Synthesis of Carbon Nanotubes (CNTs) from Poultry Litter for Removal of Chromium (Cr (VI)) from Wastewater
Abstract
:1. Introduction
2. Materials and Method
2.1. Synthesis of Catalyst
2.2. Synthesis of CNTs
2.3. Process Parameter Optimization with Response Surface Methodology
2.4. Purification of MWCNTs
2.5. Functionalization of CNT
2.6. Adsorption of Cr (VI)
2.7. Characterizations
2.7.1. X-ray Diffraction
2.7.2. Scanning Electron Microscopy (SEM)
2.7.3. Transmission Electron Microscopy (TEM)
2.7.4. Raman Spectroscopy
3. Results and Discussion
3.1. Initial Analysis of Poultry Litter
3.1.1. Moisture Content
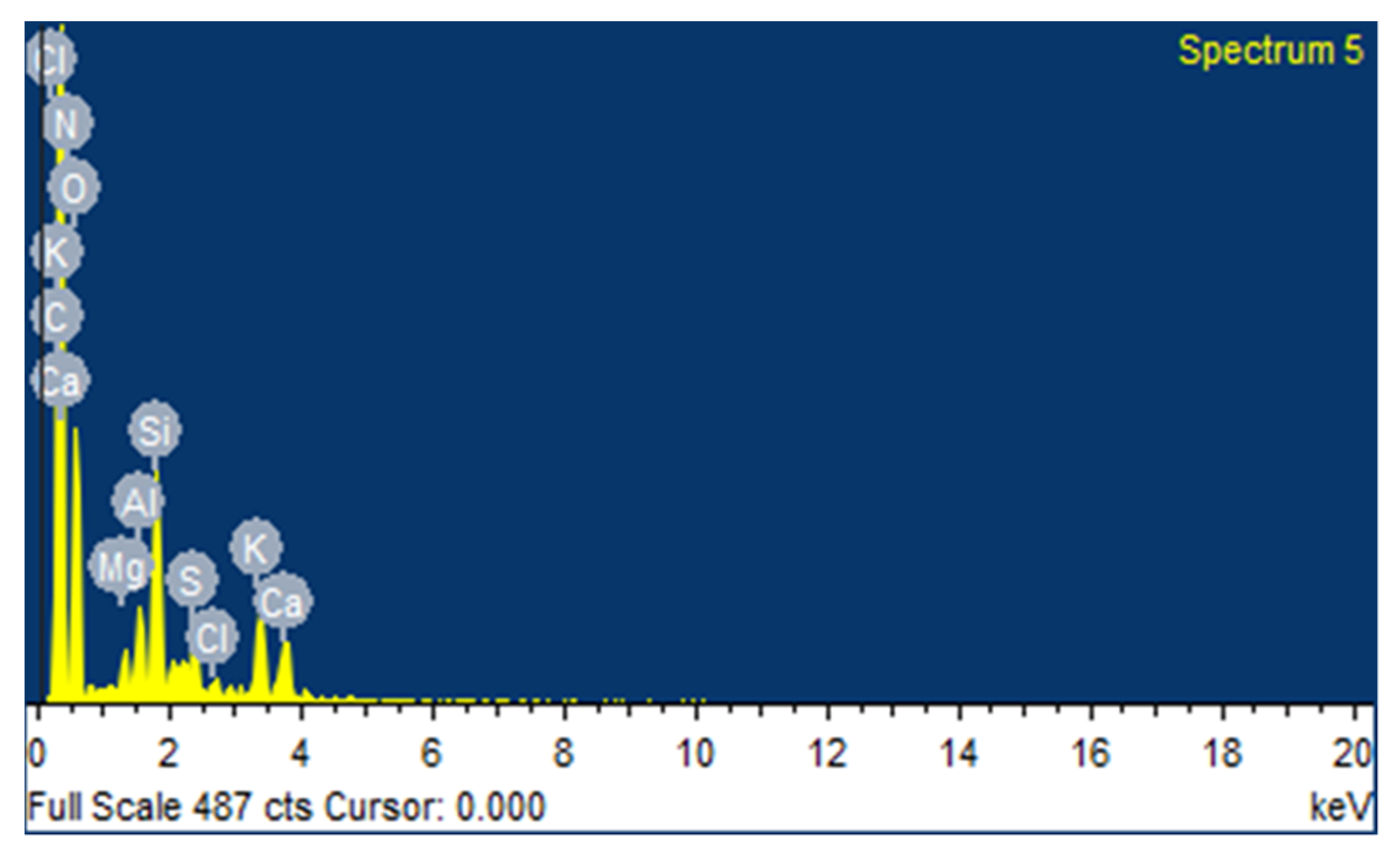
3.1.2. Elemental Analysis
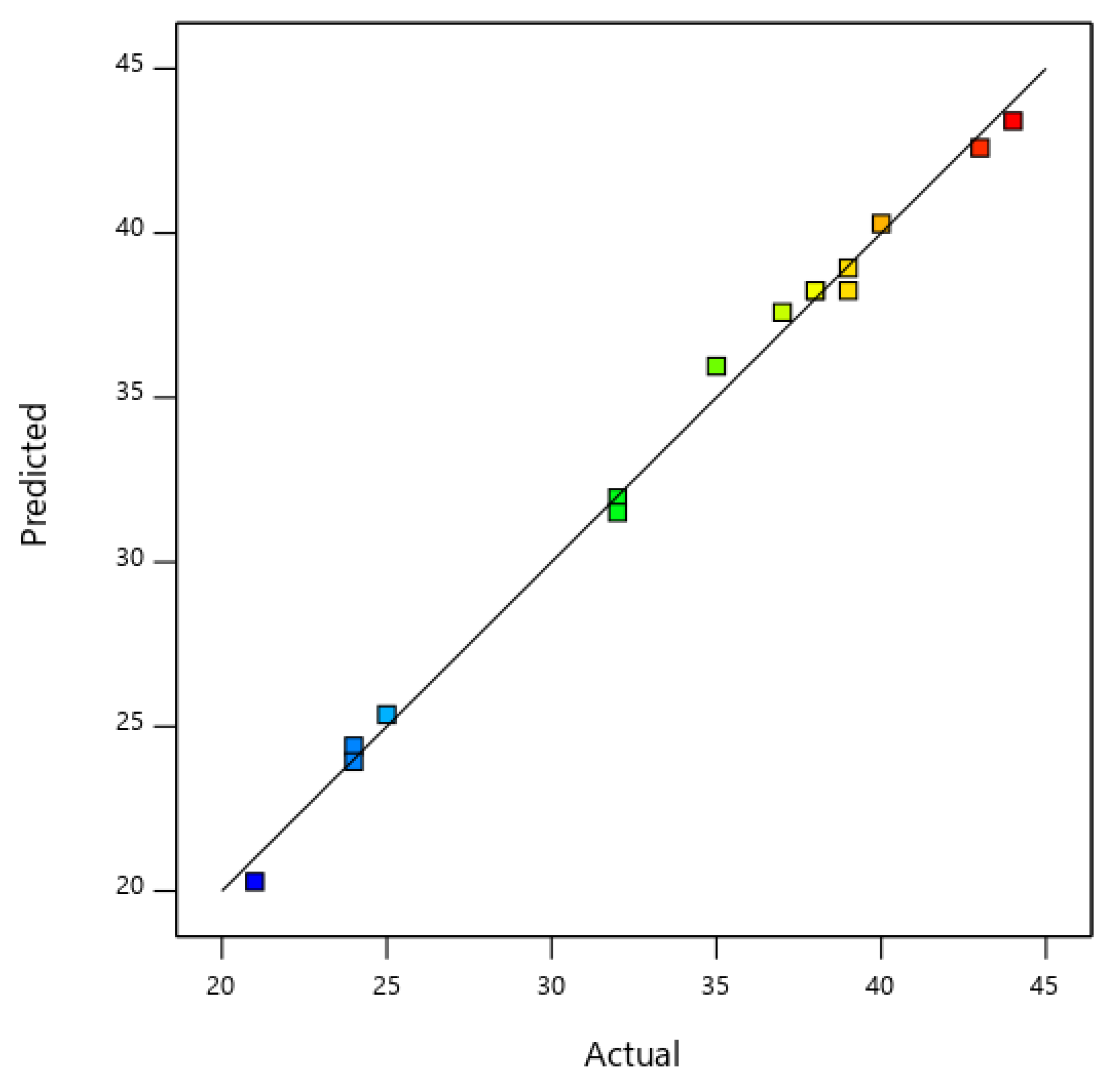
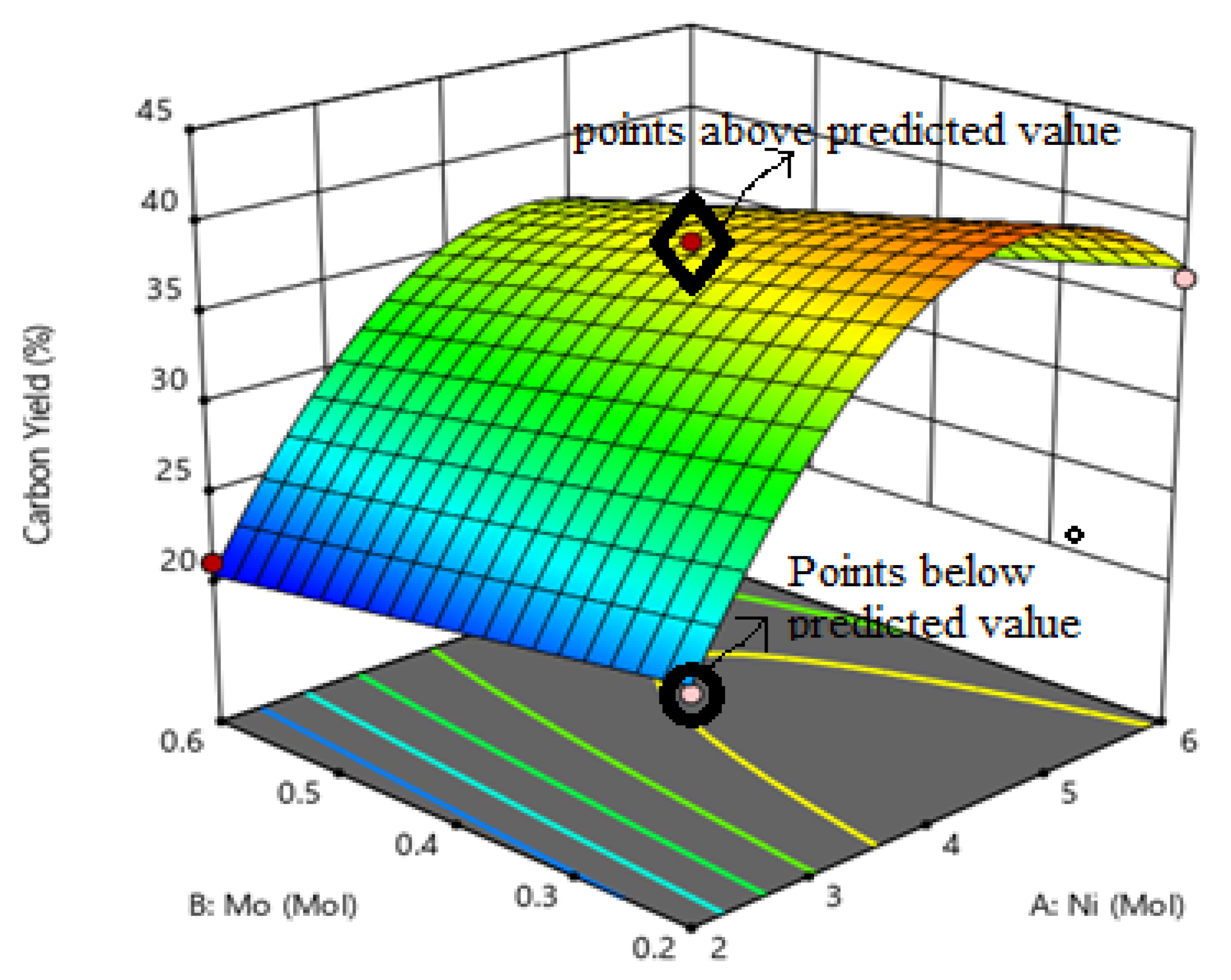
3.2. Optimization of Mole Ratio of Catalyst Precursors
Mg − 4.39474 Mo * Mg − 2.42368 Ni² + 0.065789 Mo² + 2.30526 Mg²
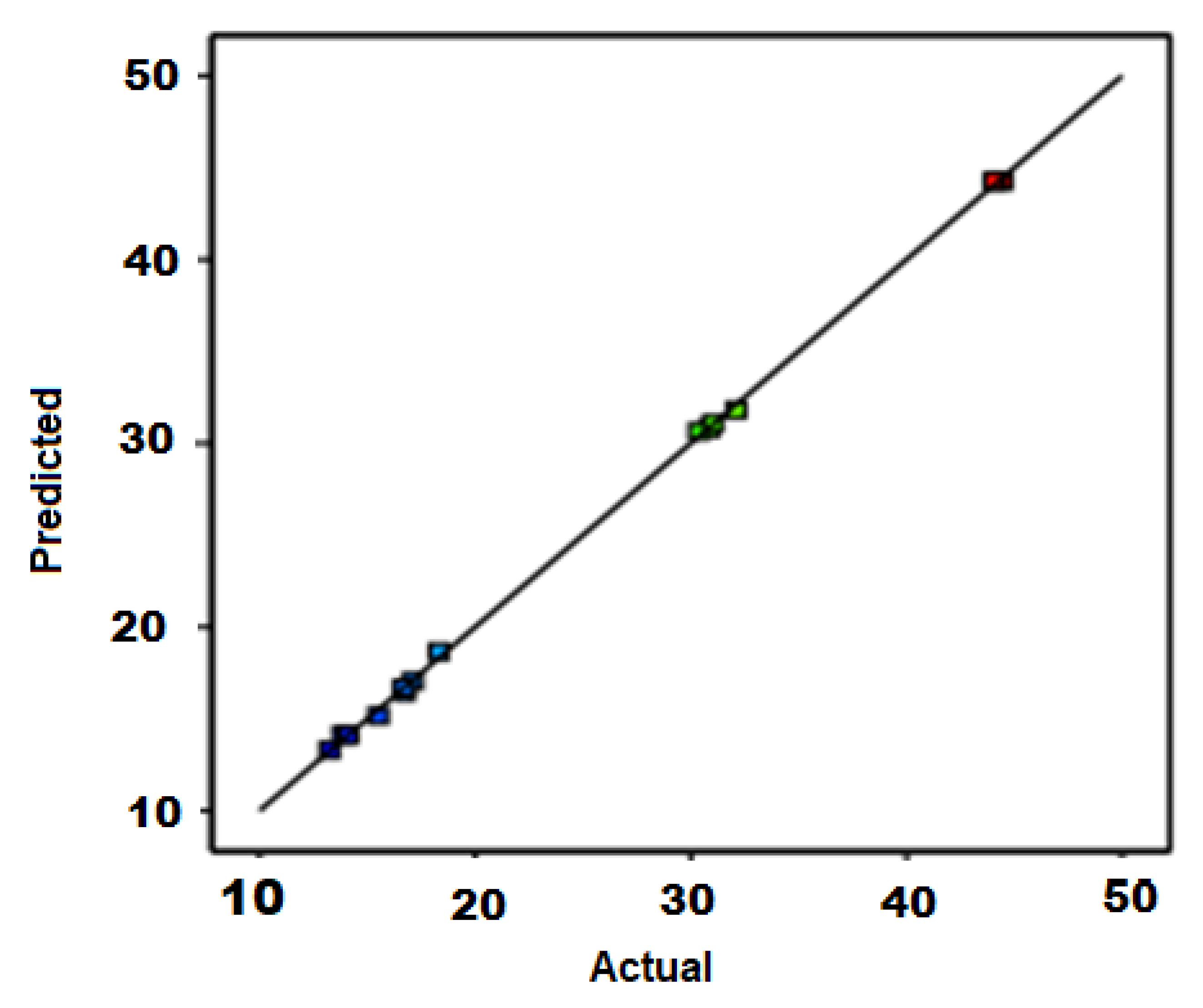
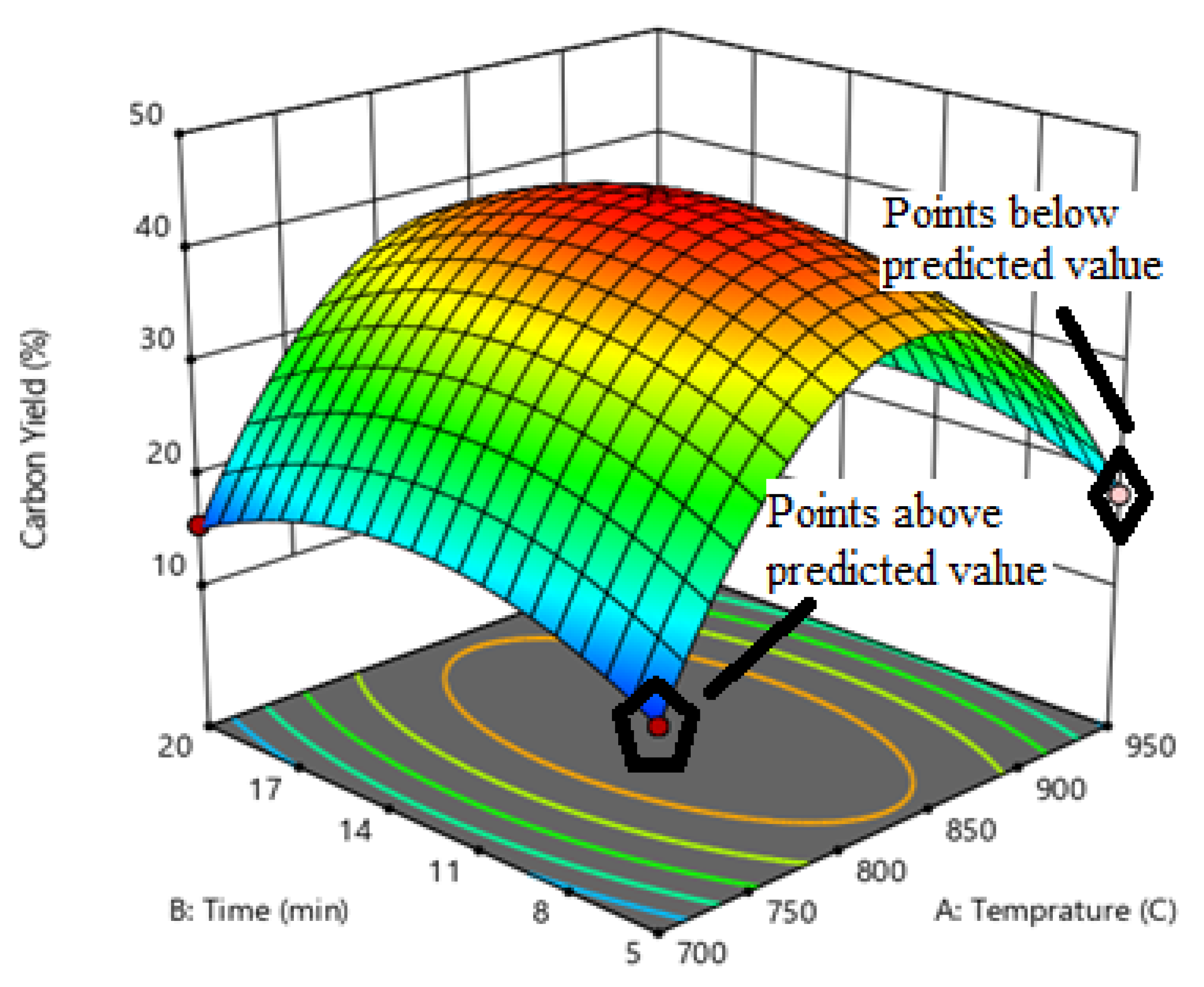
3.3. Statistical Analysis and Modeling for CNTs Growth
−0.000803 Temperature * Time − 0.000021 Temperature * Catalyst Weight − 0.000983 Time * Catalyst Weight −0.001407 Temperature² − 0.108970 Time² − 0.017649 Catalyst Weight²
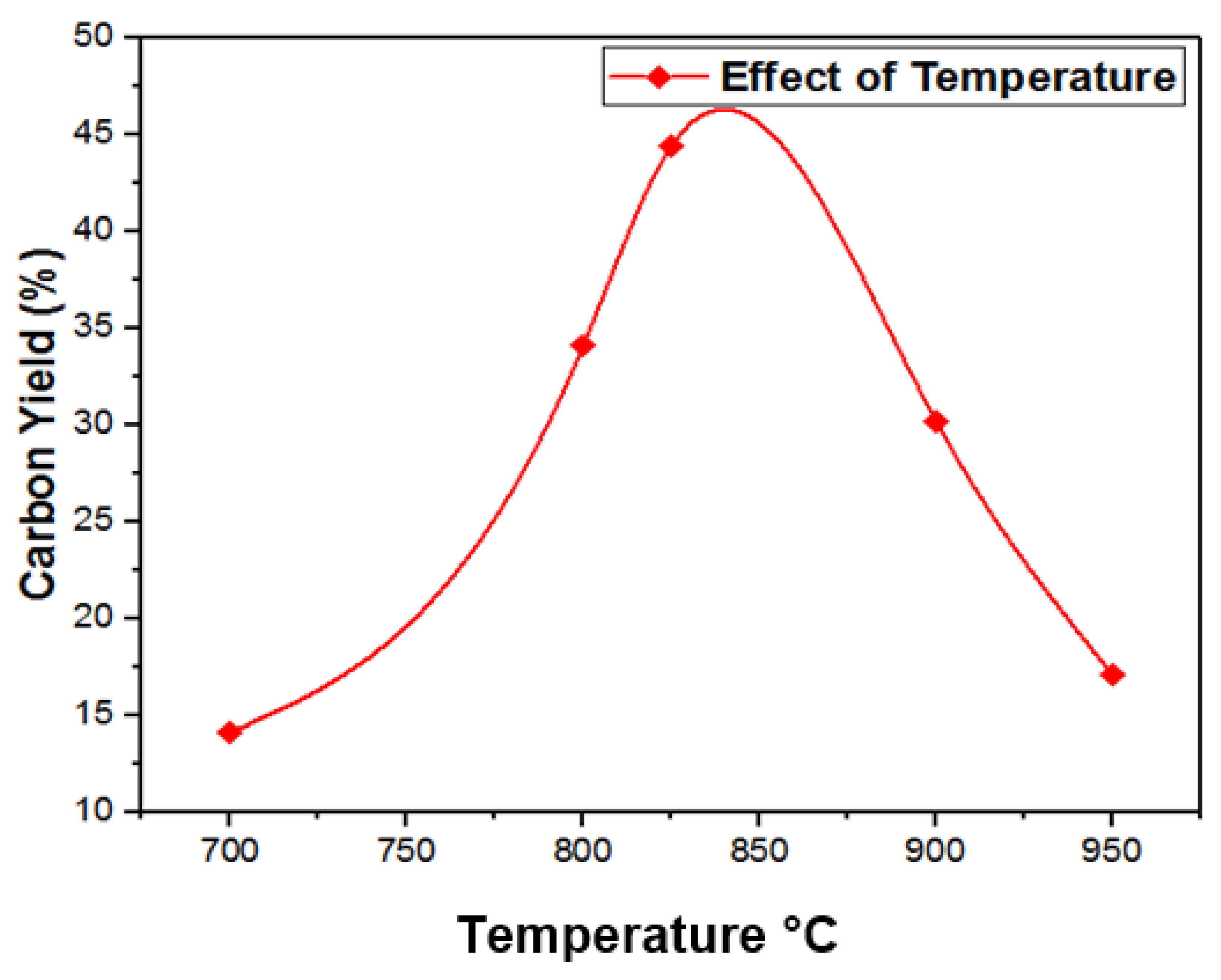
3.4. Temperature Effects on Growth of CNTs
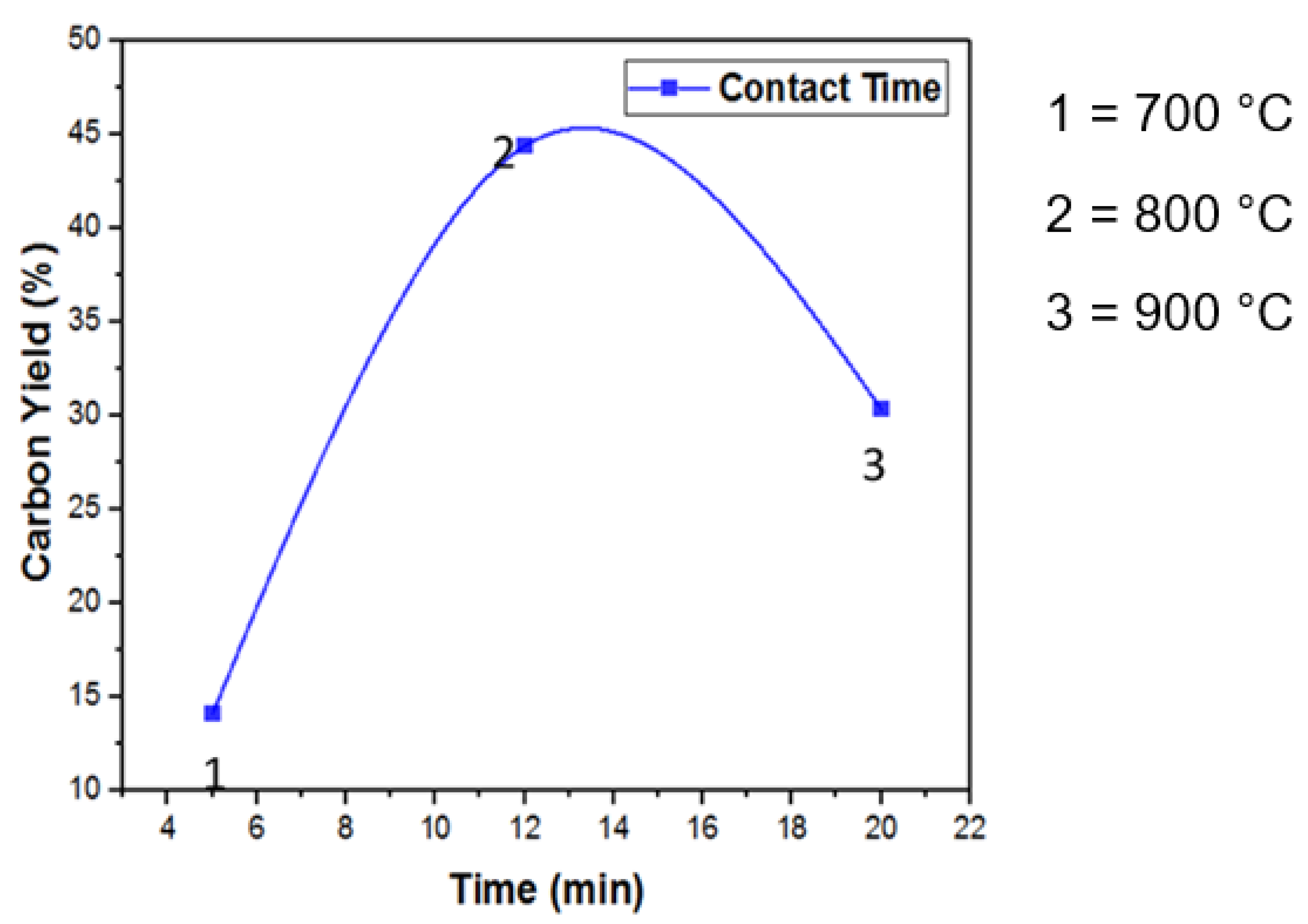
3.5. Reaction Time and CNTs Growth
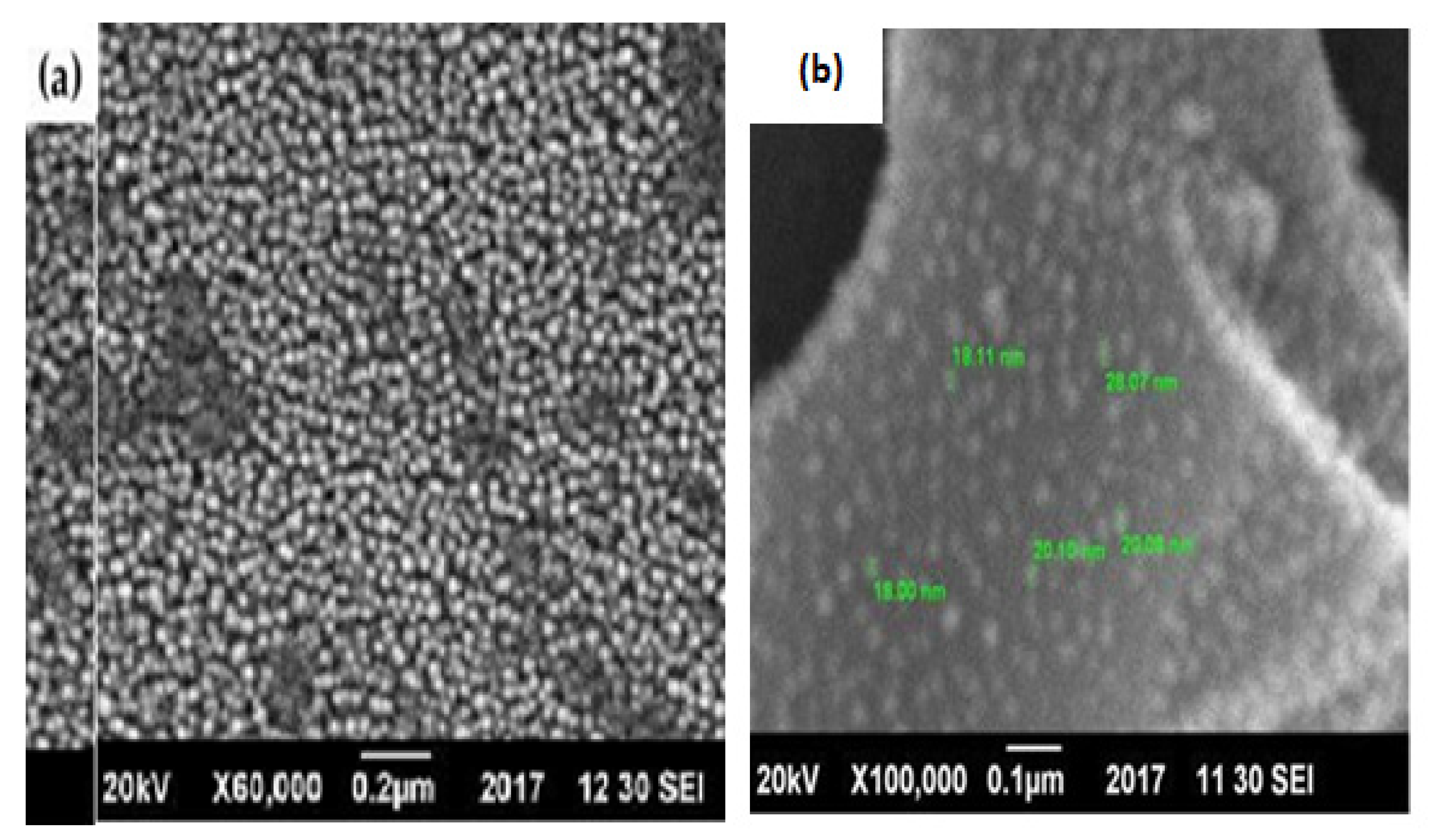
3.6. Catalyst Characterization
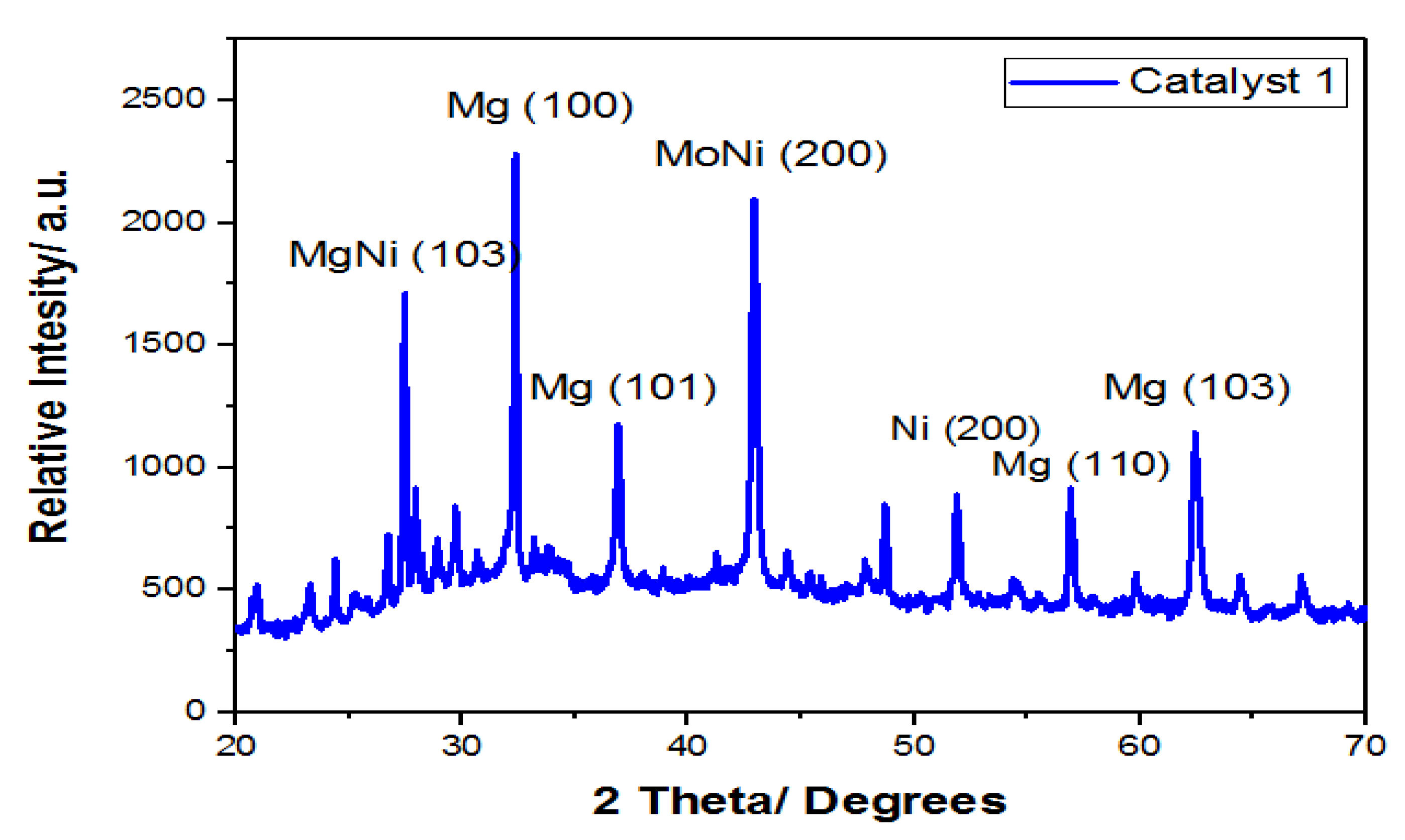
3.6.1. X-ray Diffraction (XRD)
3.6.2. Morphological Analysis of the Catalyst
3.7. Characterization of CNTs
3.7.1. X-ray Diffraction (XRD)
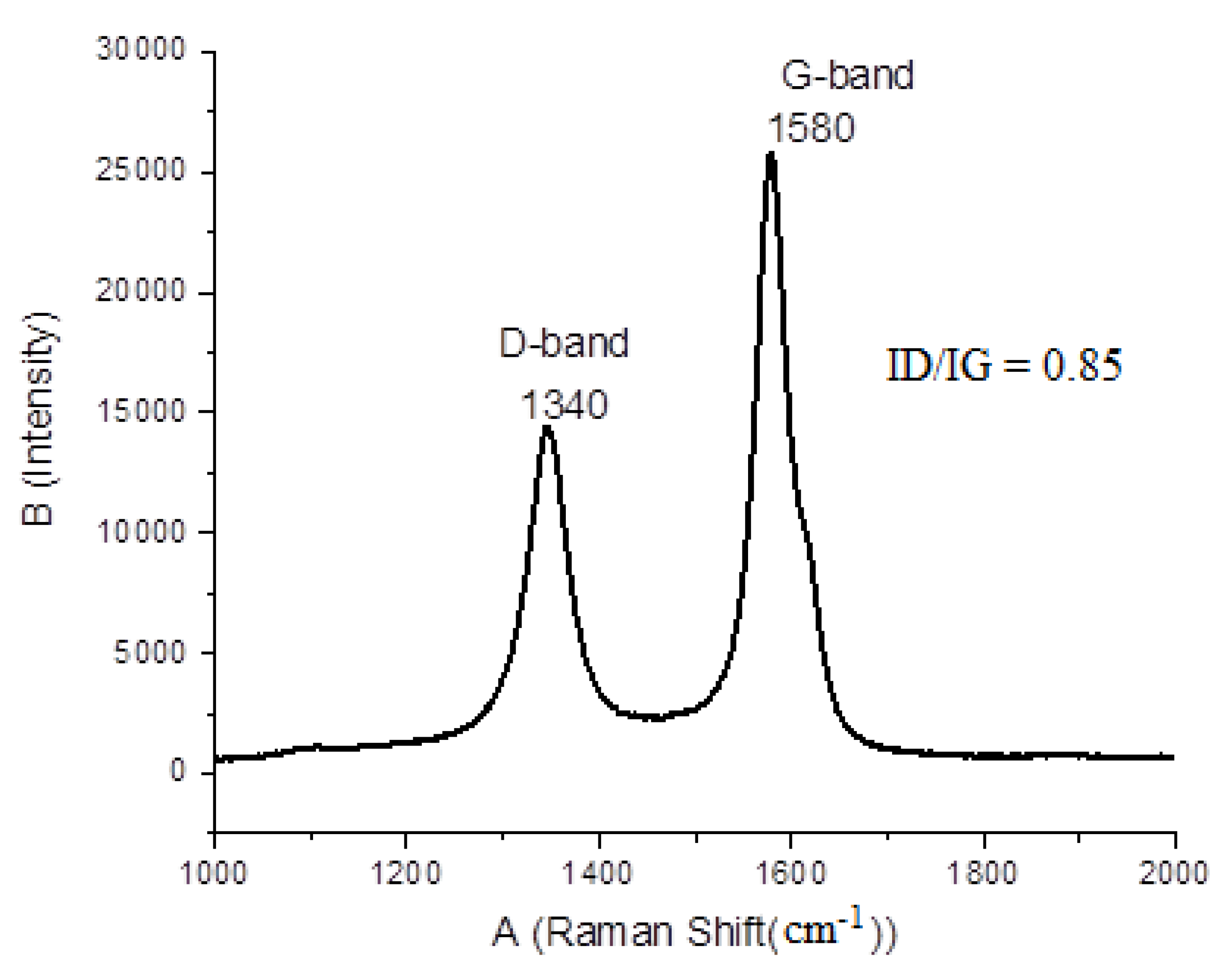
3.7.2. Raman Spectroscopy
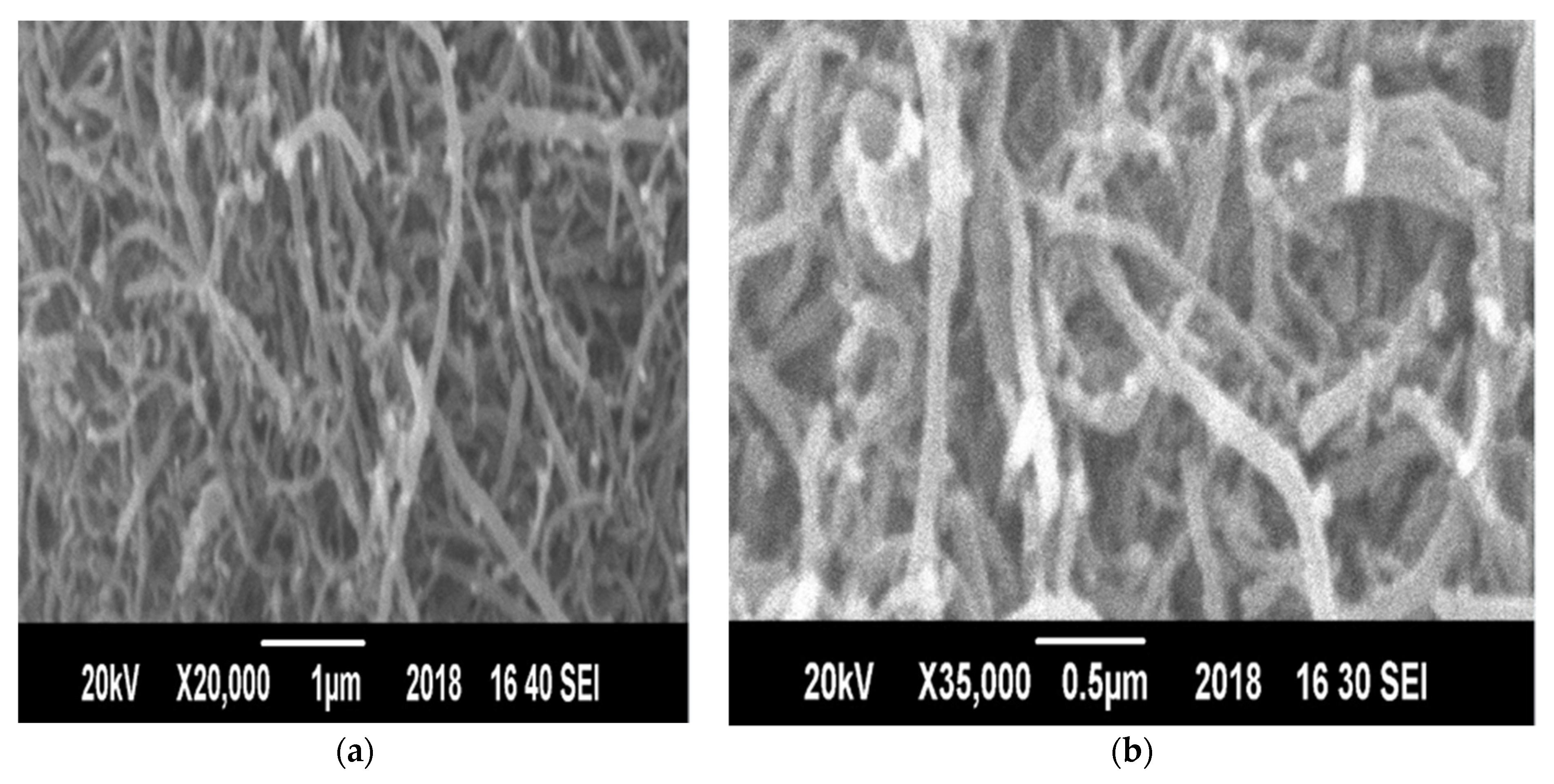
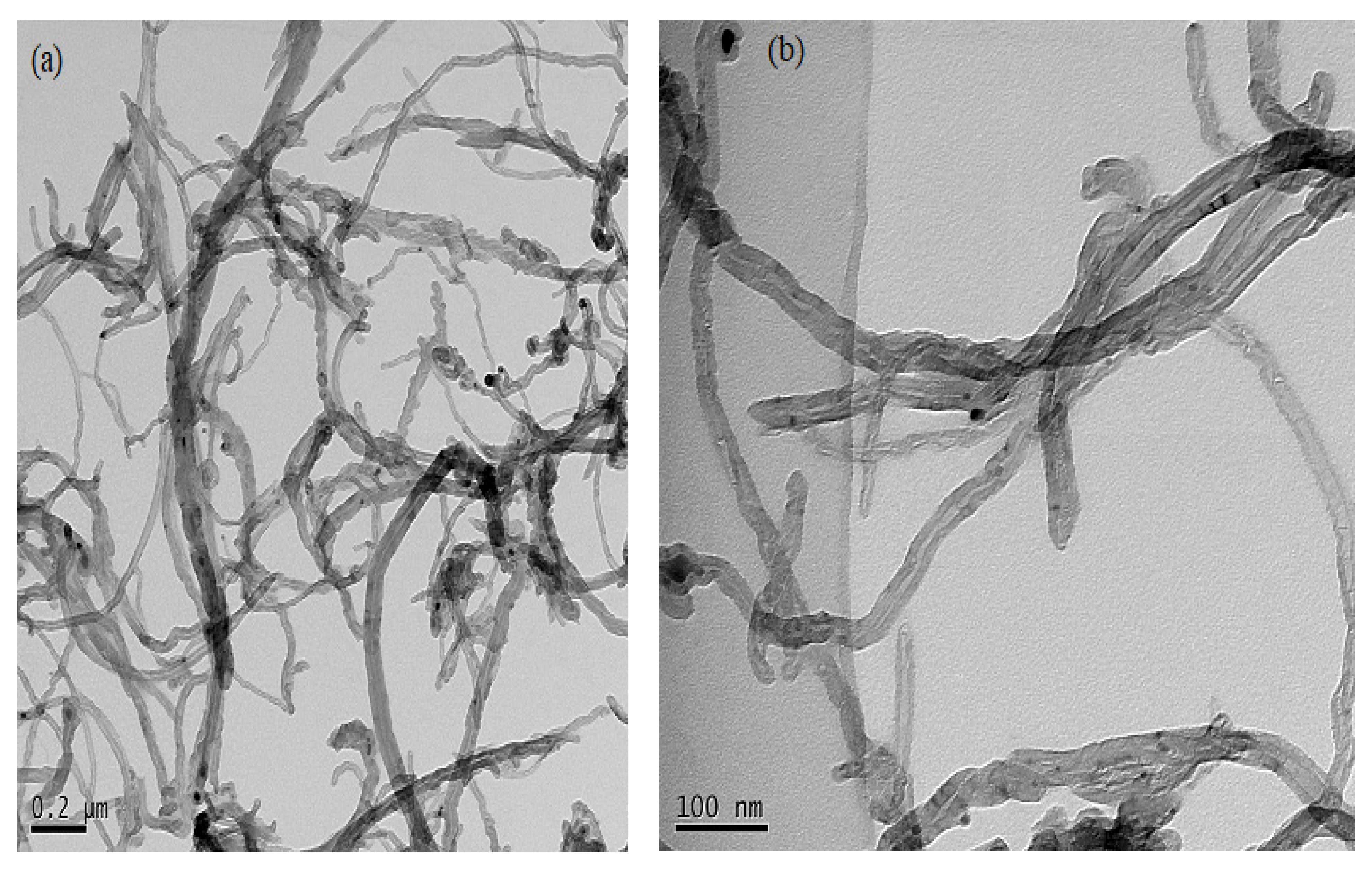
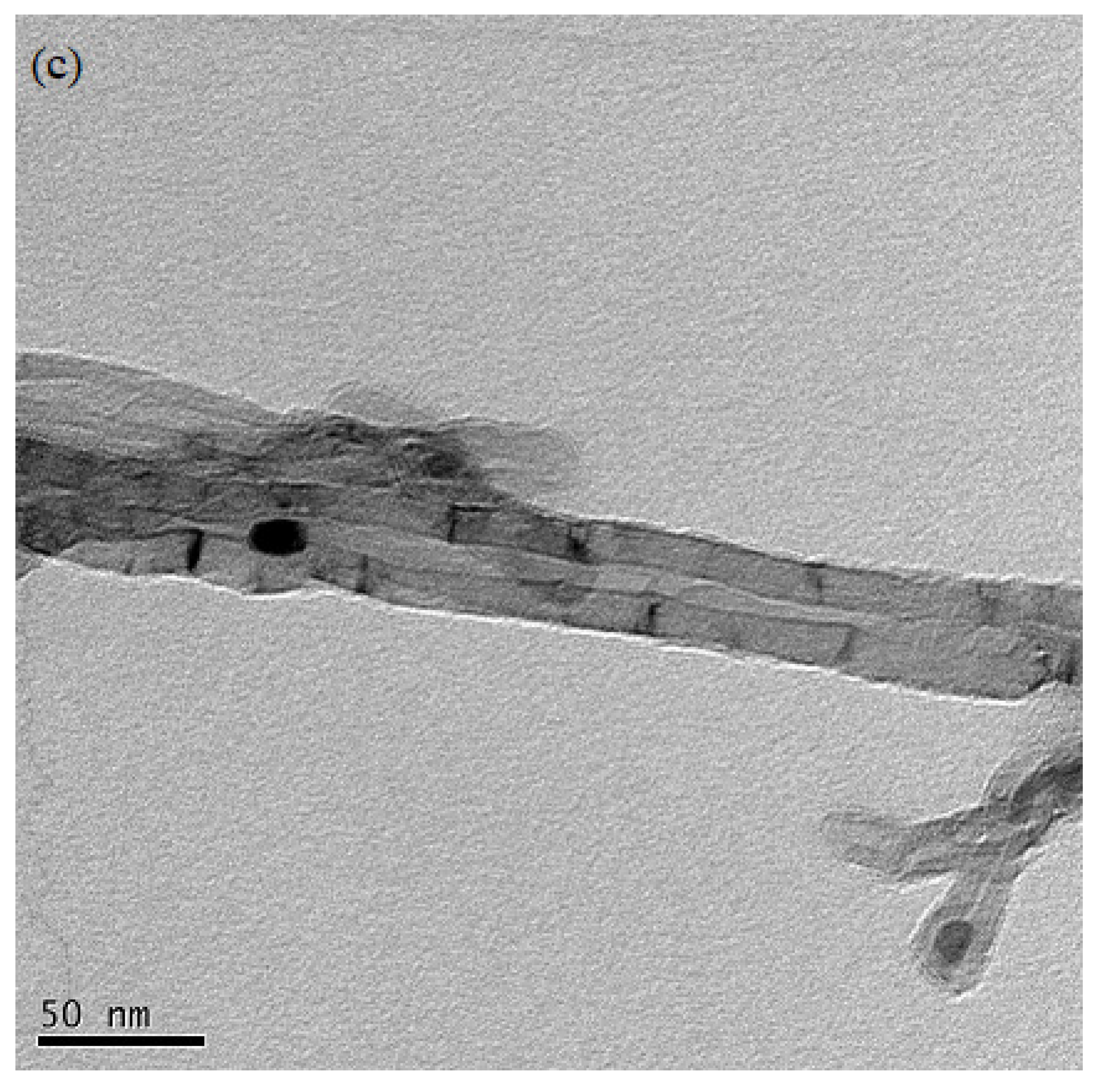
3.7.3. Morphological Analysis of CNTs
3.8. Adsorption of Cr (VI)
3.8.1. Effect of pH
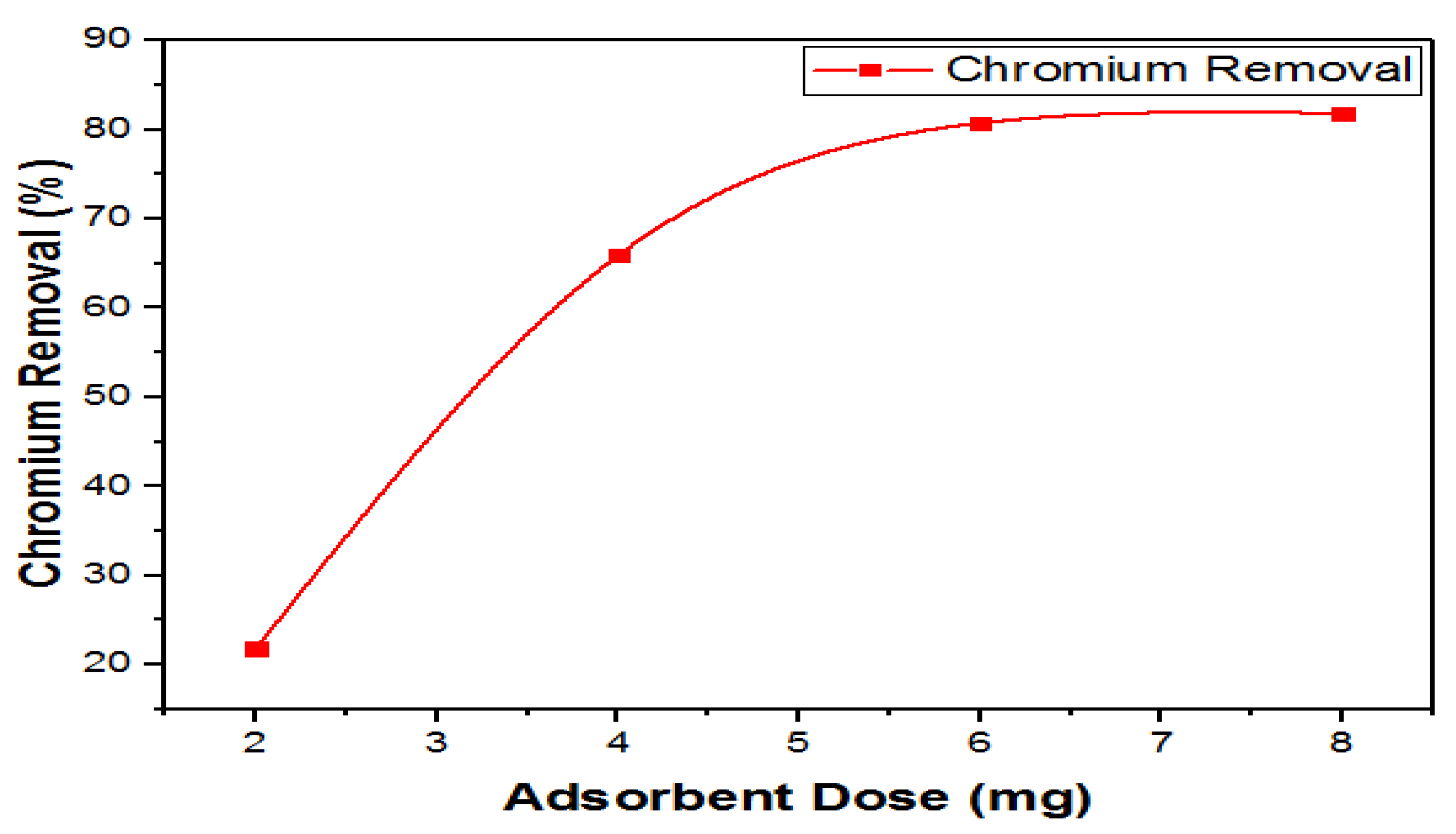
3.8.2. Effect of Adsorbent Dosage on Chromium Removal
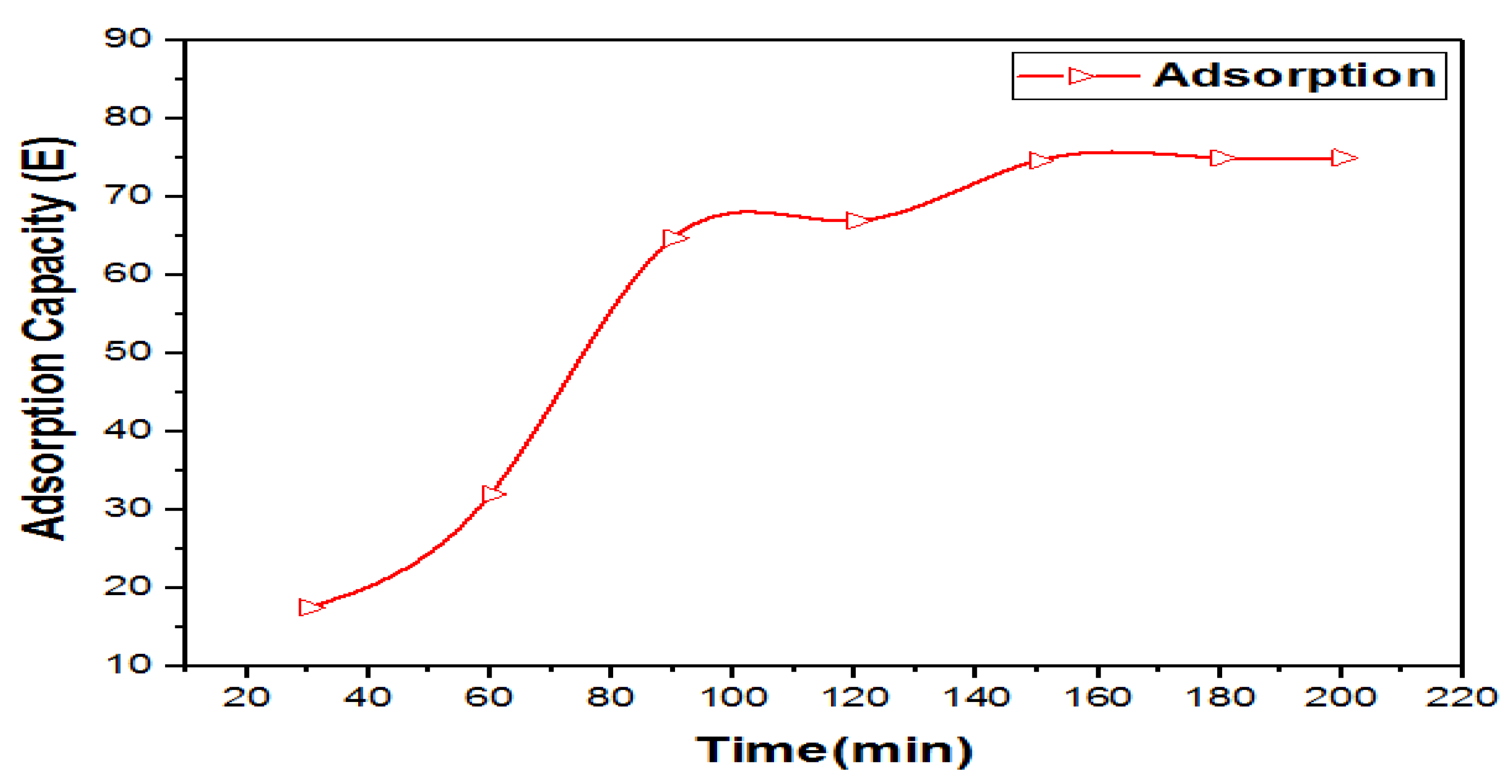
3.8.3. Effect of Adsorption Contact Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liaqat, I. Pakistan poultry industry growth and challenges. Approach Poult Dairy Veterin Sci. 2018, 2, 174–175. [Google Scholar] [CrossRef]
- Hussain, J.; Rabbani, I.; Aslam, S.; Ahmad, H.A. An overview of poultry industry in Pakistan. World’s Poult. Sci. J. 2015, 71, 689–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos Dalólio, F.; da Silva, J.N.; Carneiro de Oliveira, A.C.; Ferreira Tinôco, I.d.F.; Christiam Barbosa, R.; Resende, M.D.O.; Teixeira Albino, L.F.; Teixeira Coelho, S. Poultry litter as biomass energy: A review and future perspectives. Renew. Sustain. Energy Rev. 2017, 76, 941–949. [Google Scholar] [CrossRef]
- Ali, S.; Ali, S.; Riaz, B. Estimation of Technical Efficiency of Open Shed Broiler Farmers in Punjab, Pakistan: A Stochastic Frontier Analysis. J. Econ. Sustain. Dev. 2014, 5, 79–88. [Google Scholar]
- Leytem, A.B.; Plumstead, P.W.; Maguire, R.O.; Kwanyuen, P.; Burton, J.W.; Brake, J. Interaction of Calcium and Phytate in Broiler Diets. 2. Effects on Total and Soluble Phosphorus Excretion. Poult. Sci. 2008, 87, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Ali, S.; Ullah Khan, S.; Sajjad, M. Assessment of technical efficiency of open shed broiler farms: The case study of Khyber Pakhtunkhwa province Pakistan. J. Saudi Soc. Agric. Sci. 2019, 18, 361–366. [Google Scholar] [CrossRef]
- Singh, A.A.; Alhattab, M.K. Drying poultry manure for pollution potential reduction and production of organic fertilizer. Am. J. Environ. Sci. 2013, 9, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.C.A.X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Lima, I.; Marshall, W.E. Utilization of turkey manure as granular activated carbon: Physical, chemical and adsorptive properties. Waste Manag. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Ng, S.W.L.; Yilmaz, G.; Ong, W.L.; Ho, G.W. One-step activation towards spontaneous etching of hollow and hierarchical porous carbon nanospheres for enhanced pollutant adsorption and energy storage. Appl. Catal. B 2018, 220, 533–541. [Google Scholar] [CrossRef]
- Kim, S.-W.; Behera, S.K.; Jamal, Y.; Park, H.-S. Optimization of Sodium Hydrosulfide Synthesis for Metal Recovery from Wastewater Using Flue Gas Containing H2S. J. Environ. Eng. 2016, 142, C40150091–C40150097. [Google Scholar] [CrossRef] [Green Version]
- Khoso, W.A.; Haleem, N.; Baig, M.A.; Jamal, Y. Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s). Sci. Rep. 2021, 11, 3790. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Haleem, N.; Baig, M.A.; Jamal, Y. Phytoaccumulation of heavy metals from municipal solid waste leachate using different grasses under hydroponic condition. Sci. Rep. 2020, 10, 15802. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Sels, B.F. Sulfonated mesoporous carbon and silica-carbon nanocomposites for biomass conversion. Appl. Catal. B 2018, 236, 518–545. [Google Scholar] [CrossRef]
- Hoyos-Palacio, L.M.; García, A.G.; Pérez-Robles, J.F.; González, J.; Martínez-Tejada, H.V. Catalytic effect of Fe, Ni, Co and Mo on the CNTs production. IOP Conf. Ser. Mater. Sci. Eng. 2014, 59, 012005. [Google Scholar] [CrossRef] [Green Version]
- Mamalis, A.G.; Vogtländer, L.O.G.; Markopoulos, A. Nanotechnology and nanostructured materials: Trends in carbon nanotubes. Precis. Eng. 2004, 28, 16–30. [Google Scholar] [CrossRef]
- Zhu, Z.; Chan, Y.-C.; Chen, Z.; Gan, C.-L.; Wu, F. Effect of the size of carbon nanotubes (CNTs) on the microstructure and mechanical strength of CNTs-doped composite Sn0.3Ag0.7Cu-CNTs solder. Mater. Sci. Eng. A 2018, 727, 160–169. [Google Scholar] [CrossRef]
- Yao, Y.; Lian, C.; Wu, G.; Hu, Y.; Wei, F.; Yu, M.; Wang, S. Synthesis of “sea urchin”-like carbon nanotubes/porous carbon superstructures derived from waste biomass for treatment of various contaminants. Appl. Catal. B 2017, 219, 563–571. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Durbach, S.; Coville, N.J. Synthesis of Multi-Walled Carbon Nanotubes from Plastic Waste Using a Stainless-Steel CVD Reactor as Catalyst. Nanomaterials 2017, 7, 284. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Liang, Y.; Wu, S. Carbon Nanomaterials for Enhancing the Thermal, Physical and Rheological Properties of Asphalt Binders. Materials 2021, 14, 2585. [Google Scholar] [CrossRef]
- Wang, J.; Shen, B.; Lan, M.; Kang, D.; Wu, C. Carbon nanotubes (CNTs) production from catalytic pyrolysis of waste plastics: The influence of catalyst and reaction pressure. Catal. Today 2020, 351, 50–57. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, R.; Ding, L.; Qu, D.; Zhang, Y.; Han, Q.; Liu, N.; Piao, Y. Catalytic performance and mechanism of biochars for dechlorination of tetrachloroethylene in sulfide aqueous solution. Appl. Catal. B 2020, 278, 119285. [Google Scholar] [CrossRef]
- Melchionna, M.; Beltram, A.; Stopin, A.; Montini, T.; Lodge, R.W.; Khlobystov, A.N.; Bonifazi, D.; Prato, M.; Fornasiero, P. Magnetic shepherding of nanocatalysts through hierarchically-assembled Fe-filled CNTs hybrids. Appl. Catal. B 2018, 227, 356–365. [Google Scholar] [CrossRef]
- De Luca, P.; Siciliano, C.; Macario, A.; Nagy, J.B. The Role of Carbon Nanotube Pretreatments in the Adsorption of Benzoic Acid. Materials 2021, 14, 2118. [Google Scholar] [CrossRef]
- Jia, Z.; Kou, K.; Qin, M.; Wu, H.; Puleo, F.; Liotta, F.L. Controllable and Large-Scale Synthesis of Carbon Nanostructures: A Review on Bamboo-Like Nanotubes. Catalysts 2017, 7, 256. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Vivekchand, S.R.C.; Govindaraj, A.; Seikh, M.M.; Rao, C.N.R. New Method of Purification of Carbon Nanotubes Based on Hydrogen Treatment. J. Phys. Chem. B 2004, 108, 6935–6937. [Google Scholar] [CrossRef]
- Naseh, M.V.; Khodadadi, A.A.; Mortazavi, Y.; Sahraei, O.A.; Pourfayaz, F.; Mosadegh, S. Functionalization of carbon nanotubes using nitric acid oxidation and DBD plasma. World Acad. Sci. Eng. Technol. 2009, 49, 177–179. [Google Scholar]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Gong, Y.; Liu, H.; Jin, C.; Guo, H.; He, J. An efficient Co-WN/CNTs composite catalyst with multiple active sites for oxygen reduction reaction activity. Chem. Phys. Lett. 2021, 770, 138452. [Google Scholar] [CrossRef]
- Modekwe, H.U.; Mamo, M.; Moothi, K.; Daramola, M.O. Synthesis of bimetallic NiMo/MgO catalyst for catalytic conversion of waste plastics (polypropylene) to carbon nanotubes (CNTs) via chemical vapour deposition method. Mater. Today Proc. 2021, 38, 549–552. [Google Scholar] [CrossRef]
- Memon, I.N.; Kumbhar, M.I.; Noonari, S. Economics of Poultry Waste Use as a Fertilizer in Sindh Pakistan. J. Fish. Livest Prod. 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Bajad, G.S.; Tiwari, S.K.; Vijayakumar, R.P. Synthesis and characterization of CNTs using polypropylene waste as precursor. Mater. Sci. Eng. B 2015, 194, 68–77. [Google Scholar] [CrossRef]
- Şahan, T.; Öztürk, D. Investigation of Pb(II) adsorption onto pumice samples: Application of optimization method based on fractional factorial design and response surface methodology. Clean Technol. Environ. Policy 2014, 16, 819–831. [Google Scholar] [CrossRef]
- Arena, U.; Mastellone, M.L.; Perugini, F. The environmental performance of alternative solid waste management options: A life cycle assessment study. Chem. Eng. J. 2003, 96, 207–222. [Google Scholar] [CrossRef]
- Silva-Rodrigo, R.; Hernández-López, F.; Martinez-Juarez, K.; Castillo-Mares, A.; Melo Banda, J.A.; Olivas-Sarabia, A.; Ancheyta, J.; Rana, M.S. Synthesis, characterization and catalytic properties of NiMo/Al2O3–MCM-41 catalyst for dibenzothiophene hydrodesulfurization. Catal. Today 2008, 130, 309–319. [Google Scholar] [CrossRef]
- Stamatin, I.; Morozan, A.; Dumitru, A.; Ciupina, V.; Prodan, G.; Niewolski, J.; Figiel, H. The synthesis of multi-walled carbon nanotubes (MWNTs) by catalytic pyrolysis of the phenol-formaldehyde resins. Physica E 2007, 37, 44–48. [Google Scholar] [CrossRef]
- Das, R.; Abd Hamid, S.B.; Ali, M.; Ramakrishna, S.; Yongzhi, W. Carbon Nanotubes Characterization by X-ray Powder Diffraction—A Review. Curr. Nanosci. 2015, 11, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Saad, N.A.; Ramya, E.; Saikiran, V.; Naraharisetty, S.R.G.; Narayana Rao, D. Novel synthesis and study of nonlinear absorption and surface-enhanced Raman scattering of carbon nanotubes decorated with silver nanoparticles. Chem. Phys. 2020, 533, 110703. [Google Scholar] [CrossRef]
- Safarova, K.; Dvorak, A.; Kubinek, R.; Vujtek, M.; Rek, A. Usage of AFM, SEM and TEM for the research of carbon nanotubes. In Modern Research and Educational Topics in Microscopy; Méndez-Vilas, A., Díaz, J., Eds.; Formatex: Badajoz, Spain, 2007; Volume 1, pp. 513–519. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.618.9072&rep=rep1&type=pdf (accessed on 6 September 2021).
- Burakov, A.E.; Burakova, I.V.; Galunin, E.V.; Kucherova, A.E. New Carbon Nanomaterials for Water Purification from Heavy Metals. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–20. [Google Scholar] [CrossRef]
- Jiang, W.; Pelaez, M.; Dionysiou, D.D.; Entezari, M.H.; Tsoutsou, D.; O’Shea, K. Chromium(VI) removal by maghemite nanoparticles. Chem. Eng. J. 2013, 222, 527–533. [Google Scholar] [CrossRef]
- Li, Z.; Bowman, R.S. Retention of inorganic oxyanions by organo-kaolinite. Water Res. 2001, 35, 3771–3776. [Google Scholar] [CrossRef]
- Padmavathy, K.S.; Madhu, G.; Haseena, P.V. A study on Effects of pH, Adsorbent Dosage, Time, Initial Concentration and Adsorption Isotherm Study for the Removal of Hexavalent Chromium (Cr (VI)) from Wastewater by Magnetite Nanoparticles. Procedia Eng. 2016, 24, 585–594. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Y.M.; Hu, W.B.; Ahmad, I.; Zhu, Y.Q.; Peng, X.J.; Luan, Z.K. Carbon nanotubes—The promising adsorbent in wastewater treatment. J. Phys. Conf. Ser. 2007, 61, 698–702. [Google Scholar] [CrossRef]



| Sr. No | Weight of PL before Drying (W2) (gm) | Weight of PL after Drying (W1) (gm) | Moisture Content (%) |
|---|---|---|---|
| 1. | 200 | 89.64 | 55.18 |
| 2. | 200 | 82.28 | 58.88 |
| 3. | 200 | 103.7 | 48.5 |
| Mean = 91.87 S.D = 10.88 | Mean = 54.18 S.D = 5.26 |
| Elements | C | N | O | Na | Mg | Si | S | Cl | K | Ca |
|---|---|---|---|---|---|---|---|---|---|---|
| Conc. | 65.17 | 1.55 | 18.77 | 0.70 | 0.50 | 0.04 | 0.08 | 0.72 | 1.71 | 0.98 |
| Run | A: Nickel (Mol) | B: Molybdenum (Mol) | C: Magnesium Oxide (Mol) | Carbon Yield (%) |
|---|---|---|---|---|
| 1. | 2.00 | 0.20 | 2.00 | 24 |
| 2. | 2.00 | 0.40 | 2.00 | 21 |
| 3. | 2.00 | 0.60 | 2.00 | 24 |
| 4. | 2.00 | 0.40 | 1.00 | 25 |
| 5. | 4.00 | 0.40 | 2.00 | 39 |
| 6. | 4.00 | 0.60 | 1.00 | 40 |
| 7. | 4.00 | 0.20 | 3.00 | 43 |
| 8. | 4.00 | 0.40 | 2.00 | 38 |
| 9. | 4.00 | 0.40 | 2.00 | 38 |
| 10. | 4.00 | 0.60 | 3.00 | 35 |
| 11. | 4.00 | 0.20 | 1.00 | 44 |
| 12. | 6.00 | 0.20 | 2.00 | 37 |
| 13. | 6.00 | 0.60 | 2.00 | 32 |
| 14. | 6.00 | 0.60 | 3.00 | 32 |
| 15. | 6.00 | 0.40 | 1.00 | 39 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 763.34 | 9 | 84.82 | 117.97 | <0.0001 | significant |
| A-Ni | 277.32 | 1 | 277.32 | 385.72 | <0.0001 | – |
| B-Mo | 49.16 | 1 | 49.16 | 68.38 | 0.0004 | – |
| C-Mg | 11.93 | 1 | 11.93 | 16.59 | 0.0096 | – |
| AB | 0.6131 | 1 | 0.6131 | 0.8528 | 0.3981 | – |
| AC | 1.08 | 1 | 1.08 | 1.51 | 0.2743 | – |
| BC | 3.30 | 1 | 3.30 | 4.59 | 0.0851 | – |
| A² | 335.36 | 1 | 335.36 | 466.45 | <0.0001 | – |
| B² | 0.0000 | 1 | 0.0000 | 0.0000 | 0.9959 | – |
| C² | 18.96 | 1 | 18.96 | 26.37 | 0.0037 | – |
| Residual | 3.59 | 5 | 0.7189 | – | – | – |
| Lack of Fit | 2.93 | 3 | 0.9760 | 2.93 | 0.2649 | not significant |
| Pure Error | 0.6667 | 2 | 0.3333 | – | – | – |
| Cor Total | 766.93 | 14 | – | – | – | – |
| Run | A: Temperature (°C) | B: Time (min) | C: Catalyst Weight (mg) | D: Carbon Yield (%) |
|---|---|---|---|---|
| 1. | 700.00 | 12.50 | 80.00 | 13.24 |
| 2. | 700.00 | 12.50 | 120.00 | 13.85 |
| 3. | 700.00 | 5.00 | 100.00 | 14.12 |
| 4. | 700.00 | 20.00 | 100.00 | 15.51 |
| 5. | 825.00 | 20.00 | 80.00 | 30.37 |
| 6. | 825.00 | 5.00 | 80.00 | 30.86 |
| 7. | 825.00 | 20.00 | 120.00 | 31.02 |
| 8. | 825.00 | 5.00 | 120.00 | 32.1 |
| 9. | 825.00 | 12.50 | 100.00 | 44.04 |
| 10. | 825.00 | 12.50 | 100.00 | 44.38 |
| 11. | 825.00 | 12.50 | 100.00 | 44.41 |
| 12. | 950.00 | 20.00 | 100.00 | 16.7 |
| 13. | 950.00 | 12.50 | 80.00 | 16.72 |
| 14. | 950.00 | 12.50 | 120.00 | 17.12 |
| 15. | 950.00 | 5.00 | 100.00 | 18.32 |
| Scheme | Sum of Squares | Df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1975.25 | 9 | 219.47 | 1722.93 | <0.0001 | significant |
| A-Temperature | 18.42 | 1 | 18.42 | 144.62 | <0.0001 | – |
| B-Time | 0.4050 | 1 | 0.4050 | 3.18 | 0.1347 | – |
| C-Catalyst Weight | 1.05 | 1 | 1.05 | 8.25 | 0.0349 | – |
| AB | 2.27 | 1 | 2.27 | 17.78 | 0.0084 | – |
| AC | 0.0110 | 1 | 0.0110 | 0.0865 | 0.7804 | – |
| BC | 0.0870 | 1 | 0.0870 | 0.6832 | 0.4461 | – |
| A² | 1784.57 | 1 | 1784.57 | 14,009.47 | <0.0001 | – |
| B² | 138.73 | 1 | 138.73 | 1089.05 | <0.0001 | – |
| C² | 184.02 | 1 | 184.02 | 1444.59 | <0.0001 | – |
| Residual | 0.6369 | 5 | 0.1274 | – | – | – |
| Lack of Fit | 0.5524 | 3 | 0.1841 | 4.36 | 0.1922 | not significant |
| Pure Error | 0.0845 | 2 | 0.0422 | – | – | – |
| Cor Total | 1975.89 | 14 | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haleem, N.; Jamal, Y.; Khan, S.N.; Baig, M.A.; Wahab, M.; Yang, X. Synthesis of Carbon Nanotubes (CNTs) from Poultry Litter for Removal of Chromium (Cr (VI)) from Wastewater. Materials 2021, 14, 5195. https://doi.org/10.3390/ma14185195
Haleem N, Jamal Y, Khan SN, Baig MA, Wahab M, Yang X. Synthesis of Carbon Nanotubes (CNTs) from Poultry Litter for Removal of Chromium (Cr (VI)) from Wastewater. Materials. 2021; 14(18):5195. https://doi.org/10.3390/ma14185195
Chicago/Turabian StyleHaleem, Noor, Yousuf Jamal, Shahid Nawaz Khan, Muhammad Anwar Baig, Maryam Wahab, and Xufei Yang. 2021. "Synthesis of Carbon Nanotubes (CNTs) from Poultry Litter for Removal of Chromium (Cr (VI)) from Wastewater" Materials 14, no. 18: 5195. https://doi.org/10.3390/ma14185195
APA StyleHaleem, N., Jamal, Y., Khan, S. N., Baig, M. A., Wahab, M., & Yang, X. (2021). Synthesis of Carbon Nanotubes (CNTs) from Poultry Litter for Removal of Chromium (Cr (VI)) from Wastewater. Materials, 14(18), 5195. https://doi.org/10.3390/ma14185195







