Formation of Solid Solutions and Physicochemical Properties of the High-Entropy Ln1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ (Ln = La, Pr, Nd, Sm or Gd) Perovskites
Abstract
:1. Introduction
- long range antiferromagnetic (AFM) order is present despite the extreme ionic disorder and the high degree of dilution with non-magnetic ions;
- gradual magnetic transitions are spaced over a wide range of temperatures;
- short-range magnetic correlation is present well above Néel temperature (TN);
- Based on such characteristics it can be anticipated that the further development of perovskite-structured HEOx might result in obtaining of a number of potentially unique magnetic materials.
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Structure of Ln(Co,Cr,Fe,Mn,Ni)O3−δ
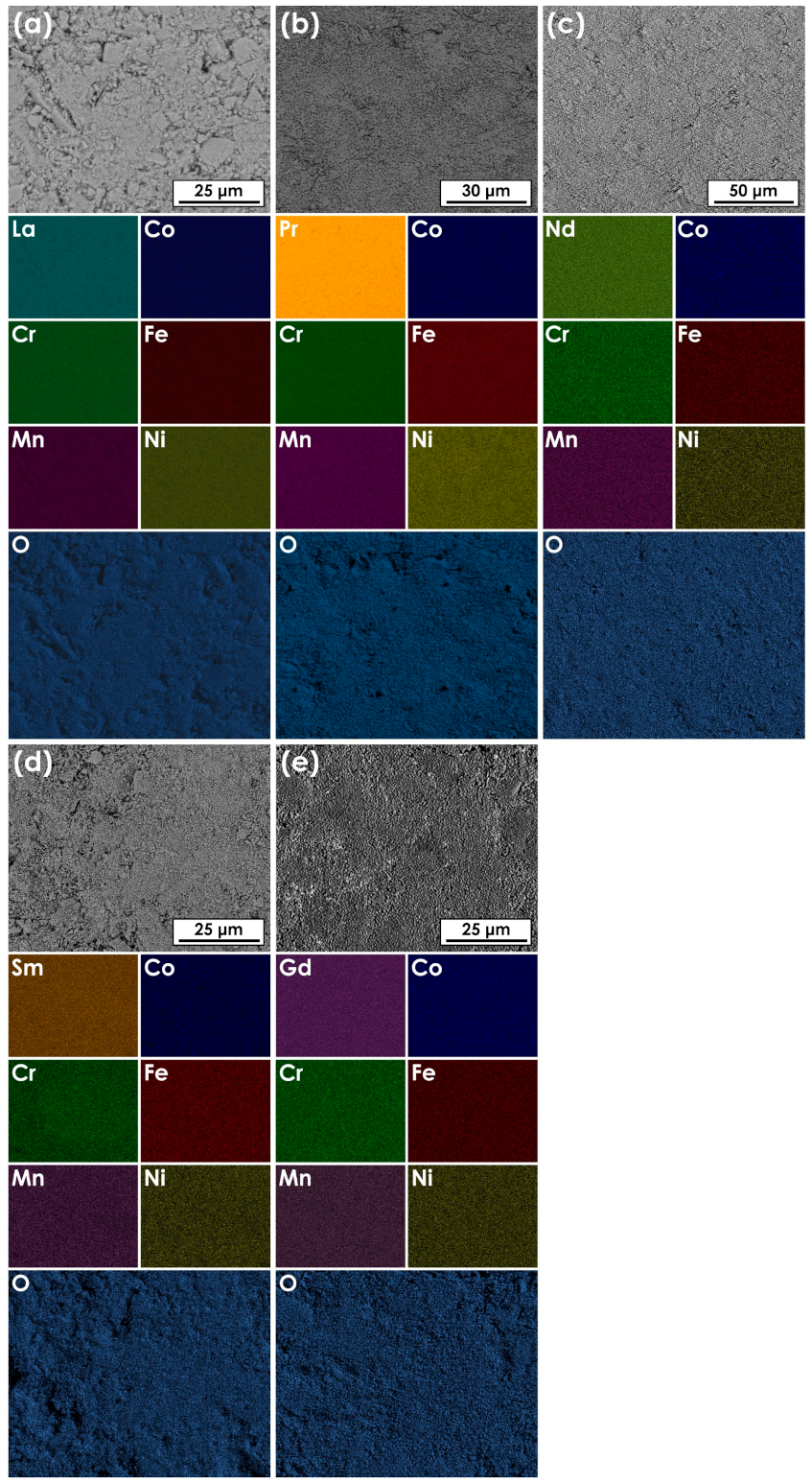
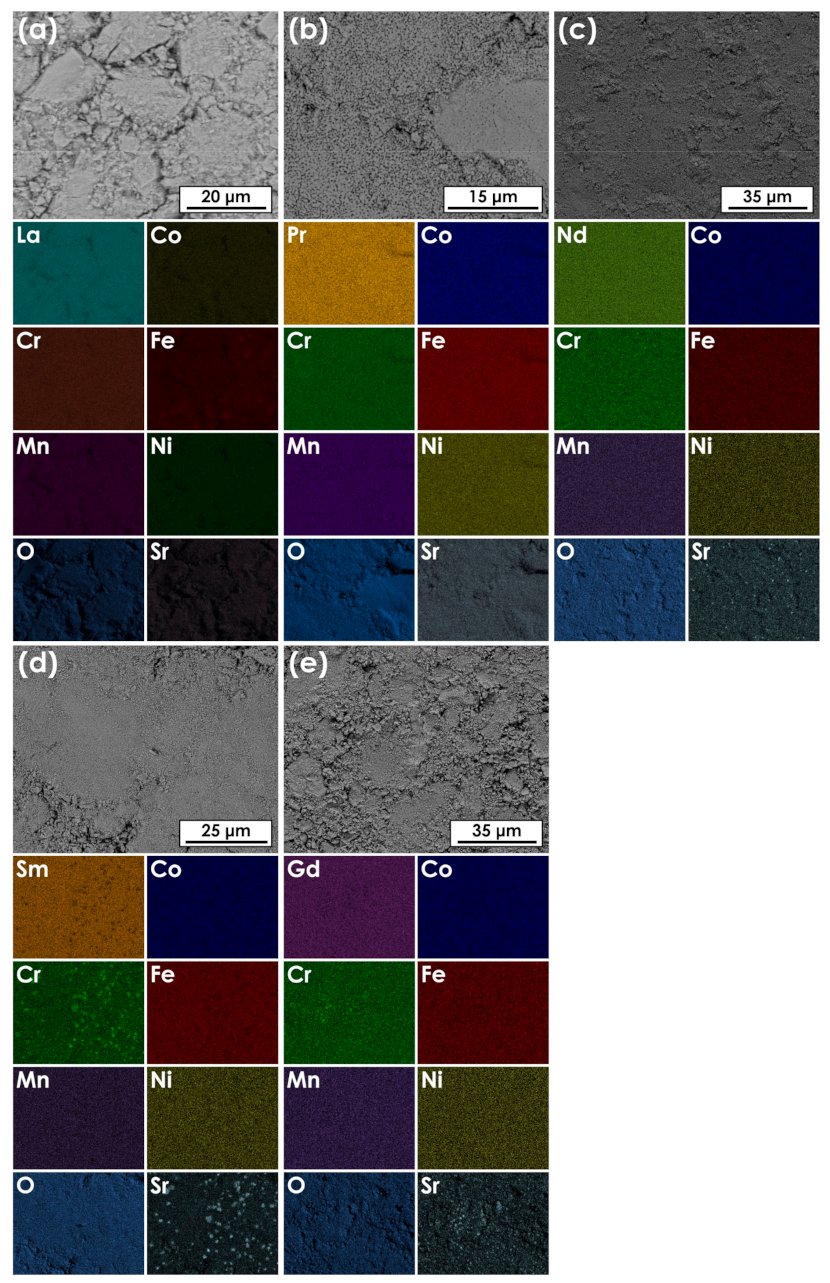
3.2. Scanning Electron Microscopy Plus Energy-Dispersive X-ray Spectroscopy (SEM+EDS) Analysis of the Ln(Co,Cr,Fe,Mn,Ni)O3−δ Series
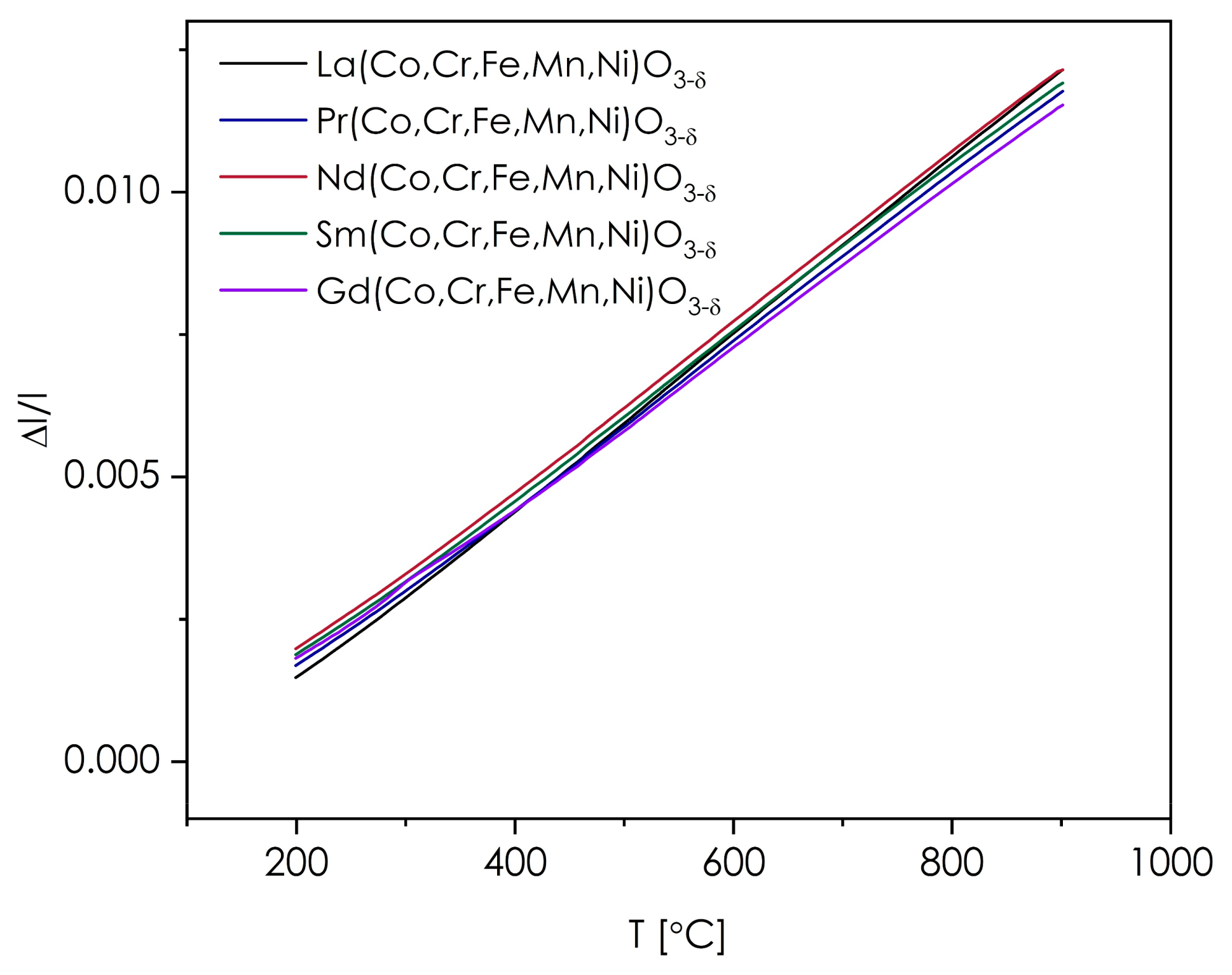
3.3. Thermomechanical Behavior of the Ln(Co,Cr,Fe,Mn,Ni)O3−δ Series
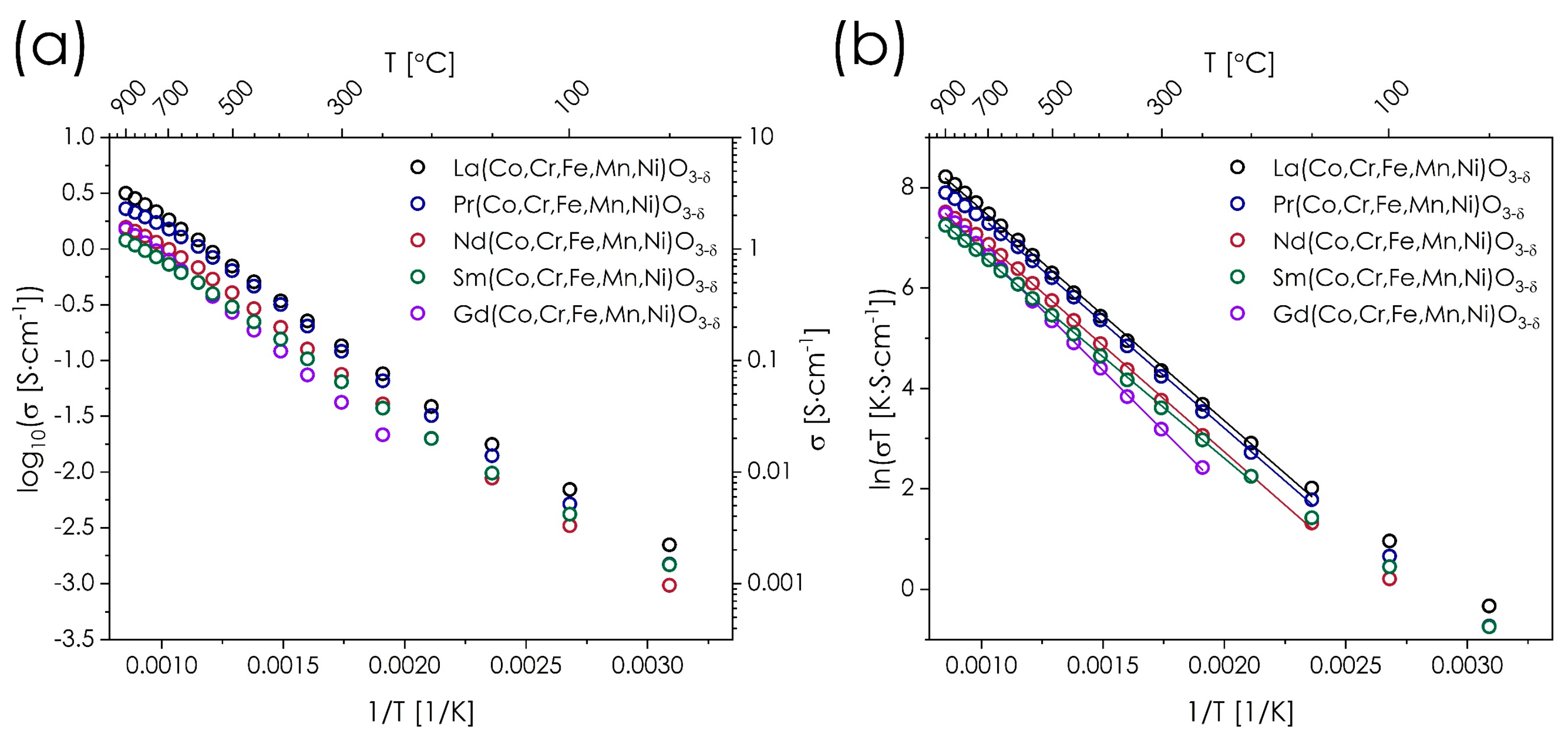
3.4. Electrical Conductivity of the Ln(Co,Cr,Fe,Mn,Ni)O3−δ Series
3.5. Strontium Solubility in Ln1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ Series
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rost, C.M.; Schaet, R.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy charcterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Mao, A.; Xiang, H.Z.; Zhang, Z.G.; Koji, K.; Zhang, H.; Jia, Y. A new class of spinel high-entropy oxides with controllable magnetic properties. J. Magn. Magn. Mater. 2020, 497, 165884. [Google Scholar] [CrossRef]
- Musicó, B.; Wright, Q.; Ward, T.Z.; Grutter, A.; Arenholz, E.; Gilbert, D.; Mandrus, D.; Keppens, V. Tunable magnetic ordering through cation selection in entropic spinel oxides. Phys. Rev. Mater. 2019, 3, 104416. [Google Scholar] [CrossRef]
- Stygar, M.; Dąbrowa, J.; Moździerz, M.; Zajusz, M.; Skubida, W.; Mroczka, K.; Berent, K.; Świerczek, K.; Danielewski, M. Formation and properties of high entropy oxides in Co-Cr-Fe-Mg-Mn-Ni-O system: Novel (Cr,Fe,Mg,Mn,Ni)3O4 and (Co,Cr,Fe,Mg,Mn)3O4 high entropy spinels. J. Eur. Ceram. Soc. 2019, 40, 1644–1650. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskite type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Braun, L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent equiatomic rare earth oxides. Mater. Res. Lett. 2016, 5, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowa, J.; Szymczak, M.; Zajusz, M.; Mikuła, A.; Moździerz, M.; Berent, K.; Wytrwal-Sarna, M.; Bernasik, A.; Stygar, M.; Świerczek, K. Stabilizing fluorite structure in ceria-based high-entropy oxides: Influence of Mo addition on crystal structure and transport properties. J. Eur. Ceram. Soc. 2020, 40, 5870–5881. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Dell’Agli, G. A simple and effective predictor to design novel fluorite-structured High Entropy Oxides (HEOs). Acta Mater. 2021, 202, 181–189. [Google Scholar] [CrossRef]
- Tseng, K.-P.; Yang, Q.; McCormack, S.J.; Kriven, W.M. High-entropy, phase-constrained, lanthanide sesquioxide. J. Am. Ceram. Soc. 2020, 103, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Zaitseva, O.V.; Gudkova, S.A.; Starikov, A.Y.; Zherebtosov, D.A.; Kirsanova, A.A.; Habner, M.; Niewa, R. High-entropy oxide phases with magnetoplumbite structure. Ceram. Int. 2019, 45, 12942–12948. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Peng, Z.; Zhou, Y. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: A novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate. J. Mater. Sci. Technol. 2019, 35, 2647–2651. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieślak, J.; Zajusz, M.; Moździerz, M.; Berent, K.; Mikuła, A.; Stępień, A.; Świerczek, K. Structure and transport properties of the novel (Dy,Er,Gd,Ho,Y)3Fe5O12 and (Dy,Gd,Ho,Sm,Y)3Fe5O12 high entropy garnets. J. Eur. Ceram. Soc. 2021, 41, 3844–3849. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Alonso, J.A.; Bian, J. Recent Advances in Perovskite-Type Oxides for Energy Conversion and Storage Applications. Adv. Energy Mater. 2021, 11, 2000459. [Google Scholar] [CrossRef]
- Kundu, A.K. Magnetic Perovskites; Springer: New Delhi, India, 2016. [Google Scholar]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Witte, R.; Sarkar, A.; Kruk, R.; Eggert, B.; Brand, R.A.; Wende, H.; Hahn, H. High-entropy oxides: An emerging prospect for magnetic rare-earth transition metal perovskites. Phys. Rev. Mater. 2019, 3, 034406. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Kruk, R.; Hahn, H. Magnetic properties of high entropy oxides. Dalton Trans. 2021, 50, 1973–1982. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Olszewska, A.; Falkenstein, A.; Schwab, C.; Szymczak, M.; Zajusz, M.; Moździerz, M.; Mikuła, A.; Zielińska, K.; Berent, K.; et al. An innovative approach to design SOFC air electrode materials: High entropy La1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ (x = 0, 0.1, 0.2, 0.3) perovskites synthesized by the sol–gel method. J. Mater. Chem. A 2020, 8, 24455–24468. [Google Scholar] [CrossRef]
- Fujimori, A. Electronic structure of metallic oxides: Band-gap closure and valence control. J. Phys. Chem. Solids 1992, 53, 1595–1602. [Google Scholar] [CrossRef]
- Jiang, S.P.; Chen, X. Chromium deposition and poisoning of cathodes of solid oxide fuel cells—A review. Int. J. Hydrog. Energy 2014, 39, 505–531. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Liao, Y.-C.; Lin, C.-C.; Su, Y.-H.; Ting, J.-M. Advanced High Entropy Perovskite Oxide Electrocatalyst for Oxygen Evolution Reaction. Adv. Func. Mater. 2021, 31, 2101632. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Basi, L.; Kubel, C.; Brezesisnki, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Du, Z.; Zhang, Y.; Dong, X.; Zhao, H. Medium-Entropy perovskites Sr(FeαTiβCoγMnζ)O3−δ as promising cathodes for intermediate temperature solid oxide fuel cell. Appl. Catal. B Environ. 2021, 295, 120264. [Google Scholar] [CrossRef]
- Yang, Y.; Bao, H.; Ni, H.; Ou, X.; Wang, S.; Lin, B.; Feng, P.; Ling, Y. A novel facile strategy to suppress Sr segregation for high-entropy stabilized La0.8Sr0.2MnO3−δ cathode. J. Power Sources 2021, 482, 228959. [Google Scholar] [CrossRef]
- Nikonov, A.V.; Kuterbekov, K.A.; Bekmyrza, K.Z.; Pavzderin, N.B. A brief review of conductivity and thermal expansion of perovskite-related oxides for SOFC cathode. Eurasian J. Phys. Funct. Mater. 2018, 2, 274–292. [Google Scholar]
- Koo, B.; Kim, K.; Kim, J.K.; Kwon, H.; Han, J.W.; Jung, W. Sr Segregation in Perovskite Oxides: Why It Happens and How It Exists. Joule 2018, 2, 1476–1499. [Google Scholar] [CrossRef] [Green Version]
- Dimesso, L. Pechini Processes: An Alternate Approach of the Sol–Gel Method, Preparation, Properties, and Applications. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitanu, A., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [Green Version]
- Mitchel, R.H. Perovskites Modern and Ancient; Almaz Press Inc.: Thunder Bay, ON, Canada, 2002. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Olszewska, A.; Świerczek, K.; Skubida, W.; Du, Z.; Zhao, H.; Dabrowski, B. Versatile Application of Redox Processes for REBaCoMnO5+δ (RE: La, Pr, Nd, Sm, Gd, and Y) Oxides. J. Phys. Chem. C 2019, 123, 48–61. [Google Scholar] [CrossRef]
- Fossdal, A.; Menon, M.; Wærnhus, I.; Wiik, K.; Einarsrud, M.-A.; Grande, T. Crystal Structure and Thermal Expansion of La1−xSrxFeO3−δ Materials. J. Am. Ceram. Soc. 2004, 87, 1952–1958. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical properties of La1−xSrxCo1−yFeyO3. Part 1. The system La0.8Sr0.2Co1−yFeyO3. Solid State Ion. 1995, 76, 259–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; He, T. La0.7Ca0.3CrO3–Ce0.8Gd0.2O1.9 composites as symmetrical electrodes for solid-oxide fuel cells. J. Power Sources 2011, 196, 76–83. [Google Scholar] [CrossRef]
- Stevenson, J.W.; Hasinska, K.; Canfield, N.L.; Armstrong, T.R. Influence of Cobalt and Iron Additions on the Electrical and Thermal Properties of (La,Sr)(Ga,Mg)O3−d. J. Electrochem. Soc. 2000, 147, 3213–3218. [Google Scholar] [CrossRef]
- Raffaelle, R.; Anderson, H.U.; Sparlin, D.M.; Parris, P.E. Transport anomalies in the high-temperature hopping conductivity and thermopower of Sr-doped La(Cr,Mn)O3. Phys. Rev. B 1991, 43, 7991–7999. [Google Scholar] [CrossRef]
- Druding, L.F.; Corbett, J.D. Lower Oxidation States of the Lanthanides. Neodymium(II) Chloride and Iodide1. J. Am. Chem. Soc. 1961, 83, 2462–2467. [Google Scholar] [CrossRef]
- Stefanik, T.S. Electrical Properties and Defect Structures of Praseodymium-Cerium Oxide Solid Solutions. Ph.D Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2003. [Google Scholar]
- Świerczek, K.; Dabrowski, B.; Suescun, L.; Kolesnik, S. Crystal structure and magnetic properties of high-oxygen pressure annealed Sr1−xLaxCo0.5Fe0.5O3−δ (0 ≤ x ≤ 0.5). J. Solid State Chem. 2009, 182, 280–288. [Google Scholar] [CrossRef]
- Dabrowski, B.; Chmaissem, O.; Mais, J.; Kolesnik, S.; Jorgensen, J.D.; Short, S. Tolerance factor rules for Sr1−x−yCaxBayMnO3 perovskites. J. Solid State Chem. 2003, 170, 154–164. [Google Scholar] [CrossRef]
- Han, X.; Yang, Y.; Fan, Y.; Ni, H.; Guo, Y.; Chen, Y.; Ou, X.; Ling, Y. New approach to enhance Sr-free cathode performance by high-entropy multi-component transition metal coupling. Ceram. Int. 2021, 47, 17383–17390. [Google Scholar] [CrossRef]
- Mizusaki, J.; Sasamoto, T.; Cannon, W.R.; Bowen, H.K. Electronic Conductivity, Seebeck Coefficient, and Defect Structure of LaFeO3. J. Am. Ceram. Soc. 1982, 65, 363–368. [Google Scholar] [CrossRef]
- Teraoka, Y.; Zhang, H.M.; Okamoto, K.; Yamazoe, N. Mixed ionic-electronic conductivity of La1−xSrxCo1−yFeyO3−δ perovskite-type oxides. Mater. Res. Bull. 1988, 23, 51–58. [Google Scholar] [CrossRef]
- Kirkpatrick, S. Percolation and Conduction. Rev. Mod. Phys. 1973, 45, 574–588. [Google Scholar] [CrossRef]
- Fuks, D.; Bakaleinikov, L.; Kotomin, E.A.; Felsteiner, J.; Gordon, A.; Evarestov, R.A.; Gryaznov, D.; Maier, J. Thermodynamic stability and disordering in LacSr1−cMnO3 solid solutions. Solid State Ion. 2006, 177, 217–222. [Google Scholar] [CrossRef]
- Weizman, A.; Fuks, D.; Kotomin, E.A.; Gryaznov, D. Ab initio study of phase competition in (La1−c,Src)CoO3 solid solutions. Solid State Ion. 2013, 230, 32–36. [Google Scholar] [CrossRef]
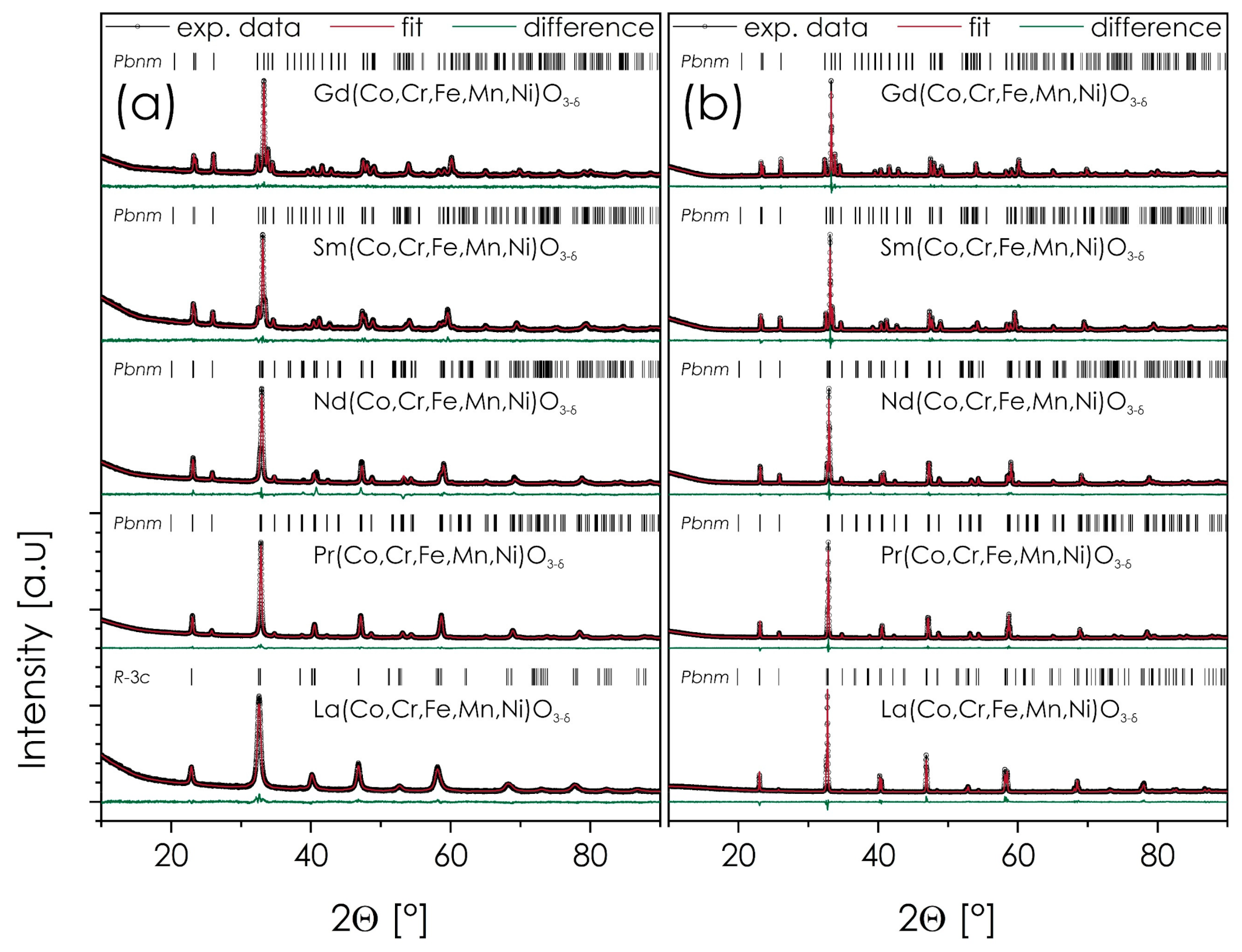
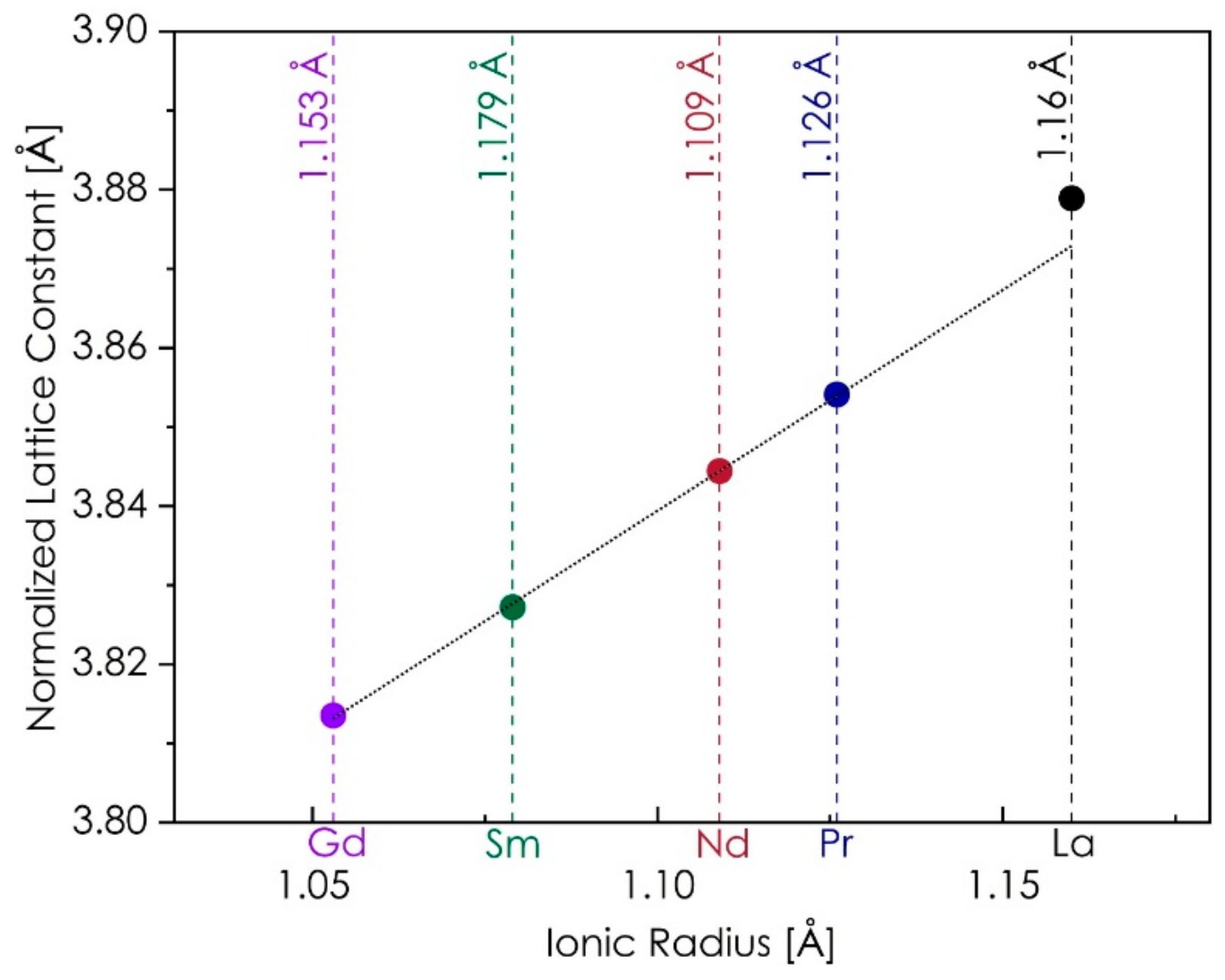
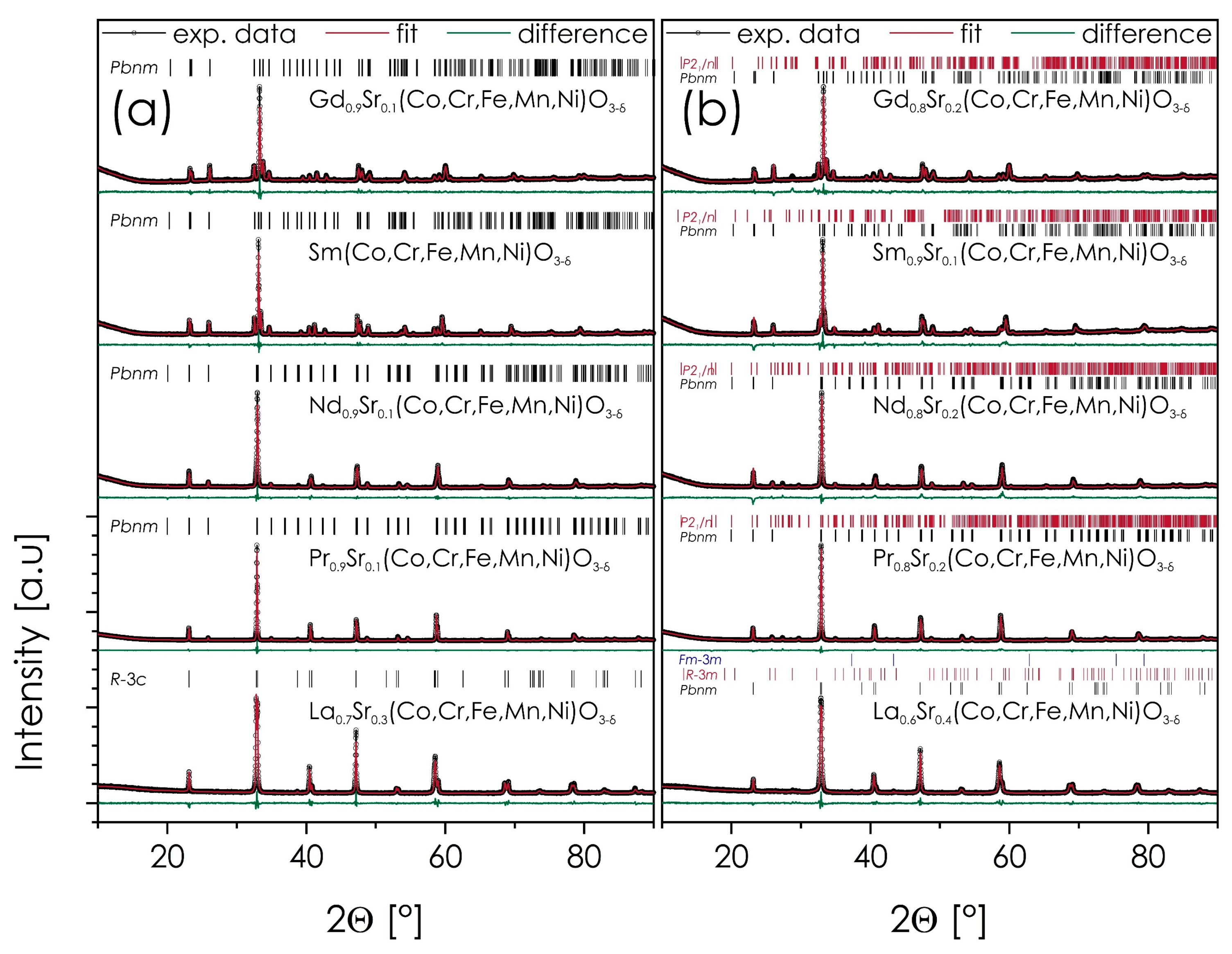

| As-Calcined Powders (Calcined at 700 °C for 6 h, Cooled Down with the Furnace) | |||||||||||
| Phase | ρtheo (g/cm3) | a (Å) | b (Å) | c (Å) | V (Å3) | a0 (Å) | Rwp (%) | Gof | |||
| La(Co,Cr,Fe,Mn,Ni)O3−δ | R-3c | 6.91 | 5.5086(2) | - | 13.371(1) | 58.563(6) | 3.8834(1) | 2.82 | 1.05 | - | - |
| Pr(Co,Cr,Fe,Mn,Ni)O3−δ | Pbnm | 7.10 | 7.6965(3) | 5.4709(2) | 5.4411(2) | 57.277(4) | 3.8547(1) | 3.18 | 3.25 | 1.0053 | 0.9998 |
| Nd(Co,Cr,Fe,Mn,Ni)O3−δ | Pbnm | 7.26 | 7.6703(4) | 5.4742(3) | 5.4090(3) | 56.779(6) | 3.8435(1) | 5.01 | 3.52 | 1.0093 | 0.9973 |
| Sm(Co,Cr,Fe,Mn,Ni)O3−δ | Pbnm | 7.53 | 7.6169(4) | 5.5062(3) | 5.3525(3) | 56.121(5) | 3.8266(1) | 2.69 | 1.33 | 1.0223 | 0.9938 |
| Gd(Co,Cr,Fe,Mn,Ni)O3−δ | Pbnm | 7.81 | 7.5724(3) | 5.5370(3) | 5.2068(3) | 55.522(4) | 3.8149(1) | 2.13 | 1.25 | 1.0341 | 0.9724 |
| Sinters (sintered at 1000 °C for 20 h, cooled down by quenching) | |||||||||||
| Phase | ρtheo (g/cm3) | a (Å) | b (Å) | c (Å) | V (Å3) | a0 (Å) | Rwp (%) | Gof | |||
| La(Co,Cr,Fe,Mn,Ni)O3−δ | Pbnm | 6.92 | 7.7472(2) | 5.5116(1) | 5.4671(1) | 58.360(2) | 3.8789(1) | 7.17 | 2.01 | 1.0061 | 0.9980 |
| Pr(Co,Cr,Fe,Mn,Ni)O3−δ | Pbnm | 7.10 | 7.6976(1) | 5.4673(1) | 5.4413(1) | 57.250(1) | 3.8541(1) | 3.77 | 5.52 | 1.0045 | 0.9997 |
| Nd(Co,Cr,Fe,Mn,Ni)O3−δ | Pbnm | 7.26 | 7.6728(1) | 5.4751(1) | 5.4099(1) | 56.817(1) | 3.8444(1) | 4.06 | 2.60 | 1.0091 | 0.9971 |
| Sm(Co,Cr,Fe,Mn,Ni) O3−δ | Pbnm | 7.54 | 7.6178(1) | 5.4987(1) | 5.3532(1) | 56.059(1) | 3.8272(1) | 3.05 | 1.96 | 1.0208 | 0.9938 |
| Gd(Co,Cr,Fe,Mn,Ni) O3−δ | Pbnm | 7.82 | 7.5726(1) | 5.5293(1) | 5.2980(1) | 55.458(1) | 3.8135(1) | 2.53 | 1.89 | 1.0326 | 0.9894 |
| Average Composition (at.%) | |||||||
| Ln | Co | Cr | Fe | Mn | Ni | O | |
| La(Co,Cr,Fe,Mn,Ni)O3−δ | 22.9 | 4.6 | 6.0 | 4.8 | 4.3 | 4.4 | 53 |
| Pr(Co,Cr,Fe,Mn,Ni)O3−δ | 24.1 | 4.6 | 4.5 | 4.7 | 4.9 | 4.8 | 53 |
| Nd(Co,Cr,Fe,Mn,Ni)O3−δ | 25.6 | 4.6 | 5.1 | 5.4 | 5.2 | 4.7 | 49 |
| Sm(Co,Cr,Fe,Mn,Ni)O3−δ | 25.8 | 5.1 | 5.0 | 5.6 | 5.2 | 4.4 | 49 |
| Gd(Co,Cr,Fe,Mn,Ni)O3−δ | 23.5 | 5.7 | 4.8 | 5.1 | 5.4 | 5.0 | 51 |
| Cations’ ratios | |||||||
| Ln | Co | Cr | Fe | Mn | Ni | O | |
| La(Co,Cr,Fe,Mn,Ni)O3−δ | - | 0.191 | 0.249 | 0.199 | 0.178 | 0.182 | - |
| Pr(Co,Cr,Fe,Mn,Ni)O3−δ | - | 0.196 | 0.191 | 0.200 | 0.209 | 0.204 | - |
| Nd(Co,Cr,Fe,Mn,Ni)O3−δ | - | 0.184 | 0.204 | 0.216 | 0.208 | 0.188 | - |
| Sm(Co,Cr,Fe,Mn,Ni)O3−δ | - | 0.202 | 0.198 | 0.221 | 0.206 | 0.174 | - |
| Gd(Co,Cr,Fe,Mn,Ni)O3−δ | - | 0.219 | 0.185 | 0.196 | 0.208 | 0.192 | - |
| Composition | TEC (10−6 K−1) ((Heating) Run) | TEC (10−6 K−1) ((Cooling) Run) |
|---|---|---|
| La(Co,Cr,Fe,Mn,Ni)O3−δ | 15.4(1) | 15.4(1) |
| Pr(Co,Cr,Fe,Mn,Ni)O3−δ | 14.6(1) | 14.4(1) |
| Nd(Co,Cr,Fe,Mn,Ni)O3−δ | 14.8(1) | 14.7(1) |
| Sm(Co,Cr,Fe,Mn,Ni)O3−δ | 14.6(1) | 14.4(1) |
| Gd(Co,Cr,Fe,Mn,Ni)O3−δ | 14.1(1) | 14.0(1) |
| Composition | Ea (eV) | T (°C) | σmax (S·cm−1) |
|---|---|---|---|
| La(Co,Cr,Fe,Mn,Ni)O3−δ | 0.363(3) | 150–900 | 3.2 |
| Pr(Co,Cr,Fe,Mn,Ni)O3−δ | 0.362(3) | 150–700 | 2.3 |
| Nd(Co,Cr,Fe,Mn,Ni)O3−δ | 0.365(3) | 150–700 | 1.6 |
| Sm(Co,Cr,Fe,Mn,Ni)O3−δ | 0.349(2) | 200–900 | 1.2 |
| Gd(Co,Cr,Fe,Mn,Ni)O3−δ | 0.417(2) | 250–900 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowa, J.; Zielińska, K.; Stępień, A.; Zajusz, M.; Nowakowska, M.; Moździerz, M.; Berent, K.; Szymczak, M.; Świerczek, K. Formation of Solid Solutions and Physicochemical Properties of the High-Entropy Ln1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ (Ln = La, Pr, Nd, Sm or Gd) Perovskites. Materials 2021, 14, 5264. https://doi.org/10.3390/ma14185264
Dąbrowa J, Zielińska K, Stępień A, Zajusz M, Nowakowska M, Moździerz M, Berent K, Szymczak M, Świerczek K. Formation of Solid Solutions and Physicochemical Properties of the High-Entropy Ln1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ (Ln = La, Pr, Nd, Sm or Gd) Perovskites. Materials. 2021; 14(18):5264. https://doi.org/10.3390/ma14185264
Chicago/Turabian StyleDąbrowa, Juliusz, Klaudia Zielińska, Anna Stępień, Marek Zajusz, Margarita Nowakowska, Maciej Moździerz, Katarzyna Berent, Maria Szymczak, and Konrad Świerczek. 2021. "Formation of Solid Solutions and Physicochemical Properties of the High-Entropy Ln1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ (Ln = La, Pr, Nd, Sm or Gd) Perovskites" Materials 14, no. 18: 5264. https://doi.org/10.3390/ma14185264
APA StyleDąbrowa, J., Zielińska, K., Stępień, A., Zajusz, M., Nowakowska, M., Moździerz, M., Berent, K., Szymczak, M., & Świerczek, K. (2021). Formation of Solid Solutions and Physicochemical Properties of the High-Entropy Ln1−xSrx(Co,Cr,Fe,Mn,Ni)O3−δ (Ln = La, Pr, Nd, Sm or Gd) Perovskites. Materials, 14(18), 5264. https://doi.org/10.3390/ma14185264








