M(II)Al4 Type Layered Double Hydroxides—Preparation Using Mechanochemical Route, Structural Characterization and Catalytic Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanical Pretreatment of Gibbsite
2.3. Preparation of the Magnesium-, Copper-, Nickel-, Cobalt- and Zinc-Poor M(II)Al4-LDHs
2.4. Carbon Monoxide Oxidation in a Continuous Flow Reactor
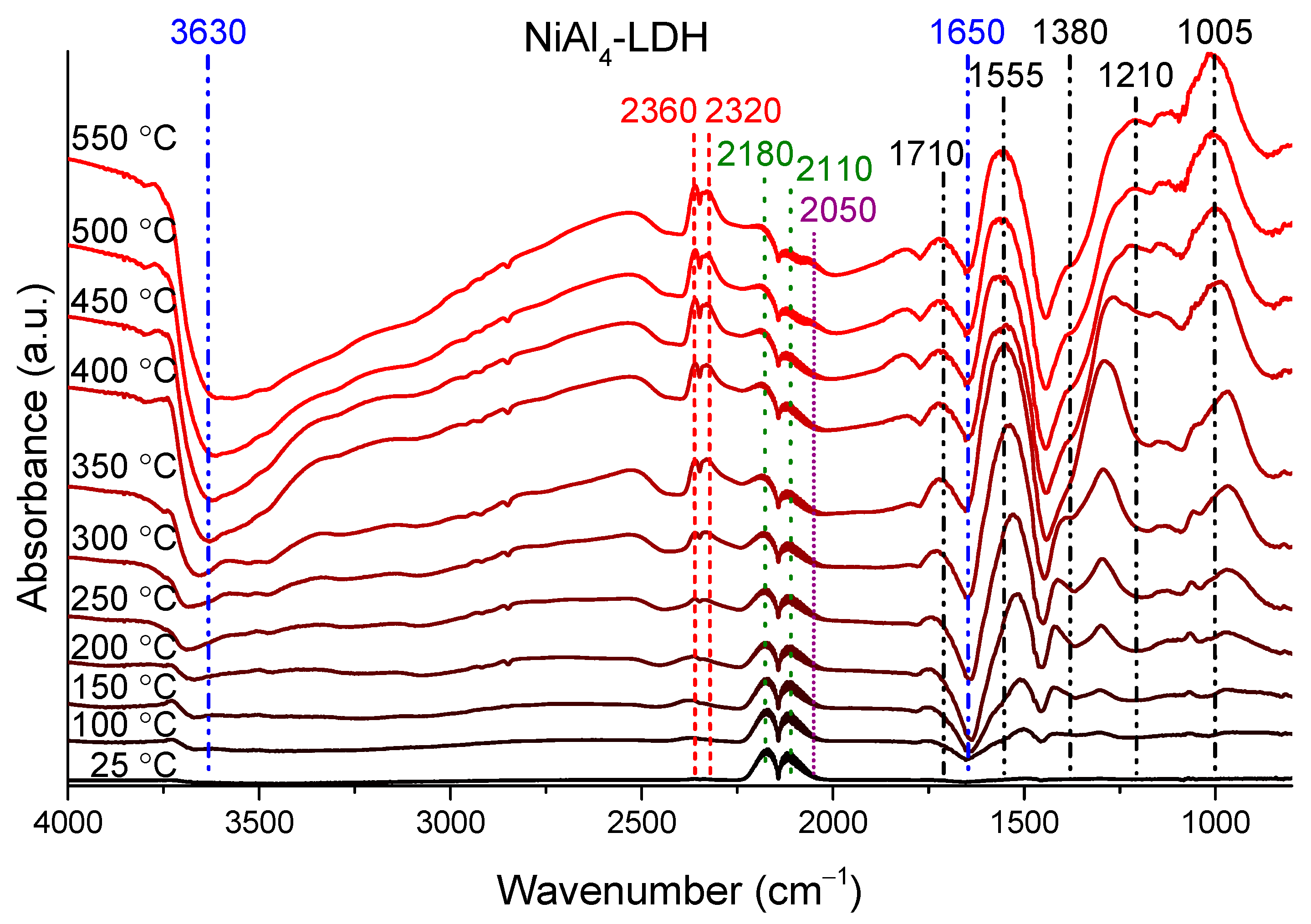
2.5. Methods of Structural Characterization
3. Results and Discussion
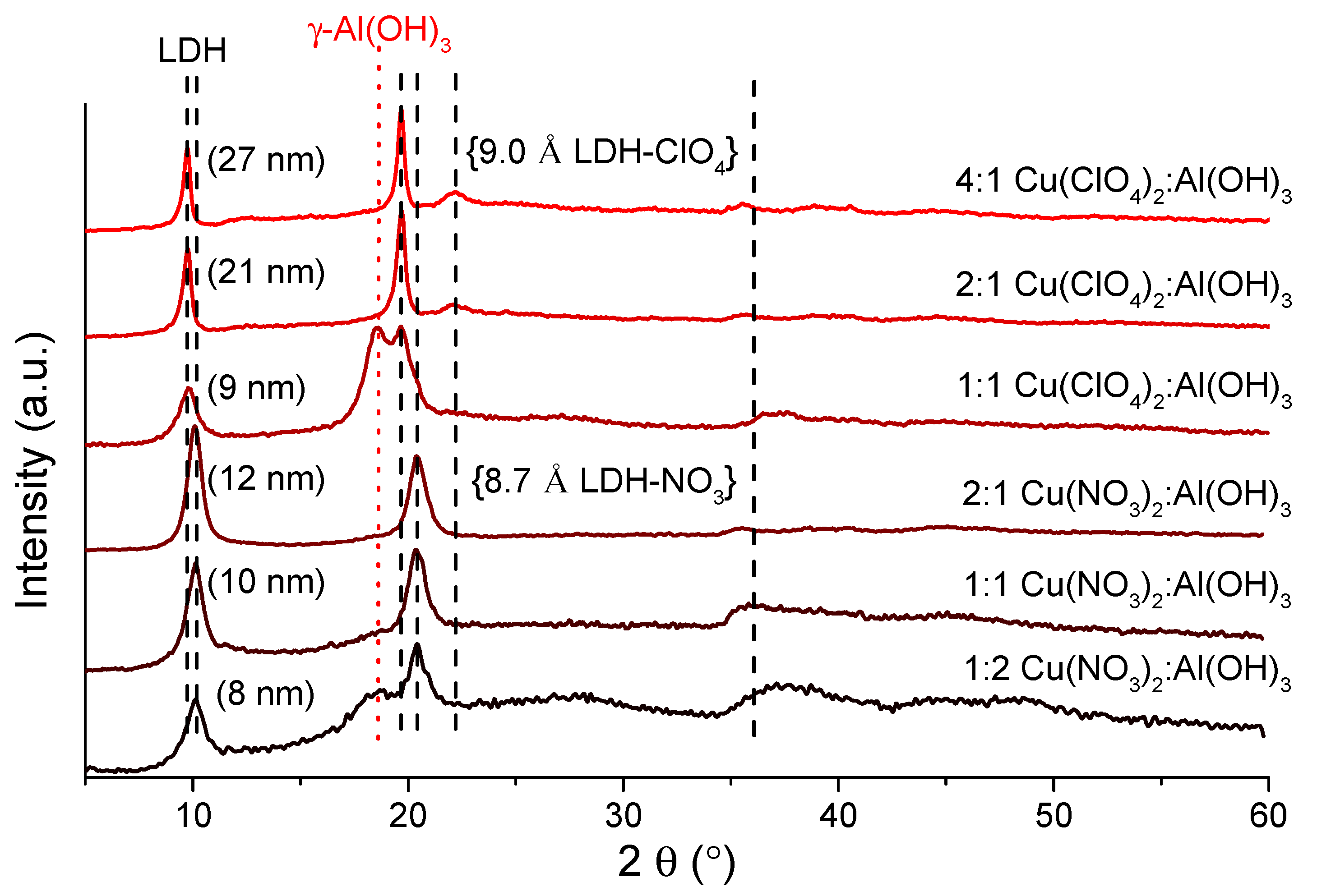
3.1. Optimization of the Synthesis Parameters for the Preparation of CuAl4–LDHs
3.2. Preparation of Layered Triple- and Multiple Hydroxide Systems
3.3. Optical Properties and Morphology of the Solids
3.4. Catalytic Oxidation of Carbon Monoxide over the Aluminum-Rich LDHs as Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Hoyo, C. Layered double hydroxides and human health: An overview. Appl. Clay Sci. 2007, 36, 103–121. [Google Scholar] [CrossRef]
- Hafez, I.H.; Berber, M.R.; Minagawa, K.; Mori, T.; Tanaka, M. Formulation of polyacrylic acid-layered double hydroxide composite system as a soil conditioner for water management. Int. J. Mod. Phys: Conf. Ser. 2012, 48, 138–143. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Wang, Q.; Wilkie, C.A.; O’Hare, D. Flame retardant polymer/layered double hydroxide nanocomposites. J. Mater. Chem. A 2014, 2, 10996–11016. [Google Scholar] [CrossRef]
- Szabados, M.; Ádám, A.A.; Traj, P.; Muráth, S.; Baán, K.; Bélteky, P.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Mechanochemical and wet chemical syntheses of CaIn-layered double hydroxide and its performance in a transesterification reaction compared to those of other Ca2M(III) hydrocalumites (M: Al, Sc, V, Cr, Fe, Ga) and Mg(II)-, Ni(II)-, Co(II)- or Zn(II)-based hydrotalcites. J. Catal. 2020, 391, 282–297. [Google Scholar]
- Prescott, H.A.; Li, Z.J.; Kemnitz, E.; Trunschke, A.; Deutsch, J.; Lieske, H.; Auroux, A. Application of calcined Mg–Al hydrotalcites for Michael additions: An investigation of catalytic activity and acid–base properties. J. Catal. 2005, 234, 119–130. [Google Scholar] [CrossRef]
- Wu, M.J.; Wu, J.Z.; Zhang, J.; Chen, H.; Zhou, J.Z.; Qian, G.R.; Xu, Z.P.; Du, Z.; Rao, Q.L. A review on fabricating heterostructures from layered double hydroxides for enhanced photocatalytic activities. Catal. Sci. Technol. 2018, 8, 1207–1228. [Google Scholar] [CrossRef]
- Evans, G.D.; Slade, R.C.T. Structural aspects of layered double hydroxides. Struct. Bond. 2006, 119, 1–87. [Google Scholar]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Forano, C.; Hibino, T.; Leroux, F.; Taviot-Guého, C. Layered double hydroxides. Dev. Clay Sci. 2006, 1, 1021–1095. [Google Scholar]
- Williams, G.R.; Dunbar, G.; Beer, A.J.; Fogg, A.M.; O’Hare, D. Intercalation chemistry of the novel layered double hydroxides [MAl4(OH)12](NO3)2·yH2O (M = Zn, Cu, Ni and Co). 1: New organic intercalates and reaction mechanisms. J. Mater. Chem. 2006, 16, 1222–1230. [Google Scholar] [CrossRef]
- Britto, S.; Kamath, P.V. Polytypism, disorder, and anion exchange properties of divalent ion (Zn, Co) containing bayerite-derived layered double hydroxides. Inorg. Chem. 2010, 49, 11370–11377. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, D.D.; Banerjee, S. Lithium aluminium layered double hydroxides: Synthesis and application in poly (vinyl chloride). Int. J. Polym. Mater. 2012, 61, 985–998. [Google Scholar] [CrossRef]
- Fogg, A.M.; O’Hare, D. Study of the intercalation of lithium salt in gibbsite using time-resolved in situ X-ray diffraction. Chem. Mater. 1999, 11, 1771–1775. [Google Scholar] [CrossRef]
- Su, L.W.; Lin, D.J.; Uan, J.Y. Novel dental resin composites containing LiAl-F layered double hydroxide (LDH) filler: Fluoride release/recharge, mechanical properties, color change, and cytotoxicity. Dent. Mater. 2019, 35, 663–672. [Google Scholar] [CrossRef]
- Fogg, A.M.; Williams, G.R.; Chester, R.; O’Hare, D. A novel family of layered double hydroxides—[MAl4(OH)12](NO3)2·xH2O (M = Co, Ni, Cu, Zn). J. Mater. Chem. 2004, 14, 2369–2371. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Sonoda, A.; Hirotsu, T. Synthesis of a novel layered double hydroxides [MgAl4(OH)12](Cl)2·2.4H2O and its anion-exchange properties. J. Hazard. Mater. 2011, 185, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.R.; Burden, C.S.; Fogg, A.M. New layered double hydroxides by prepared by the intercalation of gibbsite. J. Solid State Chem. 2015, 224, 36–39. [Google Scholar] [CrossRef]
- Pushparaj, S.S.C.; Jensen, N.D.; Forano, C.; Rees, G.J.; Prevot, V.; Hanna, J.V.; Ravnsbæk, D.B.; Bjerring, M.; Nielsen, U.G. Structural investigation of Zn(II) insertion in bayerite, an aluminum hydroxide. Inorg. Chem. 2016, 55, 9306–9315. [Google Scholar] [CrossRef]
- Williams, G.R.; Moorhous, S.J.; Prior, T.J.; Fogg, A.M.; Rees, N.H.; O’Hare, D. New insights into the intercalation chemistry of Al(OH)3. Dalton Trans. 2011, 40, 6012–6022. [Google Scholar] [CrossRef]
- Jensen, N.D.; Duong, N.T.; Bolanz, R.; Nishiyama, Y.; Rasmussen, C.A.; Gottlicher, J.; Steininger, R.; Prevot, V.; Nielsen, U.G. Synthesis and structural characterization of a pure ZnAl4(OH)12(SO4)·2.6H2O layered double hydroxide. Inorg. Chem. 2019, 58, 6114–6122. [Google Scholar] [CrossRef]
- Isupov, V.P. Intercalation compound of aluminium hydroxide. J. Struct. Chem. 1999, 40, 672–685. [Google Scholar] [CrossRef]
- Barnard, B.A.; Labuschagné, F.J.W.J. Exploring the wet mechanochemical synthesis of Mg-Al, Ca-Al, Zn-Al and Cu-Al layered double hydroxides from oxides, hydroxides and basic carbonates. Crystals 2020, 10, 954. [Google Scholar] [CrossRef]
- Ferencz, Z.; Szabados, M.; Adok-Sipiczki, M.; Kukovecz, A.; Konya, Z.; Sipos, P.; Palinko, I. Mechanochemical assisted synthesis of pristine Ca(II)Sn(IV)-layered double hydroxides and their amino acid intercalated composites. Mater. Sci. 2014, 49, 8479–8486. [Google Scholar] [CrossRef]
- Szabados, M.; Szabados, T.; Mucsi, R.; Baán, K.; Sápi, A.; Kónya, Z.; Kukovecz, Á.; Pálinkó, I.; Sipos, P. Mechanochemically induced gibbsite intercalation for preparing NiAl4-layered double hydroxides with interlayer halide and oxoanions (Cl−, Br−, I−, NO3−, NH2SO3−, SO42−, ClO4−) and their application in CO2 methanation reaction. J. Colloid Interface Sci. 2021. submitted. [Google Scholar]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; pp. 111–114. [Google Scholar]
- Huang, L.; Wang, J.; Gao, Y.; Qiao, Y.; Zheng, Q.; Gou, Z.; Zhao, Y.; O’Hare, D.; Wang, Q. Synthesis of LiAl2-layered double hydroxides for CO2 capture over a wide temperature range. J. Mater. Chem. A 2014, 2, 18454–18462. [Google Scholar] [CrossRef]
- Britto, S.; Kamath, P.V. Synthesis, structure refinement and chromate sorption characteristics of an Al-rich bayerite-based layered double hydroxide. J. Solid State Chem. 2014, 215, 206–210. [Google Scholar] [CrossRef][Green Version]
- Kloprogge, J.T.; Ruan, H.D.; Frost, R.L. Thermal decomposition of bauxite minerals: Infrared emission spectroscopy of gibbsite, boehmite and diaspore. J. Mater. Sci. 2002, 37, 1121–1129. [Google Scholar] [CrossRef]
- Song, J.; Leng, M.; Fu, X.; Liu, J. Synthesis and characterization of nanosized zinc aluminate spinel from a novel Zn–Al layered double hydroxide precursor. J. Alloy. Compd. 2012, 543, 142–146. [Google Scholar] [CrossRef]
- Miyata, S. The syntheses of hydrotalcite-like compounds and their structures and physico-chemical properties I: The systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clay Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Smith, D.W. Ionic hydration enthalpies. J. Chem. Educ. 1977, 54, 540–542. [Google Scholar] [CrossRef]
- Lincoln, S.F. Mechanistic studies of metal aqua ions: A semi-historical perspective. Helv. Chim. Acta 2005, 88, 523–545. [Google Scholar] [CrossRef]
- Graham, R.T.; Hu, J.Z.; Zhang, X.; Dembowski, M.; Jaegers, N.R.; Wan, C.; Bowden, M.; Lipton, A.S.; Felmy, R.A.; Clark, S.B.; et al. Unraveling gibbsite transformation pathways into LiAl-LDH in concentrated lithium hydroxide. Inorg. Chem. A 2019, 58, 12385–12394. [Google Scholar] [CrossRef]
- Kim, H.; Lee, B.I.; Jeong, H.; Byeon, S.H. Relationship between interlayer anions and photoluminescence of layered rare earth hydroxides. J. Mater. Chem. C 2015, 3, 7437–7445. [Google Scholar] [CrossRef]
- Lorenzi, G.; Baldi, G.; Di Benedetto, F.; Faso, V.; Lattanzi, P.; Romanelli, M. Spectroscopic study of a Ni-bearing gahnite pigment. J. Eur. Ceram. Soc. 2006, 26, 317–321. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Boukha, Z.; de Rivas, J.J.; Delgado, M.Á.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Structural characterisation of Ni/alumina reforming catalysts activated at high temperatures. Appl. Catal. A Gen. 2013, 466, 9–20. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Hashimoto, S.; Satoh, N.; Ohashi, F.; Tomura, S. The effect of cobalt on the structural properties and reducibility of CuCoZnAl layered double hydroxides and their thermally derived mixed oxides. J. Mater. Chem. 2001, 11, 2049–2060. [Google Scholar] [CrossRef]
- Dondi, M.; Ardit, M.; Cruciani, G.; Zanelli, C. Tetrahedrally coordinated Co2+ in oxides and silicates: Effect of local environment on optical properties. Am. Mineral. 2014, 99, 1736–1745. [Google Scholar] [CrossRef]
- Besserguenev, A.V.; Frogg, A.M.; Francis, R.J.; Price, S.J.; O’Hare, D. Synthesis and structure of the gibbsite intercalation compounds [LiAl2(OH)6]X {X = Cl, Br, NO3} and [LiAl2(OH)6]Cl·H2O using synchrotron X-ray and neutron powder diffraction. Chem. Mater. 1997, 9, 241–247. [Google Scholar] [CrossRef]
- Qu, J.; Li, X.; Lei, Z.; Li, Z.; Chen, M.; Zhang, Q. Mechano-hydrothermal synthesis of tetraborate pillared Li–Al layered double hydroxides. J. Am. Ceram. Soc. 2016, 99, 1151–1154. [Google Scholar] [CrossRef]
- Ramesh, K.; Chen, L.; Chen, F.; Liu, Y.; Whang, Z.; Han, Y.F. Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts. Catal. Today 2008, 131, 477–482. [Google Scholar] [CrossRef]
- Soubaihi, R.M.A.; Saoud, K.M.; Dutta, J. Critical review of low-temperature CO oxidation and hysteresis phenomenon on heterogeneous catalysts. Catalysts 2018, 8, 660. [Google Scholar] [CrossRef]
- Gabrovska, M.; Edreva-Kardjieva, R.; Crişan, D.; Tzvetkov, P.; Shopska, M.; Shtereva, I. Ni–Al layered double hydroxides as catalyst precursors for CO2 removal by methanation. React. Kinet. Mech. Cat. 2012, 105, 79–99. [Google Scholar] [CrossRef]
- Subramanian, N.D.; Kumar, C.S.S.R.; Watanabe, K.; Fischer, P.; Tanaka, R.; Spivey, J.L. A DRIFTS study of CO adsorption and hydrogenation on Cu-based core–shell nanoparticles. Catal. Sci. Technol. 2012, 2, 621–631. [Google Scholar] [CrossRef]
- Kitla, A.; Safonova, O.V.; Föttinger, K. Infrared studies on bimetallic copper/nickel catalysts supported on zirconia and ceria/zirconia. Catal. Lett. 2013, 143, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Németh, M.; Srankó, D.; Károlyi, J.; Somodi, F.; Schay, Z.; Sáfrán, G.; Sajó, I.; Horváth, A. Na-promoted Ni/ZrO2 dry reforming catalyst with high efficiency: Details of Na2O–ZrO2–Ni interaction controlling activity and coke formation. Catal. Sci. Technol. 2017, 7, 5386–5401. [Google Scholar] [CrossRef]


| Samples (LDH-NO3) | Initial Molar Ratios 1 | Measured Molar Ratios | Direct Band Gap (eV) | Indirect Band Gap (eV) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | Ni | Co | Cu | Zn | Mg | Ni | Co | Cu | Zn | |||
| NiCu-Al | - | 0.125 | - | 1 | - | - | 1 | - | 2.21 | - | 4.71 | 3.97 |
| - | 0.17 | - | 1 | - | - | 1 | - | 1.33 | - | 4.77 | 4.08 | |
| - | 0.20 | - | 1 | - | - | 1 | - | 1.18 | - | 4.66 | 3.90 | |
| - | 0.25 | - | 1 | - | - | 1.35 | - | 1 | - | 4.72 | 4.05 | |
| - | 0.50 | - | 1 | - | - | 2.77 | - | 1 | - | 4.73 | 4.09 | |
| - | 1 | - | 1 | - | - | 5.05 | - | 1 | - | 4.93 | 4.19 | |
| - | 1 | - | 2 | - | - | 3.61 | - | 1 | - | 4.95 | 4.36 | |
| - | 1 | - | 4 | - | - | 1.85 | - | 1 | - | 4.96 | 4.49 | |
| NiCo-Al | - | 1 | 1 | - | - | - | 21.24 | 1 | - | - | 4.96 | 4.37 |
| NiZn-Al | - | 1 | - | - | 1 | - | 16.70 | - | - | 1 | 5.01 | 4.60 |
| CoZn-Al | - | - | 1 | - | 1 | - | - | 1 | - | 1.48 | 5.10 | 4.76 |
| CoCu-Al | - | - | 1 | 1 | - | - | - | 1 | 8.76 | - | 4.56 | 3.78 |
| CuZn-Al | - | - | - | 4 | 4 | - | - | - | 7.01 | 1 | 4.62 | 3.87 |
| NiCoCu-Al | - | 1 | 1 | 1 | - | - | 22.11 | 1 | 6.15 | - | 4.97 | 4.39 |
| NiCuZn-Al | - | 1 | - | 1 | 1 | - | 15.80 | - | 3.73 | 1 | 4.95 | 4.39 |
| NiCoZn-Al | - | 1 | 1 | - | 1 | - | 21.84 | 1 | - | 1.33 | 4.92 | 4.49 |
| CoCuZn-Al | - | - | 1 | 1 | 1 | - | - | 1 | 11.24 | 1.45 | 4.80 | 4.06 |
| NiCoCuZn-Al | - | 1 | 1 | 1 | 1 | - | 17.67 | 1 | 5.23 | 1.44 | 4.95 | 4.46 |
| MgNiCoCuZn-Al | 1 | 1 | 1 | 1 | 1 | 0.03 | 17.03 | 1 | 5.31 | 1.36 | 5.03 | 4.61 |
| MgCoCu-Al | 1 | - | 1 | 1 | - | 0.06 | - | 1 | 7.81 | - | 4.87 | 4.21 |
| MgCoZn-Al | 4 | - | 4 | - | 4 | 0.11 | - | 1 | - | 1.32 | 5.21 | 4.91 |
| MgCuZn-Al | 1 | - | - | 1 | 1 | 0.06 | - | - | 6.15 | 1 | 4.83 | 4.20 |
| MgCo-Al | 4 | - | 4 | - | - | 1 | - | 22.47 | - | - | 5.00 | 4.34 |
| MgCu-Al | 1 | - | - | 1 | - | 1 | - | - | 122.2 | - | 4.88 | 4.07 |
| MgZn-Al | 4 | - | - | - | 4 | 1 | - | - | - | 45.63 | 5.14 | 4.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabados, M.; Ádám, A.A.; Kása, Z.; Baán, K.; Mucsi, R.; Sápi, A.; Kónya, Z.; Kukovecz, Á.; Sipos, P. M(II)Al4 Type Layered Double Hydroxides—Preparation Using Mechanochemical Route, Structural Characterization and Catalytic Application. Materials 2021, 14, 4880. https://doi.org/10.3390/ma14174880
Szabados M, Ádám AA, Kása Z, Baán K, Mucsi R, Sápi A, Kónya Z, Kukovecz Á, Sipos P. M(II)Al4 Type Layered Double Hydroxides—Preparation Using Mechanochemical Route, Structural Characterization and Catalytic Application. Materials. 2021; 14(17):4880. https://doi.org/10.3390/ma14174880
Chicago/Turabian StyleSzabados, Márton, Adél Anna Ádám, Zsolt Kása, Kornélia Baán, Róbert Mucsi, András Sápi, Zoltán Kónya, Ákos Kukovecz, and Pál Sipos. 2021. "M(II)Al4 Type Layered Double Hydroxides—Preparation Using Mechanochemical Route, Structural Characterization and Catalytic Application" Materials 14, no. 17: 4880. https://doi.org/10.3390/ma14174880
APA StyleSzabados, M., Ádám, A. A., Kása, Z., Baán, K., Mucsi, R., Sápi, A., Kónya, Z., Kukovecz, Á., & Sipos, P. (2021). M(II)Al4 Type Layered Double Hydroxides—Preparation Using Mechanochemical Route, Structural Characterization and Catalytic Application. Materials, 14(17), 4880. https://doi.org/10.3390/ma14174880










