Insights into the Intrinsic Factors Affecting the NIR Reflectance Based on Rylene Diimide Molecules
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental Section
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mazhar, M.; Abdouss, M.; Zarifi, F.; Zargaran, M. Effectiveness of perylene pigment on the reduction of energy demand of a building. Pigm. Resin. Technol. 2020, 49, 265–271. [Google Scholar] [CrossRef]
- Levinson, R.; Berdahl, P.; Akbari, H.; Miller, W.; Joedicke, I.; Reilly, J.; Suzuki, Y.; Vondran, M. Methods of creating solar-reflective nonwhite surfaces and their application to residential roofing materials. Sol. Energy Mater. Sol. Cells 2007, 91, 304–314. [Google Scholar] [CrossRef]
- Kolokotroni, M.; Giannitsaris, I.; Watkins, R. The effect of the London urban heat island on building summer cooling demand and night ventilation strategies. Sol. Energy 2006, 80, 383–392. [Google Scholar] [CrossRef]
- Wong, N.H.; Jusuf, S.K.; Syafii, N.I.; Chen, Y.; Hajadi, N.; Sathyanarayanan, H.; Manickavasagam, Y.V. Evaluation of the impact of the surrounding urban morphology on building energy consumption. Sol. Energy 2011, 85, 57–71. [Google Scholar] [CrossRef]
- Jose, S.; Joshy, D.; Narendranath, S.B.; Periyat, P. Recent advances in infrared reflective inorganic pigments. Sol. Energy Mater. Sol. Cells 2019, 194, 7–27. [Google Scholar] [CrossRef]
- Wong, A.; Daoud, W.A.; Liang, H.-H.; Szeto, Y.S. Application of rutile and anatase onto cotton fabric and their effect on the NIR reflection/surface temperature of the fabric. Sol. Energy Mater. Sol. Cells 2015, 134, 425–437. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Mei, S.-G.; Byon, Y.-J.; Wang, J.-L.; Zhang, G.-L. Highly solar radiation reflective Cr2O3–3TiO2 orange nanopigment prepared by a polymer-pyrolysis method. ACS Sustain. Chem. Eng. 2013, 2, 318–321. [Google Scholar] [CrossRef]
- Sreeram, K.J.; Aby, C.P.; Nair, B.U.; Ramasami, T. Colored cool colorants based on rare earth metal ions. Sol. Energy Mater. Sol. Cells 2008, 92, 1462–1467. [Google Scholar] [CrossRef]
- Yamamiya, S.; Horiguchi, S.; Ohira, S.; Abe, Y.; Zama, Y.; Nishikatsu, H. Inventors Dainichi Color & Chem MFG Co LTD Assignee. Infrared Reflective Material. Japanese Patent JPS6230202, 9 February 1987. [Google Scholar]
- Bäbler, F. Inventor Cibaspeciality, Assignee. IR Reflective Pigment Composition. U.S. Patent 6989056, 2006. [Google Scholar]
- Graser, F. Inventor BASF AG Assignee. N-Substd. Perylenetetracarboxylic Acid Diimides—As Black Pigments for Paints, Plastics and aq. Compsns. German Patent DE2451780, 12 February 1976. [Google Scholar]
- Erk, P.; Stohr, A.; Boehm, A.; Kurtz, W.; Mizuguchi, J.; Sens, B. Inventors BASF Aktiengesellschaft Assignee. Black Perylene Pigments. U.S. Patent 7416601, 26 August 2008. [Google Scholar]
- Faulkner, E.B.; Schwartz, R.J. High Performance Pigments; Wiley-VCH: Weinheim, Germany, 2009; p. 272. [Google Scholar]
- Song, R.B.; Wu, Y.; Lin, Z.Q.; Xie, J.; Tan, C.H.; Loo, J.S.C.; Cao, B.; Zhang, J.R.; Zhu, J.J.; Zhang, Q. Living and conducting: Coating individual bacterial cells with in situ formed polypyrrole. Angew. Chem. Int. Ed. Engl. 2017, 56, 10516–10520. [Google Scholar] [CrossRef]
- Sonmez, G.; Meng, H.; Zhang, Q.; Wudl, F. A Highly Stable, New electrochromic polymer: Poly(1,4-bis(2-(3′,4′-ethylenedioxy) thienyl)-2-methoxy-5-2″-ethylhexyloxybenzene). Adv. Funct. Mater. 2003, 13, 726–731. [Google Scholar] [CrossRef]
- Yao, C.J.; Zhang, H.L.; Zhang, Q. Recent progress in thermoelectric materials based on conjugated polymers. Polymers 2019, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q. Shooting flexible electronics. Front. Phys. 2020, 16, 13602. [Google Scholar] [CrossRef]
- Roes, A.L.; Alsema, E.A.; Blok, K.; Patel, M.K. Ex-ante environmental and economic evaluation of polymer photovoltaics. Prog Photovolt 2009, 17, 372–393. [Google Scholar] [CrossRef]
- Brass, D.M.; Palmer, S.M. Models of toxicity of diacetyl and alternative diones. Toxicology 2017, 388, 15–20. [Google Scholar] [CrossRef]
- Chen, W.; Song, Y.; Zhang, L.; Liu, M.; Hu, X.; Zhang, Q. Thiophene-fused-heteroaromatic diones as promising NIR reflectors for radiative cooling. Angew. Chem. Int. Ed. Engl. 2018, 57, 6289–6293. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, M.; Gullo, G.; Battisti, A.; Martini, F.; Borsacchi, S.; Geppi, M.; Ruggeri, G.; Pucci, A. Thermochromic polyethylene films doped with perylene chromophores: Experimental evidence and methods for characterization of their phase behaviour. Polym. Chem. 2015, 6, 4003–4012. [Google Scholar] [CrossRef]
- Donati, F.; Pucci, A.; Cappelli, C.; Mennucci, B.; Ruggeri, G. Modulation of the optical response of polyethylene films containing luminescent perylene chromophores. J. Phys. Chem. B 2008, 112, 3668–3679. [Google Scholar] [CrossRef]
- Wurthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 14, 1564–1579. [Google Scholar] [CrossRef]
- Raj, M.R.; Margabandu, R.; Mangalaraja, R.V.; Anandan, S. Influence of imide-substituents on the H-type aggregates of perylene diimides bearing cetyloxy side-chains at bay positions. Soft Matter 2017, 13, 9179–9191. [Google Scholar] [CrossRef]
- Kaur, B.; Quazi, N.; Ivanov, I.; Bhattacharya, S.N. Near-infrared reflective properties of perylene derivatives. Dyes Pigm. 2012, 92, 1108–1113. [Google Scholar] [CrossRef]
- Mazhar, M.; Abdouss, M.; Gharanjig, K.; Teimuri-Mofrad, R. Synthesis, characterization and near infra-red properties of perylenebisimide derivatives. Prog. Org. Coat. 2016, 101, 297–304. [Google Scholar] [CrossRef]
- Kaur, B.; Bhattacharya, S.N.; Henry, D.J. Interpreting the near-infrared reflectance of a series of perylene pigments. Dyes Pigm. 2013, 99, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Mazhar, M.; Abdouss, M.; Gharanjig, K.; Teimuri-Mofrad, R.; Zargaran, M. Effects of isomerism on near infrared properties of perylene bisimide derivatives. J. Coat. Technol Res. 2016, 14, 207–214. [Google Scholar] [CrossRef]
- Mahmoudi Meymand, F.; Mazhar, M.; Abdouss, M. Investigation of substituent effect on cool activity of perylene bisimide pigments. J. Coat. Technol Res. 2018, 16, 439–447. [Google Scholar] [CrossRef]
- Martini, F.; Geppi, M.; Barcaro, G.; Monti, S.; Contiero, L.; Ruggeri, G.; Lessi, M.; Pucci, A.; Bellina, F.; Borsacchi, S. Structure and dynamics of perylene bisimide pigments for “cool” organic coatings by solid-state NMR: A combined experimental and DFT study. J. Phys. Chem. C 2020, 124, 17971–17980. [Google Scholar] [CrossRef]
- Martini, F.; Minei, P.; Lessi, M.; Contiero, L.; Borsacchi, S.; Ruggeri, G.; Geppi, M.; Bellina, F.; Pucci, A. Structural order and NIR reflective properties of perylene bisimide pigments: Experimental evidences from a combined multi-technique study. Dyes Pigm. 2020, 179, 108401. [Google Scholar] [CrossRef]
- Minei, P.; Lessi, M.; Contiero, L.; Borsacchi, S.; Martini, F.; Ruggeri, G.; Geppi, M.; Bellina, F.; Pucci, A. Boosting the NIR reflective properties of perylene organic coatings with thermoplastic hollow microspheres: Optical and structural properties by a multi-technique approach. Sol. Energy 2020, 198, 689–695. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Lim, X.M.; Hu, X.; Liu, M.; Zhang, Q. Electronic configuration in outset orbitals of doping elements plays as a key factor in tuning near infrared reflection of YMn0.9M0.1O3 (M = Cr, Mn, Fe, Co, Al, Ga and In). J. Solid State Chem. 2019, 273, 81–84. [Google Scholar] [CrossRef]
- Seckin, T.; Özdemir, I.; Köytepe, S.; Gürbüz, N. Preparation and Catalytic Properties of a Ru(II) Coordinated Polyimide Supported by a Ligand Containing Terpyridine Units. J. Inorg. Organomet. Polym. Mater. 2009, 19, 143–151. [Google Scholar] [CrossRef]
- Yang, T.F.; Huang, S.H.; Chiu, Y.P.; Chen, B.H.; Shih, Y.W.; Chang, Y.C.; Yao, J.Y.; Lee, Y.J.; Kuo, M.Y. Pyromellitic dithioimides: Thionation improves air-stability and electron mobility of N-type organic field-effect transistors. Chem. Commun. 2015, 51, 13772–13775. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tang, H.; Jiang, S.; Kayser, L.V.; Li, M.; Liu, F.; Ji, F.; Lipomi, D.J.; Ong, S.P.; Chen, Z. Understanding the electrochemical properties of naphthalene diimide: Implication for stable and high-rate lithium-ion battery electrodes. Chem. Mater. 2018, 30, 3508–3517. [Google Scholar] [CrossRef]
- Jung, B.J.; Lee, K.; Sun, J.; Andreou, A.G.; Katz, H.E. Air-operable, high-mobility organic transistors with semifluorinated side chains and unsubstituted naphthalenetetracarboxylic diimide cores: High mobility and environmental and bias stress stability from the perfluorooctylpropyl side chain. Adv. Funct. Mater. 2010, 20, 2930–2944. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Long, G.; Liu, Y.; Zhang, Q. From non-detectable to decent: Replacement of oxygen with sulfur in naphthalene diimide boosts electron transport in organic thin-film transistors (OTFT). J. Mater. Chem. C 2015, 3, 8219–8224. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Long, G.; Wan, X.; Chen, Y.; Zhang, Q. A perylene diimide (PDI)-based small molecule with tetrahedral configuration as a non-fullerene acceptor for organic solar cells. J. Mater. Chem. C 2015, 3, 4698–4705. [Google Scholar] [CrossRef] [Green Version]
- Black, D.B.; Lovering, E.G. Estimation of the degree of crystallinity in digoxin by X-ray and infrared methods. J. Pharm. Pharmacol. 1977, 29, 684–687. [Google Scholar] [CrossRef]
- Shah, B.; Kakumanu, V.K.; Bansal, A.K. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J. Pharmacol. Sci. 2006, 95, 1641–1665. [Google Scholar] [CrossRef]

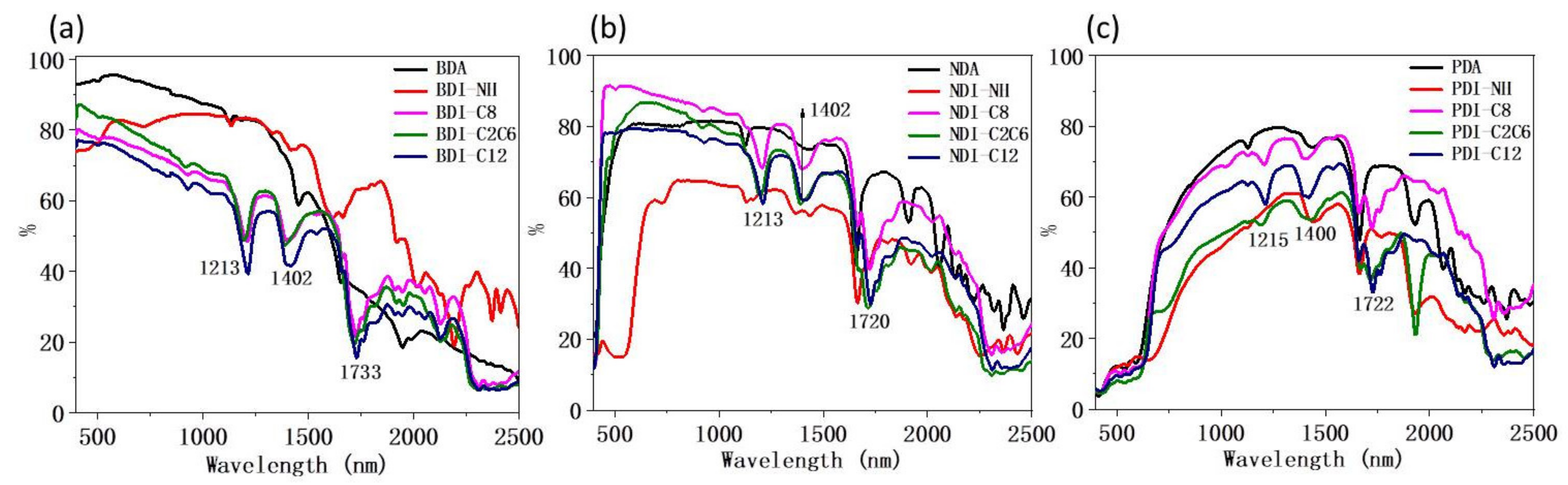
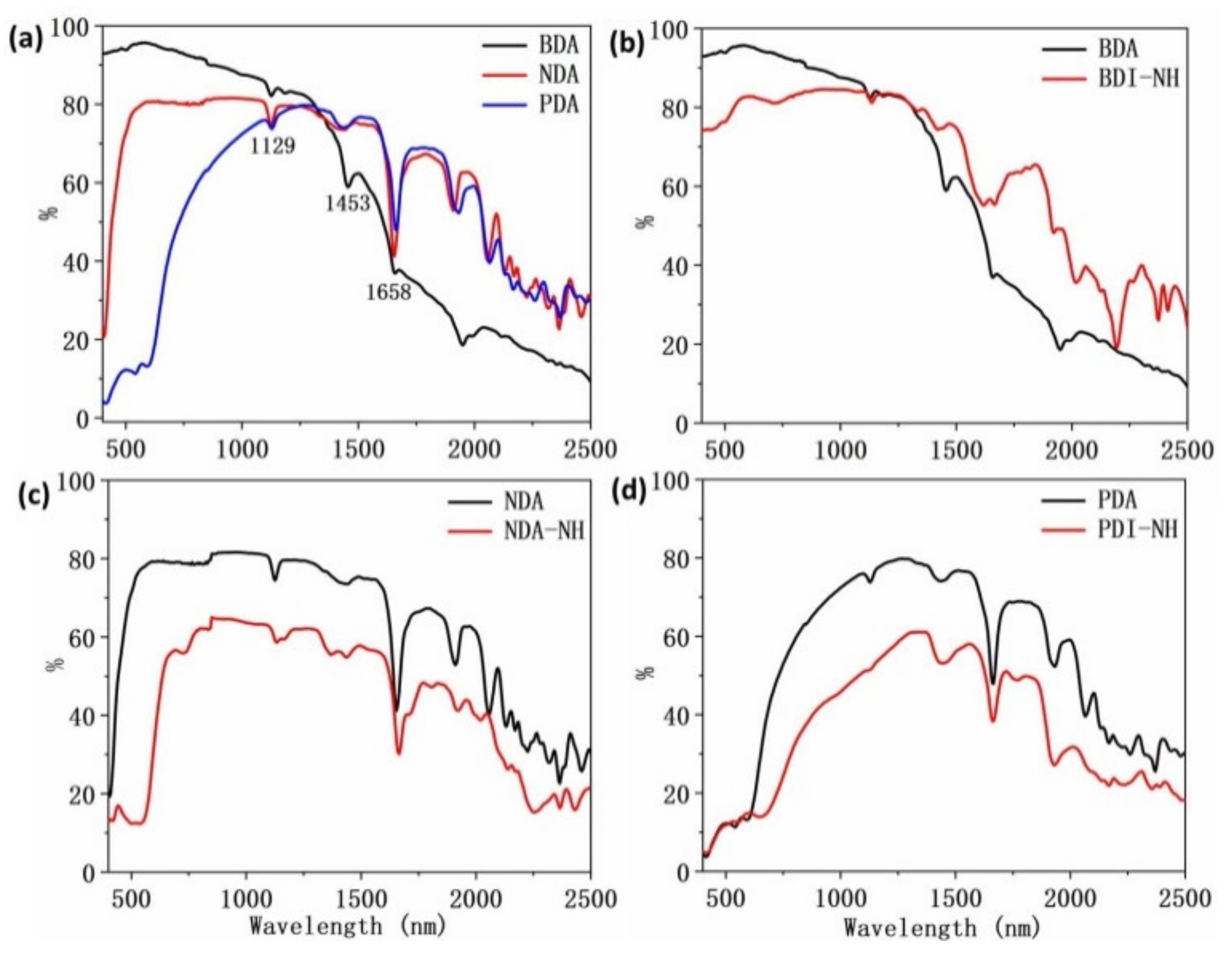

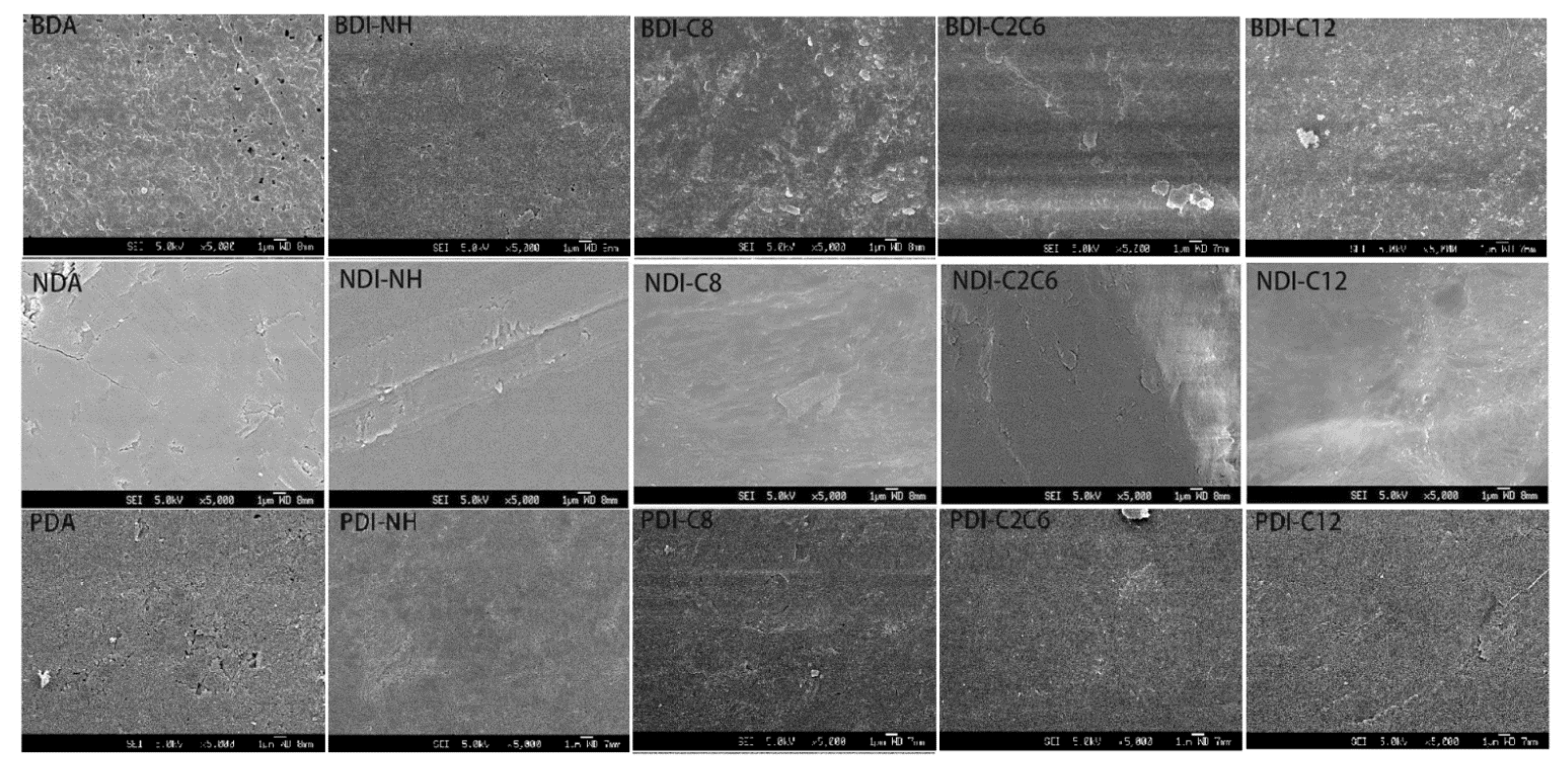
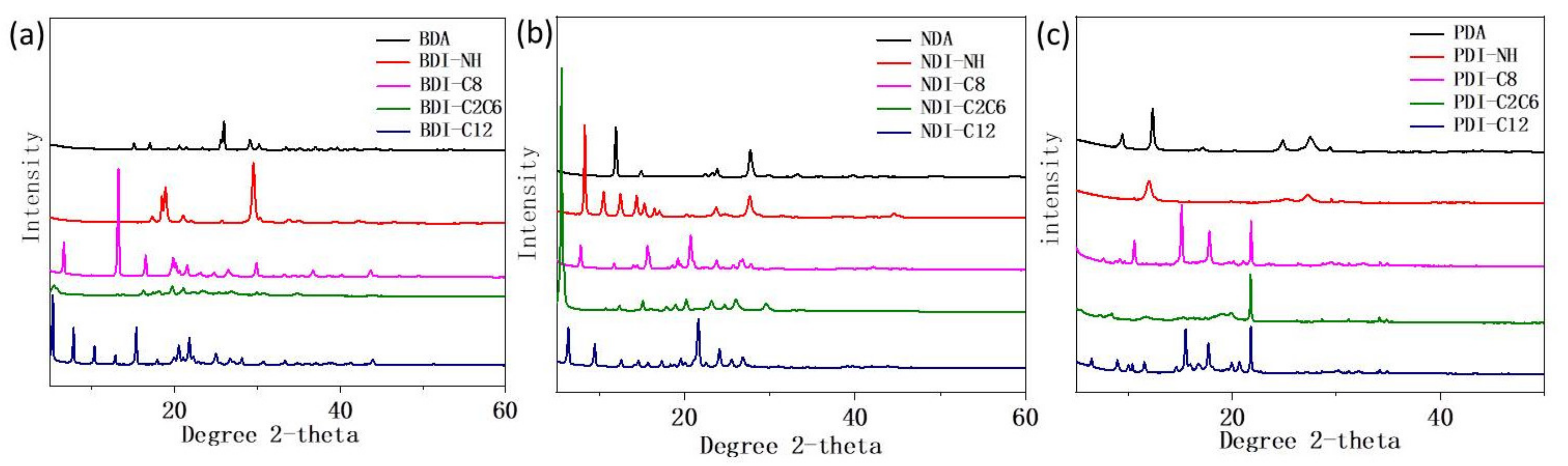

| Compounds | Wavelength (nm) | Anhydride (%) | NH (%) | C8 (%) | C2C6 (%) | C12 (%) |
|---|---|---|---|---|---|---|
| BDI- | 700–1750 | 75 | 77 | 59 | 57 | 54 |
| 1750–2500 | 23 | 41 | 25 | 21 | 20 | |
| NDI- | 700–1750 | 76 | 58 | 77 | 69 | 68 |
| 1750–2500 | 45 | 31 | 40 | 28 | 32 | |
| PDI- | 700–1750 | 70 | 49 | 68 | 50 | 59 |
| 1750–2500 | 45 | 29 | 48 | 31 | 32 |
| Compounds | Factors | Anhydride | NH | C8 | C2C6 | C12 |
|---|---|---|---|---|---|---|
| BDI- | Particle Size (nm) | 34 | 24 | 40 | 16 | 42 |
| Crystallinity (%) | 42 | 64 | 60 | 65 | 70 | |
| NDI- | Particle Size (nm) | 27 | 30 | 31 | 41 | 34 |
| Crystallinity (%) | 57 | 71 | 61 | 71 | 68 | |
| PDI- | Particle Size (nm) | 29 | 12 | 53 | 75 | 57 |
| Crystallinity (%) | 43 | 40 | 64 | 53 | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Song, Y.; Zhang, J.; Chen, H.; Liu, M.; Chen, W. Insights into the Intrinsic Factors Affecting the NIR Reflectance Based on Rylene Diimide Molecules. Materials 2021, 14, 5269. https://doi.org/10.3390/ma14185269
Zeng W, Song Y, Zhang J, Chen H, Liu M, Chen W. Insights into the Intrinsic Factors Affecting the NIR Reflectance Based on Rylene Diimide Molecules. Materials. 2021; 14(18):5269. https://doi.org/10.3390/ma14185269
Chicago/Turabian StyleZeng, Weili, Yujie Song, Jianning Zhang, Hong Chen, Ming Liu, and Wangqiao Chen. 2021. "Insights into the Intrinsic Factors Affecting the NIR Reflectance Based on Rylene Diimide Molecules" Materials 14, no. 18: 5269. https://doi.org/10.3390/ma14185269
APA StyleZeng, W., Song, Y., Zhang, J., Chen, H., Liu, M., & Chen, W. (2021). Insights into the Intrinsic Factors Affecting the NIR Reflectance Based on Rylene Diimide Molecules. Materials, 14(18), 5269. https://doi.org/10.3390/ma14185269










