Cytotoxic Effects on Gingival Mesenchymal Stromal Cells and Root Surface Modifications Induced by Some Local Antimicrobial Products Used in Periodontitis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Gingival Mesenchymal Stromal Cell (gMSC) Isolation and Characterization
2.3. Preparation of Experimental Culture Media
2.4. Cell Proliferation (CCK-8)
2.5. Cell Survival by MTT Assay
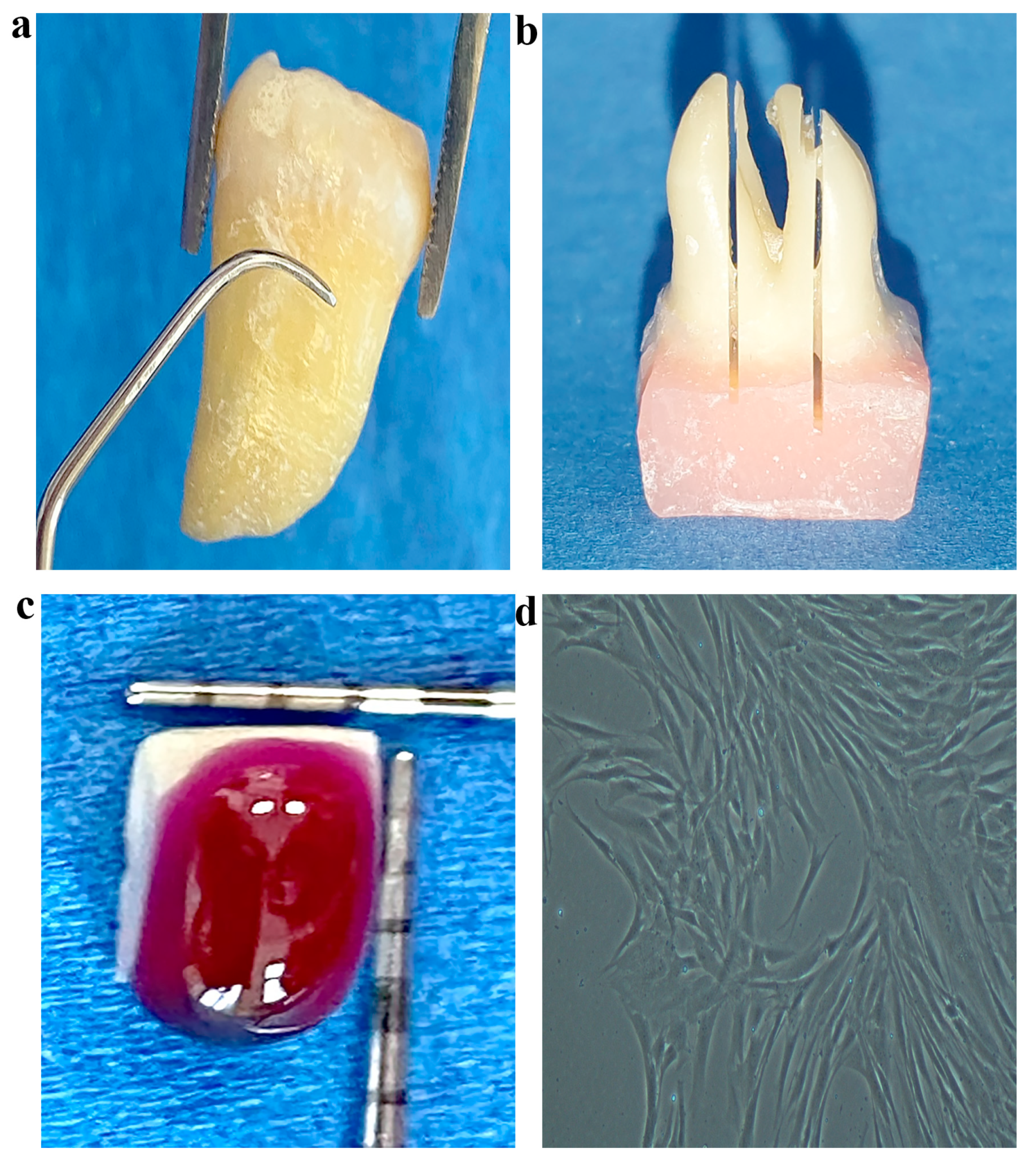
2.6. Cell Culture on Treated Root Samples
2.7. Statistical Analysis
3. Results
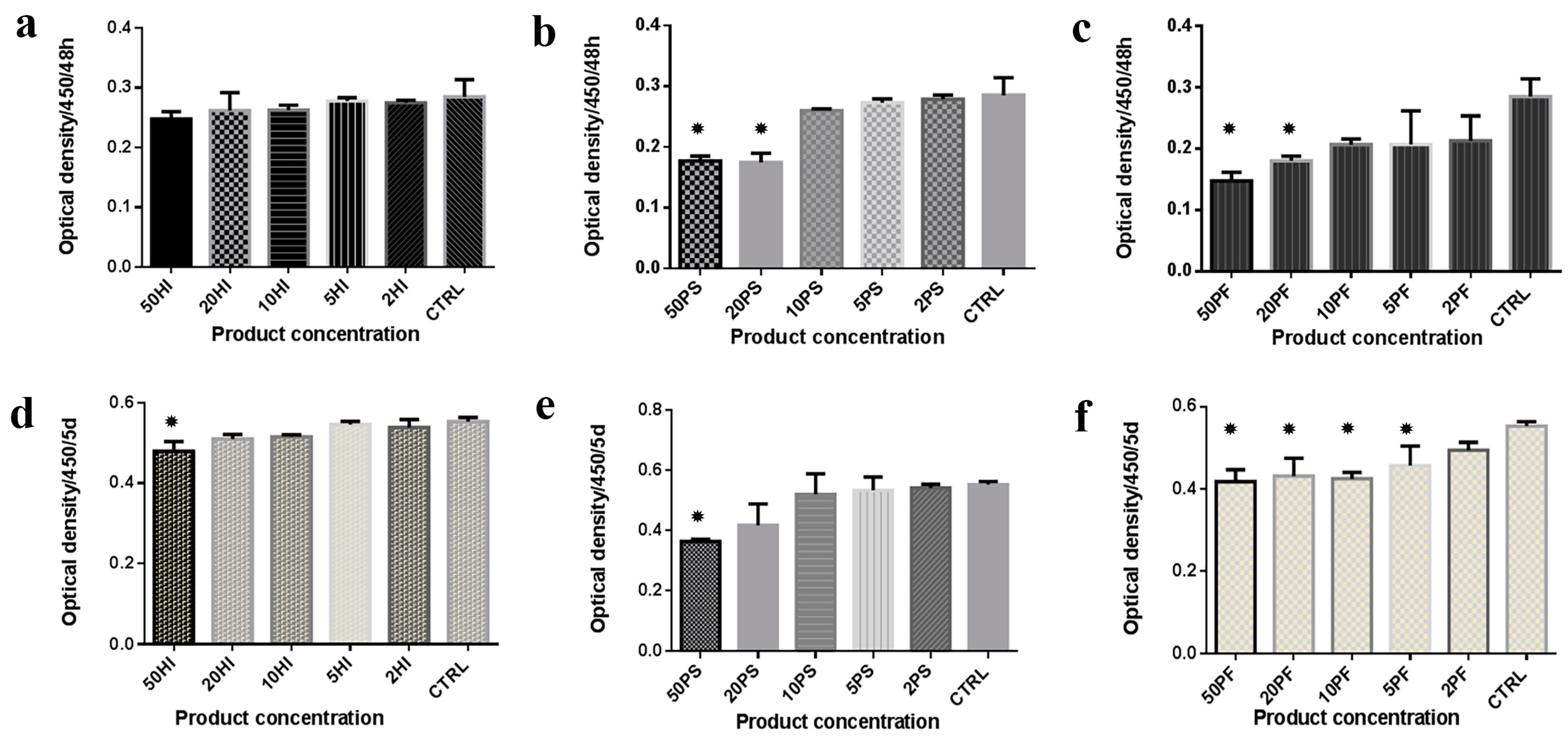
3.1. CCK Analysis
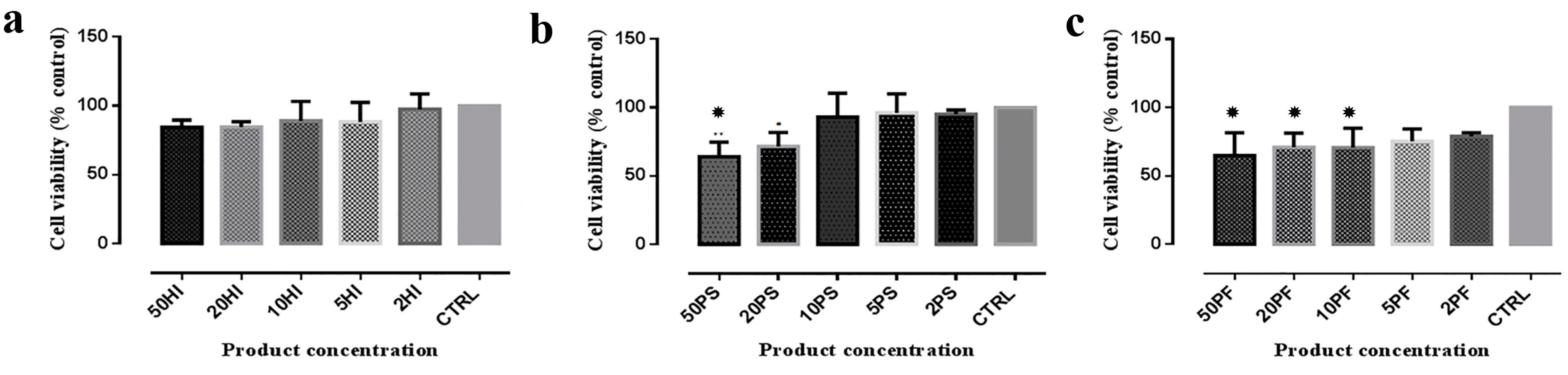
3.2. MTT Analysis
3.3. SEM Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savage, A.; Eaton, K.A.; Moles, D.R.; Needleman, I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J. Clin. Periodontol. 2009, 36, 458–467. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontol. 2000 2017, 75, 152–188. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Merete Aass, A.; Aimetti, M.; et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Gomes, E.W.B.; Casarin, M.; Martins, T.M.; da Silva, A.F. Local delivery therapies as adjuvants to non-surgical periodontal treatment of periodontitis grade C: A systematic review. Clin. Oral Investig. 2000, 24, 4213–4224. [Google Scholar] [CrossRef]
- Hallmon, W.W.; Rees, T.D. Local anti-infective therapy: Mechanical and physical approaches. A systematic review. Ann. Periodontol. 2003, 8, 99–114. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524.e5. [Google Scholar] [CrossRef] [PubMed]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Nagarakanti, S.; Gunupati, S.; Chava, V.K.; Ramesh Reddy, B.V. Effectiveness of subgingival irrigation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A systematic review. J. Clin. Diagn. Res. 2015, 9, ZE06–ZE09. [Google Scholar] [CrossRef]
- Antonelli, A.; Giovannini, L.; Baccani, I.; Giuliani, V.; Pace, R.; Rossolini, G.M. In vitro antimicrobial activity of the decontaminant hybenx® compared to chlorhexidine and sodium hypochlorite against common bacterial and yeast pathogens. Antibiotics 2019, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Pini-Prato, G.; Magnani, C.; Rotundo, R. Treatment of Acute Periodontal Abscesses Using the Biofilm Decontamination Approach: A Case Report Study. Int. J. Periodontics Restor. Dent. 2016, 36, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.; Al-Johani, K.; Fedele, S.; Moles, D. Randomised controlled trial of the efficacy of HybenX in the symptomatic treatment of recurrent aphthous stomatitis. Oral Dis. 2009, 15, 155–161. [Google Scholar] [CrossRef]
- Babich, H.; Tipton, D.A. In vitro response of human gingival epithelioid S-G cells to minocycline. Toxicol. In Vitro 2002, 16, 11–21. [Google Scholar] [CrossRef]
- Cabral, C.T.; Fernandes, M.H. In vitro comparison of chlorhexidine and povidone-iodine on the long-term proliferation and functional activity of human alveolar bone cells. Clin. Oral Investig. 2007, 11, 155–164. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.G.; Si, Y.M.; Chen, B.; Meng, J. The difference on the osteogenic differentiation between periodontal ligament stem cells and bone marrow mesenchymal stem cells under inflammatory microenviroments. Differentiation 2014, 88, 97–105. [Google Scholar] [CrossRef]
- Ramanauskaite, E.; Machiulskiene, V.; Eliezer, M.; Sculean, A. Sodium Hypochlorite as an Adjunct to Nonsurgical Treatment of Periodontitis: A Systematic Review. Oral Health Prev. Dent. 2020, 18, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, P.; Imfeld, T.; Ronay, V.; Attin, T.; Schmidlin, P.R. Povidone-iodine gel and solution as adjunct to ultrasonic debridement in nonsurgical periodontitis treatment: An RCT pilot study. Quintessence Int. 2014, 45, 281–290. [Google Scholar] [CrossRef]
- Lauritano, D.; Pazzi, D.; Iapichino, A.; Gaudio, R.M.; Di Muzio, M.; Lo Russo, L.; Pezzetti, F. Evaluation of the efficacy of a new oral gel containing carvacrol and thymol for home oral care in the management of chronic periodontitis using PCR analysis: A microbiological pilot study. J. Biol. Regul. Homeost. Agents 2016, 30, 129–134. [Google Scholar]
- Bracke, J.; Basara, M.; Savor, E.; Dunaway, A.; Watkins, M. Pilot evaluation of a simple adjunctive method for improved removal of oral biofilm during conventional scaling and root planing therapy. J. Biol. Regul. Homeost. Agents 2015, 29, 6–9. [Google Scholar] [PubMed]
- Isola, G.; Matarese, G.; Williams, R.C.; Siciliano, V.I.; Alibrandi, A.; Cordasco, G.; Ramaglia, L. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin. Oral Investig. 2018, 22, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Signoriello, A.; Corrocher, G.; Signoretto, C.; Burlacchini, G.; Pardo, A.; Nocini, P.F. A Topical Desiccant Agent in Association with Manual Debridement in the Initial Treatment of Peri-Implant Mucositis: A Clinical and Microbiological Pilot Study. Antibiotics 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Althumairy, R.I.; Diogenes, A. Direct and Indirect Effect of Chlorhexidine on Survival of Stem Cells from the Apical Papilla and Its Neutralization. J. Endod. 2019, 45, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Farhad Mollashahi, N.; Saberi, E.; Karkehabadi, H. Evaluation of cytotoxic effects of various endodontic irrigation solutions on the survival of stem cell of human apical papilla. Iran. Endod. J. 2016, 11, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, S.J.; Gawlitta, D.; Heemstra, K.A.; Poolman, R.W.; Vogely, H.C.; Kruyt, M.C. Selection of an optimal antiseptic solution for intraoperative irrigation: An in vitro study. J. Bone Jt. Surg. Am. 2014, 96, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.B.; Zilinski, G.S.; Kirkpatrick, T.C.; Himel, V.T.; Sabey, K.A.; Lallier, T.E. The Effects of Irrigants on the Survival of Human Stem Cells of the Apical Papilla, Including Endocyn. J. Endod. 2018, 44, 263–268. [Google Scholar] [CrossRef]
- Alkahtani, A.; Alkahtany, S.M.; Anil, S. An in vitro evaluation of the cytotoxicity of varying concentrations of sodium hypochlorite on human mesenchymal stem cells. J. Contemp. Dent. Pract. 2014, 15, 473–481. [Google Scholar] [CrossRef]
- Regedent, A.G. PERISOLV®. Available online: http://www.regedent.com/en/professionals/products/perisolv/ (accessed on 15 March 2021).
- Liu, J.X.; Werner, J.A.; Buza, J.A., III; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Povidone-iodine solutions inhibit cell migration and survival of osteoblasts, fibroblasts, and myoblasts. Spine 2017, 42, 1757–1762. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Hernández-Sierra, J.F.; Galicia-Cruz, O.; Angélica, S.A.; Ruiz, F.; Pierdant-Pérez, M.; Pozos-Guillén, A.J. In vitro cytotoxicity of silver nanoparticles as a hydroxyapatite modifier on human fibroblasts. Front. Bioeng. J. Clin. Pediatr. Dent. 2011, 36, 37–42. [Google Scholar] [CrossRef]
- Chan, E.L.; Zhang, C.; Cheung, G.S.P. Cytotoxicity of a novel nano-silver particle endodontic irrigant. Clin. Cosmet. Investig. Dent. 2015, 7, 65–74. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- HYBENX—Oral Tissue Decontaminant. Available online: http://hybenx.it/ (accessed on 15 March 2021).
- Lombardo, G.; Signoretto, C.; Corrocher, G.; Pardo, A.; Pighi, J.; Rovera, A.; Caccuri, F.; Nocini, P.F. A Topical Desiccant Agent in Association with Ultrasonic Debridement in the Initial Treatment of Chronic Periodontitis: A Clinical and Microbiological Study. New Microbiol. 2015, 38, 393–407. [Google Scholar] [PubMed]
- Dental Life Sciences. Perioflush. Available online: https://www.dentallifesciences.com/products/perioflush/ (accessed on 15 March 2021).
- Feist, I.S.; De Micheli, G.; Carneiro, S.R.S.; Eduardo, C.P.; Miyagi, S.P.H.; Marques, M.M. Adhesion and Growth of Cultured Human Gingival Fibroblasts on Periodontally Involved Root Surfaces Treated by Er:YAG Laser. J. Periodontol. 2003, 74, 1368–1375. [Google Scholar] [CrossRef]
- Blomlöf, J.; Kansson, L.; Blomlöf, L.; Lindskog, S. Root surface etching at neutral pH promotes periodontal healing. J. Clin. Periodontol. 1996, 23, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, M.D.; Prasad, H.S.; Savord, E.G. A histologic assessment of a Hybenix ® oral tissue decontaminant in vital pulp therapy in dogs. J. Biol. Regul. Homeost. Agents 2016, 30, 189–197. [Google Scholar]
- Lopez, M.A.; Passarelli, P.C.; Godino, E.; Lombardo, N.; Altamura, F.R.; Speranza, A.; Lopez, A.; Papi, P.; Pompa, G.; D’Addona, A. The treatment of peri-implant diseases: A new approach using HYBENX® as a decontaminant for implant surface and oral tissues. Antibiotics 2021, 10, 512. [Google Scholar] [CrossRef]
- Pace, R.; Morecchiato, F.; Giovannini, L.; Di Nasso, L.; Giuliani, V.; Franceschi, D.; Pagavino, G.; Rossolini, G.M.; Antonelli, A. In vitro alteration by dentine and protein of the antimicrobial activity of two endodontic irrigants: HybenX® and sodium hypochlorite. Antibiotics 2020, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.H.; Fan, B.; Purcell, W.; Meghil, M.M.; Cutler, C.W.; Bergeron, B.E.; Ma, J.Z.; Tay, F.R.; Niu, L.N. Anti-biofilm efficacy of root canal irrigants against in-situ Enterococcus faecalis biofilms in root canals, isthmuses and dentinal tubules. J. Dent. 2018, 79, 68–76. [Google Scholar] [CrossRef]
- Veena, H.R.; Mahantesha, S.; Joseph, P.A.; Patil, S.R.; Patil, S.H. Dissemination of aerosol and splatter during ultrasonic scaling: A pilot study. J. Infect. Public Health 2015, 8, 260–265. [Google Scholar] [CrossRef]
- Bajrami, D.; Hoxha, V.; Gorduysus, O.; Muftuoglu, S.; Zeybek, N.D.; Küçükkaya, S. Cytotoxic effect of endodontic irrigants in vitro. Med. Sci. Monit. Basic Res. 2014, 20, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.R.; Fujioka-Kobayashi, M.; Mueller, H.D.; Sculean, A.; Lussi, A.; Miron, R.J. Effects of air polishing and an amino acid buffered hypochlorite solution to dentin surfaces and periodontal ligament cell survival, attachment, and spreading. Clin. Oral Investig. 2017, 21, 1589–1598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beeley, J.A.; Yip, H.K.; Stevenson, A.G. Chemo-mechanical caries removal: A review of the techniques and latest developments. Ned. Tijdschr. Tandheelkd 2001, 108, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, B.; He, J.; Cui, Z.; Liu, Y. Silver nanoparticles promote osteogenic differentiation of human periodontal ligament fibroblasts by regulating the RhoA–TAZ axis. Cell Biol. Int. 2019, 43, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Illeperuma, R.P.; Park, Y.J.; Kim, J.M.; Bae, J.Y.; Che, Z.M.; Son, H.K.; Han, M.R.; Kim, K.M.; Kim, J. Immortalized gingival fibroblasts as a cytotoxicity test model for dental materials. J. Mater. Sci. Mater. Med. 2012, 23, 753–762. [Google Scholar] [CrossRef]
- Flury, S.; Hayoz, S.; Peutzfeldt, A.; Hüsler, J.; Lussi, A. Depth of cure of resin composites: Is the ISO 4049 method suitable for bulk fill materials? Dent. Mater. 2012, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.C.; Nattuthurai, G.S.; Sundararajan, S.K.; Sujith, I.; Joseph, J.; Syedshah, Y.P. Gingival Crevicular Fluid: An Overview Title. J. Pharm. Bioallied Sci. 2019, 11, S135–S139. [Google Scholar] [CrossRef]
- Mengel, R.; Stelzel, M.; Mengel, C.; Flores-de-Jacoby, L.; Diekwisch, T. An in vitro study of various instruments for root planing. Int. J. Periodontics Restor. Dent. 1997, 17, 592–599. [Google Scholar]
- Maritato, M.; Orazi, L.; Laurito, D.; Formisano, G.; Serra, E.; Lollobrigida, M.; Molinari, A.; De Biase, A. Root surface alterations following manual and mechanical scaling: A comparative study. Int. J. Dent. Hyg. 2018, 16, 553–558. [Google Scholar] [CrossRef]
- Oberholzer, R.; Rateitschak, K.H. Root cleaning or root smoothing. An in vivo study. J. Clin. Periodontol. 1996, 23, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.J.; Cho, C.M.; Jeong, S.N. Characteristics of the molar surface after removal of cervical enamel projections: Comparison of three different rotating instruments. J. Periodontal Implant Sci. 2016, 46, 107–115. [Google Scholar] [CrossRef]
- Ballal, V.; Khandelwal, D.; Yegneswaran, P.P.; Varghese, J.; Al-Haj Husain, N.; Özcan, M. Evaluation of Smear Layer Removal and Antimicrobial Efficacy of HybenX Against Enterococcus Faecalis Biofilm. Eur. J. Prosthodont. Restor. Dent. 2021, 29, 6–13. [Google Scholar] [CrossRef]
- Damante, C.A.; Ducati, P.; Ferreira, R.; Salmeron, S.; Zangrando, M.S.R.; de Rezende, M.L.R.; Sant’Ana, A.C.P.; Greghi, S.L.A.; Magalhães, A.C. In vitro evaluation of adhesion/proliferation of human gingival fibroblasts on demineralized root surfaces by toluidine blue O in antimicrobial photodynamic therapy. Photodiagn. Photodyn. Ther. 2016, 13, 303–307. [Google Scholar] [CrossRef] [PubMed]

| Product | Manufacturer | Composition | Indication |
|---|---|---|---|
| HYBENX® [35,36] (HI) | Epien Medical, St Paul, MN, USA | Hygroscopic solution Sulfonated phenolics (60%) Sulfuric acid (28%) Water (12%) | Oral Tissue Decontaminant Removal of plaque biofilm Topical irrigation during scaling and root planing Topical rinse/solution for oral cavity Adjunctive rinse of tooth root canal systems in endodontic treatments |
| Perisolv® [29] (PS) | Regedent AG, Zurich, Switzerland | Gel: Amino acids (glutamic acid, leucine, lysine) Sodium chloride, Sodium carboxymethyl cellulose Ultrapure water Liquid: Sodium hypochlorite solution 0.95% | Adjunctive therapy to scaling and root planing in periodontitis and peri-implantitis due to inhibitory bacterial effects and biofilm elimination |
| Perioflush® [37] (PF) | Dental Life Sciences LTD, Wigan, UK | Water based solution: Ultrapurified water Silver nanocolloid solution Sodium nitrate Apple, mint flavours Lactic acid Phosphoric acid | Rinsing and cleaning the gums and oral mucosa Rinsing around bridge spans, erupting teeth, around implant areas, overhanging fillings, interdental spaces, after scaling |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupșe, I.; Pall, E.; Barbu Tudoran, L.; Bulboacă, A.E.; Ciurea, A.; Micu, I.C.; Roman, A.; Delean, A.G.; Muntean, A.; Soancă, A. Cytotoxic Effects on Gingival Mesenchymal Stromal Cells and Root Surface Modifications Induced by Some Local Antimicrobial Products Used in Periodontitis Treatment. Materials 2021, 14, 5049. https://doi.org/10.3390/ma14175049
Lupșe I, Pall E, Barbu Tudoran L, Bulboacă AE, Ciurea A, Micu IC, Roman A, Delean AG, Muntean A, Soancă A. Cytotoxic Effects on Gingival Mesenchymal Stromal Cells and Root Surface Modifications Induced by Some Local Antimicrobial Products Used in Periodontitis Treatment. Materials. 2021; 14(17):5049. https://doi.org/10.3390/ma14175049
Chicago/Turabian StyleLupșe, Irina, Emoke Pall, Lucian Barbu Tudoran, Adriana Elena Bulboacă, Andreea Ciurea, Iulia Cristina Micu, Alexandra Roman, Ada Gabriela Delean, Alexandrina Muntean, and Andrada Soancă. 2021. "Cytotoxic Effects on Gingival Mesenchymal Stromal Cells and Root Surface Modifications Induced by Some Local Antimicrobial Products Used in Periodontitis Treatment" Materials 14, no. 17: 5049. https://doi.org/10.3390/ma14175049
APA StyleLupșe, I., Pall, E., Barbu Tudoran, L., Bulboacă, A. E., Ciurea, A., Micu, I. C., Roman, A., Delean, A. G., Muntean, A., & Soancă, A. (2021). Cytotoxic Effects on Gingival Mesenchymal Stromal Cells and Root Surface Modifications Induced by Some Local Antimicrobial Products Used in Periodontitis Treatment. Materials, 14(17), 5049. https://doi.org/10.3390/ma14175049











