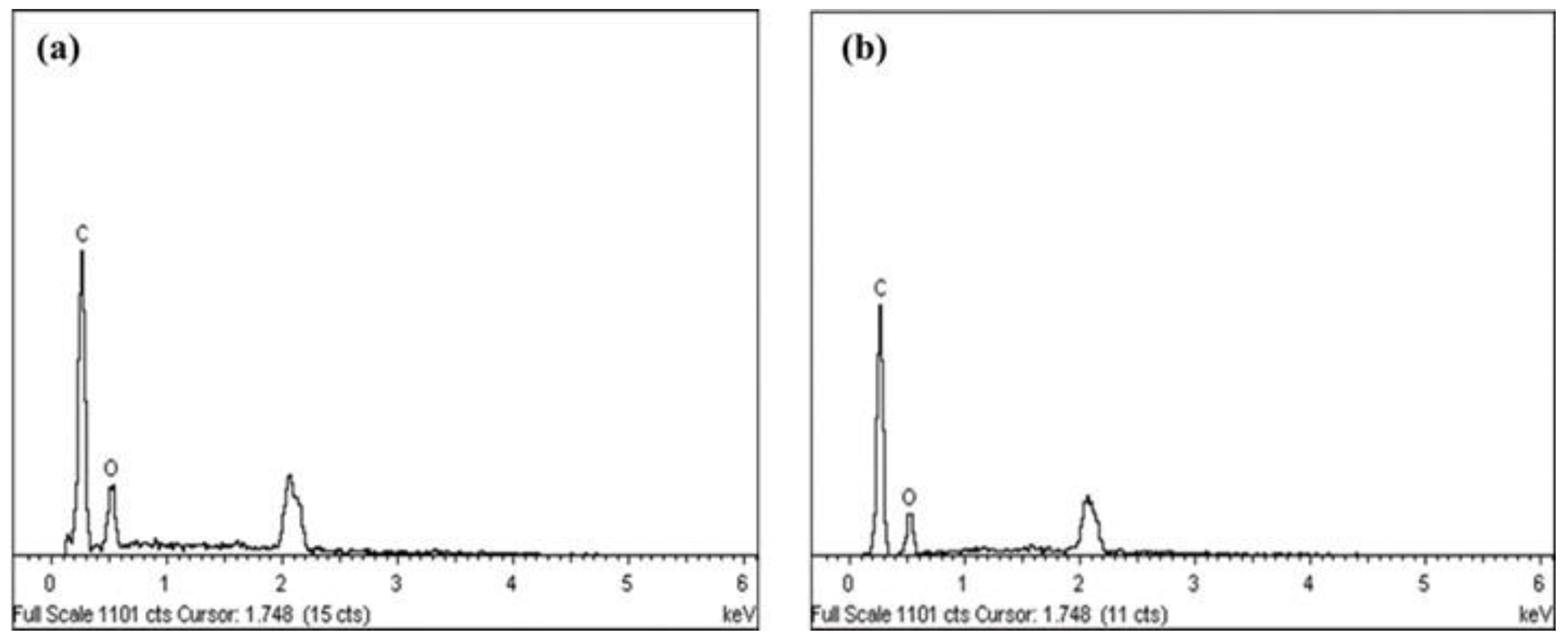
2.3.3. Structure, Elemental Composition, Particle Size Distribution and Zeta Potential Analysis
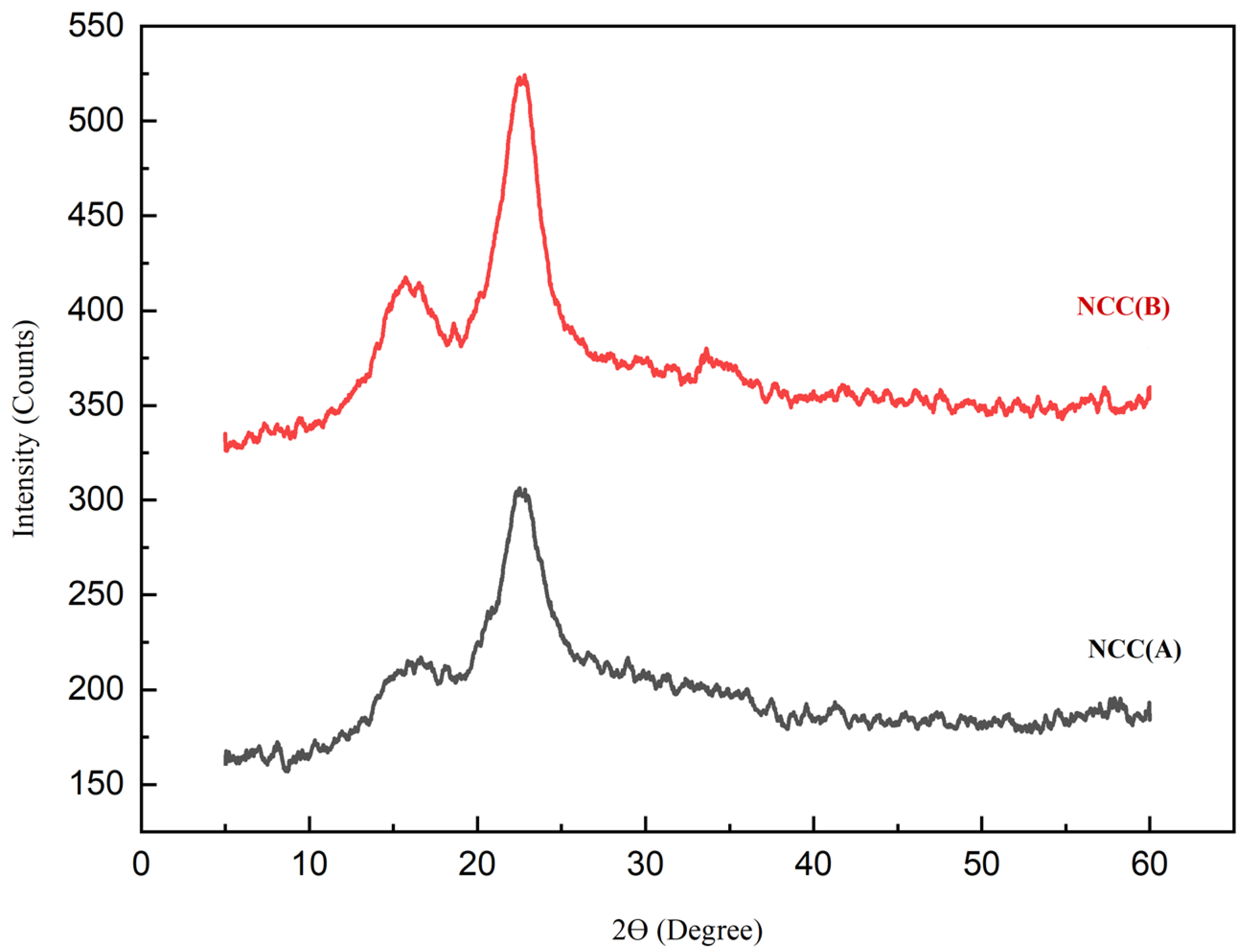
2.3.4. Crystallinity Analysis
2.3.5. Thermal Analysis
3. Results and Discussion
3.1. The Yield and Infrared Spectroscopy
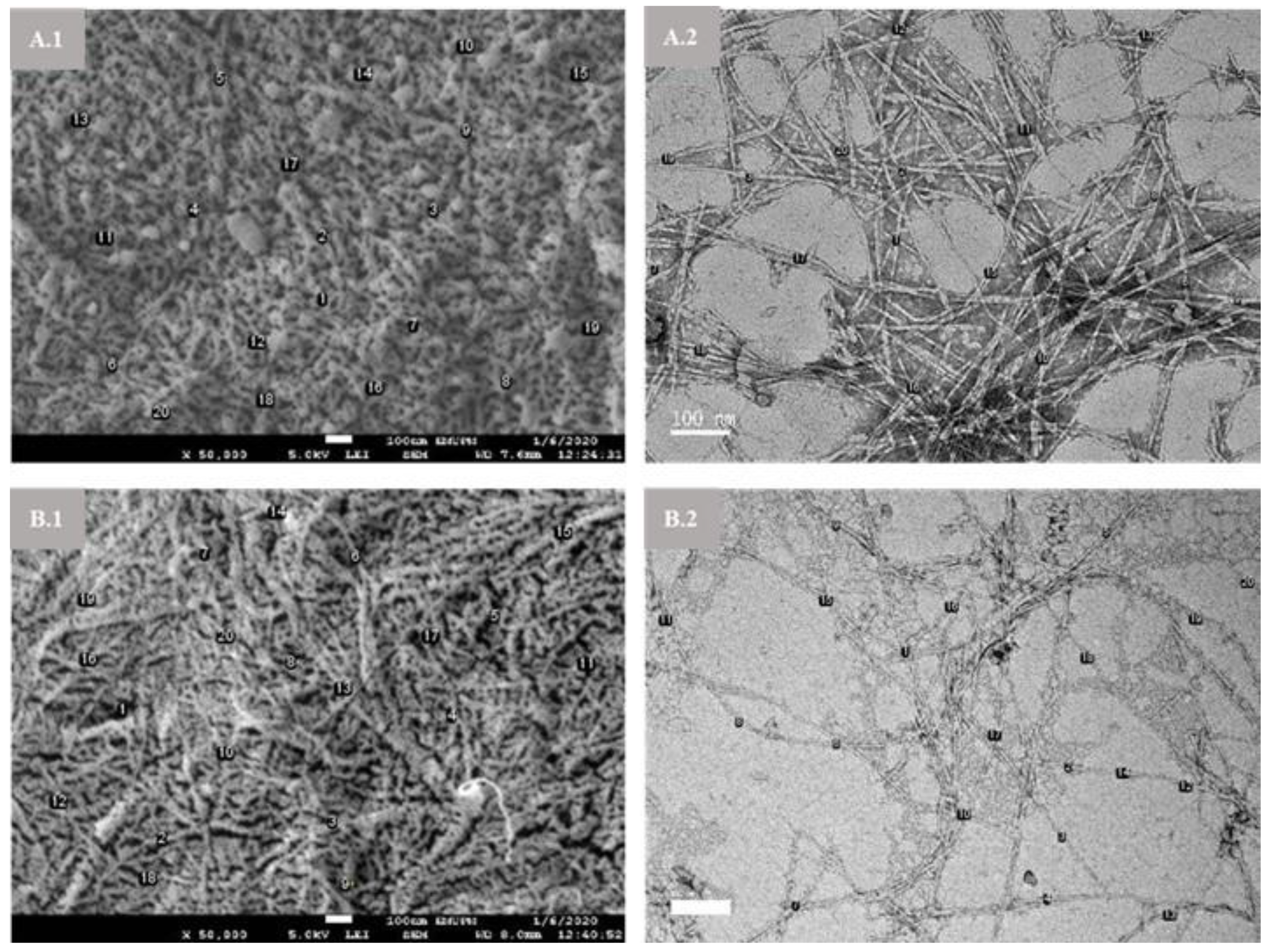
3.2. Morphology, Particle Size, Element Composition, and Zeta Potential Analysis
3.3. X-ray Diffraction (XRD)
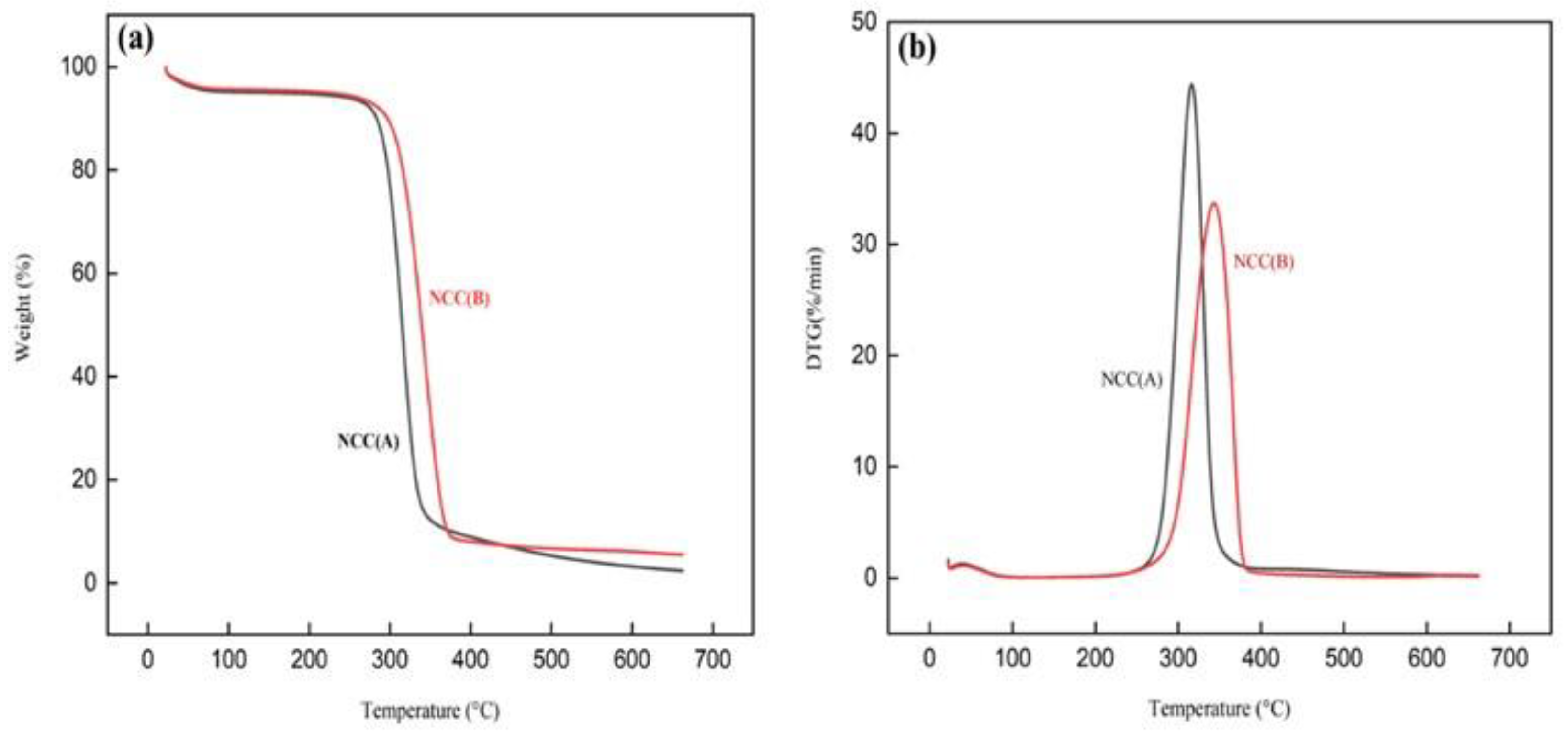
3.4. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasheed, M.; Jawaid, M.; Parveez, B.; Zuriyati, A.; Khan, A. Morphological, chemical and thermal analysis of cellulose nanocrystals extracted from bamboo fibre. Int. J. Biol. Macromol. 2020, 160, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Karim, Z. Isolation and characterization of nanocrystalline cellulose from roselle-derived microcrystalline cellulose. Int. J. Biol. Macromol. 2018, 114, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Taban, E.; Amininasab, S.; Soltani, P.; Berardi, U.; Abdi, D.D.; Samaei, S.E. Use of date palm waste fibers as sound absorption material. J. Build. Eng. 2021, 41, 102752. [Google Scholar] [CrossRef]
- Bedjaoui, H.; Benbouza, H. Assessment of phenotypic diversity of local Algerian date palm (Phoenix dactylifera L.) cultivars. J. Saudi Soc. Agri. Sci. 2020, 19, 65–75. [Google Scholar] [CrossRef]
- Faci, M. Typology and varietal biodiversity of date palm farms in the North-East of Algerian Sahara. J. Taibah Uni. Sci. 2019, 13, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Hachaichi, A.; Kouini, B.; Kian, L.K.; Asim, M.; Jawaid, M. Extraction and characterization of microcrystalline cellulose from date palm fibers using successive chemical treatments. J. Polym. Environ. 2021, 29, 1990–1999. [Google Scholar] [CrossRef]
- Amroune, S.; Bezazi, A.; Belaadi, A.; Zhu, C.; Scarpa, F.; Rahatekar, S.; Imad, A. Tensile mechanical properties and surface chemical sensitivity of technical fibres from date palm fruit branches (Phoenix dactylifera L.). Compos. Part A Appl. Sci. Manuf. 2015, 71, 95–106. [Google Scholar] [CrossRef]
- Karimi, S.; Tahir, P.M.; Karimi, A.; Dufresne, A.; Abdulkhani, A. Kenaf bast cellulosic fibers hierarchy: A comprehensive approach from micro to nano. Carbohydr. Polym. 2014, 101, 878–885. [Google Scholar] [CrossRef]
- Santos, F.A.; Iulianelli, G.C.V.; Tavares, M.I.B. Effect of microcrystalline and nanocrystals cellulose fillers in materials based on PLA matrix. Polym. Test. 2017, 61, 280–288. [Google Scholar] [CrossRef]
- Pan, M.; Zhou, X.; Chen, M. Cellulose nanowhiskers isolation and properties from acid hydrolysis combined with high pressure homogenization. Bioresources 2013, 8, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Haafiz, M.K.M.; Hassan, A.; Zakaria, Z.; Inuwa, I.M. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr. Polym. 2014, 103, 119–125. [Google Scholar] [CrossRef]
- Mehanny, S.; Magd, E.E.A.; Ibrahim, M.; Farag, M.; Millan, R.G.S.; Navarro, J.; Habbak, A.E.H.E.; Kashifa, E.E. Extraction and characterization of nanocellulose from three types of palm residues. J. Mater. Res. Technol. 2021, 10, 526–537. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Derradji, M.; Bessa, W. Ecofriendly isolation and characterization of microcrystalline cellulose from giant reed using various acidic media. Cellulose 2019, 26, 7635–7651. [Google Scholar] [CrossRef]
- Faria, L.U.S.; Pacheco, B.J.S.; Oliveira, G.C.; Silva, J.L. Production of cellulose nanocrystals from pineapple crown fibers through alkaline pretreatment and acid hydrolysis under different conditions. J. Mater. Res. Technol. 2020, 9, 12346–12353. [Google Scholar] [CrossRef]
- Kian, L.K.; Saba, N.; Jawaid, M.; Alothman, O.Y.; Fouad, H. Properties and characteristics of nanocrystalline cellulose isolated from olive fiber. Carbohydr. Polym. 2020, 241, 116423. [Google Scholar] [CrossRef] [PubMed]
- Prathapan, R.; Thapa, R.; Garnier, G.; Tabor, R.F. Modulating the zeta potential of cellulose nanocrystals using salts and surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 11–18. [Google Scholar] [CrossRef]
- Hachaichi, A.; Jawaid, M.; Asim, M.; Kouini, B. Nanocellulose Reinforced Polylactic Acid Bionanocomposites. In Eco-Friendly Adhesives for Wood and Natural Fiber Composites. Composites Science and Technology; Jawaid, M., Khan, T.A., Nasir, M., Asim, M., Eds.; Springer: Singapore, 2021. [Google Scholar]
- Foo, M.L.; Ooi, C.W.; Tan, K.W.; Chew, I.M.L. A step closer to sustainable industrial production: Tailor the properties of nanocrystalline cellulose from oil palm empty fruit bunch. J. Environ. Chem. Eng. 2020, 8, 104058. [Google Scholar] [CrossRef]
- Qian, M.; Lei, H.; Villota, E.; Zhao, Y.; Wang, C.; Huo, E.; Zhang, Q.; Mateo, W.; Lin, X. High yield production of nanocrystalline cellulose by microwave-assisted dilute-acid pretreatment combined with enzymatic hydrolysis. Chem. Eng. Process. Process Intensif. 2021, 160, 108292. [Google Scholar] [CrossRef]
- Tuerxun, D.; Pulingam, T.; Nordin, N.I.; Chen, Y.W.; Kamaldin, J.B.; Julkapli, N.B.M.; Lee, H.V.; Leo, B.F.; Johan, M.R.B. Synthesis, characterization and cytotoxicity studies of nanocrystalline cellulose from the production waste of rubber-wood and kenaf-bast fibers. Euro. Polym. J. 2019, 116, 352–360. [Google Scholar] [CrossRef]
- Ogundare, S.A.; Moodley, V.; Zyl, W.E. Nanocrystalline cellulose isolated from discarded cigarette filters. Carbohydr. Polym. 2017, 175, 273–281. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Xie, W.; Deng, H. Extraction, isolation and characterization of nanocrystalline cellulose from industrial kelp (Laminaria japonica) waste. Carbohydr. Polym. 2017, 173, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.J.; Acharya, B.; Bissessur, R. Isolation of nanocrystalline cellulose from tunicates. J. Environ. Chem. Eng. 2018, 6, 4408–4412. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Puglia, D.; Petrucci, R.; Kenny, J.M.; Torre, L. Modulation of acid hydrolysis reaction time for the extraction of cellulose nanocrystals from Posidonia oceanica leaves. J. Renew. Mater. 2016, 4, 190–198. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Il Park, S.; Lee, Y.S. Microwave-assisted step reduced extraction of seaweed (Gelidiella aceroso) cellulose nanocrystals. Int. J. Biol. Macromol. 2017, 99, 506–510. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; Filho, M.S.M.S.; Rosa, M.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Chen, H.H. Physical properties of bacterial cellulose composites for wound dressings. Food Hydrocoll. 2016, 53, 75–83. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Jasmani, L.; Adnan, S. Preparation and characterization of nanocrystalline cellulose from Acacia mangium and its reinforcement potential. Carbohydr. Polym. 2017, 161, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, H.S.; Chan, C.H.; Chia, C.H.; Zakaria, S.; Jaafar, S.N.S. Isolation and fractionation of cellulose nanocrystals from kenaf core. Sains Malays. 2015, 44, 1635–16432. [Google Scholar]
- Fillat, U.; Wicklein, B.; Sampedro, R.M.; Ibarra, D.; Hitzky, E.R.; Valencia, C.; Sarrión, A.; Castro, E.; Eugenio, M.E. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohydr. Polym. 2018, 179, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guo, X.; Wang, S.; Yin, Y. Effects of ultrasonic treatment during acid hydrolysis on the yield, particle size and structure of cellulose nanocrystals. Carbohydr. Polym. 2016, 135, 248–255. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Mohamad, A.B.; Zain, M.F.M.; Awang, N.A.; Hir, Z.A.M. Physicochemical characterization of cellulose nanocrystal and nanoporous self-assembled CNC membrane derived from Ceiba pentandra. Carbohydr. Polym. 2017, 157, 1892–1902. [Google Scholar] [CrossRef]
- Chan, C.H.; Chia, C.H.; Zakaria, S.; Ahmad, I.; Dufresne, A. Production and characterisation of cellulose and nano- crystalline cellulose from kenaf core wood. Bioresources 2013, 8, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, Z.Z.; Hamid, S.B.A. Preparation and characterization of nanocrystalline cellulose using ultrasonication combined with a microwave-assisted pretreatment process. Bioresources 2016, 11, 3397–3415. [Google Scholar] [CrossRef]
- Mariano, M.; Cercená, R.; Soldi, V. Thermal characterization of cellulose nanocrystals isolated from sisal fibers using acid hydrolysis. Ind. Crops Prod. 2016, 94, 454–462. [Google Scholar] [CrossRef]
- Salam, A.; Lucia, L.A.; Jameel, H. A novel cellulose nanocrystals-based approach to improve the mechanical properties of recycled paper. ACS Sustain. Chem. Eng. 2013, 1, 1584–1592. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S. Studies on cellulose nanocrystals isolated from groundnut shells. Carbohydr. Polym. 2017, 157, 1041–1049. [Google Scholar] [CrossRef]
- Shanmugarajah, B.; Kiew, P.L.; Chew, I.M.L.; Choong, T.S.Y.; Tan, K.W. Isolation of nanocrystalline cellulose (NCC) from palm oil empty fruit bunch (EFB): Preliminary result on FTIR and DLS analysis. Chem. Eng. Trans. 2015, 45, 1705–1710. [Google Scholar]
- French, A.D.; Cintrón, M.S. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Achaby, M.E.; Miri, N.E.; Aboulkas, A.; Zahouily, M.; Bilal, E.; Barakat, A.; Solhya, A. Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse. Int. J. Biol. Macromol. 2017, 96, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Chang, Y.; Han, J. Development of polylactic acid nanocomposite films reinforced with cellulose nanocrystals derived from coffee silverskin. Carbohydr. Polym. 2017, 169, 495–503. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Zhang, Q.; Zhao, G.; Heng, L.; Chen, D.; Liu, D. Preparation of cellulose nanocrystals from Humulus japonicus stem and the influence of high temperature pretreatment. Carbohydr. Polym. 2017, 164, 284–293. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, Z.; Lin, F.; Tang, L.; Wang, S.; Huang, B. Extraction of cellulose nanocrystals with a high yield of 88% by simultaneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Taflick, T.; Schwendler, L.A.; Rosa, S.M.L.; Bica, C.I.D.; Nachtigall, S.M.B. Cellulose nanocrystals from acacia bark–Influence of solvent extraction. Int. J. Biol. Macromol. 2017, 101, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Espino, E.; Cakir, M.; Domenek, S.; Román-Gutiérrez, A.D.; Belgacem, N.; Bras, J. Isolation and characterization of cellulose nanocrystals from industrial by-products of Agave tequilana and barley. Ind. Crops Prod. 2014, 62, 552–559. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, X.; Zhang, J.; Guo, D.; Kong, F.; Zhang, N. Extraction of cellulose nano-crystals from old corrugated container fiber using phosphoric acid and enzymatic hydrolysis followed by sonication. Carbohydr. Polym. 2015, 125, 360–366. [Google Scholar] [CrossRef]

| Designation of NCC Product | Hydrolysis Reaction Times (min) | Yield (%) |
|---|---|---|
| NCC (A) | 60 | 22 |
| NCC (B) | 90 | 25 |
| Samples | Width Size Average (nm) | Length Size Average (nm) | Carbon (%) | Oxygen (%) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| NCC (A) | 7.51 | 139.91 | 78.58 | 21.42 | −33.7 |
| NCC (B) | 4.34 | 111.51 | 82.01 | 17.99 | −27.9 |
| Samples | TGA Analysis | DSC Analysis | |||||
|---|---|---|---|---|---|---|---|
| Tinitial (°C) a | Tpeak (°C) b | Wloss (%) c | Wresidue (%) d | Tinitial (°C) e | Tpeak (°C) f | ΔH (J/g) g | |
| NCC (A) | 293.94 | 315.9 | 92.56 | 2.40 | 193.67 | 195.06 | 70.5 |
| NCC (B) | 312.23 | 344.4 | 90.08 | 5.49 | 188.20 | 189.34 | 82.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachaichi, A.; Kouini, B.; Kian, L.K.; Asim, M.; Fouad, H.; Jawaid, M.; Sain, M. Nanocrystalline Cellulose from Microcrystalline Cellulose of Date Palm Fibers as a Promising Candidate for Bio-Nanocomposites: Isolation and Characterization. Materials 2021, 14, 5313. https://doi.org/10.3390/ma14185313
Hachaichi A, Kouini B, Kian LK, Asim M, Fouad H, Jawaid M, Sain M. Nanocrystalline Cellulose from Microcrystalline Cellulose of Date Palm Fibers as a Promising Candidate for Bio-Nanocomposites: Isolation and Characterization. Materials. 2021; 14(18):5313. https://doi.org/10.3390/ma14185313
Chicago/Turabian StyleHachaichi, Amina, Benalia Kouini, Lau Kia Kian, Mohammad Asim, Hassan Fouad, Mohammad Jawaid, and Mohini Sain. 2021. "Nanocrystalline Cellulose from Microcrystalline Cellulose of Date Palm Fibers as a Promising Candidate for Bio-Nanocomposites: Isolation and Characterization" Materials 14, no. 18: 5313. https://doi.org/10.3390/ma14185313
APA StyleHachaichi, A., Kouini, B., Kian, L. K., Asim, M., Fouad, H., Jawaid, M., & Sain, M. (2021). Nanocrystalline Cellulose from Microcrystalline Cellulose of Date Palm Fibers as a Promising Candidate for Bio-Nanocomposites: Isolation and Characterization. Materials, 14(18), 5313. https://doi.org/10.3390/ma14185313







