Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair
Abstract
:1. Introduction
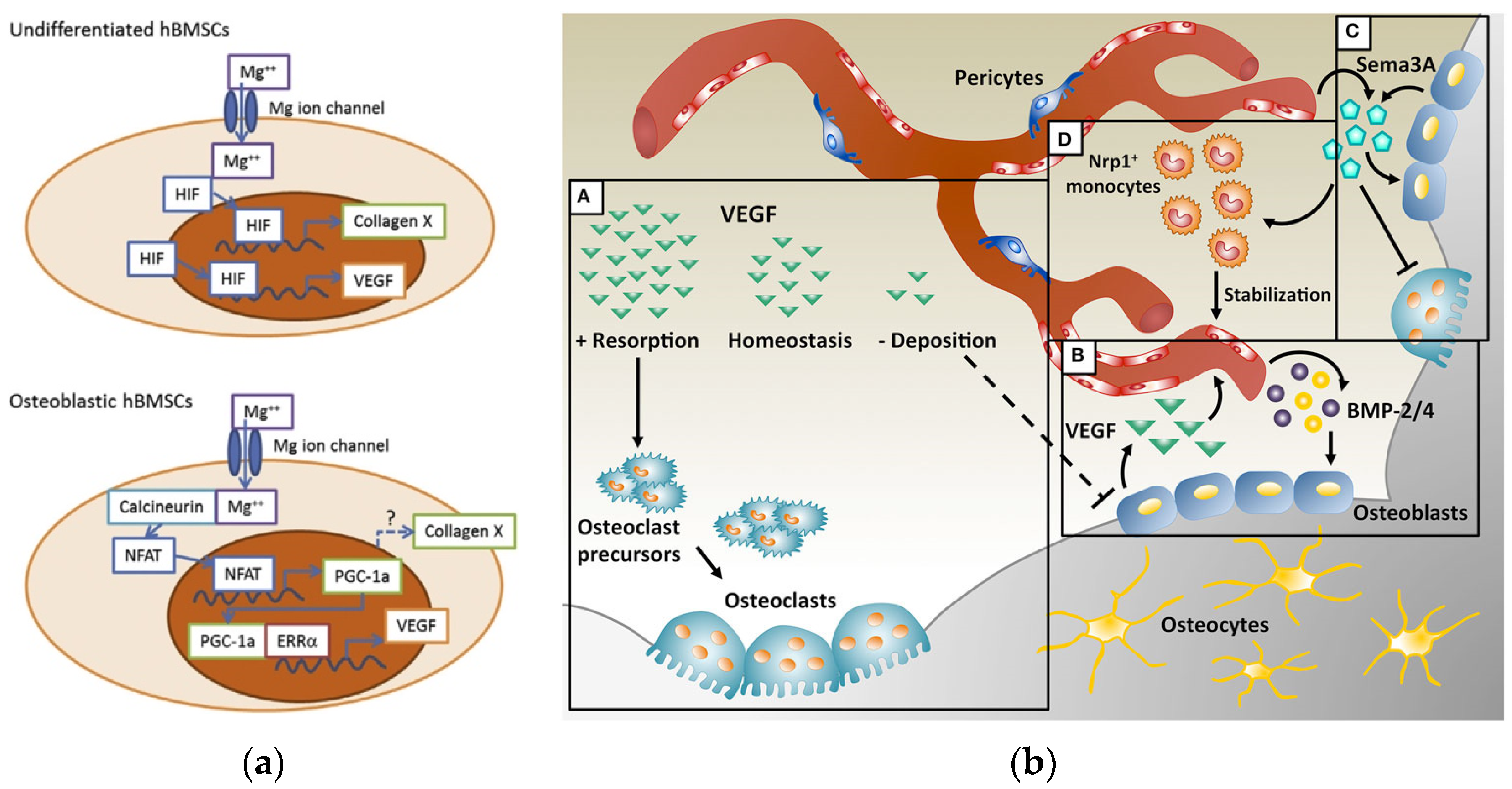
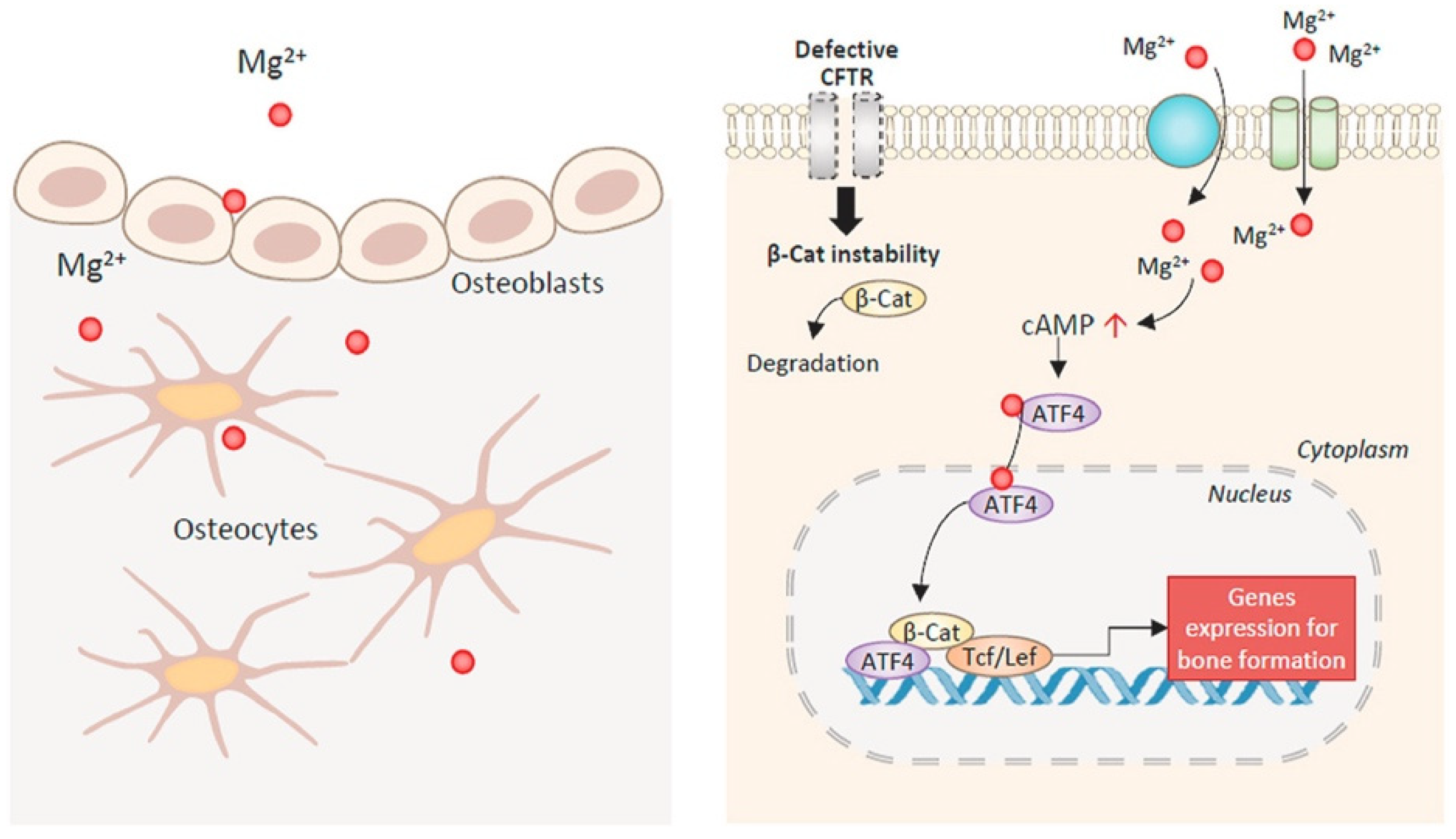
2. The Role of Magnesium in the Human Body and Its Inducing Influence on Bone Regeneration
3. Magnesium Phosphate-Based Bone Cements
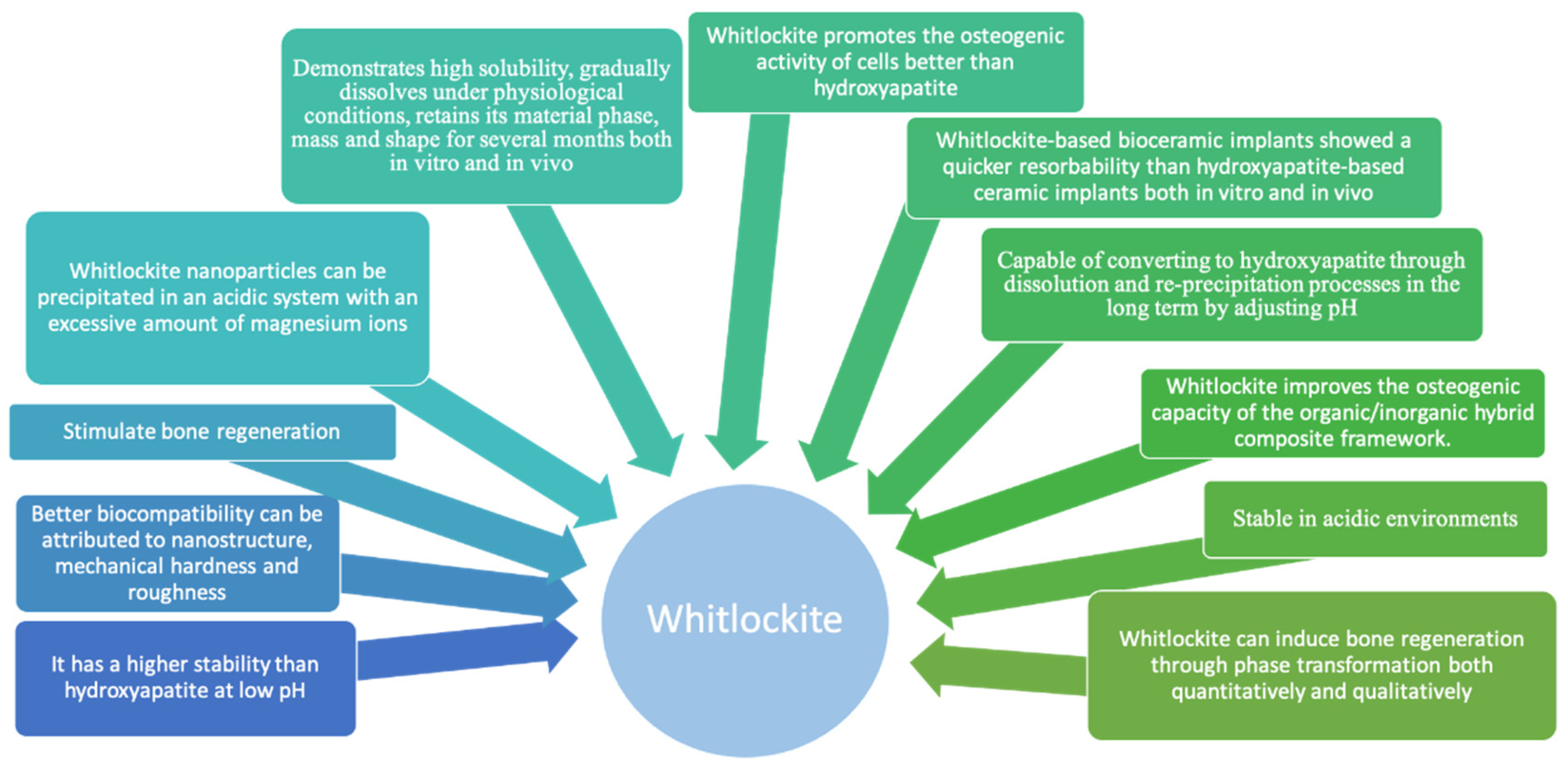
4. Whitlockite Synthesis and Its Bone Remodeling Features
5. Resorbability of Phosphate-Based Biomaterials with Different Ca/Mg Ratios
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, W. Opportunities and Challenges for the Biodegradable Magnesium Alloys as Next-Generation Biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Yuan, G.; Liao, Y.; Mao, L.; Zhang, J.; Wang, Y.; Huang, F.; Jiang, Y.; He, Y.; Ding, W. Enhanced Biocorrosion Resistance and Biocompatibility of Degradable Mg–Nd–Zn–Zr Alloy by Brushite Coating. Mater. Sci. Eng. C 2013, 33, 4833–4841. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.S. In Vitro Degradation and Mechanical Integrity of Calcium-Containing Magnesium Alloys in Modified-Simulated Body Fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Lukyanova, E.; Anisimova, N.; Martynenko, N.; Kiselevsky, M.; Dobatkin, S.; Estrin, Y. Features of In Vitro and In Vivo Behaviour of Magnesium Alloy WE43. Mater. Lett. 2018, 215, 308–311. [Google Scholar] [CrossRef]
- Wang, H.X.; Guan, S.K.; Wang, X.; Ren, C.X.; Wang, L.G. In Vitro Degradation and Mechanical Integrity of Mg–Zn–Ca Alloy Coated with Ca-Deficient Hydroxyapatite by the Pulse Electrodeposition Process. Acta Biomater. 2010, 6, 1743–1748. [Google Scholar] [CrossRef]
- Wolff, M.; Luczak, M.; Schaper, J.G.; Wiese, B.; Dahms, M.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. In Vitro Biodegradation Testing of Mg-Alloy EZK400 and Manufacturing of Implant Prototypes Using PM (Powder Metallurgy) Methods. Bioact. Mater. 2018, 3, 213–217. [Google Scholar] [CrossRef]
- Urban, R.M.; Jacobs, J.J.; Gilbert, J.L.; Galante, J.O. Migration of Corrosion Products from Modular Hip Prostheses. Particle Microanalysis and Histopathological Findings. JBJS 1994, 76, 1345–1359. [Google Scholar] [CrossRef]
- Cooper, H.J.; Urban, R.M.; Wixson, R.L.; Meneghini, R.M.; Jacobs, J.J. Adverse Local Tissue Reaction Arising from Corrosion at the Femoral Neck-Body Junction in a Dual-Taper Stem with a Cobalt-Chromium Modular Neck. JBJS 2013, 95, 865. [Google Scholar] [CrossRef]
- Kirkpatrick, C.J.; Alves, A.; Köhler, H.; Kriegsmann, J.; Bittinger, F.; Otto, M.; Williams, D.F.; Eloy, R. Biomaterial-Induced Sarcoma: A Novel Model to Study Preneoplastic Change. Am. J. Pathol. 2000, 156, 1455–1467. [Google Scholar] [CrossRef]
- Kavalar, R.; Fokter, S.K.; Lamovec, J. Total Hip Arthroplasty-Related Osteogenic Osteosarcoma: Case Report and Review of the Literature. Eur. J. Med. Res. 2016, 21, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable Biomaterials Based on Magnesium Corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Lu, H.; Sun, J. Long-Term in Vivo Evolution of High-Purity Mg Screw Degradation—Local and Systemic Effects of Mg Degradation Products. Acta Biomater. 2018, 71, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, R.; Ahmadi, S.M.; Lietaert, K.; Tümer, N.; Li, Y.; Amin Yavari, S.; Zadpoor, A.A. Fatigue and Quasi-Static Mechanical Behavior of Bio-Degradable Porous Biomaterials Based on Magnesium Alloys. J. Biomed. Mater. Res. Part A 2018, 106, 1798–1811. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Herrera, J.A.; Reina-Romo, E.; Boccaccini, A.R. In Silico Design of Magnesium Implants: Macroscopic Modeling. J. Mech. Behav. Biomed. Mater. 2018, 79, 181–188. [Google Scholar] [CrossRef]
- Trisvetova, E. Magnesium Homeostasis and Aging. Meditsinskie Nov. 2018, 2, 45–50. [Google Scholar]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-Based Biodegradable Alloys: Degradation, Application, and Alloying Elements. Interv. Med. Appl. Sci. Interv. Med. Appl. Sci. 2017, 9, 27–38. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X. Surface Modification of Biodegradable Magnesium and Its Alloys for Biomedical Applications. Regen. Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Zhu, D. Endothelial Responses of Magnesium and Other Alloying Elements in Magnesium-Based Stent Materials. Metallomics 2015, 7, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.F.; Randall, J.T. The Fine Structure of Bone. Nature 1956, 178, 798. [Google Scholar] [CrossRef] [PubMed]
- Scotchford, C.A.; Vickers, M.; Yousuf Ali, S. The Isolation and Characterization of Magnesium Whitlockite Crystals from Human Articular Cartilage. Osteoarthr. Cartil. 1995, 3, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, M.; Dominguez, L. Magnesium and Aging. Curr. Pharm. Des. 2010, 16, 832–839. [Google Scholar] [CrossRef]
- Neuman, W.F.; Mulryan, B.J. Synthetic Hydroxyapatite Crystals. Calcif. Tissue Res. 1971, 7, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Weisinger, J.R.; Bellorín-Font, E. Magnesium and Phosphorus. Lancet 1998, 352, 391–396. [Google Scholar] [CrossRef]
- Wolf, F.I.; Cittadini, A. Chemistry and Biochemistry of Magnesium. Mol. Asp. Med. 2003, 24, 3–9. [Google Scholar] [CrossRef]
- Boskey, A.L.; Rimnac, C.M.; Bansal, M.; Federman, M.; Lian, J.; Boyan, B.D. Effect of Short-Term Hypomagnesemia on the Chemical and Mechanical Properties of Rat Bone. J. Orthop. Res. 1992, 10, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Dimai, H.-P.; Porta, S.; Wirnsberger, G.; Lindschinger, M.; Pamperl, I.; Dobnig, H.; Wilders-Truschnig, M.; Lau, K.-H.W. Daily Oral Magnesium Supplementation Suppresses Bone Turnover in Young Adult Males. J. Clin. Endocrinol. Metab. 1998, 83, 2742–2748. [Google Scholar] [CrossRef]
- Visly, A.A. Role of Magnesium in the Regulation of Physiological Processes in Organism. J. Med. Pharm. News 2008, 6, 14–15. Available online: http://www.mif-ua.com/archive/article/4762 (accessed on 24 August 2021). (In Russian).
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J.C. Effects of Extracellular Magnesium Extract on the Proliferation and Differentiation of Human Osteoblasts and Osteoclasts in Coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef]
- Classen, H.G.; Baier, S.; Schimatschek, H.F.; Classen, C.U. Clinically Relevant Interactions between Hormones and Magnesium Metabolism—A Review. Magnes. Bull. 1995, 17, 96–103. [Google Scholar]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium Ion Stimulation of Bone Marrow Stromal Cells Enhances Osteogenic Activity, Simulating the Effect of Magnesium Alloy Degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF Couples Hypertrophic Cartilage Remodeling, Ossification and Angiogenesis during Endochondral Bone Formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Carlevaro, M.F.; Cermelli, S.; Cancedda, R.; Descalzi Cancedda, F. Vascular Endothelial Growth Factor (VEGF) in Cartilage Neovascularization and Chondrocyte Differentiation: Auto-Paracrine Role during Endochondral Bone Formation. J. Cell Sci. 2000, 113, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. Osteoblast-Derived VEGF Regulates Osteoblast Differentiation and Bone Formation during Bone Repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, J.I.; Shields, D.J.; Barillas, S.G.; Acevedo, L.M.; Murphy, E.; Huang, J.; Scheppke, L.; Stockmann, C.; Johnson, R.S.; Angle, N.; et al. A Role for VEGF as a Negative Regulator of Pericyte Function and Vessel Maturation. Nature 2008, 456, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Schönmeyr, B.H.; Soares, M.; Avraham, T.; Clavin, N.W.; Gewalli, F.; Mehrara, B.J. Vascular Endothelial Growth Factor Inhibits Bone Morphogenetic Protein 2 Expression in Rat Mesenchymal Stem Cells. Tissue Eng. Part A 2010, 16, 653–662. [Google Scholar] [CrossRef]
- Song, X.; Liu, S.; Qu, X.; Hu, Y.; Zhang, X.; Wang, T.; Wei, F. BMP2 and VEGF Promote Angiogenesis but Retard Terminal Differentiation of Osteoblasts in Bone Regeneration by Up-Regulating Id1. Acta Biochim. Biophys. Sin. 2011, 43, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Helmrich, U.; Di Maggio, N.; Güven, S.; Groppa, E.; Melly, L.; Largo, R.D.; Heberer, M.; Martin, I.; Scherberich, A.; Banfi, A. Osteogenic Graft Vascularization and Bone Resorption by VEGF-Expressing Human Mesenchymal Progenitors. Biomaterials 2013, 34, 5025–5035. [Google Scholar] [CrossRef]
- Xie, H.; Cui, Z.; Wang, L.; Xia, Z.; Hu, Y.; Xian, L.; Li, C.; Xie, L.; Crane, J.; Wan, M.; et al. PDGF-BB Secreted by Preosteoclasts Induces Angiogenesis during Coupling with Osteogenesis. Nat. Med. 2014, 20, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.-C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The Role of Magnesium Ions in Bone Regeneration Involves the Canonical Wnt Signaling Pathway. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Hamushan, M.; Cai, W.; Zhang, Y.; Ren, Z.; Du, J.; Zhang, S.; Zhao, C.; Cheng, P.; Zhang, X.; Shen, H.; et al. High-Purity Magnesium Pin Enhances Bone Consolidation in Distraction Osteogenesis via Regulating Ptch Protein Activating Hedgehog-Alternative Wnt Signaling. Bioact. Mater. 2021, 6, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; de Lamare, J.; Stiles, P. Noise Effect on Core Bias Power Supply for Klystron in LCLS. In Proceedings of the 2017 IEEE 18th Workshop on Control and Modeling for Power Electronics (COMPEL), Stanford, CA, USA, 9–12 July 2017; pp. 1–4. [Google Scholar]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-Derived Magnesium Induces Local Neuronal Production of CGRP to Improve Bone-Fracture Healing in Rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Xu, J.; Hu, P.; Zhang, X.; Chen, J.; Wang, J.; Zhang, J.; Chen, Z.; Yu, M.K.; Chung, Y.W.; Wang, Y.; et al. Magnesium Implantation or Supplementation Ameliorates Bone Disorder in CFTR-Mutant Mice through an ATF4-Dependent Wnt/β-Catenin Signaling. Bioact. Mater. 2021, in press. [Google Scholar] [CrossRef]
- Belluci, M.M.; Schoenmaker, T.; Rossa-Junior, C.; Orrico, S.R.; de Vries, T.J.; Everts, V. Magnesium Deficiency Results in an Increased Formation of Osteoclasts. J. Nutr. Biochem. 2013, 24, 1488–1498. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Mills, B.G. Bone Loss Induced by Dietary Magnesium Reduction to 10% of the Nutrient Requirement in Rats Is Associated with Increased Release of Substance P and Tumor Necrosis Factor-α. J. Nutr. 2004, 134, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.; Qu, X.; Li, H.; Yang, K.; Wan, P.; Tan, L.; Ouyang, Z.; Liu, X.; Tian, B.; Xiao, F.; et al. The Effect of Metallic Magnesium Degradation Products on Osteoclast-Induced Osteolysis and Attenuation of NF-ΚB and NFATc1 Signaling. Biomaterials 2014, 35, 6299–6310. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared Mechanisms and Crosstalk between the Immune and Bone Systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture Healing under Healthy and Inflammatory Conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory Properties of Magnesium Scaffolds Coated with β-Tricalcium Phosphate. Biomaterials 2014, 35, 8553–8565. [Google Scholar] [CrossRef]
- Libako, P.; Nowacki, W.; Castiglioni, S.; Mazur, A.; Jeanette, A.M. Maier Extracellular Magnesium and Calcium Blockers Modulate Macrophage Activity. Magnes. Res. 2016, 29, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Rubin, H. Central Role for Magnesium in Coordinate Control of Metabolism and Growth in Animal Cells. Proc. Natl. Acad. Sci. USA 1975, 72, 3551–3555. [Google Scholar] [CrossRef] [Green Version]
- Wolf, F.I.; Trapani, V. Cell (Patho)Physiology of Magnesium. Clin. Sci. 2007, 114, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, K.; Kocot, J.; Horecka, A. Biochemistry of Magnesium. J. Elementol. 2010, 3, 601–616. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Salimi, M.H.; Heughebaert, J.C.; Nancollas, G.H. Crystal Growth of Calcium Phosphates in the Presence of Magnesium Ions. Langmuir 1985, 1, 119–122. [Google Scholar] [CrossRef]
- Grünewald, T.A.; Rennhofer, H.; Hesse, B.; Burghammer, M.; Stanzl-Tschegg, S.E.; Cotte, M.; Löffler, J.F.; Weinberg, A.M.; Lichtenegger, H.C. Magnesium from Bioresorbable Implants: Distribution and Impact on the Nano- and Mineral Structure of Bone. Biomaterials 2016, 76, 250–260. [Google Scholar] [CrossRef]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-Substituted Hydroxyapatite: From Synthesis to in Vivo Behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.-S.; Zhou, W.-S.; He, X.-W.; Liu, W.; Bai, B.-L.; Zhou, Q.; Huang, Z.-L.; Tu, K.; Li, H.; Sun, T.; et al. A Comparative Study of Zinc, Magnesium, Strontium-Incorporated Hydroxyapatite-Coated Titanium Implants for Osseointegration of Osteopenic Rats. Mater. Sci. Eng. C 2016, 62, 226–232. [Google Scholar] [CrossRef]
- Santos, G.; Nunes, V.; Marinho, S.; Santos, S.; Rossi, A.; Miguel, F. Biological Behavior of Magnesium-Substituted Hydroxyapatite during Bone Repair-PubMed. Braz. J. Biol. 2021, 1, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, E. Magnesium Screw and Nail Transfixion in Fractures. South. Med. J. 1938, 31, 508–514. [Google Scholar] [CrossRef]
- Liu, C.; Xin, Y.; Tang, G.; Chu, P.K. Influence of Heat Treatment on Degradation Behavior of Bio-Degradable Die-Cast AZ63 Magnesium Alloy in Simulated Body Fluid. Mater. Sci. Eng. A 2007, 456, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Brar, H.S.; Wong, J.; Manuel, M.V. Investigation of the Mechanical and Degradation Properties of Mg–Sr and Mg–Zn–Sr Alloys for Use as Potential Biodegradable Implant Materials. J. Mech. Behav. Biomed. Mater. 2012, 7, 87–95. [Google Scholar] [CrossRef]
- Wu, L.; Luthringer, B.J.C.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of Extracellular Magnesium on the Differentiation and Function of Human Osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef] [Green Version]
- Golafshan, N.; Vorndran, E.; Zaharievski, S.; Brommer, H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Gbureck, U.; van Weeren, R.; Castilho, M.; Malda, J. Tough Magnesium Phosphate-Based 3D-Printed Implants Induce Bone Regeneration in an Equine Defect Model. Biomaterials 2020, 261, 120302. [Google Scholar] [CrossRef]
- Zhai, Q.; Han, F.; He, Z.; Shi, C.; Zhou, P.; Zhu, C.; Guo, Q.; Zhu, X.; Yang, H.; Li, B. The “Magnesium Sacrifice” Strategy Enables PMMA Bone Cement Partial Biodegradability and Osseointegration Potential. Int. J. Mol. Sci. 2018, 19, 1746. [Google Scholar] [CrossRef] [Green Version]
- Kanter, B.; Vikman, A.; Brückner, T.; Schamel, M.; Gbureck, U.; Ignatius, A. Bone Regeneration Capacity of Magnesium Phosphate Cements in a Large Animal Model. Acta Biomater. 2018, 69, 352–361. [Google Scholar] [CrossRef]
- O’Neill, R.; McCarthy, H.O.; Montufar, E.B.; Ginebra, M.-P.; Wilson, D.I.; Lennon, A.; Dunne, N. Critical Review: Injectability of Calcium Phosphate Pastes and Cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium Phosphate Cements for Bone Substitution: Chemistry, Handling and Mechanical Properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhou, H.; Wei, J.; Jiang, X.; Hua, H.; Chen, F.; Wei, S.; Shin, J.-W.; Liu, C. Development of Magnesium Calcium Phosphate Biocement for Bone Regeneration. J. R. Soc. Interface 2010, 7, 1171–1180. [Google Scholar] [CrossRef]
- Wang, S.; Xu, C.; Yu, S.; Wu, X.; Jie, Z.; Dai, H. Citric Acid Enhances the Physical Properties, Cytocompatibility and Osteogenesis of Magnesium Calcium Phosphate Cement. J. Mech. Behav. Biomed. Mater. 2019, 94, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Jia, J.; Wu, F.; Wei, S.; Zhou, H.; Zhang, H.; Shin, J.-W.; Liu, C. Hierarchically Microporous/Macroporous Scaffold of Magnesium–Calcium Phosphate for Bone Tissue Regeneration. Biomaterials 2010, 31, 1260–1269. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, S.; Wu, X.; Dai, H.; Liu, W.; Tu, R.; Goto, T. Construction of Macroporous Magnesium Phosphate-Based Bone Cement with Sustained Drug Release. Mater. Des. 2021, 200, 109466. [Google Scholar] [CrossRef]
- Fan, C.; Zhan, S.-H.; Dong, Z.-X.; Yang, W.; Deng, W.-S.; Liu, X.; Wang, D.-A.; Sun, P. Cross-Linked Gelatin Microsphere-Based Scaffolds as a Delivery Vehicle of MC3T3-E1 Cells: In Vitro and in Vivo Evaluation. Mater. Sci. Eng. C 2020, 108, 110399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, A.; Chen, C.; Tang, J.; Fan, H.; Sun, J.; Fan, H. An Efficient Two-Step Preparation of Photocrosslinked Gelatin Microspheres as Cell Carriers to Support MC3T3-E1 Cells Osteogenic Performance. Colloids Surf. B Biointerfaces 2020, 188, 110798. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y.; Li, Y.-T. Influence of Cross-Linker Concentration on the Functionality of Carbodiimide Cross-Linked Gelatin Membranes for Retinal Sheet Carriers. J. Biomater. Sci. 2011, 22, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S. Calcium Phosphate Ceramic Systems in Growth Factor and Drug Delivery for Bone Tissue Engineering: A Review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Chen, G.; Jiang, Z.; Zhou, H.; Yao, H.; Zhou, R.; Ren, W. Rheological Properties of Magnesium Phosphate Cement with Different M/P Ratios. Constr. Build. Mater. 2021, 282, 122657. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, L.; Gong, C.; Li, W.; Zhao, Y.; Guo, W. A Bioactive Magnesium Phosphate Cement Incorporating Chondroitin Sulfate for Bone Regeneration. Biomed. Mater. 2021, 16, 035034. [Google Scholar] [CrossRef]
- Manton, K.J.; Leong, D.F.M.; Cool, S.M.; Nurcombe, V. Disruption of Heparan and Chondroitin Sulfate Signaling Enhances Mesenchymal Stem Cell-Derived Osteogenic Differentiation via Bone Morphogenetic Protein Signaling Pathways. Stem Cells 2007, 25, 2845–2854. [Google Scholar] [CrossRef]
- Koutsoukos, P.; Amjad, Z.; Tomson, M.B.; Nancollas, G.H. Crystallization of Calcium Phosphates. A Constant Composition Study. J. Am. Chem. Soc. 1980, 102, 1553–1557. [Google Scholar] [CrossRef]
- Sader, M.S.; LeGeros, R.Z.; Soares, G.A. Human Osteoblasts Adhesion and Proliferation on Magnesium-Substituted Tricalcium Phosphate Dense Tablets. J. Mater. Sci. Mater. Med. 2009, 20, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Bizios, R. Hydroxylapatite with Substituted Magnesium, Zinc, Cadmium, and Yttrium. II. Mechanisms of Osteoblast Adhesion. J. Biomed. Mater. Res. 2002, 59, 312–317. [Google Scholar] [CrossRef]
- Bracci, B.; Torricelli, P.; Panzavolta, S.; Boanini, E.; Giardino, R.; Bigi, A. Effect of Mg2+, Sr2+, and Mn2+ on the Chemico-Physical and in Vitro Biological Properties of Calcium Phosphate Biomimetic Coatings. J. Inorg. Biochem. 2009, 103, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Massa-Schlueter, E.A.; Smith, J.L.; Slamovich, E.B. Osteoblast Response to Hydroxyapatite Doped with Divalent and Trivalent Cations. Biomaterials 2004, 25, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Jin, K.; Lee, J.; Kim, Y.; Nahm, S.H.; Hong, K.S.; Nam, K.T. Revisiting Whitlockite, the Second Most Abundant Biomineral in Bone: Nanocrystal Synthesis in Physiologically Relevant Conditions and Biocompatibility Evaluation. ACS Nano 2014, 8, 634–641. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504. [Google Scholar] [CrossRef]
- Blair, H.; Teitelbaum, S.; Ghiselli, R.; Gluck, S. Osteoclastic Bone Resorption by a Polarized Vacuolar Proton Pump. Science 1989, 245, 855. [Google Scholar] [CrossRef]
- Meinke, D.K.; Skinner, H.C.W.; Thomson, K.S. X-Ray Diffraction of the Calcified Tissues InPolypterus. Calcif. Tissue Int. 1979, 28, 37–42. [Google Scholar] [CrossRef]
- Quint, P.; Althoff, J.; Höhling, H.J.; Boyde, A.; Laabs, W.A. Characteristic Molar Ratios of Magnesium, Carbon Dioxide, Calcium and Phosphorus in the Mineralizing Fracture Callus and Predentine. Calcif. Tissue Int. 1980, 32, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Jang, H.L.; Ahn, H.-Y.; Lee, H.K.; Park, J.; Lee, E.; Lee, E.A.; Jeong, Y.-H.; Kim, D.-G.; Nam, K.T.; et al. Biomimetic Whitlockite Inorganic Nanoparticles-Mediated in Situ Remodeling and Rapid Bone Regeneration. Biomaterials 2017, 112, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Driessens, F.C.M.; Verbeeck, R. (viaf)37087491 Biominerals; CRC Press: Boca Raton, FL, USA, 1990; ISBN 0-8493-5280-0. [Google Scholar]
- Terpstra, R.A.; Driessens, F.C.M. Magnesium in Tooth Enamel and Synthetic Apatites. Calcif. Tissue Int. 1986, 39, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Lee, H.K.; Jin, K.; Ahn, H.-Y.; Lee, H.-E.; Nam, K.T. Phase Transformation from Hydroxyapatite to the Secondary Bone Mineral, Whitlockite. J. Mater. Chem. B 2015, 3, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Zheng, G.B.; Park, J.; Kim, H.D.; Baek, H.-R.; Lee, H.K.; Lee, K.; Han, H.N.; Lee, C.-K.; Hwang, N.S.; et al. In Vitro and In Vivo Evaluation of Whitlockite Biocompatibility: Comparative Study with Hydroxyapatite and β-Tricalcium Phosphate. Adv. Healthc. Mater. 2016, 5, 128–136. [Google Scholar] [CrossRef]
- Cheng, H.; Chabok, R.; Guan, X.; Chawla, A.; Li, Y.; Khademhosseini, A.; Jang, H.L. Synergistic Interplay between the Two Major Bone Minerals, Hydroxyapatite and Whitlockite Nanoparticles, for Osteogenic Differentiation of Mesenchymal Stem Cells. Acta Biomater. 2018, 69, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Maxian, S.H.; Zawadsky, J.P.; Dunn, M.G. Mechanical and Histological Evaluation of Amorphous Calcium Phosphate and Poorly Crystallized Hydroxyapatite Coatings on Titanium Implants. J. Biomed. Mater. Res. 1993, 27, 717–728. [Google Scholar] [CrossRef]
- Abbona, F.; Lundager Madsen, H.E.; Boistelle, R. The Final Phases of Calcium and Magnesium Phosphates Precipitated from Solutions of High to Medium Concentration. J. Cryst. Growth 1988, 89, 592–602. [Google Scholar] [CrossRef]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Cacciotti, I.; Bianco, A.; Lombardi, M.; Montanaro, L. Mg-Substituted Hydroxyapatite Nanopowders: Synthesis, Thermal Stability and Sintering Behaviour. J. Eur. Ceram. Soc. 2009, 29, 2969–2978. [Google Scholar] [CrossRef]
- Batra, U.; Kapoor, S.; Sharma, S. Influence of Magnesium Ion Substitution on Structural and Thermal Behavior of Nanodimensional Hydroxyapatite. J. Mater. Eng. Perform. 2013, 22, 1798–1806. [Google Scholar] [CrossRef]
- Marchi, J.; Dantas, A.C.S.; Greil, P.; Bressiani, J.C.; Bressiani, A.H.A.; Müller, F.A. Influence of Mg-Substitution on the Physicochemical Properties of Calcium Phosphate Powders. Mater. Res. Bull. 2007, 42, 1040–1050. [Google Scholar] [CrossRef]
- Li, X.; Ito, A.; Sogo, Y.; Wang, X.; LeGeros, R.Z. Solubility of Mg-Containing β-Tricalcium Phosphate at 25 °C. Acta Biomater. 2009, 5, 508–517. [Google Scholar] [CrossRef] [Green Version]
- Enderle, R.; Götz-Neunhoeffer, F.; Göbbels, M.; Müller, F.A.; Greil, P. Influence of Magnesium Doping on the Phase Transformation Temperature of β-TCP Ceramics Examined by Rietveld Refinement. Biomaterials 2005, 26, 3379–3384. [Google Scholar] [CrossRef]
- Yasukawa, A.; Ouchi, S.; Kandori, K.; Ishikawa, T. Preparation and Characterization of Magnesium–Calcium Hydroxyapatites. J. Mater. Chem. 1996, 6, 1401–1405. [Google Scholar] [CrossRef]
- Bigi, A.; Falini, G.; Foresti, E.; Ripamonti, A.; Gazzano, M.; Roveri, N. Magnesium Influence on Hydroxyapatite Crystallization. J. Inorg. Biochem. 1993, 49, 69–78. [Google Scholar] [CrossRef]
- Amjad, Z.; Koutsoukos, P.G.; Nancollas, G.H. The Crystallization of Hydroxyapatite and Fluorapatite in the Presence of Magnesium Ions. J. Colloid Interface Sci. 1984, 101, 250–256. [Google Scholar] [CrossRef]
- Eanes, E.D.; Rattner, S.L. The Effect of Magnesium on Apatite Formation in Seeded Supersaturated Solutions at PH 7.4. J. Dent Res. 1981, 60, 1719–1723. [Google Scholar] [CrossRef]
- Chu, W.; Li, T.; Jia, G.; Chang, Y.; Liu, Z.; Pei, J.; Yu, D.; Zhai, Z. Exposure to High Levels of Magnesium Disrupts Bone Mineralization in Vitro and in Vivo. Ann. Transl. Med. 2020, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Posner, A.S. Magnesium Stabilization of Amorphous Calcium Phosphate: A Kinetic Study. Mater. Res. Bull. 1974, 9, 907–916. [Google Scholar] [CrossRef]
- Zhou, H.; Bhaduri, S. Novel Microwave Synthesis of Amorphous Calcium Phosphate Nanospheres. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1142–1150. [Google Scholar] [CrossRef]
- Zhou, H.; Luchini, T.J.F.; Bhaduri, S.B. Microwave Assisted Synthesis of Amorphous Magnesium Phosphate Nanospheres. J. Mater. Sci. Mater. Med. 2012, 23, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Tung, M.S.; Tomazic, B.; Brown, W.E. The Effects of Magnesium and Fluoride on the Hydrolysis of Octacalcium Phosphate. Arch. Oral Biol. 1992, 37, 585–591. [Google Scholar] [CrossRef]
- Krause, A.; von der Höh, N.; Bormann, D.; Krause, C.; Bach, F.-W.; Windhagen, H.; Meyer-Lindenberg, A. Degradation Behaviour and Mechanical Properties of Magnesium Implants in Rabbit Tibiae. J. Mater. Sci. 2010, 45, 624–632. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.F.; Staiger, M.P.; Dias, G.J. The in Vitro and in Vivo Evaluation of the Biocompatibility of Mg Alloys. Biomed. Mater. 2013, 9, 015006. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Le Gars Santoni, B.; Douillard, T.; Zhang, F.; Gremillard, L.; Dolder, S.; Hofstetter, W.; Meille, S.; Bohner, M.; Chevalier, J.; et al. Effect of Grain Orientation and Magnesium Doping on β-Tricalcium Phosphate Resorption Behavior. Acta Biomater. 2019, 89, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kumta, P.N. Chemical Synthesis and Stabilization of Magnesium Substituted Brushite. Mater. Sci. Eng. C 2010, 30, 934–943. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Hong, K.S.; Lee, J.-K.; Kim, D.J. Variations of Structure and Composition in Magnesium Incorporated Hydroxyapatite/ß-Tricalcium Phosphate. J. Mater. Res. 2006, 21, 428–436. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic Magnesium Incorporated into PLGA/TCP Porous Scaffold by 3D Printing for Repairing Challenging Bone Defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- McCarthy, W.J.; Smith, D.M.A.; Adamowicz, L.; Saint-Martin, H.; Ortega-Blake, I. An Ab Initio Study of the Isomerization of Mg− and Ca−Pyrophosphates. J. Am. Chem. Soc. 1998, 120, 6113–6120. [Google Scholar] [CrossRef]
- Generosi, A.; Smirnov, V.V.; Rau, J.V.; Albertini, V.R.; Ferro, D.; Barinov, S.M. Phase Development in the Hardening Process of Two Calcium Phosphate Bone Cements: An Energy Dispersive X-Ray Diffraction Study. Mater. Res. Bull. 2008, 43, 561–571. [Google Scholar] [CrossRef]
- Boistelle, R.; Lopez-Valero, I.; Abbona, F. Crystallization of calcium phosphate in the presence of magnesium. Nephrologie 1993, 14, 265–269. [Google Scholar]
- Cheng, P.; Grabher, J.; LeGeros, R. Effects of Magnesium on Calcium Phosphate Formation. Magnesium 1988, 7, 123–132. [Google Scholar] [PubMed]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-Setting Bioactive Calcium–Magnesium Phosphate Cement with High Strength and Degradability for Bone Regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Klammert, U.; Reuther, T.; Blank, M.; Reske, I.; Barralet, J.E.; Grover, L.M.; Kübler, A.C.; Gbureck, U. Phase Composition, Mechanical Performance and in Vitro Biocompatibility of Hydraulic Setting Calcium Magnesium Phosphate Cement. Acta Biomater. 2010, 6, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F. Reaction Mechanisms of Magnesium Potassium Phosphate Cement and Its Application. Ph.D. Thesis, Hong Kong University of Science and Technology, Hong Kong, China, 2010. [Google Scholar]
- Goldberg, M.A.; Krohicheva, P.A.; Fomin, A.S.; Khairutdinova, D.R.; Antonova, O.S.; Baikin, A.S.; Smirnov, V.V.; Fomina, A.A.; Leonov, A.V.; Mikheev, I.V.; et al. In Situ Magnesium Calcium Phosphate Cements Formation: From One Pot Powders Precursors Synthesis to in Vitro Investigations. Bioact. Mater. 2020, 5, 644–658. [Google Scholar] [CrossRef]
- Kowalewicz, K.; Vorndran, E.; Feichtner, F.; Waselau, A.-C.; Brueckner, M.; Meyer-Lindenberg, A. In-Vivo Degradation Behavior and Osseointegration of 3D Powder-Printed Calcium Magnesium Phosphate Cement Scaffolds. Materials 2021, 14, 946. [Google Scholar] [CrossRef]
- Daniels, Y.; Lyczko, N.; Nzihou, A.; Alexandratos, S.D. Modification of Hydroxyapatite with Ion-Selective Complexants: 1-Hydroxyethane-1,1-Diphosphonic Acid. Ind. Eng. Chem. Res. 2015, 54, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Lijuan, X.; Liuyun, J.; Chengdong, X.; Lixin, J. Effect of Different Synthesis Conditions on the Microstructure, Crystallinity and Solubility of Mg-Substituted Hydroxyapatite Nanopowder. Adv. Powder Technol. 2014, 25, 1142–1146. [Google Scholar] [CrossRef]
- Safronova, T.V. Inorganic Materials for Regenerative Medicine. Inorg. Mater. 2021, 57, 443–474. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Smirnov, V.V.; Antonova, O.S.; Tut’kova, Y.B.; Obolkina, T.O.; Khairutdinova, D.R.; Krokhicheva, P.A.; Barinov, S.M.; Komlev, V.S. Ceramic Materials in the Tricalcium Phosphate–Trimagnesium Phosphate System. Inorg. Mater. 2020, 56, 314–320. [Google Scholar] [CrossRef]
- Kazakova, G.; Safronova, T.; Putlayev, V.; Knot’ko, A. Synthesis and Properties of Powders for the Preparation of Bioresorbable Ceramics Containing Calcium and Magnesium Orthophosphates. In Proceedings of the Third All-Russian Scientific Conference (With International Participation): “Success of Synthesis and Complex Formation”, RUDN University, Moscow, Russia, 21–25 April 2014; p. 100, ISBN 978-5-209-05765-9. Available online: https://www.elibrary.ru/item.asp?id=24638131 (accessed on 24 August 2021). (In Russian).
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis Methods for Nanosized Hydroxyapatite with Diverse Structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Lazić, S.; Zec, S.; Miljević, N.; Milonjić, S. The Effect of Temperature on the Properties of Hydroxyapatite Precipitated from Calcium Hydroxide and Phosphoric Acid. Thermochim. Acta 2001, 374, 13–22. [Google Scholar] [CrossRef]
- Pang, Y.X.; Bao, X. Influence of Temperature, Ripening Time and Calcination on the Morphology and Crystallinity of Hydroxyapatite Nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 1697–1704. [Google Scholar] [CrossRef] [Green Version]
- Yelten, A.; Yilmaz, S. Various Parameters Affecting the Synthesis of the Hydroxyapatite Powders by the Wet Chemical Precipitation Technique. Mater. Today Proc. 2016, 3, 2869–2876. [Google Scholar] [CrossRef]
- Kitikova, N.; Ivanets, A.; Shashkova, I.; Shareiko, A. Modeling of the Synthesis Conditions Impact on the Structure of Calcium Magnesium Phosphates. Mater. Chem. Phys. 2021, 267, 124627. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials 2021, 14, 4857. https://doi.org/10.3390/ma14174857
Kazakova G, Safronova T, Golubchikov D, Shevtsova O, Rau JV. Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials. 2021; 14(17):4857. https://doi.org/10.3390/ma14174857
Chicago/Turabian StyleKazakova, Gilyana, Tatiana Safronova, Daniil Golubchikov, Olga Shevtsova, and Julietta V. Rau. 2021. "Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair" Materials 14, no. 17: 4857. https://doi.org/10.3390/ma14174857
APA StyleKazakova, G., Safronova, T., Golubchikov, D., Shevtsova, O., & Rau, J. V. (2021). Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials, 14(17), 4857. https://doi.org/10.3390/ma14174857







