TiCoCrFeMn (BCC + C14) High-Entropy Alloy Multiphase Structure Analysis Based on the Theory of Molecular Orbitals
Abstract
:1. Introduction
- Titanium has a hexagonally compact crystal lattice at room temperature, which is characterized by higher strength properties than nickel, at a much lower density;
- Titanium easily forms a BCC-structured solid solution with elements such as V, Cr, Fe, Mo, Cu, Nb, Ta and Mn;
- The melting point of titanium is about 200 °C higher than that of nickel (1660 °C to 1453 °C);
- Titanium has paramagnetic properties, which extends its possible functional applications;
- Toxic nickel has harmful effects on human health, while titanium remains biocompatible.
2. Materials and Methods
2.1. Experimental Methods
2.2. Design of High-Entropy Alloys
3. Results and Discussion
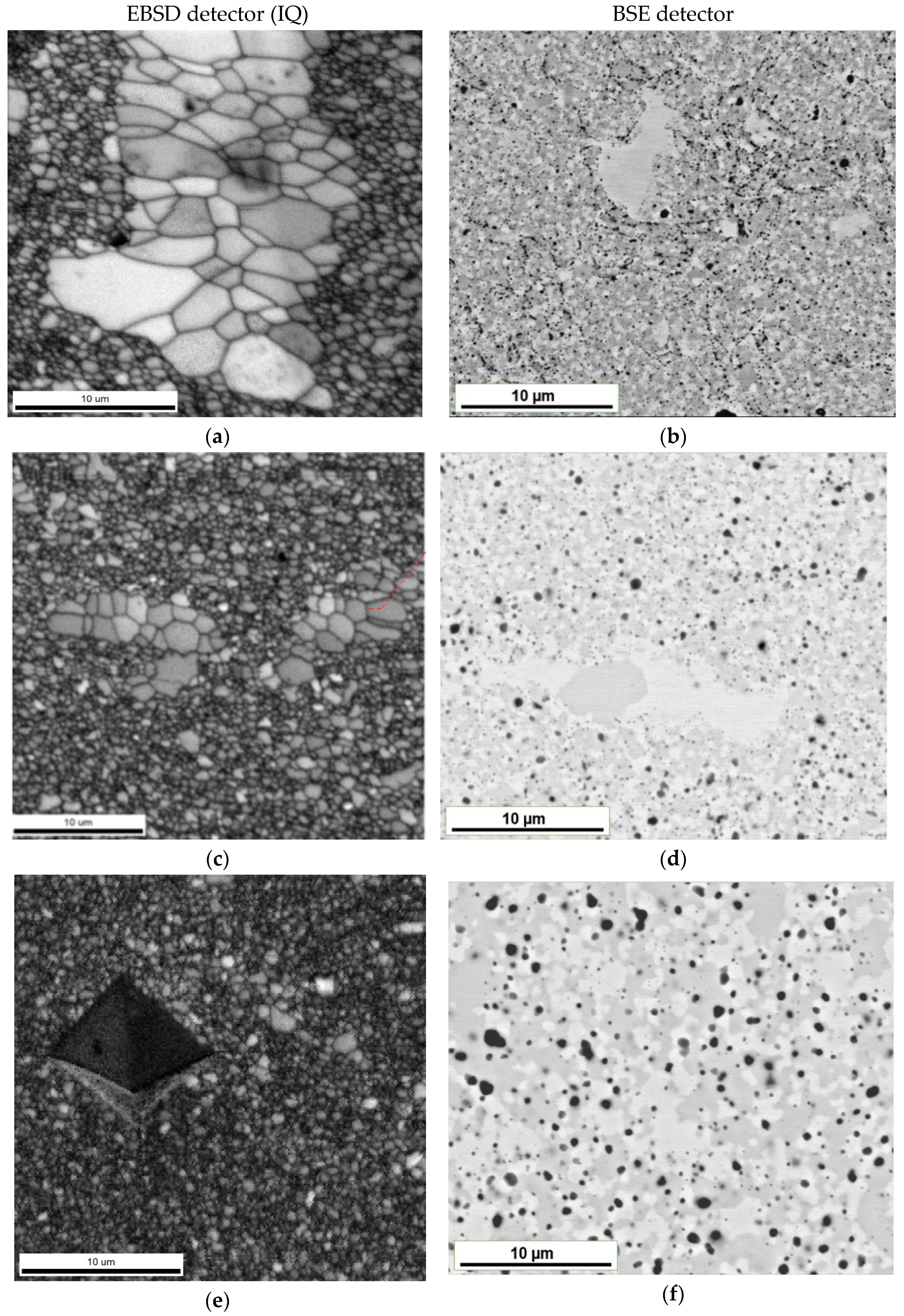
- Small bright area—sb—for areas with a bright matrix phase contrast;
- Small gray area—sg—for areas of the dark gray matrix phase contrast;
- Large bright area—lb—for areas with bright phase contrast and a grain size of about 5 µm.
4. Conclusions
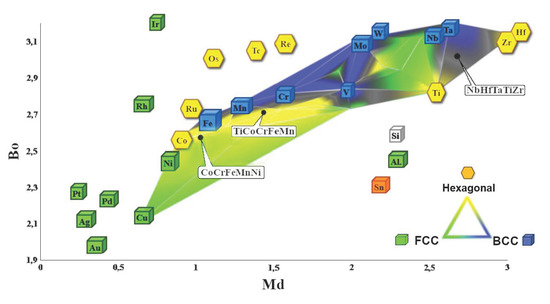
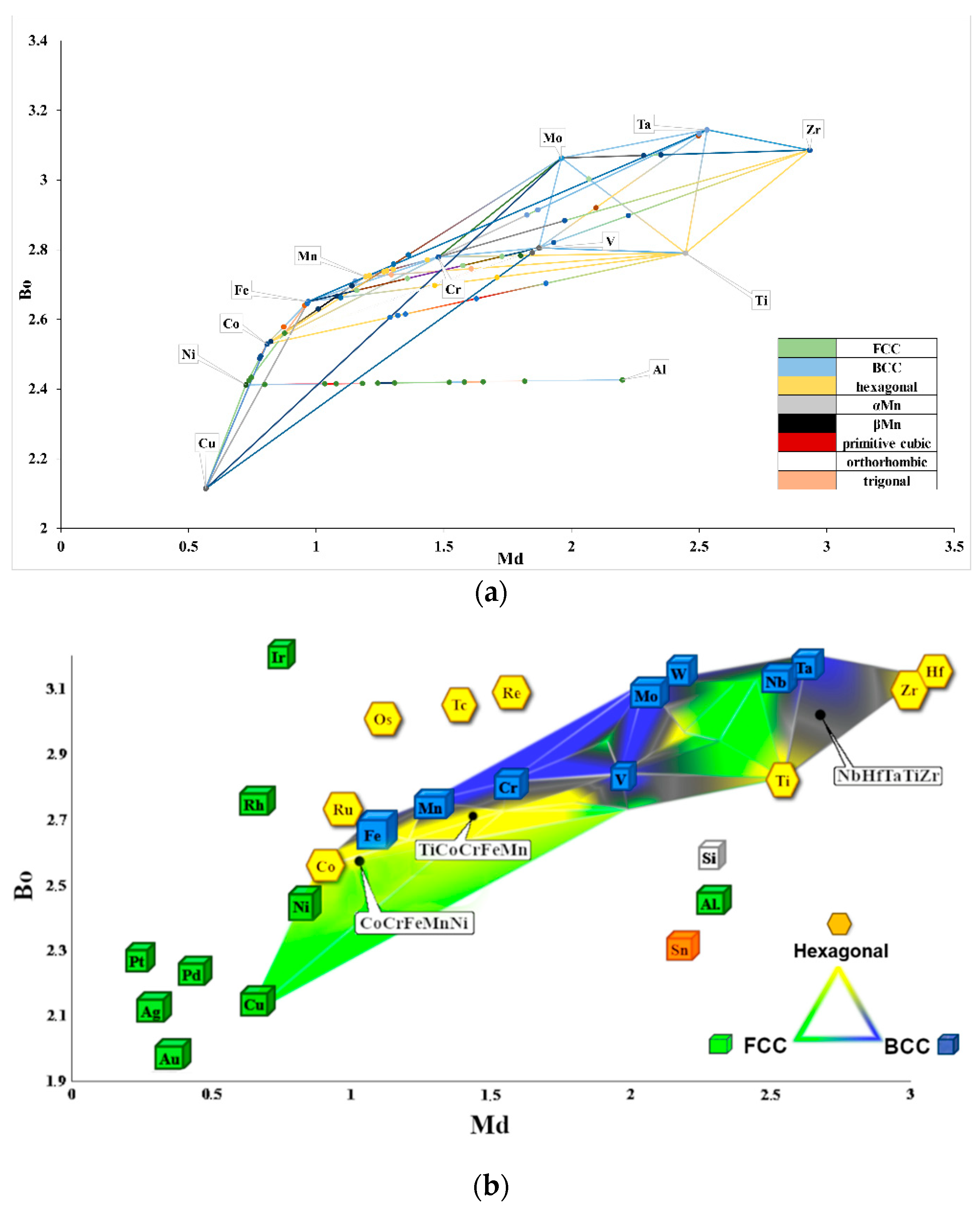
- The molecular orbitals and equilibrium systems-based methodology led to the successful development of a Bo–Md diagram that further enables us to predict the multiphase structure of high-entropy alloys at the stage of their design;
- The sintering process enabled us to form an ultra-fine-grained, two-phase TiCoCrFeMn alloy consisting of the BCC solid solution and the Laves C14 phase;
- The slow diffusion effect, typical for high-entropy alloys, results in a long incubation period of up to 10 h at 1000 °C, preceding the subsequent changes in the chemical composition;
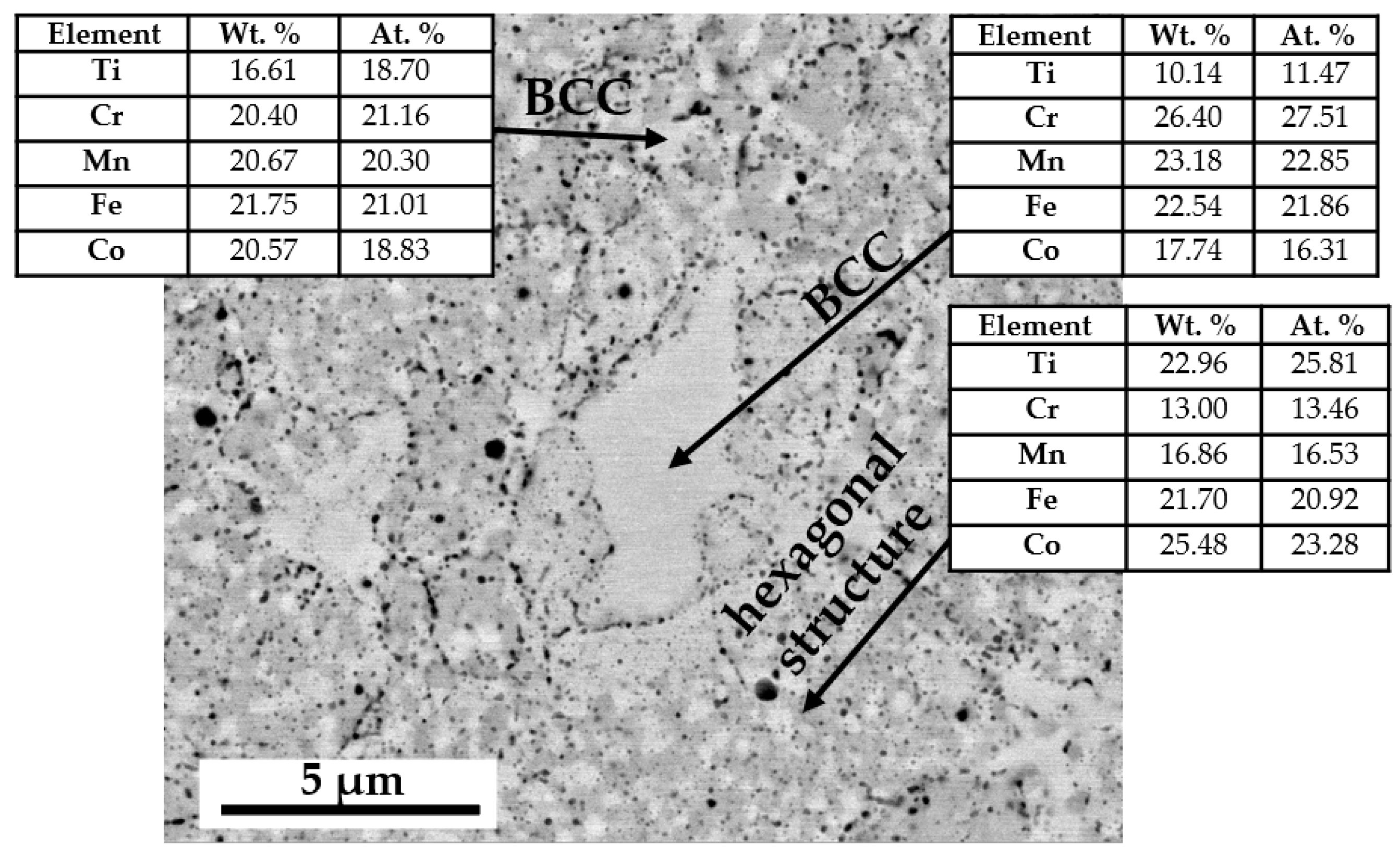
- The evolution of the chemical composition occurred during homogenization at 1000 °C for up to 1000 h, observed using the EDS method, and its impact on changes in the parameters of the crystal lattices of the identified phases are reflected in the Bo–Md diagram. One could confirm an appropriate sensitivity of the molecular orbitals model in the area of a multicomponent, multiphase alloys structural analysis.
- It was found that the description of the precipitation phenomena using the theory of molecular orbitals must consider the simultaneous changes in the parameters characterizing the forces of covalent bonding between the alloying elements used (Bo parameter) and the average energy level of the d-orbital (parameter Md), which must be determined for the dominant alloy element.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, R.X.; Zhang, Y. Entropic Alloys for Cryogenic Applications, Stainless Steels and Alloys; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Krishna, Y.V.; Jaiswal, U.K.; Rahul, M.R. Machine learning approach to predict new multiphase high entropy alloys. Scr. Mater. 2021, 197, 113804. [Google Scholar] [CrossRef]
- Kalali, D.G.; Antharam, S.; Hasan, M.; Karthik, P.S.; Phani, P.S.; Rao, K.B.S.; Rajulapati, K.V. On the origins of ultra-high hardness and strain gradient plasticity in multi-phase nanocrystalline MoNbTaTiW based refractory high-entropy alloy. Mater. Sci. Eng. A 2021, 812, 141098. [Google Scholar] [CrossRef]
- Lin, L.; Xian, X.; Liaw, P.K. A multi-phase CrMnFeCoNiAl0.75 high-entropy alloy with high strength at intermediate temperature. Intermetallics 2020, 120, 106744. [Google Scholar] [CrossRef]
- Shabani, A.; Toroghinejad, M.R. Evaluation of microstructure and texture formation during annealing of cold-rolled FeCrCuMnNi multiphase high-entropy alloy. Trans. Nonferrous Met. Soc. China 2020, 30, 449–462. [Google Scholar]
- Shabani, A.; Toroghinejad, M.R. Investigation of microstructure, texture, and mechanical properties of FeCrCuMnNi multiphase high entropy alloy during recrystallization. Mater. Charact. 2019, 154, 253–263. [Google Scholar] [CrossRef]
- Gwalani, B.; Choudhuri, D.; Banerjee, R. Interplay between single phase solid solution strengthening and multi-phase strengthening in the same high entropy alloy. Mater. Sci. Eng. A 2020, 771, 138620. [Google Scholar] [CrossRef]
- Cieslak, J.; Tobola, J.; Calvo-Dahlborg, M. Multi-phase nature of sintered vs. arc-melted CrxAlFeCoNi high entropy alloys—Experimental and theoretical study. J. Alloys Compd. 2019, 801, 511–519. [Google Scholar] [CrossRef]
- Manzoni, A.M.; Glatze, U. New multiphase compositionally complex alloys driven by the high entropy alloy approach. Mater. Charact. 2019, 147, 512–532. [Google Scholar] [CrossRef]
- Bała, P.; Górecki, K.; Bednarczyk, W.; Watroba, M.; Lecha, S.; Kawałko, J. Effect of high-temperature exposure on the microstructure and mechanical properties of the Al5Ti5Co35Ni35Fe20 high-entropy alloy. J. Mater. Res. Technol. 2020, 9, 551–559. [Google Scholar] [CrossRef]
- Rabadia, C.D.; Liu, Y.J.; Jawed, S.F.; Wang, L.; Li, Y.H.; Zhang, X.H.; Sercombe, T.B.; Sun, H.; Zhang, L.C. Improved deformation behavior in Ti-Zr-Fe-Mn alloys comprising the C14 type Laves and β phases. Mater. Des. 2018, 160, 1059–1070. [Google Scholar] [CrossRef]
- Koželj, P.; Vrtnik, S.; Jelen, A.; Jazbec, S.; Jagličić, Z.; Maiti, S.; Feuerbacher, M.; Steurer, W.; Dolinšek, J. Discovery of a Superconducting High-Entropy Alloy. Phys. Rev. Lett. 2014, 113, 107001. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cava, R.J. High-entropy alloy superconductors: Status, opportunities, and challenges. Phys. Rev. Mater. 2019, 3, 090301. [Google Scholar] [CrossRef] [Green Version]
- Ćwik, J. Magnetism and magnetocaloric effect in multicomponent Laves-phase compounds: Study and comparative analysis. J. Solid State Chem. 2014, 209, 13–22. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.S.; Yadav, T.P.; Srivastava, O.N.; Mukhopadhyay, N.K.; Biswas, K. Formation and stability of C14 type Laves phase in multi component high-entropy alloys. J. Alloys Compd. 2020, 832, 153764. [Google Scholar] [CrossRef]
- Akiba, E.; Iba, H. Hydrogen absorption by Laves phase related BCC solid solution. Intermetallics 1997, 6, 461–470. [Google Scholar] [CrossRef]
- Pickering, E.J.; Carruthers, A.W.; Barron, P.J.; Middleburgh, S.C.; Armstrong, D.E.J.; Gandy, A.S. High-Entropy Alloys for Advanced Nuclear Applications. Entropy 2021, 23, 98. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Chen, S.; Cao, W. Computational Thermodynamics Aided High-Entropy Alloy Design. JOM 2012, 64, 839–845. [Google Scholar] [CrossRef]
- Kozak, R.; Sologubenko, A.; Steurer, W. Single-phase high-entropy alloys—An overview. Z. Kristallogr. 2014, 230, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Kolli, R.P.; Devara, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Matsugi, K.; Endo, T.; Choi, Y.-B.; Sasaki, G. Alloy Design of Ti Alloys Using Ubiquitous Alloying Elements and Characteristics of Their Levitation-Melted Alloys. Mater. Trans. 2010, 51, 740–748. [Google Scholar] [CrossRef] [Green Version]
- Morinaga, M. Alloy Design Based on Molecular Orbital Method. Mater. Trans. 2016, 57, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Morinaga, M.; Yukawa, H. Alloy design with the aid of molecular orbital method. Bull. Mater. Sci. 1997, 20, 805–815. [Google Scholar] [CrossRef]
- Zhang, J. Mechanical Behavior and Microstructural Evolution in Metastable Beta TiMo Based Alloys with TRIP and TWIP Effects, Materials; Université Pierre et Marie Curie-Paris VI: Paris, France, 2014. [Google Scholar]
- Yukawa, N.; Morinaga, M. Alloy Phase Stability Index Diagram. European Patent EP0218312A1, 15 April 1987. [Google Scholar]
- Sadeghpour, S.; Abbasi, S.M.; Morakabati, M. Design of a New Multi-element Beta Titanium Alloy Based on d-Electron Methods. In Proceedings of the TMS Annual Meeting & Exhibition, Phoenix, AZ, USA, 11–15 March 2018; pp. 377–386. [Google Scholar]
- Bignon, M.; Bertrand, E.; Tancret, F.; Rivera-Díaz-Del-Castillo, P.E.J. Modelling martensitic transformation in titanium alloys: The influence of temperature and deformation. Materialia 2019, 7, 100382. [Google Scholar] [CrossRef]
- Oak, J.; Louzguine-Luzgin, D.V.; Inoue, A. Synthetic relationship between titanium and alloying elements in designing Ni-free Ti-based bulk metallic glass alloys. Appl. Phys. Lett. 2007, 91, 053106. [Google Scholar] [CrossRef]
- Lilensten, L.; Couzinié, J.P.; Bourgon, J.; Perrière, L.; Dirras, G.; Prima, F.; Guillot, I. Design and tensile properties of a bcc Ti-rich high-entropy alloy with transformation-induced plasticity. Mater. Res. Lett. 2016, 5, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Lee, K.; Hong, S.J.; Lee, J.K.; Han, J.; Kim, K.B.; Liaw, P.K.; Lee, C.; Song, G. Investigation of phase-transformation path in TiZrHf(VNbTa)x refractory high-entropy alloys and its effect on mechanical property. J. Alloys Compd. 2021, 886, 161187. [Google Scholar] [CrossRef]
- Eleti, R.R.; Klimova, M.; Tikhonovsky, M.; Stepanov, N.; Zherebtsov, S. Exceptionally high strain-hardening and ductility due to transformation induced plasticity efect in Ti-rich high-entropy alloys. Sci. Rep. 2020, 10, 13293. [Google Scholar] [CrossRef]
- Sheikh, S.; Klement, U.; Guo, S. Predicting the solid solubility limit in high-entropy alloys using the molecular orbital approach. J. Appl. Phys. 2015, 118, 194902. [Google Scholar] [CrossRef] [Green Version]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef] [Green Version]
- Akhlaghi, P.; Amirjan, M.; Parvin, N. The effect of processing parameters and heat-treatment on the microstructure and mechanical properties of PM CoCrFeMnNiTi0.1 high-entropy alloy. Mater. Chem. Phys. 2020, 257, 123722. [Google Scholar] [CrossRef]
- Qin, G.; Li, Z.; Chen, R.; Zheng, H.; Fan, C.; Wang, L.; Su, Y.; Ding, H.; Guo, J.; Fu, H. CoCrFeMnNi high-entropy alloys reinforced with Laves phase by adding Nb and Ti elements. J. Mater. Res. 2019, 34, 1011–1020. [Google Scholar] [CrossRef]
- Jain, R.; Rahul, M.R.; Samal, S.; Kumar, V.; Phanikumar, G. Hot workability of Co-Fe-Mn-Ni-Ti eutectic high entropy alloy. J. Alloys Compd. 2019, 822, 153609. [Google Scholar] [CrossRef]
- Liu, H.; Gao, W.; Liu, J.; Du, X.; Li, X.; Yang, H. Microstructure and Properties of CoCrFeNiTi High-Entropy Alloy Coating Fabricated by Laser Cladding. J. Mater. Eng. Perform. 2020, 29, 7170–7178. [Google Scholar] [CrossRef]
- Górecki, K. Wytwarzanie i Charakterystyka Nowych, Wysokoentropowych Stopów z Układu Al-Ti-Co-Ni-Fe. Ph.D. Thesis, Akademia Górniczo-Hutnicza im. S. Staszica w Krakowie, Kraków, Poland, 2018. [Google Scholar]
- Available online: https://genicore.eu/products/devices/genicore-u-fastsps-device/ (accessed on 6 August 2021).
- Carruthers, A.W.; Li, B.S.; Rigby, M.; Raquet, L.C.; Mythili, R.; Ghosh, C.; Dasgupta, A.; Armstrong, D.E.J.; Gandy, A.S.; Pickering, E.J. Novel reduced-activation TiVCrFe based high entropy alloys. J. Alloys Compd. 2021, 856, 157399. [Google Scholar] [CrossRef]
- Morinaga, M.; Yukawa, N.; Maya, T.; Sone, K.; Adachi, H. Theoretical design of titanium alloys. In Proceedings of the Sixth World Conference on Titanium, Cannes, France, 6–9 June 1988; pp. 1601–1606. [Google Scholar]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]


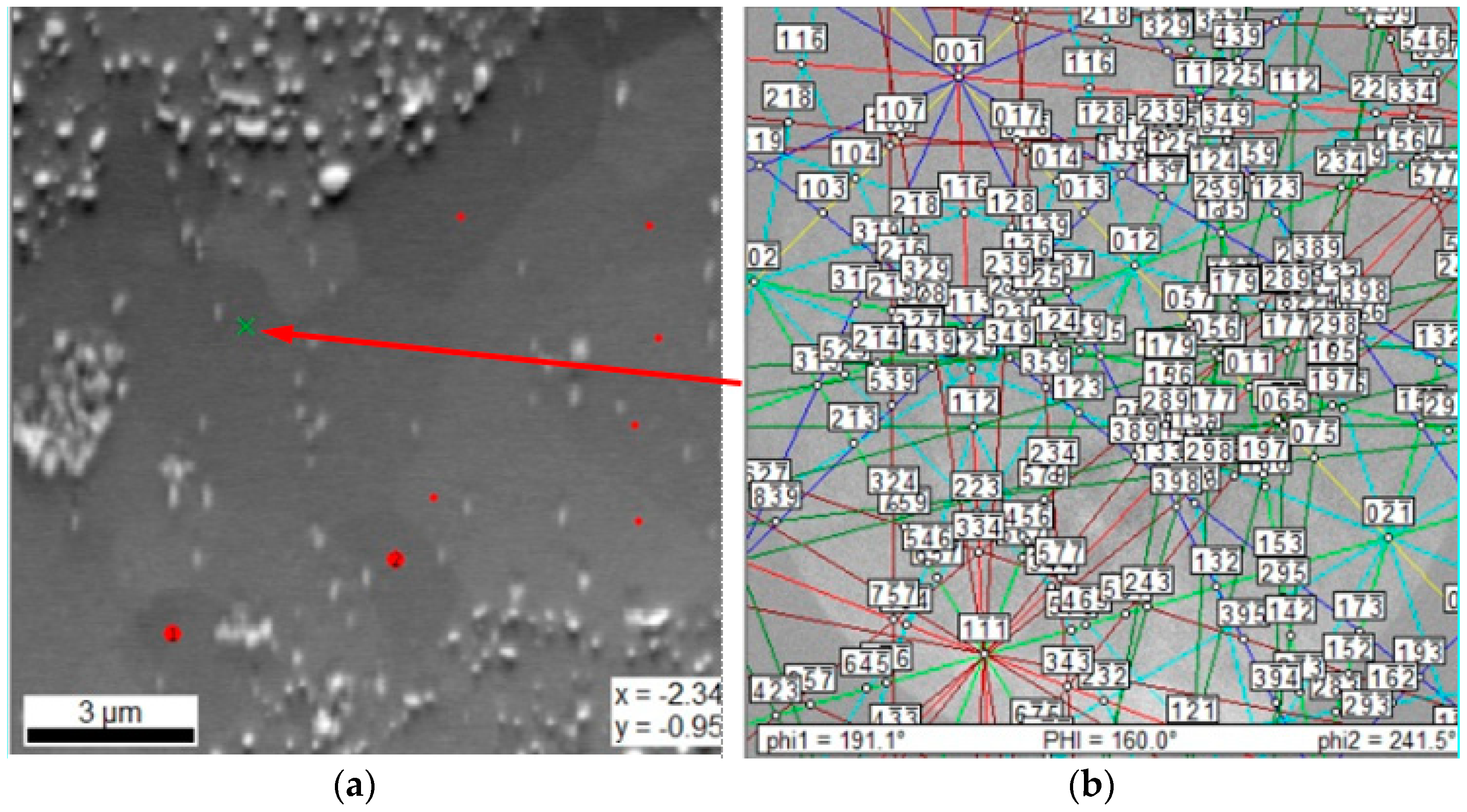
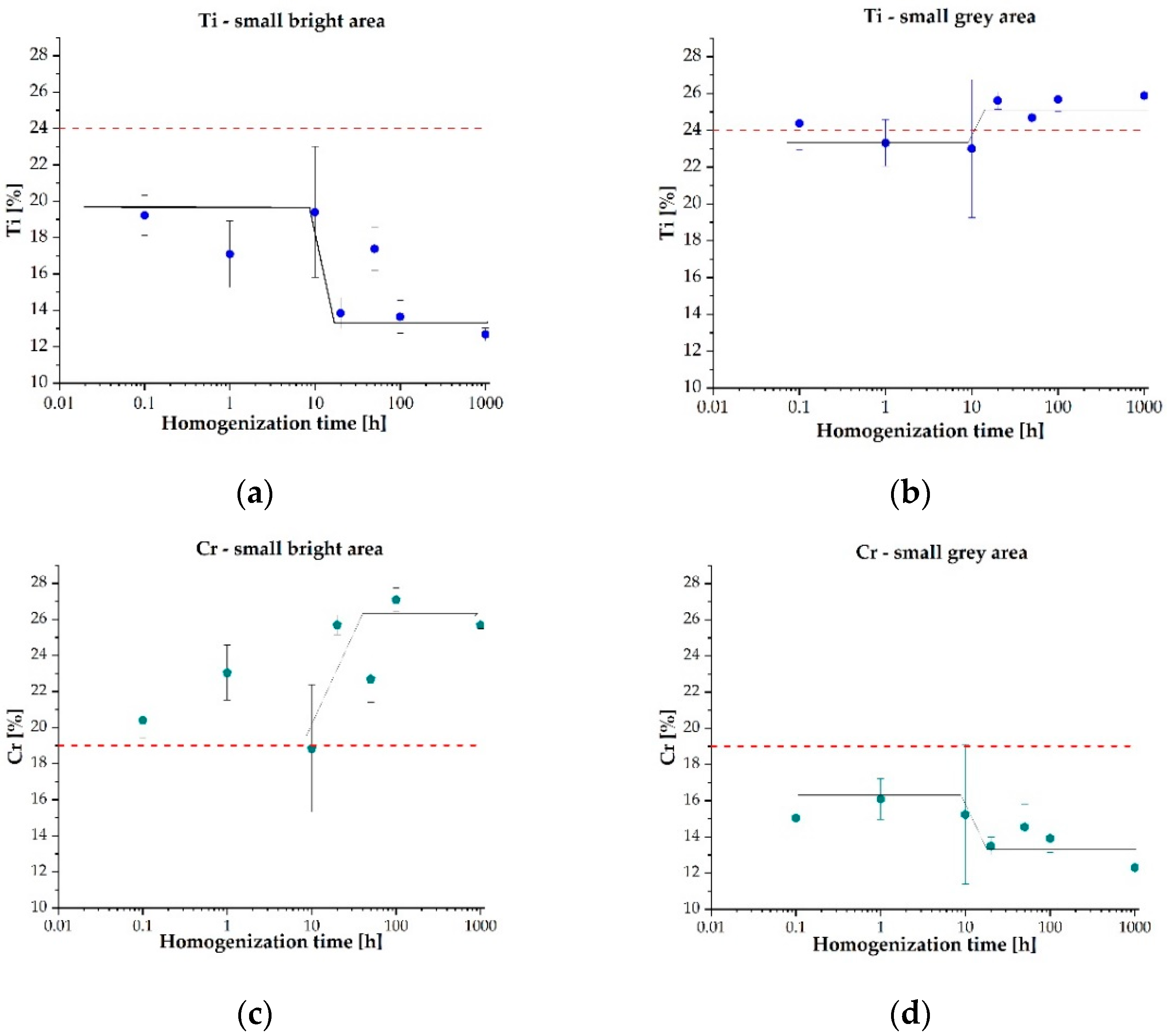
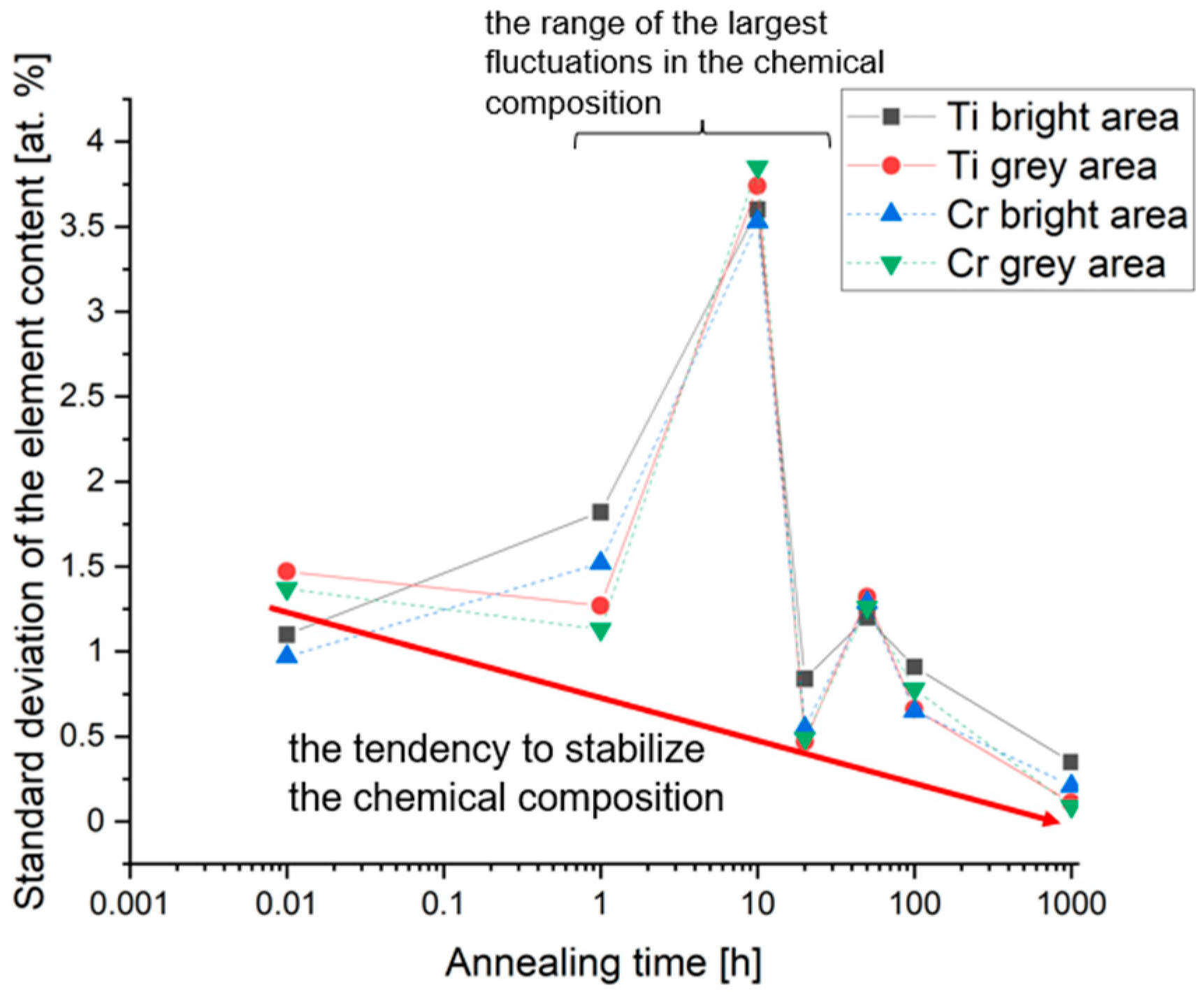
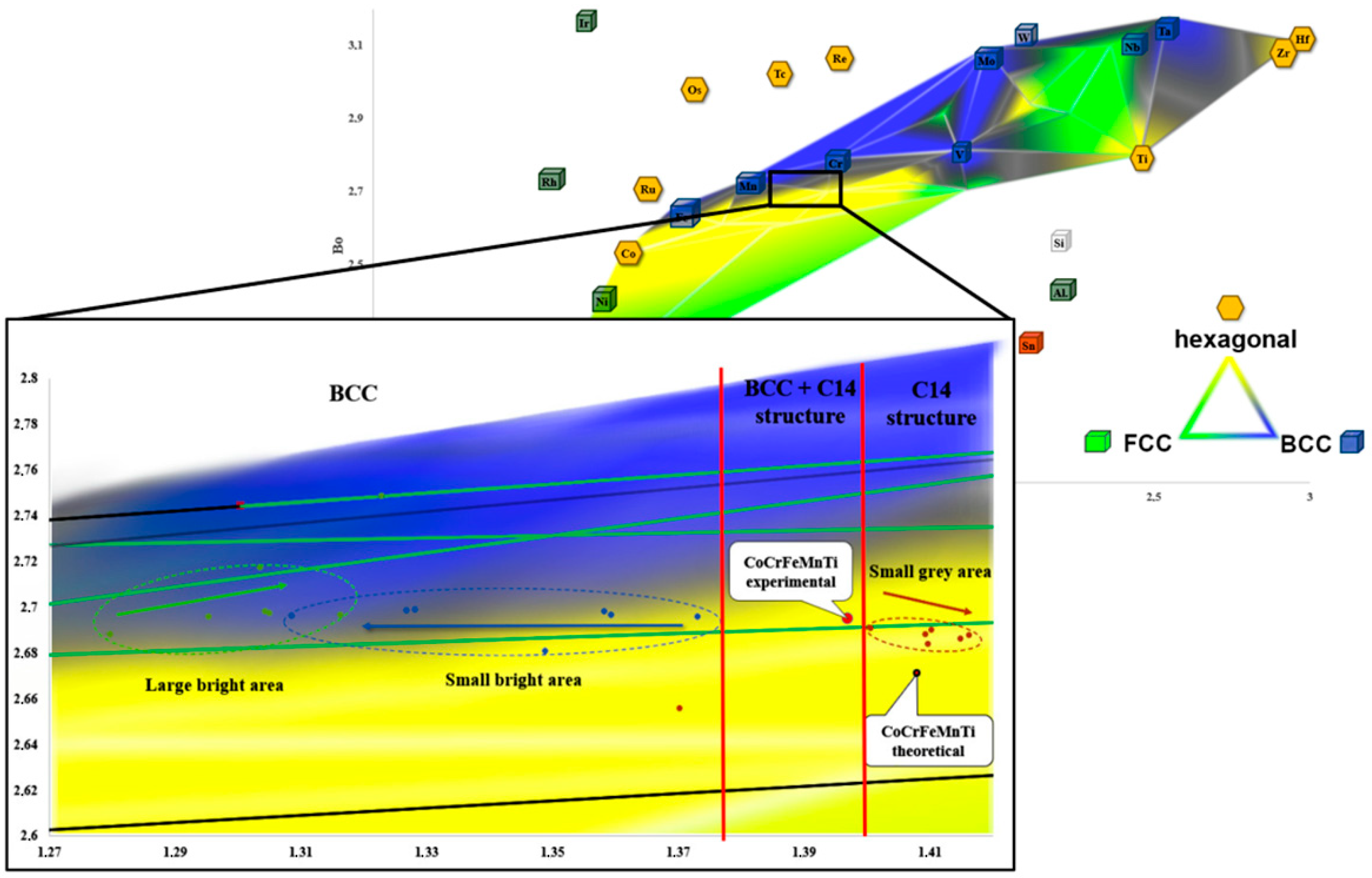
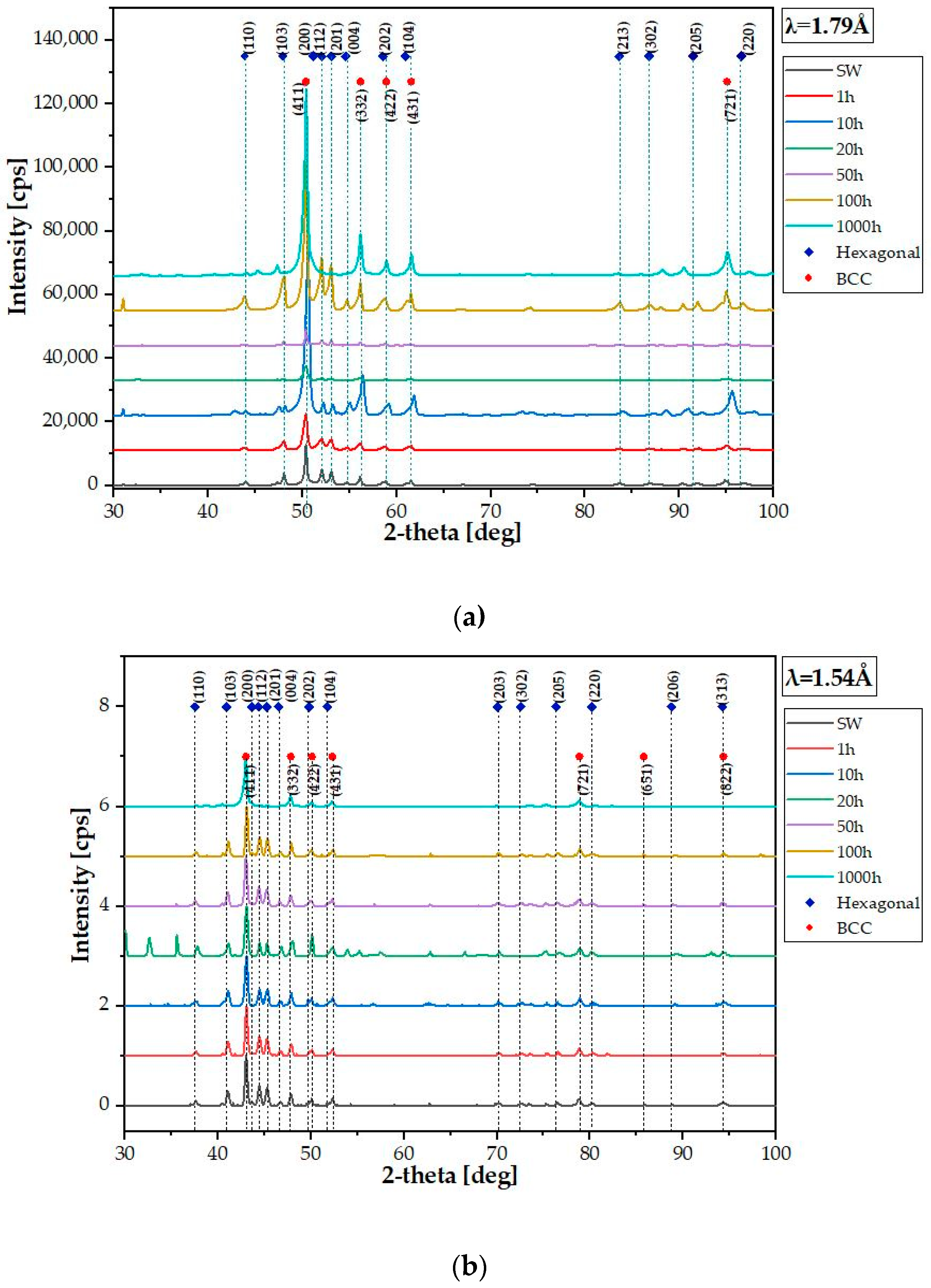
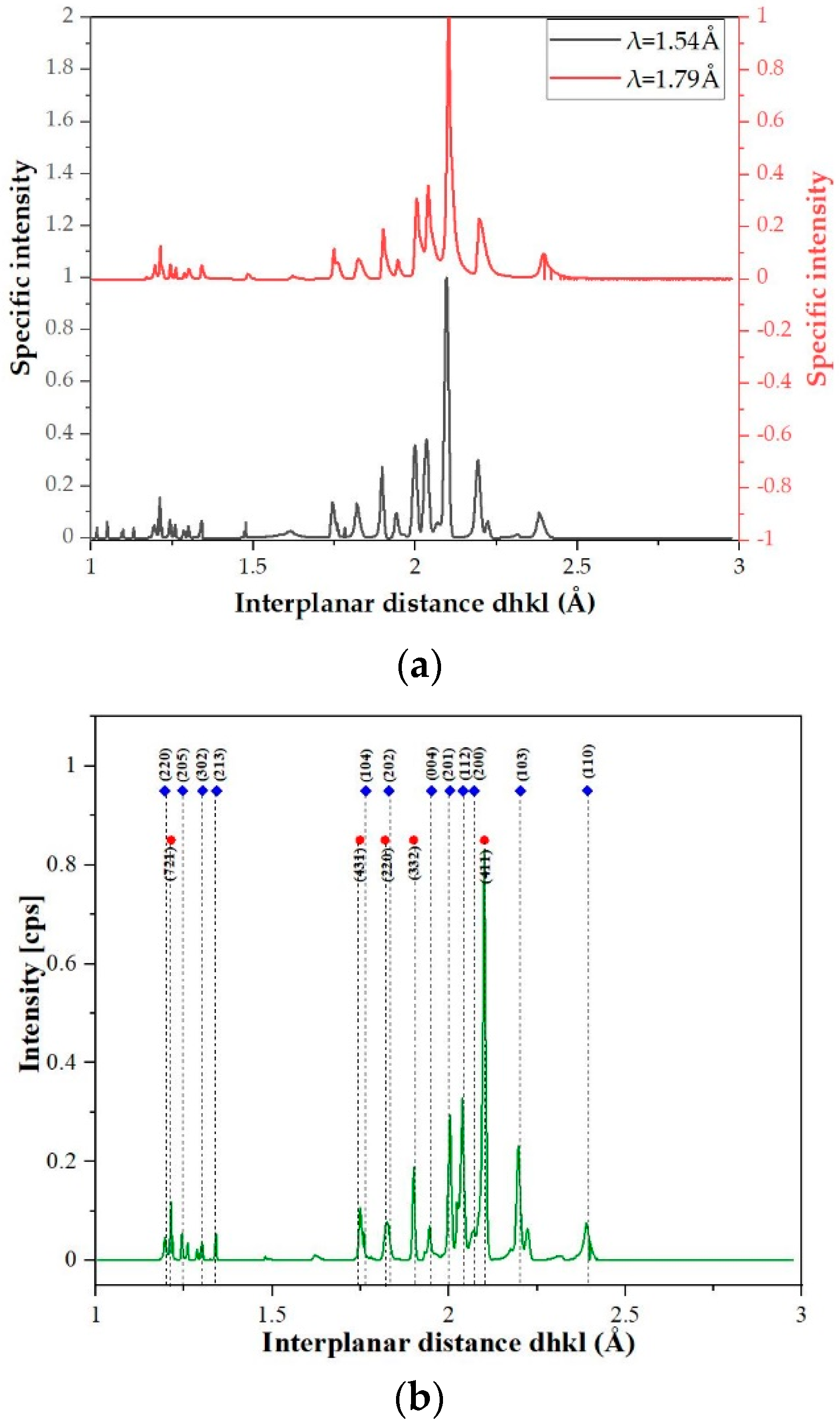
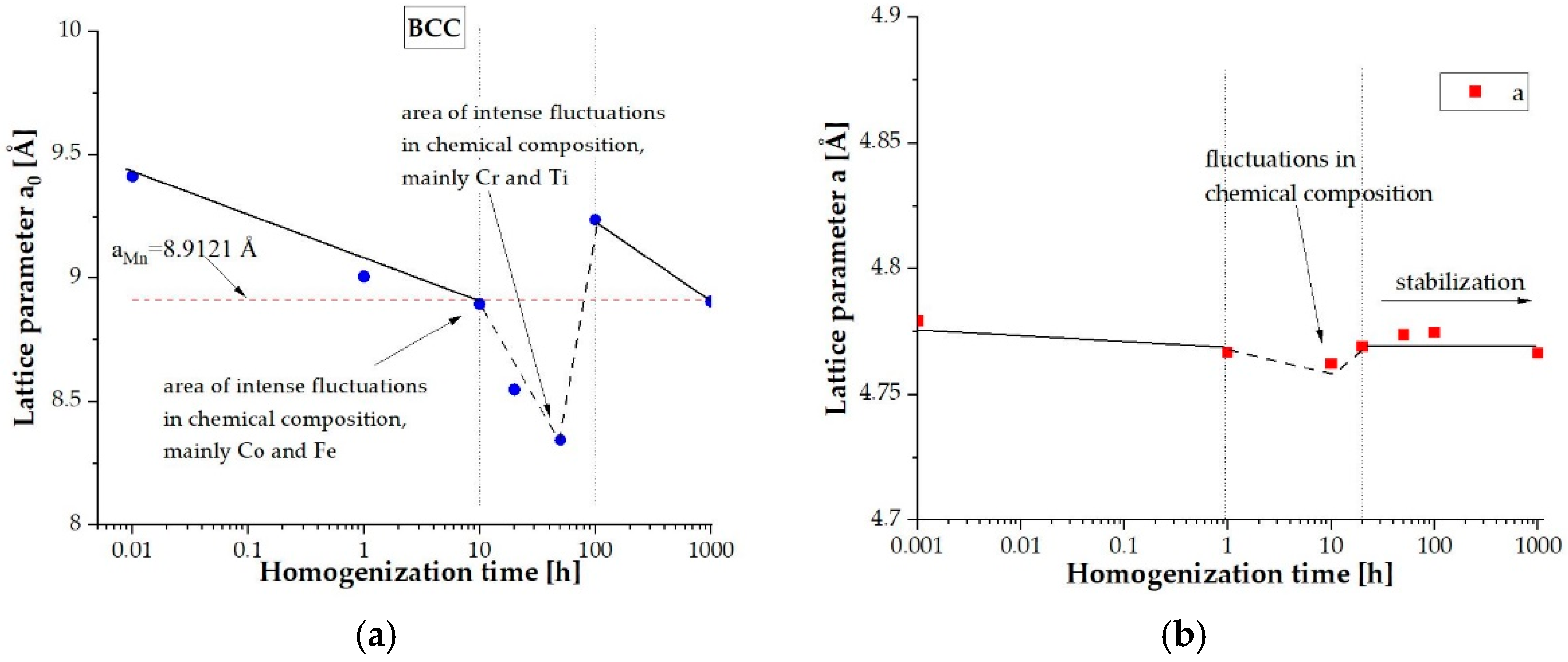
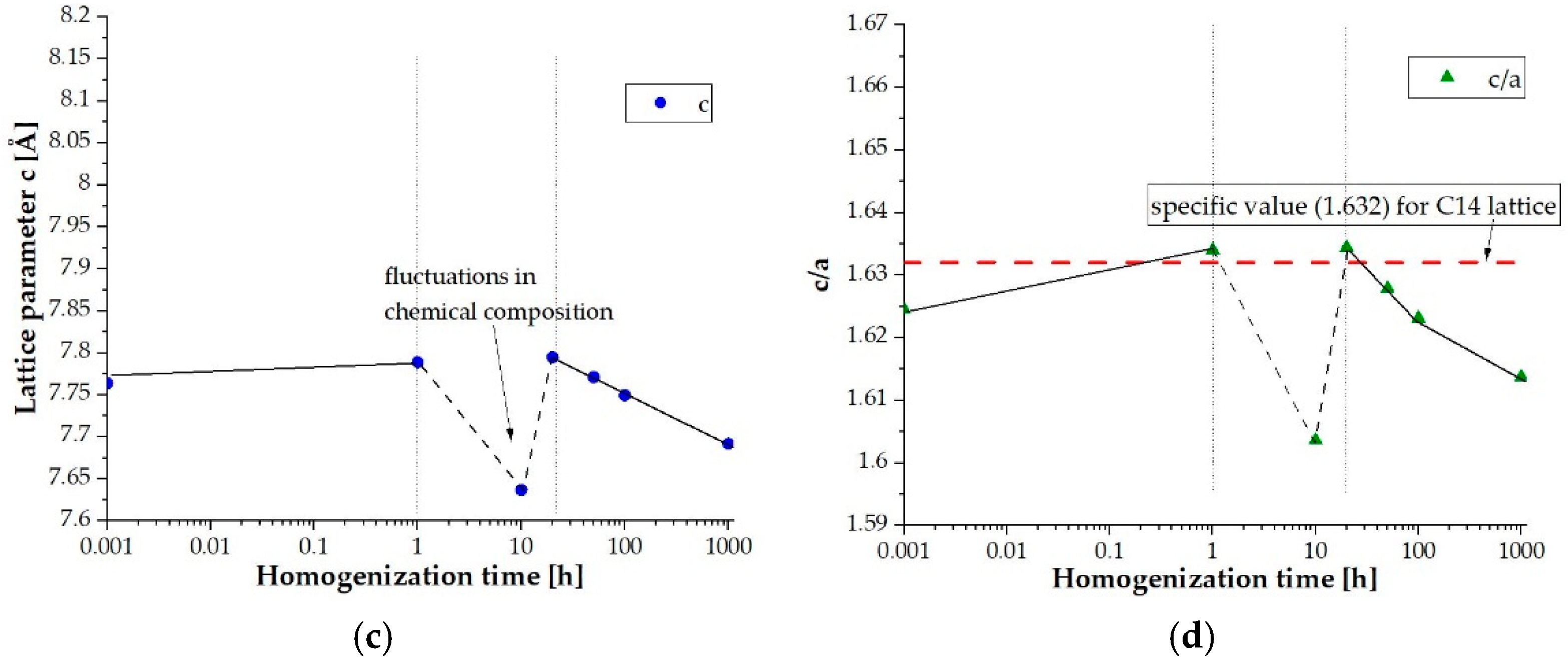
| Element | Ti | Co | Cr | Fe | Mn |
|---|---|---|---|---|---|
| At. % | 23.6 | 19.1 | 19.1 | 19.1 | 19.1 |
| Preliminary Sintering | 1 h | 10 h | 20 h | 50 h | 100 h | 1000 h | ||
|---|---|---|---|---|---|---|---|---|
| Ti | lb | 13.34 ± 0.92 | 11.81 ± 0.21 | 11.31 ± 0.20 | 11.96 ± 0.12 | 11.66 ± 0.10 | 11.85 ± 0.13 | 11.49 ± 0.12 |
| sb | 19.23 ± 1.10 | 17.09 ± 1.82 | 19.40 ± 3.60 | 13.85 ± 0.84 | 17.39 ± 1.20 | 13.65 ± 0.91 | 12.68 ± 0.35 | |
| sg | 24.38 ± 1.47 | 23.31 ± 1.27 | 23.00 ± 3.74 | 25.60 ± 0.47 | 24.68 ± 1.32 | 25.68 ± 0.66 | 25.87 ± 0.11 | |
| Co | lb | 16.35 ± 0.59 | 16.74 ± 2.92 | 21.49 ± 0.22 | 18.50 ± 4.01 | 16.50 ± 0.10 | 16.89 ± 0.06 | 17.27 ± 0.05 |
| sb | 18.43 ± 0.52 | 17.25 ± 0.74 | 25.13 ± 2.11 | 16.43 ± 0.25 | 17.93 ± 0.68 | 16.79 ± 0.24 | 17.16 ± 0.13 | |
| sg | 21.68 ± 0.82 | 21.34 ± 0.56 | 26.34 ± 3.83 | 22.84 ± 0.36 | 22.76 ± 0.62 | 23.57 ± 0.43 | 24.42 ± 0.07 | |
| Cr | lb | 25.20 ± 1.58 | 25.20 ± 2.36 | 25.83 ± 0.58 | 26.62 ± 0.17 | 27.06 ± 0.25 | 27.48 ± 0.13 | 26.29 ± 0.18 |
| sb | 20.41 ± 0.97 | 23.04 ± 1.52 | 18.84 ± 3.53 | 25.69 ± 0.55 | 22.69 ± 1.29 | 27.09 ± 0.65 | 25.68 ± 0.21 | |
| sg | 15.04 ± 1.37 | 16.09 ± 1.13 | 15.24 ± 3.85 | 13.50 ± 0.49 | 14.55 ± 1.26 | 13.92 ± 0.78 | 12.31 ± 0.09 | |
| Fe | lb | 23.46 ± 2.19 | 24.56 ± 2.87 | 20.53 ± 0.37 | 21.75 ± 0.14 | 21.67 ± 0.27 | 22.31 ± 0.07 | 22.51 ± 0.10 |
| sb | 21.60 ± 0.35 | 21.56 ± 0.19 | 19.18 ± 0.77 | 21.70 ± 0.15 | 21.06 ± 0.11 | 22.35 ± 0.14 | 22.62 ± 0.10 | |
| sg | 21.39 ± 0.50 | 21.67 ± 0.21 | 18.52 ± 0.50 | 21.56 ± 0.18 | 20.99 ± 0.17 | 21.90 ± 0.14 | 21.73 ± 0.08 | |
| Mn | lb | 21.62 ± 0.95 | 22.52 ± 0.62 | 20.83 ± 0.38 | 23.17 ± 0.12 | 23.11 ± 0.16 | 21.47 ± 0.10 | 22.45 ± 0.08 |
| sb | 20.33 ± 0.65 | 21.05 ± 0.88 | 17.34 ± 1.44 | 22.33 ± 0.31 | 20.91 ± 0.56 | 20.12 ± 0.33 | 21.86 ± 0.13 | |
| sg | 17.51 ± 0.50 | 17.60 ± 0.58 | 15.92 ± 1.47 | 16.50 ± 0.21 | 17.02 ± 0.66 | 14.93 ± 0.29 | 15.67 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorniewicz, D.; Przygucki, H.; Kopec, M.; Karczewski, K.; Jóźwiak, S. TiCoCrFeMn (BCC + C14) High-Entropy Alloy Multiphase Structure Analysis Based on the Theory of Molecular Orbitals. Materials 2021, 14, 5285. https://doi.org/10.3390/ma14185285
Gorniewicz D, Przygucki H, Kopec M, Karczewski K, Jóźwiak S. TiCoCrFeMn (BCC + C14) High-Entropy Alloy Multiphase Structure Analysis Based on the Theory of Molecular Orbitals. Materials. 2021; 14(18):5285. https://doi.org/10.3390/ma14185285
Chicago/Turabian StyleGorniewicz, Dominika, Hubert Przygucki, Mateusz Kopec, Krzysztof Karczewski, and Stanisław Jóźwiak. 2021. "TiCoCrFeMn (BCC + C14) High-Entropy Alloy Multiphase Structure Analysis Based on the Theory of Molecular Orbitals" Materials 14, no. 18: 5285. https://doi.org/10.3390/ma14185285
APA StyleGorniewicz, D., Przygucki, H., Kopec, M., Karczewski, K., & Jóźwiak, S. (2021). TiCoCrFeMn (BCC + C14) High-Entropy Alloy Multiphase Structure Analysis Based on the Theory of Molecular Orbitals. Materials, 14(18), 5285. https://doi.org/10.3390/ma14185285