Hydroxyapatite in Oral Care Products—A Review
Abstract
1. Introduction
2. Hydroxyapatite in Oral Care Products
3. Benefits of Hydroxyapatite Dentifrice/Oral Care Products
3.1. Tooth Remineralisation

3.2. Dentine Hypersensitivity
3.3. Oral Biofilm Management: Effect on Periodontal Conditions
3.4. Teeth Whitening
| Type of Study | Method | Comparison Group | Main Findings | Reference |
|---|---|---|---|---|
| Remineralisation | ||||
| In situ | Mineral loss and lesion depth of each specimen were quantified using microradiography | Fluoride toothpaste | All dentifrices were effective in reducing mineral loss and lesion depth but showed no significant differences between each other in percent mineral gain. | [17] |
| In situ | Microradiography | 500 ppm fluoride toothpaste | 10% HA achieved comparable efficacy with 500 ppm fluoride in remineralising initial caries and preventing demineralisation. | [30] |
| In vitro | %SMHR, SEM | Deionised water (negative control), NaF positive control | Nano-HA had the potential to remineralise initial enamel lesions. A concentration of 10% nano-HA may be optimal for remineralisation of early enamel caries. | [42] |
| In vitro | DIAGNOdent | Fluoride varnish | Significant remineralisation effects of both fluoride and nano-HA paste compared to the control group: no treatment. However, there were no statistically significant differences between the groups. | [44] |
| In vitro | Mineral loss was quantified using microradiography | Fluoride-based gel or artificial saliva | Both fluoride and HA showed effective remineralisation abilities but there were no statistically significant differences between the groups. | [18] |
| In vitro | Remineralisation effects were studied with Vickers Hardness Number and SEM image of the enamel surface | Nano-HA with fluoride | Significance in remineralisation for nano-HA with and without fluoride, however not statistically significant effects from each other. | [45] |
| In vitro | Remineralisation effect investigated through surface and CSMH tests and PLM | - | Nano-HA > Micro-HA. | [43] |
| In vitro | Differences in mineral loss evaluated with microradiography | Amine fluoride toothpaste | Nano-HA toothpaste showed higher remineralising effects compared to amine fluoride toothpastes in bovine dentine and enamel. | [38] |
| In vivo RCT | Remineralisation effects were investigated through comparative bitewing radiographs before and after treatment | In-office ozone therapy, or a combination of both | The smallest rate of remineralisation was seen with nano-HA treatment alone, and the highest rate was seen when nano-HA and ozone therapy were used in conjunction. | [28] |
| In vivo RCT | ICDAS ≥ code 1, ICDAS ≥ code 2, the plaque index, and the gingival index | Fluoride dentifrice | No statistical difference between nano-HA and fluoride dentifrice. | [23] |
| In vivo RCT | The development of ICDAS of more than or equal to 1 | Fluoride toothpaste | Micro-HA toothpaste was clinically non-inferior compared to fluoride toothpaste in preventing primary teeth enamel lesion progression. | [24] |
| Dentine hypersensitivity | ||||
| In vivo RCT | Airblast, tactile tests, and VAS of pain to stimuli | Fluoride dentifrice and placebo | Statistically significant lower values of sensitivity with nano-HA compared to fluoride and placebo in subsequent weeks of use. Significantly lower values of cold air and tactile sensitivity, and VAS scores. | [55] |
| In vivo RCT | Airblast, tactile, cold water, and subjective tests | Potassium nitrate/fluoride dentifrice | Both CHA and potassium nitrate/fluoride dentifrice were significantly effective in reducing dentine hypersensitivity among subjects. Statistically significant improvement in airblast score and subjective scores with CHA toothpaste. In contrast, no significant difference between groups for tactile or cold-water tests. | [56] |
| In vitro | SEM | Novamin, Proargin, normal saline | All three were effective in dentine tubule occlusion. Statistically significant difference on increasing dentinal tubule occlusion between HA and Proargin. Although HA occluded dentinal tubules more than Novamin, findings here were not statistically significant. | [54] |
| In vitro | SEM | Calcium sodium phosphosilicate, Proargin, normal saline | All three were effective in dentine tubule occlusion, but calcium sodium phosphosilicate showed significantly higher tubular occlusion compared to other groups. | [61] |
| In vivo RCT | VAS | - | Significant improvement in sensitivity between 52–76% after 48 h, and 70–84% after two weeks. | [58] |
| In vivo RCT | Airblast method using Schiff Sensitivity Scale. | - | Statistically significant differences were observed between baseline and four and eight-week intervals. | [59] |
| In vivo RCT | Questionnaire with VAS and Likert scales | - | Biomimetic zinc HA was effective in reducing dentin hypersensitivity. | [25] |
| In vivo RCT | VAS and EPT | Placebo paste | Sensitivity scores significantly decreased in HA group compared to placebo group. | [57] |
| In vitro and In vivo | Hydraulic conductance through commercially available capillary flow system. Once without saliva (in-vitro) and once with saliva and ageing to replicate biological conditions (in-vivo) | Potassium nitrate, and an arginine and calcium carbonate-containing toothpaste | —In vitro: In the absence of saliva, HA containing toothpaste was most effective in reducing dentin permeability, and arginine and calcium carbonate was the worst —In vivo: In the presence of saliva and ageing, HA was the worst, and arginine and calcium carbonate was the best. | [62] |
| Biofilm management | ||||
| In situ | DAPI and live/dead staining, SEM | Chlorhexidine | HA particles reduce initial biofilm formation on enamel surface is comparable to chlorhexidine. | [65] |
| In situ | DAPI and live/dead staining | Chlorhexidine | Mouthwash containing HA reduces initial biofilm formation comparable to chlorhexidine. | [19] |
| In vivo RCT | Plaque formation rate, plaque control record, gingival index, bleeding on probing, pocket probing depth | Amine and stannous fluoride toothpaste | HA toothpaste reduced plaque index, bleeding on probing and gingival index but did not change the plaque formation rates. This is comparable to amine and stannous fluoride toothpaste. | [63] |
| In vivo | Plaque and gingival index, DIAGNOdent | Chlorhexidine | HA is as effective as chlorhexidine in plaque reduction. | [66] |
| In vivo | Questionnaire VAS and Likert scales both at baseline and follow-up | - | Subjective feeling of tooth-smoothing can be explained by the reduction of bacterial colonisation by HA particles. A stronger feeling of freshness after toothbrushing was also reported. | [25] |
| In vivo | Levels of calcium and phosphorus of plaque samples were analysed by energy-dispersive X-ray spectroscopy | - | HA may be incorporated into the oral biofilm and/or may adhere to dental plaque. | [76] |
| In vivo RCT | Paired-end Illumina Miseq 16S rDNA sequencing | AmF/SnF2 | Toothpaste containing anti-adhesive HA did not induce statistically noticeably different changes in microbial composition compared to an antimicrobial and anti-adhesive AmF/SnF2 formulation. | [77] |
| Teeth whitening | ||||
| Combined experimental and clinical study. | Weighed using a fine balance, thickness-loss (nm) per cm2 per hour Two colourimeters (SZ-Y-90 and SE-2000) with two specially made fiberscopes (inner diameters of 3.5 and 2.5 mm) | - | 1. Different amounts of HA in toothpaste do not change the polishing properties. 2. HA toothpaste increased teeth brightness and whiteness. Has a dose-response relationship. 3. No correlation between polishing and whitening properties of HA toothpaste. | [71] |
| Combined experimental and clinical study | Photoresearch Spectra-Scan PR-650 photocolorimeter SEN (Hitachi S-4500) | An identical toothpaste without HA | HA toothpaste can alter tooth colour by at least one shade with daily brushing. However, this is less powerful than harmful bleaches such as peroxides. | [74] |
| In vitro | Dental spectrophotometer | - | Synthetic nano-HA is an alternative for tooth whitening because they have some advantages when compared to oxidising bleaching materials. All nano and micro-HA tested exhibited whitening effects of variable degrees on the enamel surfaces. | [73] |
| In vitro | Colour changes (ΔE) were measured spectrophotometrically | Whitening mouth rinse with phosphates, or negative control (distilled water) | Significantly higher ad hoc whitening effect of the HA gel compared to the mouth rinse and water after short-time application. | [27] |
| In vivo, pilot study | Questionnaire regarding their perception of their tooth colour and brightness | - | Micro-HA is a promising whitening agent for oral care formulations and represents a biomimetic alternative to other whitening agents for daily dental care. | [26] |
| In vivo RCT | Vita Easyshade (Vita 3D-master scale) and Degudent Shadepilot (Classical Vitashade scale) | Calcium peroxide and no active ingredient (placebo) | Toothpaste containing HA or calcium peroxide did not produce any reduction in tooth staining compared with a placebo fluoride toothpaste. | [70] |
| In vivo RCT | ShadeEye NCC and Vita classical shade guide, VAS scale (range, 1–5) | Hydrogen peroxide and placebo | Hydrogen peroxide-containing toothpaste caused significant lightening of tooth colouration than the HA and placebo toothpaste. | [69] |
| In vitro | VITA shade scores of Shadeeye-EX NCC Dental Chroma meter (Shofu Co. Japan) | Group 1, a new toothpaste containing (Nano-HA) Group 2: toothpaste containing silica and multi phosphate. Group 3: toothpaste containing abrasives with silica and micro-sized HA | No significant differences in shade change between each group (p > 0.05). New Nano-HA toothpaste had similar whitening efficacy to commercially available whitening toothpaste. | [72] |
| Combined In vitro and in vivo study | A spectrophotometer. Proof-of-concept clinical study was performed investigating the mixture of SAPM+HA | - | The combination of SAPM+HA particles caused optical whitening based on diffuse reflection by the HA particles on the tooth surface. The whitening effect and its magnitude observed in vitro were also seen in vivo. | [75] |
| In vitro | Dental spectrophotometer | - | Calcium phosphate-based formulations that can adhere to the enamel surface have promising tooth-whitening potential. | [78] |
4. Conclusions and Future Perspective of Hydroxyapatite Oral Care Products
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Stamm, J.W. Epidemiology of gingivitis. J. Clin. Periodontol. 1986, 13, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Enax, J.; Epple, M. Synthetic hydroxyapatite as a biomimetic oral care agent. Oral Health Prev. Dent 2018, 16, 7–19. [Google Scholar]
- Adamopoulos, O.; Papadopoulos, T. Nanostructured bioceramics for maxillofacial applications. J. Mater. Sci. Mater. Med. 2007, 18, 1587–1597. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A. A critical review of decades of research on calcium phosphate–based coatings: How far are we from their widespread clinical application? Curr. Opin. Biomed. Eng. 2019, 10, 35–44. [Google Scholar] [CrossRef]
- Habibah, T.U.; Amlani, D.V.; Brizuela, M. Hydroxyapatite Dental Material. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Domingo, C.; Arcís, R.; López-Macipe, A.; Osorio, R.; Rodríguez-Clemente, R.; Murtra, J.; Fanovich, M.; Toledano, M. Dental composites reinforced with hydroxyapatite: Mechanical behavior and absorption/elution characteristics. J. Biomed. Mater. Res. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 56, 297–305. [Google Scholar] [CrossRef]
- Meyer, F.; Amaechi, B.T.; Fabritius, H.-O.; Enax, J. Overview of calcium phosphates used in biomimetic oral care. Open Dent. J. 2018, 12, 406. [Google Scholar] [CrossRef]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Annali di Stomatologia 2014, 5, 108. [Google Scholar] [CrossRef]
- Pu’ad, N.A.S.M.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar]
- Ratnayake, J.T.B.; Gould, M.L.; Shavandi, A.; Mucalo, M.; Dias, G.J. Development and characterization of a xenograft material from New Zealand sourced bovine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1054–1062. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, A.; Sun, Z.; Ratnayake, J.T. Microwave-assisted synthesis of high purity β-tricalcium phosphate crystalline powder from the waste of Green mussel shells (Perna canaliculus). Powder Technol. 2015, 273, 33–39. [Google Scholar] [CrossRef]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A. Chapter Four—Marine Waste Utilization as a Source of Functional and Health Compounds. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 87, pp. 187–254. [Google Scholar]
- Shavandi, A.; Wilton, V.; Bekhit, A.E.-D.A. Synthesis of macro and micro porous hydroxyapatite (HA) structure from waste kina (Evechinus chloroticus) shells. J. Taiwan Inst. Chem. Eng. 2016, 65, 437–443. [Google Scholar] [CrossRef]
- Najibfard, K.; Ramalingam, K.; Chedjieu, I.; Amaechi, B. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J. Clin. Dent. 2011, 22, 139. [Google Scholar] [PubMed]
- Amaechi, B.T.; AbdulAzees, P.A.; Okoye, L.O.; Meyer, F.; Enax, J. Comparison of hydroxyapatite and fluoride oral care gels for remineralization of initial caries: A pH-cycling study. BDJ Open 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Hannig, C.; Basche, S.; Burghardt, T.; Al-Ahmad, A.; Hannig, M. Influence of a mouthwash containing hydroxyapatite microclusters on bacterial adherence in situ. Clin. Oral Investig. 2013, 17, 805–814. [Google Scholar] [CrossRef]
- Hill, R.G.; Gillam, D.G.; Chen, X. The ability of a nano hydroxyapatite toothpaste and oral rinse containing fluoride to protect enamel during an acid challenge using 19F solid state NMR spectroscopy. Mater. Lett. 2015, 156, 69–71. [Google Scholar] [CrossRef]
- Jagtap, A.M.; Kaulage, S.R.; Kanse, S.S.; Shelke, V.D.; Gavade, A.S.; Vambhurkar, G.B.; Todkar, R.R.; Dange, V.N. Preparation and Evaluation of Toothpaste. Asian J. Pharm. Anal. 2018, 8, 191–194. [Google Scholar] [CrossRef]
- Ramis, J.M.; Coelho, C.C.; Córdoba, A.; Quadros, P.A.; Monjo, M. Safety assessment of nano-hydroxyapatite as an oral care ingredient according to the EU cosmetics regulation. Cosmetics 2018, 5, 53. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Kunzelmann, K.H.; Hannig, C.; May, T.W.; Hösl, H.; Gratza, M.; Viergutz, G.; Nazet, M.; Schamberger, S.; Proff, P. Impact of a non-fluoridated microcrystalline hydroxyapatite dentifrice on enamel caries progression in highly caries-susceptible orthodontic patients: A randomized, controlled 6-month trial. J. Investig. Clin. Dent. 2019, 10, e12399. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily application of a toothpaste with biomimetic hydroxyapatite and its subjective impact on dentin hypersensitivity, tooth smoothness, tooth whitening, gum bleeding, and feeling of freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef]
- Steinert, S.; Kuchenbecker, J.; Meyer, F.; Simader, B.; Zwanzig, K.; Enax, J. Whitening Effects of a Novel Oral Care Gel with Biomimetic Hydroxyapatite: A 4-Week Observational Pilot Study. Biomimetics 2020, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Sarembe, S.; Enax, J.; Morawietz, M.; Kiesow, A.; Meyer, F. In vitro whitening effect of a hydroxyapatite-based oral care gel. Eur. J. Dent. 2020, 14, 335. [Google Scholar] [CrossRef]
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Droździk, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomasik, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Demito, C.F.; Costa, J.V.d.; Fracasso, M.d.L.C.; Ramos, A.L. Efficacy of fluoride associated with nano-hydroxyapatite in reducing enamel demineralization adjacent to orthodontic brackets: In situ study. Dent. Press J. Orthod. 2020, 24, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.P.d.C.S.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef]
- Enax, J.; Fabritius, H.-O.; Fabritius-Vilpoux, K.; Amaechi, B.T.; Meyer, F. Modes of action and clinical efficacy of particulate hydroxyapatite in preventive oral health care—State of the art. Open Dent. J. 2019, 13, 274–287. [Google Scholar] [CrossRef]
- Zafar, M.S.; Alnazzawi, A.A.; Alrahabi, M.; Fareed, M.A.; Najeeb, S.; Khurshid, Z. 18—Nanotechnology and nanomaterials in dentistry. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 477–505. [Google Scholar]
- Kuśnieruk, S.; Wojnarowicz, J.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1586–1601. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Ratnayake, J.T.B.; Moratti, S.C.; Dias, G.J. Effect of chitosan infiltration on hydroxyapatite scaffolds derived from New Zealand bovine cancellous bones for bone regeneration. Int. J. Biol. Macromol. 2020, 160, 1009–1020. [Google Scholar] [CrossRef]
- Esteves-Oliveira, M.; Santos, N.M.; Meyer-Lückel, H.; Wierichs, R.J.; Rodrigues, J.A. Caries-preventive effect of anti-erosive and nano-hydroxyapatite-containing toothpastes in vitro. Clin. Oral Investig. 2017, 21, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic aspects of restorative dentistry biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Arifa, M.K.; Ephraim, R.; Rajamani, T. Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int. J. Clin. Pediatr. Dent. 2019, 12, 139–144. [Google Scholar] [CrossRef]
- Huang, S.B.; Gao, S.S.; Yu, H.Y. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed. Mater. 2009, 4, 034104. [Google Scholar] [CrossRef]
- Gjorgievska, E.S.; Nicholson, J.W.; Slipper, I.J.; Stevanovic, M.M. Remineralization of Demineralized Enamel by Toothpastes: A Scanning Electron Microscopy, Energy Dispersive X-Ray Analysis, and Three-Dimensional Stereo-Micrographic Study. Microsc. Microanal. 2013, 19, 587–595. [Google Scholar] [CrossRef]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Daas, I.; Badr, S.; Osman, E. Comparison between Fluoride and Nano-hydroxyapatite in Remineralizing Initial Enamel Lesion: An in vitro Study. J. Contemp. Dent. Pr. 2018, 19, 306–312. [Google Scholar] [CrossRef]
- Kim, B.I.; Jeong, S.H.; Jang, S.O.; Kim, K.N.; Kwon, H.K.; Park, Y.D. Remineralization potential of new toothpaste containing nano-hydroxyapatite. In Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2006; pp. 537–540. [Google Scholar]
- Ramesh, N.; Ratnayake, J.T.B.; Dias, G.J. 11—Calcium-based ceramic biomaterials. In Structural Biomaterials; Wen, C., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 333–394. [Google Scholar]
- Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Garcia-Godoy, F.; Brambilla, E. Substituted Nano-Hydroxyapatite toothpastes reduce biofilm formation on enamel and resin-based composite surfaces. J. Funct. Biomater. 2020, 11, 36. [Google Scholar] [CrossRef]
- Hamba, H.; Nakamura, K.; Nikaido, T.; Tagami, J.; Muramatsu, T. Remineralization of enamel subsurface lesions using toothpaste containing tricalcium phosphate and fluoride: An in vitro µCT analysis. BMC Oral Health 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Jo, S.-Y.; Chong, H.-J.; Lee, E.-H.; Chang, N.-Y.; Chae, J.-M.; Cho, J.-H.; Kim, S.-C.; Kang, K.-H. Effects of various toothpastes on remineralization of white spot lesions. Korean J. Orthod. 2014, 44, 113. [Google Scholar] [CrossRef][Green Version]
- Vanichvatana, S.; Auychai, P. Efficacy of two calcium phosphate pastes on the remineralization of artificial caries: A randomized controlled double-blind in situ study. Int. J. Oral Sci. 2013, 5, 224–228. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kwon, H.K.; Choi, C.H.; Kim, B.I. Combined effects of nano-hydroxyapatite and NaF on remineralization of early caries lesion. In Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2007; pp. 1347–1350. [Google Scholar]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. Part B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Combined effects of nano-hydroxyapatite and Galla chinensis on remineralisation of initial enamel lesion in vitro. J. Dent. 2010, 38, 811–819. [Google Scholar] [CrossRef]
- Kulal, R.; Jayanti, I.; Sambashivaiah, S.; Bilchodmath, S. An in-vitro comparison of nano hydroxyapatite, novamin and proargin desensitizing toothpastes-A SEM Study. J. Clin. Diagn. Res. JCDR 2016, 10, ZC51. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Covani, U. Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: A double-blind randomized controlled trial. Quintessence Int. 2014, 45, 703–711. [Google Scholar] [PubMed]
- Maharani, D.A. Efficacy of a commercially available hydroxyapatite-containing toothpaste in reducing dentin hypersensitivity. Int. J. Clin. Prev. Dent. 2012, 8, 151–154. [Google Scholar]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Giuliodori, F.; Lorenzini, A.; Putignano, A. A double-blind randomized-controlled trial comparing the desensitizing efficacy of a new dentifrice containing carbonate/hydroxyapatite nanocrystals and a sodium fluoride/potassium nitrate dentifrice. J. Clin. Periodontol. 2010, 37, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Low, S.B.; Allen, E.P.; Kontogiorgos, E.D. Reduction in dental hypersensitivity with nano-hydroxyapatite, potassium nitrate, sodium monoflurophosphate and antioxidants. Open Dent. J. 2015, 9, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Al Asmari, D.; Khan, M.K. Evaluate efficacy of desensitizing toothpaste containing zinc-carbonate hydroxyapatite nanocrystals: Non-comparative eight-week clinical study. J. Int. Soc. Prev. Community Dent. 2019, 9, 566. [Google Scholar]
- Dhillon, P.; Govila, V.; Verma, S. Evaluation Of Various Desensitizing Agents In Reducing Dentin Hypersensitivity Using Scanning Electron Microscope: A Comparative In Vitro Study. Indian J. Dent. Sci. 2014, 6, 31–35. [Google Scholar]
- Hiller, K.-A.; Buchalla, W.; Grillmeier, I.; Neubauer, C.; Schmalz, G. In vitro effects of hydroxyapatite containing toothpastes on dentin permeability after multiple applications and ageing. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Harks, I.; Jockel-Schneider, Y.; Schlagenhauf, U.; May, T.W.; Gravemeier, M.; Prior, K.; Petersilka, G.; Ehmke, B. Impact of the daily use of a microcrystal hydroxyapatite dentifrice on de novo plaque formation and clinical/microbiological parameters of periodontal health. A randomized trial. PLoS ONE 2016, 11, e0160142. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Enax, J. Hydroxyapatite in oral biofilm management. Eur. J. Dent. 2019, 13, 287. [Google Scholar] [CrossRef]
- Kensche, A.; Holder, C.; Basche, S.; Tahan, N.; Hannig, C.; Hannig, M. Efficacy of a mouthrinse based on hydroxyapatite to reduce initial bacterial colonisation in situ. Arch. Oral Biol. 2017, 80, 18–26. [Google Scholar] [CrossRef]
- Hegazy, S.A.; Salama, R.I. Antiplaque and remineralizing effects of Biorepair mouthwash: A comparative clinical trial. Pediatr. Dent. J. 2016, 26, 89–94. [Google Scholar] [CrossRef]
- Luo, W.; Huang, Y.; Zhou, X.; Han, Q.; Peng, X.; Ren, B.; Li, J.; Li, M.; Cheng, L. The effect of disaggregated nano-hydroxyapatite on oral biofilm in vitro. Dent. Mater. 2020, 36, e207–e216. [Google Scholar] [CrossRef] [PubMed]
- Epple, M.; Meyer, F.; Enax, J. A critical review of modern concepts for teeth whitening. Dent. J. 2019, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Woo, G.J.; Kim, E.K.; Jeong, S.H.; Song, K.B.; Goo, H.J.; Jeon, E.S.; Choi, Y.H. Comparison of the whitening effect of toothpastes containing 0.25% hydroxyapatite and 0.75% hydrogen peroxide. J. Korean Acad. Oral Health 2014, 38, 3–9. [Google Scholar] [CrossRef]
- Raoufi, S.; Birkhed, D. Effect of whitening toothpastes on tooth staining using two different colour-measuring devices—A 12-week clinical trial. Int. Dent. J. 2010, 60, 419–423. [Google Scholar] [PubMed]
- Niwa, M.; Sato, T.; Li, W.; Aoki, H.; Aoki, H.; Daisaku, T. Polishing and whitening properties of toothpaste containing hydroxyapatite. J. Mater. Sci. Mater. Med. 2001, 12, 277–281. [Google Scholar] [CrossRef]
- Kim, B.I.; Jeong, S.H.; Jang, S.O.; Kim, K.N.; Kwon, H.K.; Park, Y.D. Tooth whitening effect of toothpastes containing nano-hydroxyapatite. In Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2006; pp. 541–544. [Google Scholar]
- Dabanoglu, A.; Wood, C.; Garcia-Godoy, F.; Kunzelmann, K.-H. Whitening effect and morphological evaluation of hydroxyapatite materials. Am. J. Dent. 2009, 22, 23. [Google Scholar]
- Guo, C.; Liu, H.; Katayama, I. Effect of hydroxyapatite toothpaste on vital tooth color. J. Dent. Res. 2002, 81, A254-1964. [Google Scholar]
- Bommer, C.; Flessa, H.-P.; Xu, X.; Kunzelmann, K.-H. Hydroxyapatite and Self-Assembling Peptide Matrix for Non-Oxidizing Tooth Whitening. J. Clin. Dent. 2018, 29, 57–63. [Google Scholar] [PubMed]
- Sudradjat, H.; Meyer, F.; Loza, K.; Epple, M.; Enax, J. In vivo effects of a hydroxyapatite-based oral care gel on the calcium and phosphorus levels of dental plaque. Eur. J. Dent. 2020, 14, 206. [Google Scholar] [CrossRef]
- Hagenfeld, D.; Prior, K.; Harks, I.; Jockel-Schneider, Y.; May, T.W.; Harmsen, D.; Schlagenhauf, U.; Ehmke, B. No differences in microbiome changes between anti-adhesive and antibacterial ingredients in toothpastes during periodontal therapy. J. Periodontal Res. 2019, 54, 435–443. [Google Scholar] [CrossRef]
- Jin, J.; Xu, X.; Lai, G.; Kunzelmann, K.H. Efficacy of tooth whitening with different calcium phosphate-based formulations. Eur. J. Oral Sci. 2013, 121, 382–388. [Google Scholar] [CrossRef]
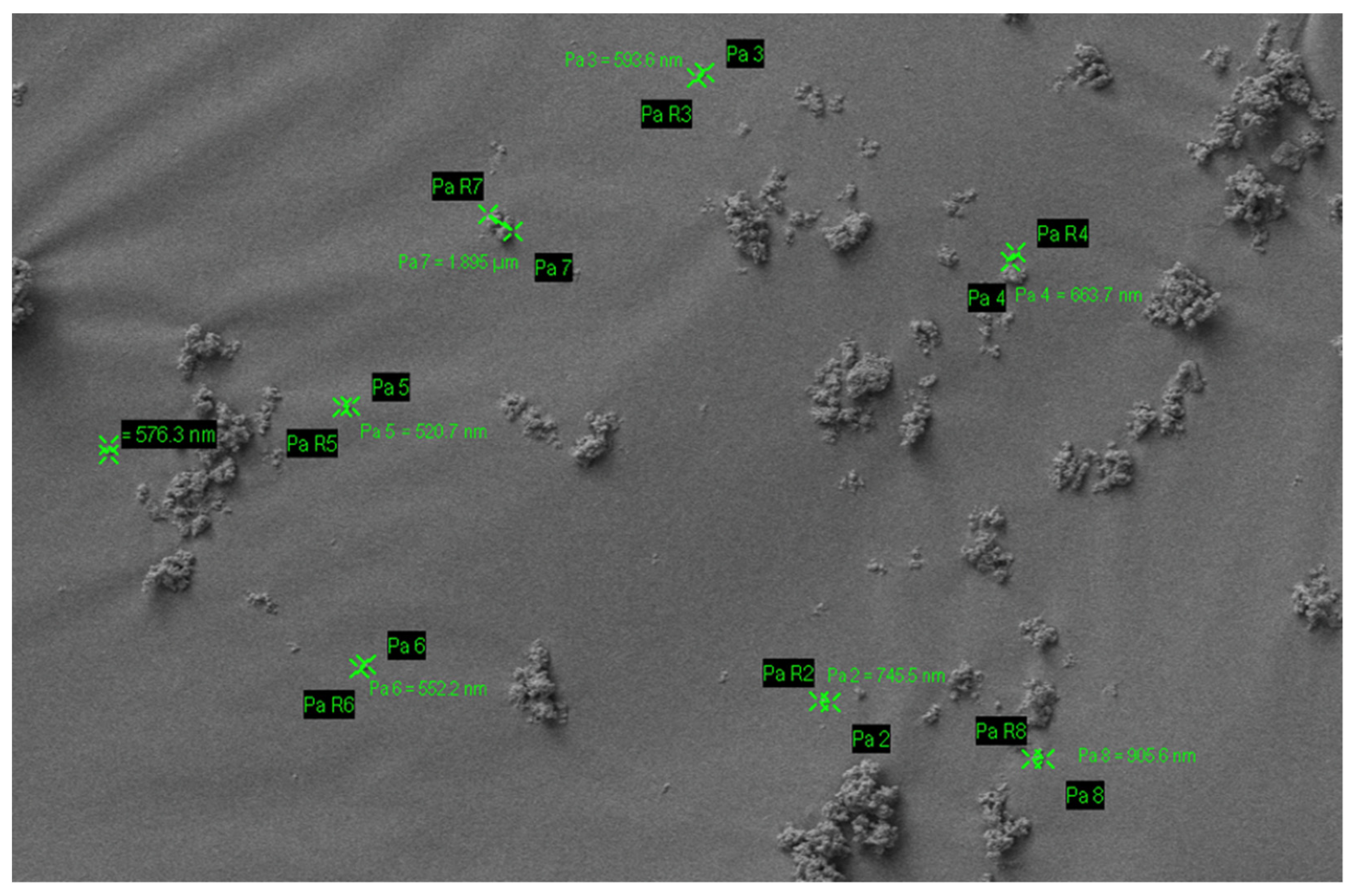
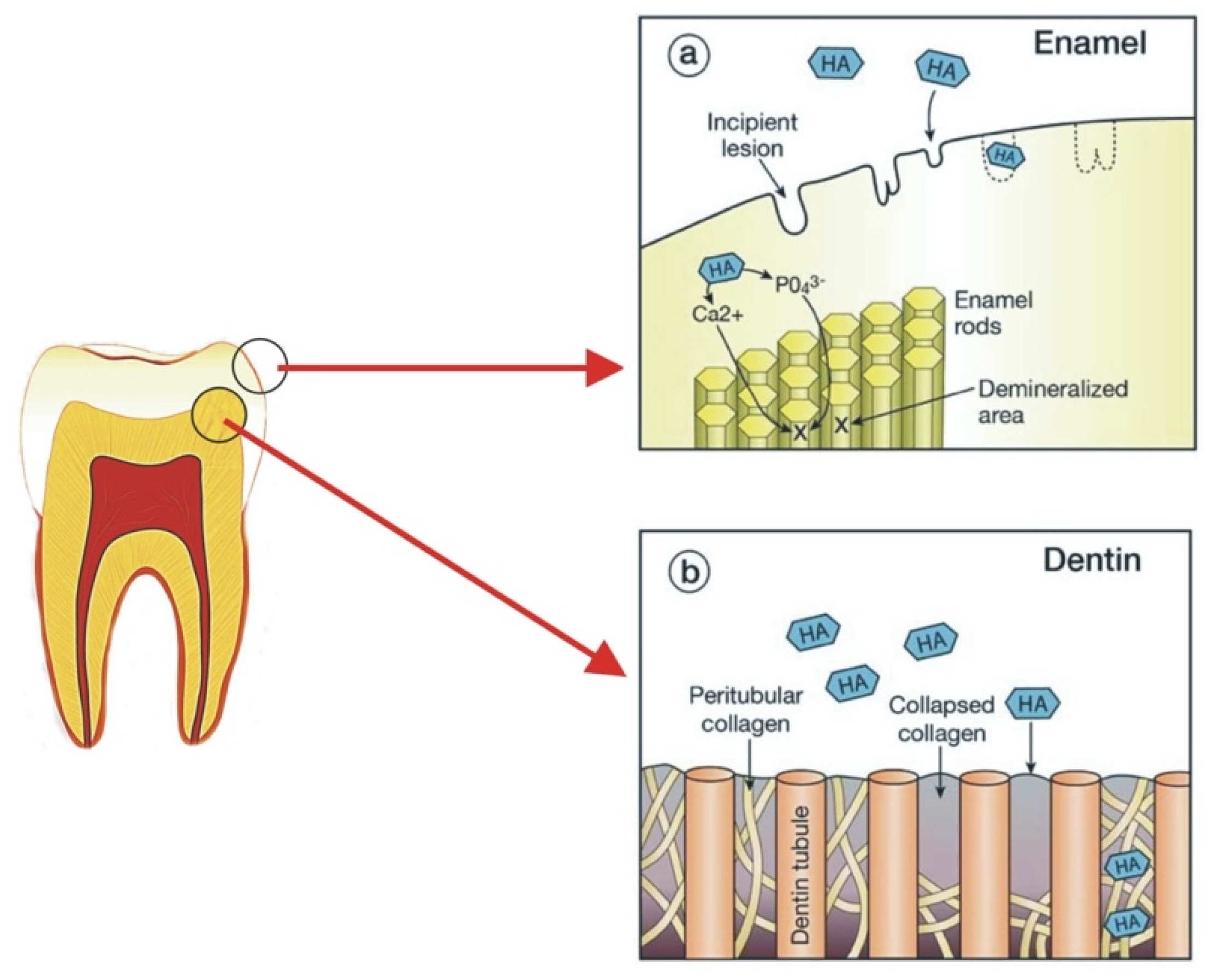
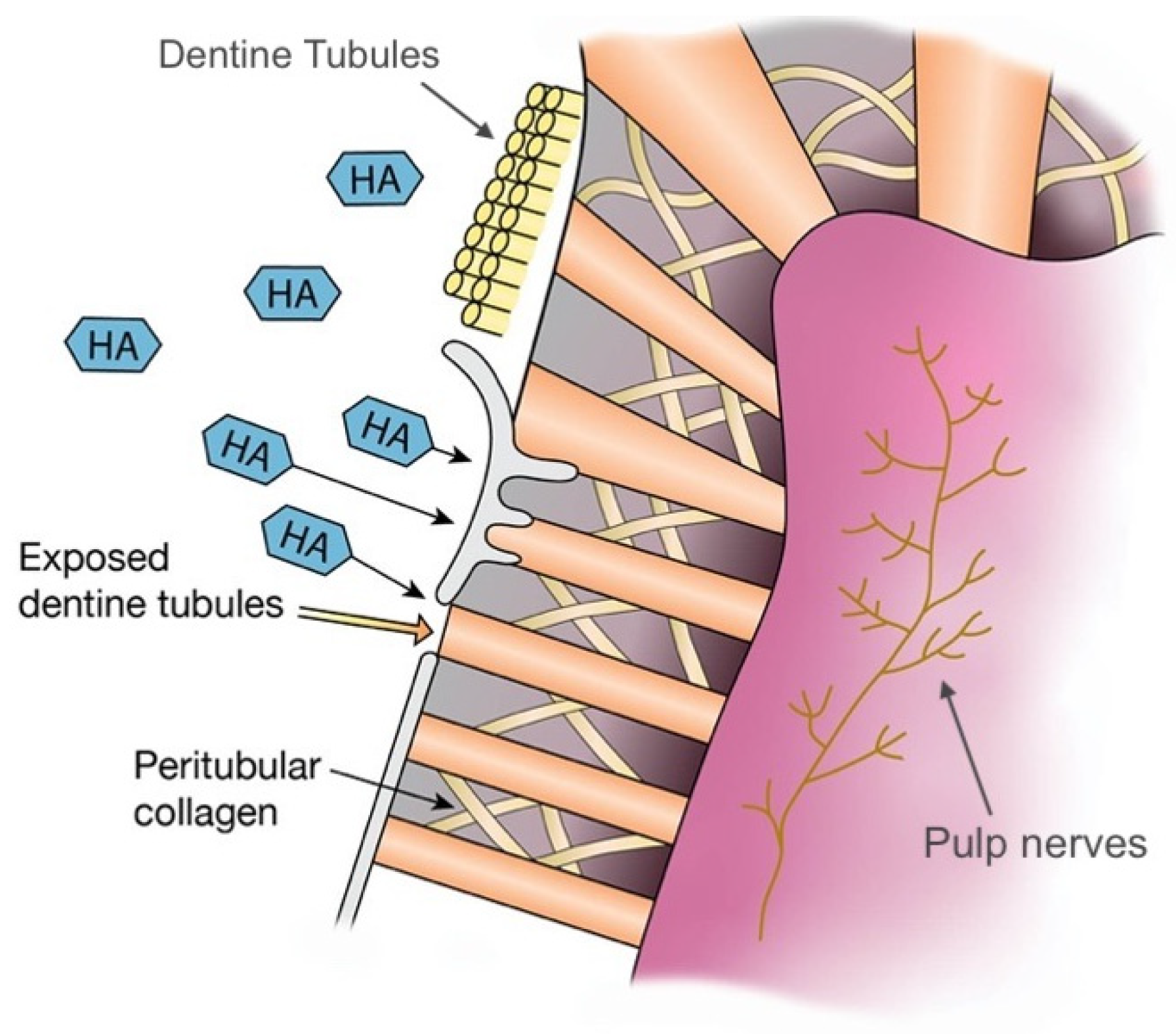
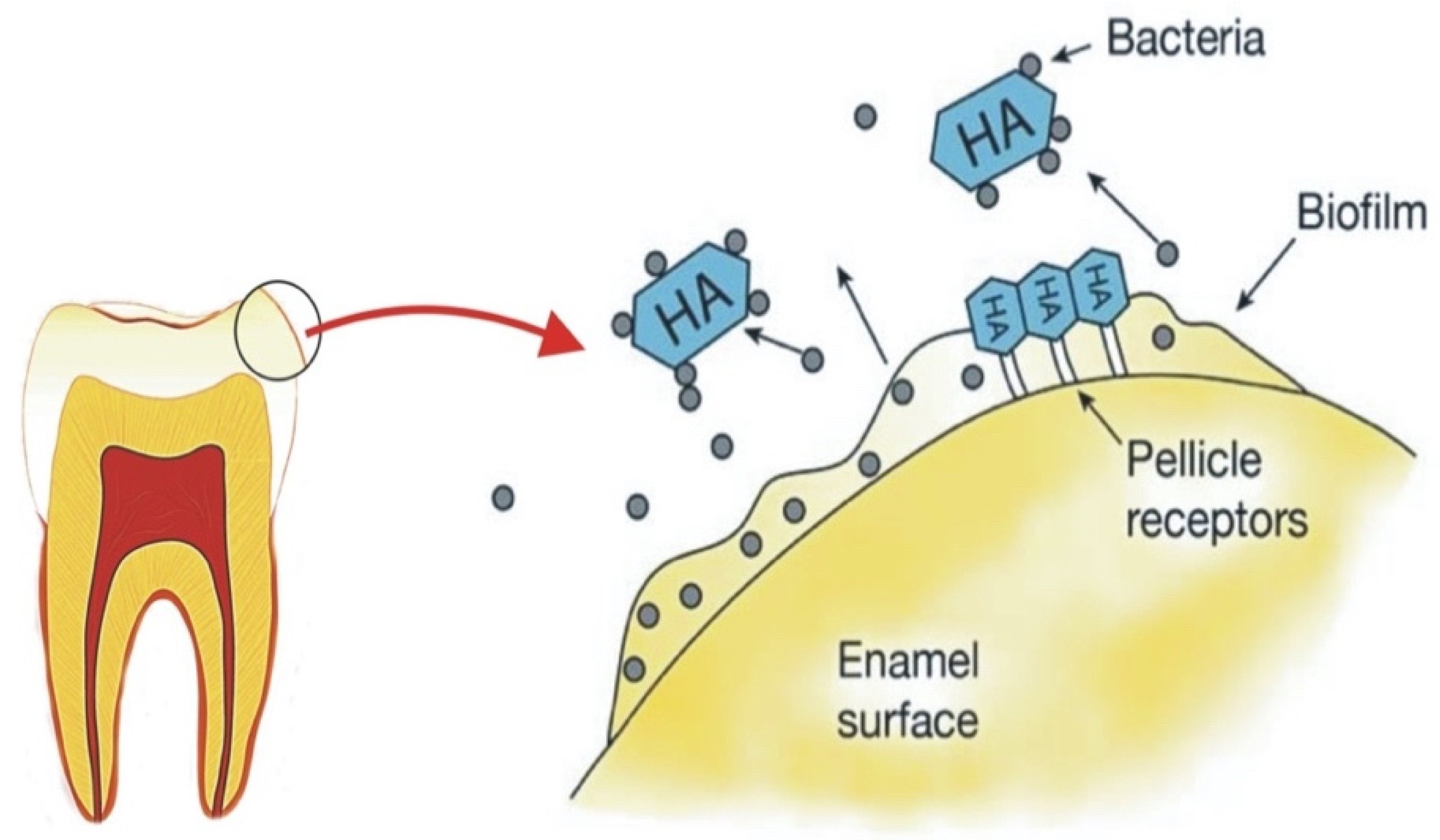
| S. No | Commercial Product | Ingredients | Country |
|---|---|---|---|
| 1 | APAGARD® PREMIO | Aqua, dicalcium phosphate, glycerin, xylitol, hydroxyapatite (nano), silica, Peg-8, sodium lauryl sulfate, cellulose gum, aroma, sodium silicate, trimagnesium phosphate, hydrolyzed conchiolin protein, sodium saccharin, glycyrrhetinic acid, cetylpyridinium chloride, lauryl diethylenediaminoglycine Hcl | Germany |
| 2 | Ela Mint Toothpaste | Water, vegetable glycerin, hydrated silica, sorbitol powder, silica, hydroxyapatite (nano), sodium benzoate, sodium lauroyl sarcosinate, mentha piperita essential (peppermint) oil, Mentha viridis (spearmint) oil, Illicium verum (star anise) oil, Gaultheria procumberis (wintergreen) oil, xylitol, xanthan gum, Stevia rebaudiana extract powder, methylsulfonylmethane, Aloe barbadensis (aloe vera) leaf juice, sodium bicarbonate, Camellia sinensis (green tea) leaf extract, Cucumis sativus (cucumber) fruit extract, Persea gratissima (avocado) fruit extract, Mangifera indica (mango) fruit extract, menthol, Elettaria cardamomum miniscula seed (cardamom), potassium chloride. | USA |
| 3 | Coco Ginger Toothpaste | Glycerin, water, hydrated silica, erythritol, silica, natural flavors (coconut and ginger), hydroxyapatite (nano), xanthan gum, sodium benzoate, Aloe barbadensis (aloe vera) leaf juice, Chamomilla recutita (chamomile) flower extract, methylsulfonylmethane (msm), potassium chloride, sodium bicarbonate, Stevia rebaudiana extract powder, sodium lauroyl sarcosinate. | USA |
| 4 | APAGARD® RIN-SU | Aqua, glycerin, xylitol, hydroxyapatite, xanthan gum, alcohol, polyglyceryl-5 stearate, lauryl diethylenediaminoglycine HCL, aroma, cethylpyridinium chloride | Germany |
| 5 | APADENT® TOTAL CARE | Aqua, dicalcium phosphate, glycerin, hydroxyapatite (nano), silica, peg-8, sodium lauryl sulphate, cellulose gum, aroma, trimagnesium phosphate, pvp, butylene glyium sodium, alcoholic acrylic, sodium sodium, sodium, sodium, sodium, sodylacride, sodylen, sodylacride, sodylacride, sodyl. pyridoxine HCL, lauryl diethylenediaminoglycine HCL, Camellia sinensis leaf extract, Chamomilla recutilla (Matricaria) extract, Salvia officinalis (Sage) leaf extract | Germany |
| 6 | Travel Size Ela Mint Toothpaste | Water, vegetable glycerin, hydrated silica, sorbitol powder, silica, hydroxyapatite (nano), sodium benzoate, sodium lauroyl sarcosinate, Mentha piperita essential (peppermint) oil, Mentha viridis (spearmint) oil, Illicium verum (star anise) oil, Gaultheria procumberis (wintergreen) oil, xylitol, xanthan gum, stevia rebaudiana extract powder, methylsulfonylmethane, Aloe barbadensis (aloe vera) leaf juice, sodium bicarbonate, camellia sinensis (green tea) leaf extract, Cucumis sativus (cucumber) fruit extract, Persea gratissima (avocado) fruit extract, Mangifera indica (mango) fruit extract, menthol, Elettaria cardamomum miniscula seed (cardamom), potassium chloride. | USA |
| 7 | Toothpaste PrevDent® nHAp™ | Aqua, hydrated silica, sorbitol, glycerin, xylitol, potassium nitrate, nano-hydroxyapatite, magnesium aluminum silicate, mentha piperita oil, sodium lauroyl sarcosinate, xanthan gum, phenoxyethanol, potassium chloride, sodium sulfate, sodium saccharin, CI 77891 | The Netherlands |
| 8 | Biorepair® | Sorbitol, Aqua, Silica, PEG32, Glycerin, Aroma, Zinc hydroxyapatite, Na-Myristoyl Sarcosinate, Celulose gum, Citric acid, Na-benzoate, Benzylalcohol, Na-methyl cocoyl taurate, Mentha peprita oil, Na-sacchrine, K-sorbate, Fragaria vesca Juice, Anethole, Phenoxyethanol, Mentho | Italy |
| 9 | X-PUR Remin® | 10% Nano Medical Hydroxyapatite 10% Xylitol, Water, macrogol 400, zeolite, polyvinylpyrrolidone, glycyrrthetinc acid, cetylpyridinium chloride, glycerin, xylitol silicic anhydride, castor oil, sodium lauroyl glutamate, carragenan, ethanol, carboxymethylcellulose sodium, titanium dioxide, flavour. | Canada |
| 10 | Biorepair® Advanced Active Shield Anti-Cavities | Aqua, zinc hydroxyapatite, hydrated silica, sorbitol, glycerin, xylitol, silica, aroma, cellulose gum, zinc pca, sodium myristoyl sarcosinate, sodium methyl cocoyl taurate, tetrapotassium pyrophosphate, sodium saccharin, zinc citrate, citric acid, ammonium acryloyldimethyltaurate/VP copolymer, benzyl alcohol, phenoxyethanol, sodium benzoate, limonene. | Italy |
| 11 | GUM SensiVital+ toothpaste | Glycerin, aqua, hydrated silica, isomalt, potassium nitrates, hydroxyapatite, PVM/MA copolymer, lauryl glucoside, PEG-40 hydrogenated castor oil, sodium monofluorophosphate, aroma, cellulose gum, sodium hydroxide, sodium saccharin, cocamidopropyl betaine, hesperidin, sucralose, sodium chloride, cetylpyridinium chloride, sodium benzoate, CI 42090. | Germany |
| 12 | Kinder Karex™ toothpaste | Aqua, hydrogenated starch hydrolysate, hydrated silica, hydroxyapatite, xylitol, silica, cellulose gum, aroma, 1,2-hexanediol, caprylyl glycol, sodium methyl cocoyl taurate, sodium sulfate, sodium cocoyl glycinate, limonene | Germany |
| 13 | NanoXIM •CarePaste | (Synthetic nano-HA water-based suspension ingredient designed to be easily incorporated in oral care products.) hydroxyapatite (nano), Potassium Chloride, Microbial content, heavy metals. | Portugal |
| 14 | VITIS® whitening toothpaste | Aqua, glycerin, sorbitol, silica, PVP, sodium lauryl sulphate, titanium dioxide, sodium monofluorophosphate, pentasodium triphosphate, perlite, sodium hexametaphosphate, xanthan gum, xylitol, tetrapotassium pyrophosphate, hydroxylapatite (nano), menthone glycerin acetal, sodium saccharin, potassium chloride, sodium methylparaben, potassium acesulfame, aroma. | Spain |
| 15 | INNOVA | Aqua, hydrated silica, hydrogenated starch hydrolysate, glycerin, PEG-8, hydroxyapatite, cellulose gum, aroma, sodium monofluorophosphate, cocamidopropyl betaine, sodium lauroyl sarcosinate, xylitol, propylene glycol, olaflur, Stevia rebaudiana leaf extract, anethole, citric acid, eucalyptol, o-cymen-5-ol, tocopheryl acetate, CI 77891, thymol, calcium lactate, Vitis vinifera seed extract, disodium EDTA, aspergillus/tannic acid ferment extract, glucose, inositol, sodium benzoate, potassium sorbate, limonene. Fluoride content—0,15% (1500 ppm). | Russian |
| 16 | MEGASONEX | Sorbitol, glycerin, hydroxyapatite (nano), water (aqua), silica, xylitol, tetrasodium pyrophosphate, sodium methyl cocoyl taurate, mica, titanium dioxide, sodium carboxymethylcellulose, citric acid, sodium saccharin, aroma | USA |
| 17 | WhiteWashLaboratories | Glycerin, aqua, calcium carbonate, xylitol, hydroxyapatite, potassium nitrate, hydrated silica, tetrasodium pyrophosphate, kaolin, sodium bicarbonate, pentasodium triphosphate, pvp, sodium monofluorophosphate, cocamidopropyl betaine, potassium chloride, xanthan gum, stevioside, Mentha piperita oil, bromelain, l-menthol, papain, urea peroxide, Eucalyptus globulus leaf oil, limonene. | UK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in Oral Care Products—A Review. Materials 2021, 14, 4865. https://doi.org/10.3390/ma14174865
Chen L, Al-Bayatee S, Khurshid Z, Shavandi A, Brunton P, Ratnayake J. Hydroxyapatite in Oral Care Products—A Review. Materials. 2021; 14(17):4865. https://doi.org/10.3390/ma14174865
Chicago/Turabian StyleChen, Lijie, Suma Al-Bayatee, Zohaib Khurshid, Amin Shavandi, Paul Brunton, and Jithendra Ratnayake. 2021. "Hydroxyapatite in Oral Care Products—A Review" Materials 14, no. 17: 4865. https://doi.org/10.3390/ma14174865
APA StyleChen, L., Al-Bayatee, S., Khurshid, Z., Shavandi, A., Brunton, P., & Ratnayake, J. (2021). Hydroxyapatite in Oral Care Products—A Review. Materials, 14(17), 4865. https://doi.org/10.3390/ma14174865








