Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Preparation and Electrospinning Procedure
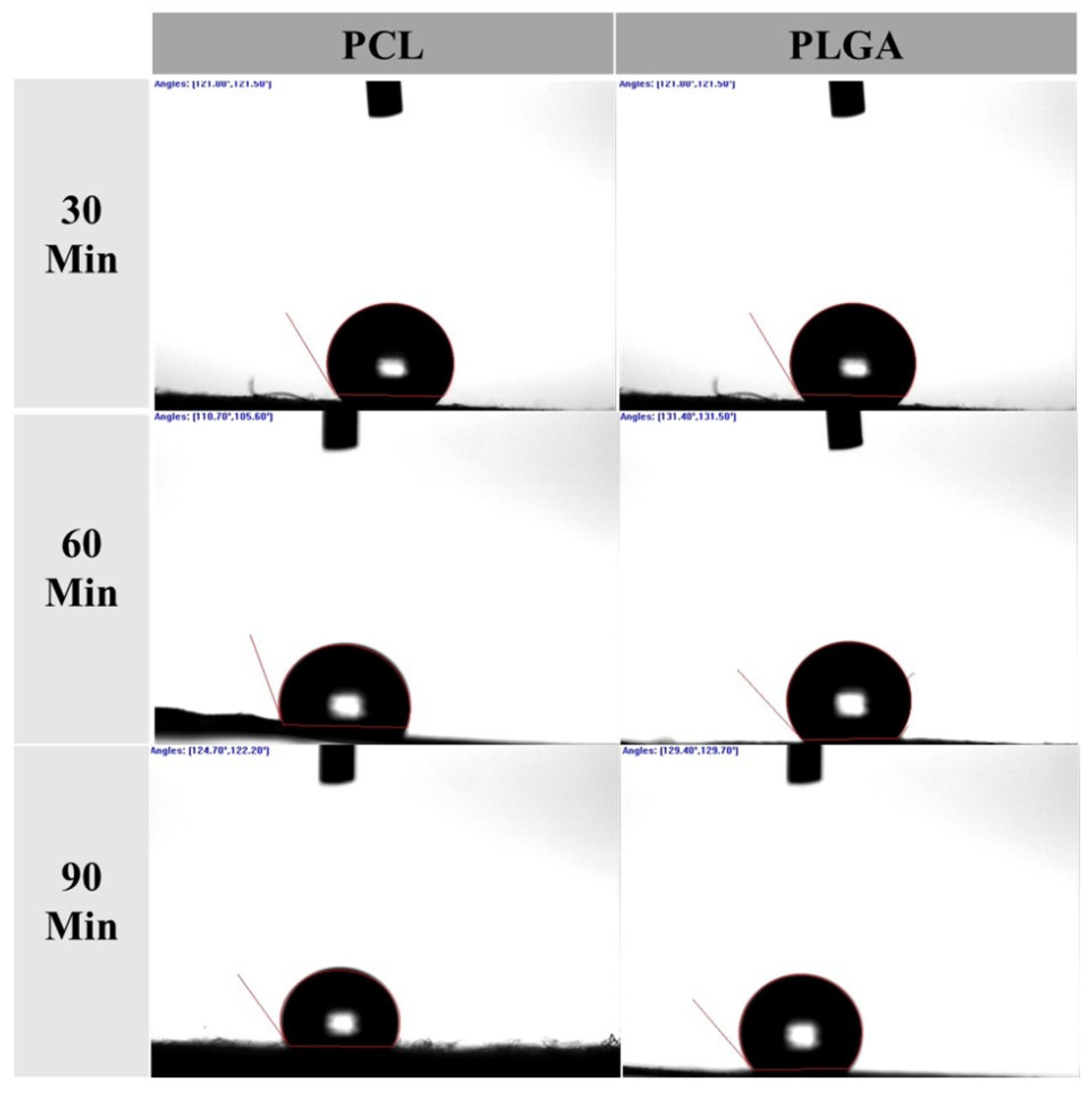
2.3. Wettability Test
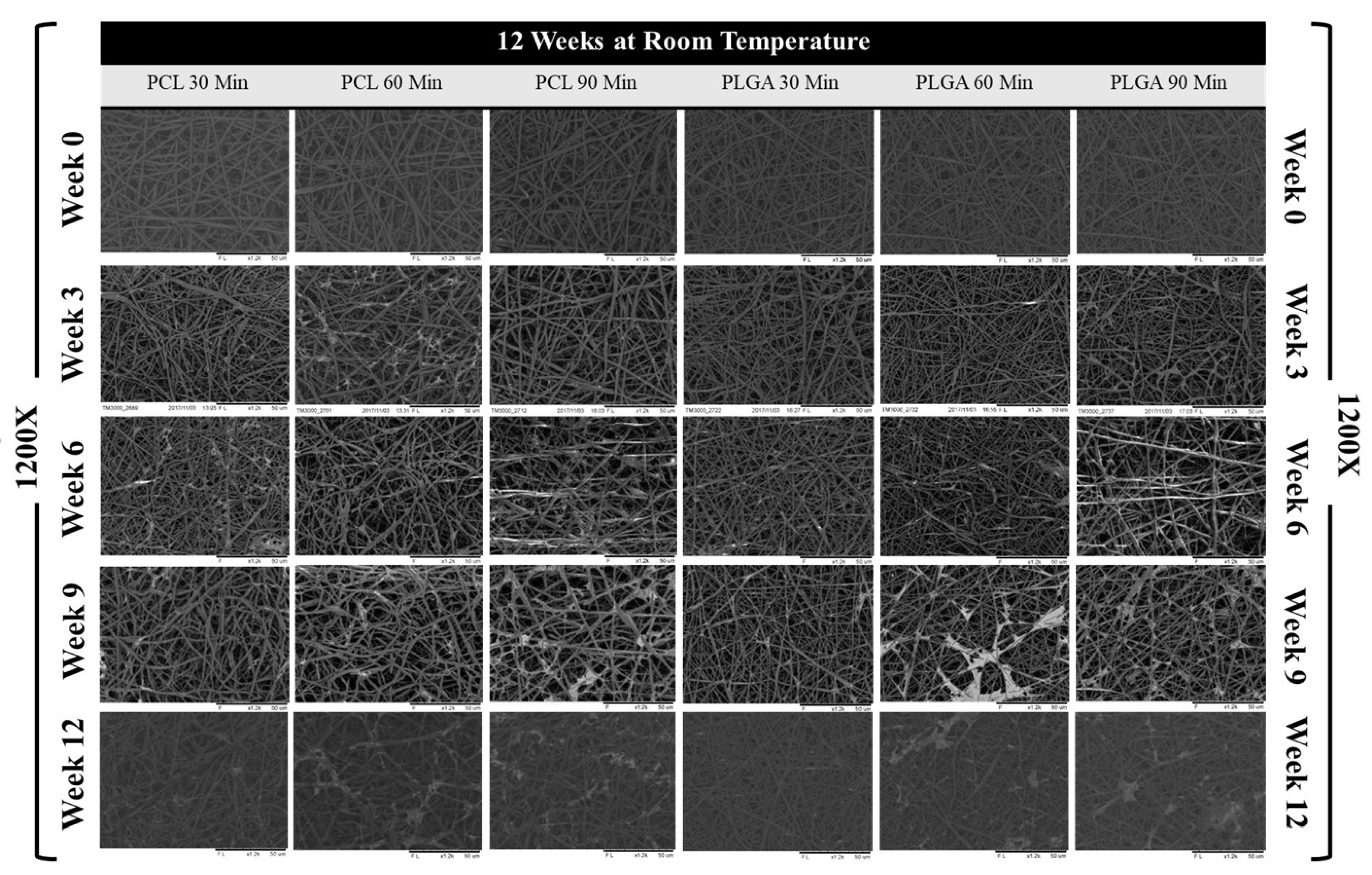
2.4. Scaffold Morphology Characterisation
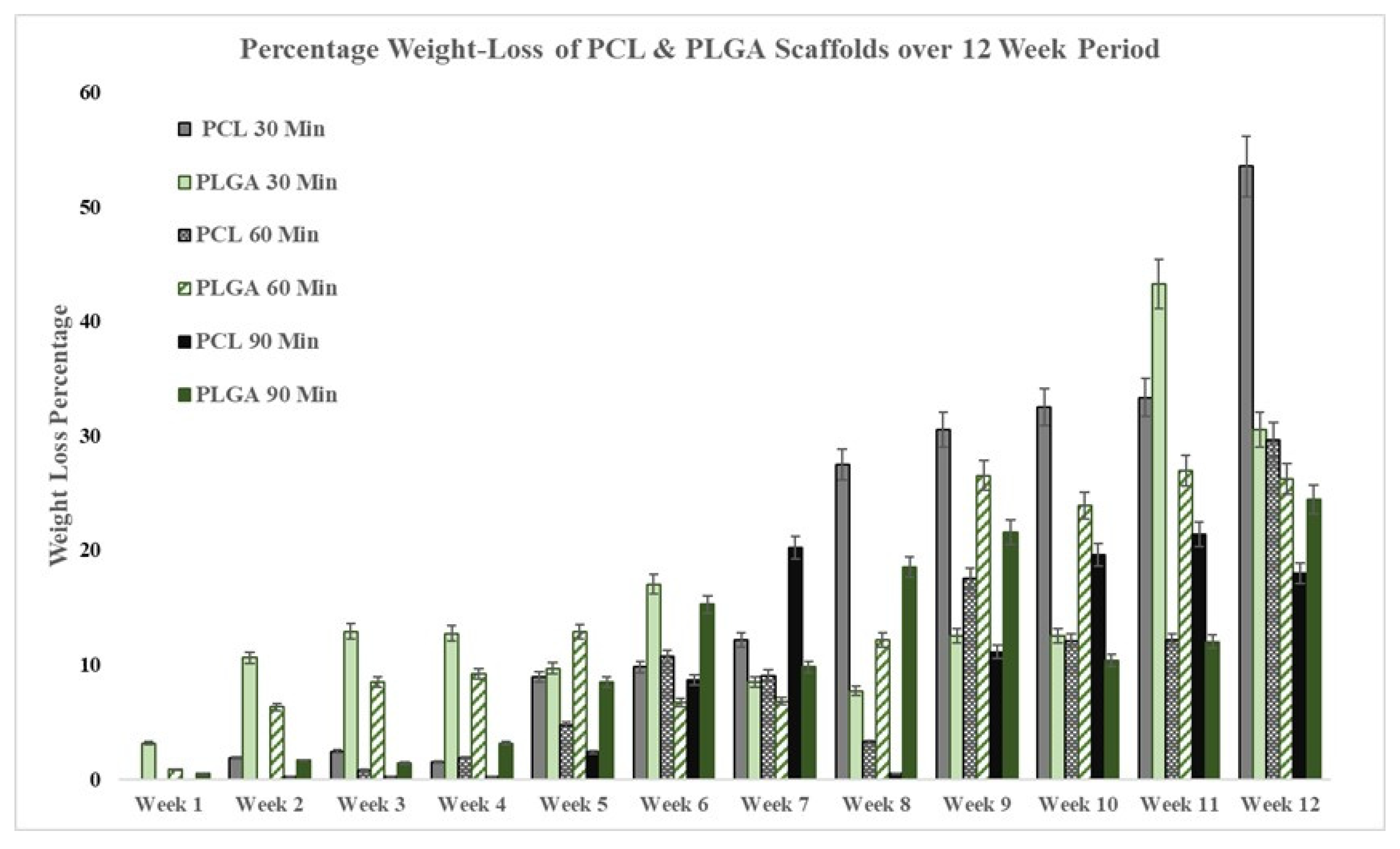
2.5. Degradation Procedure
2.6. Tensile Testing Procedure
2.7. Statistical Analysis
3. Results
3.1. Water Contact Angle
3.2. Degradation Rate
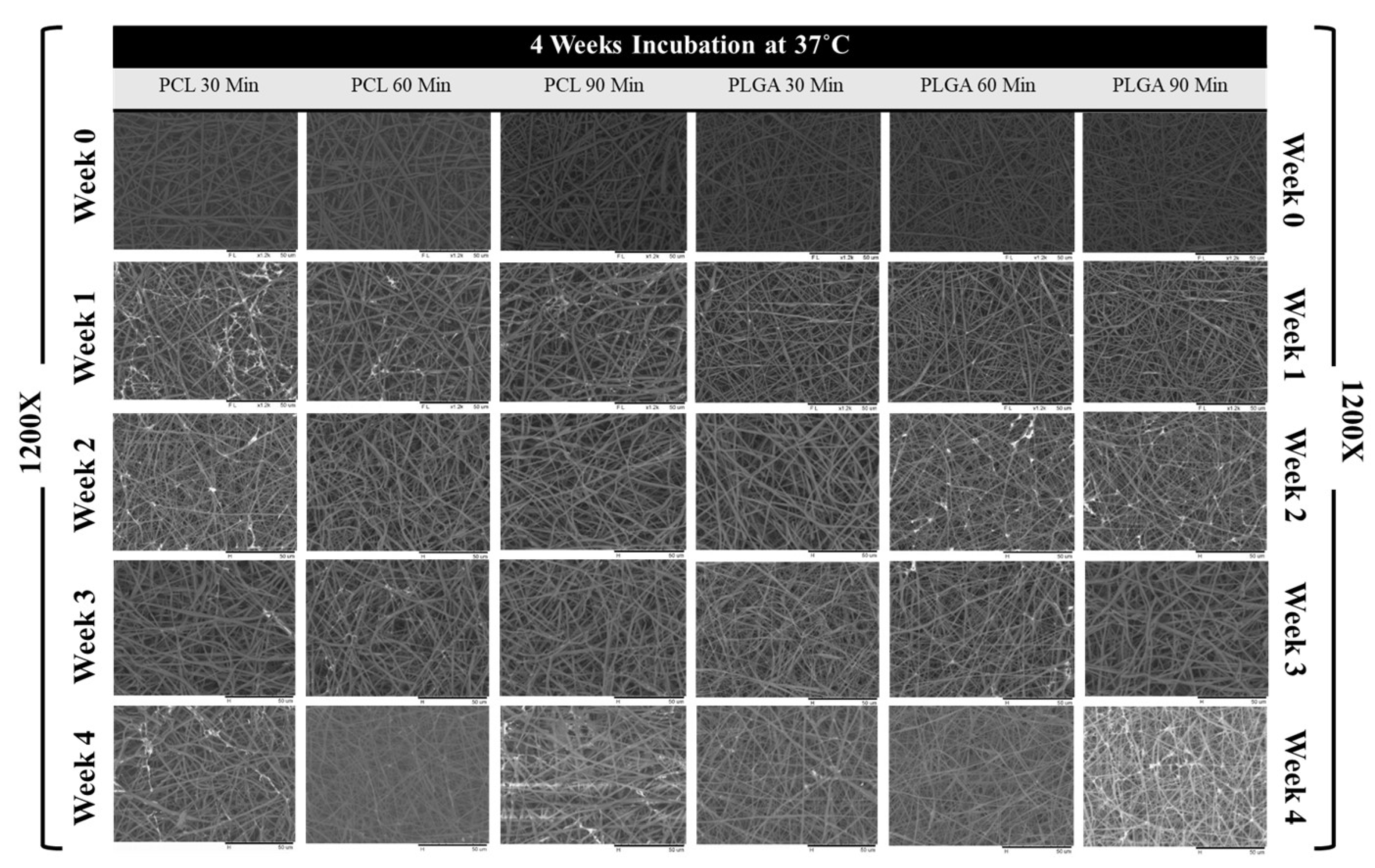
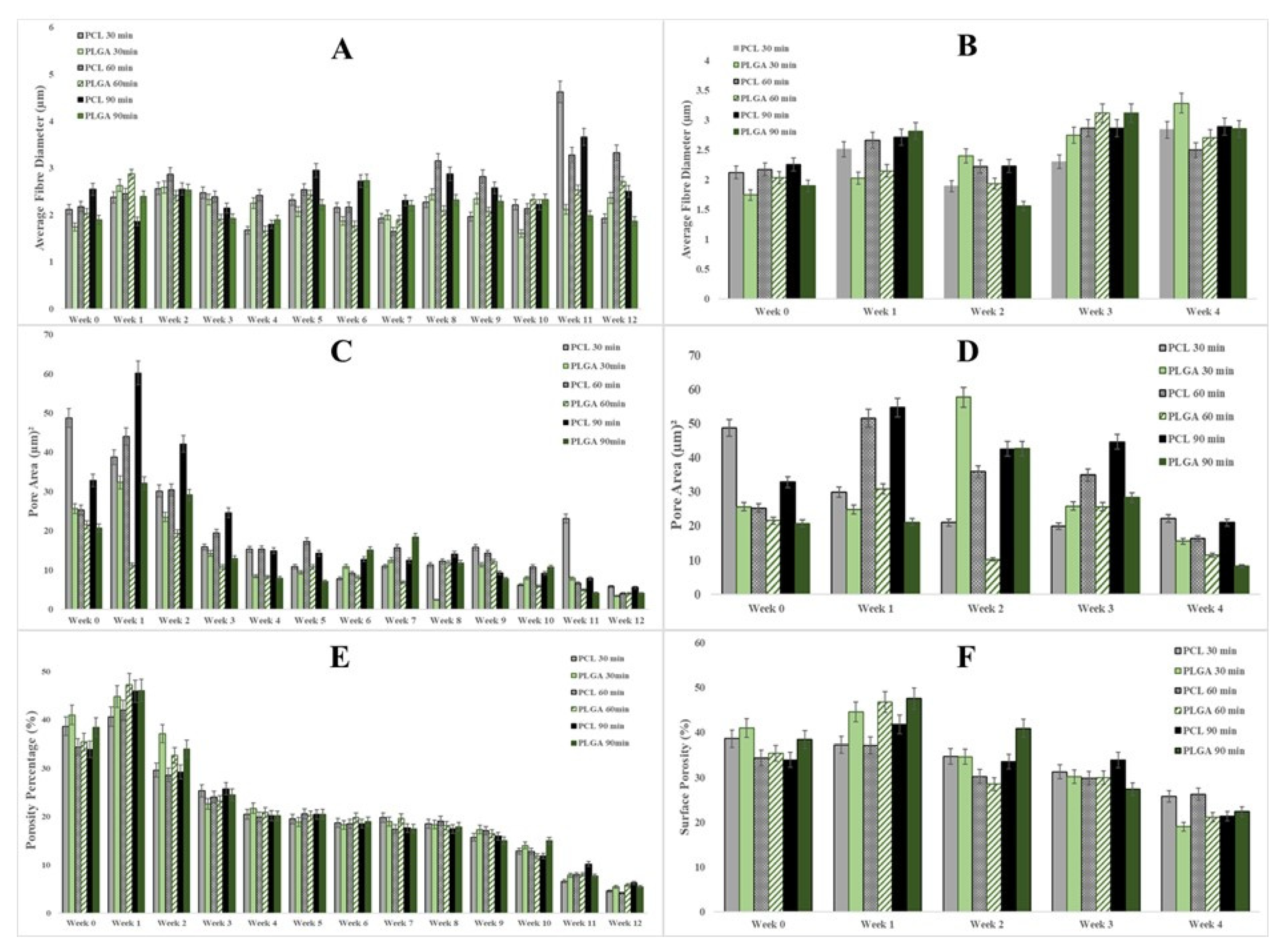
3.3. Scaffold Morphology
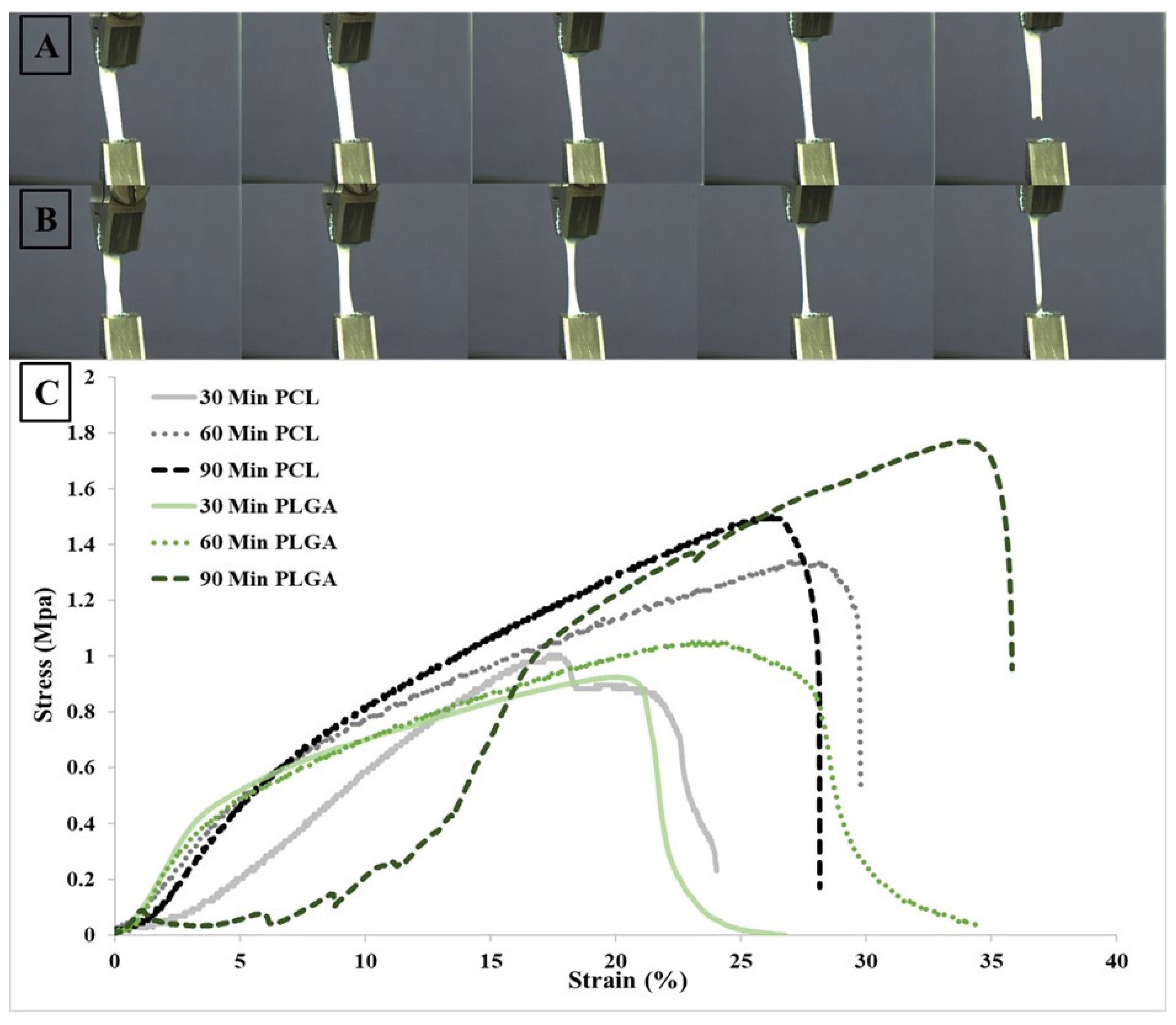
3.4. Mechanical Properties
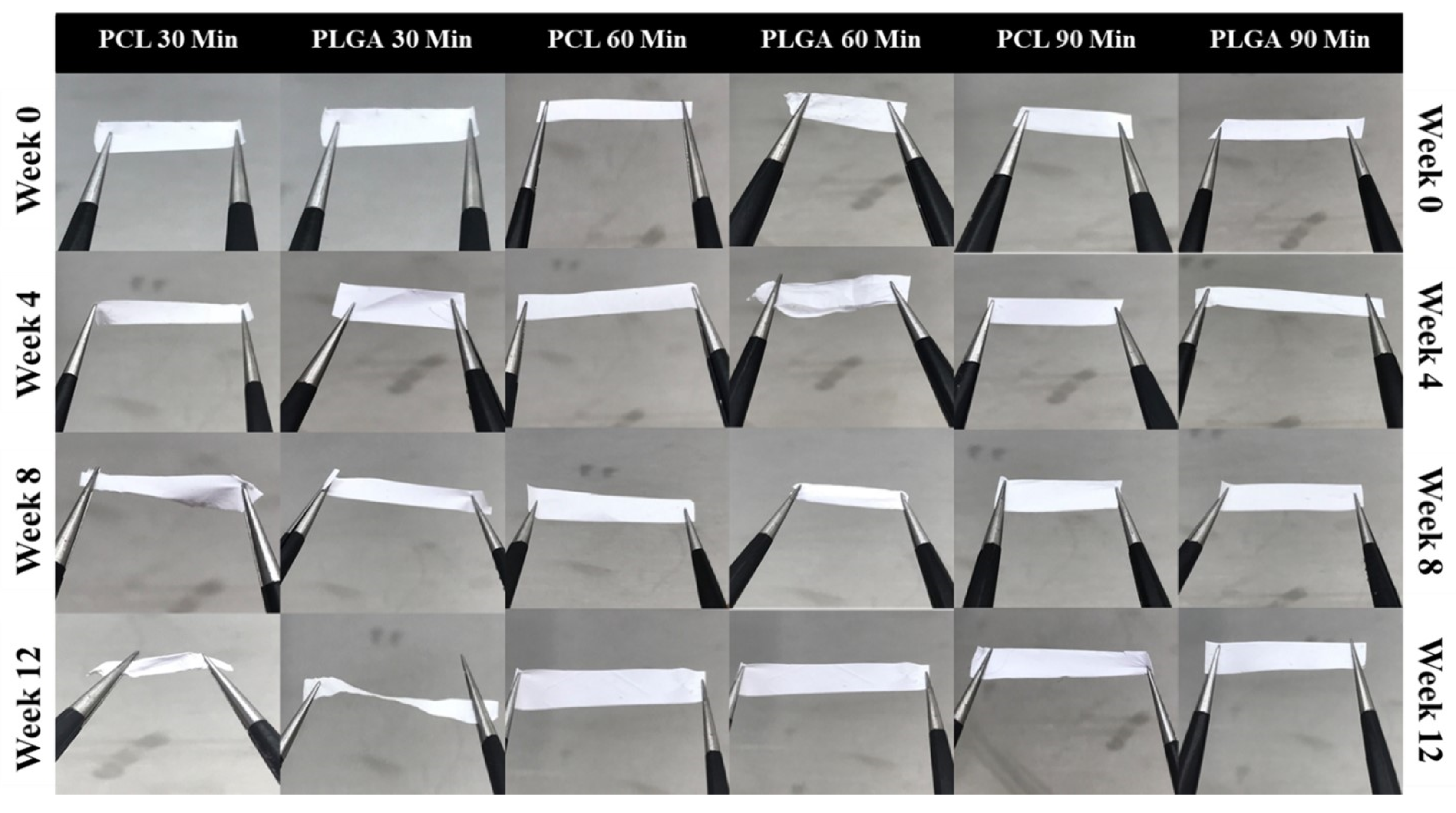
3.5. Handleability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Fabrication, characterization and in vitro evaluation of aligned PLGA–PCL nanofibers for neural regeneration. Ann. Biomed. Eng. 2012, 40, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, V.M. Natural History of Autografts and Allografts; Springer: London, UK, 1992; pp. 9–12. [Google Scholar]
- Jackson, D.W.; Corsetti, J.; Simon, T.M. Biologic incorporation of allograft anterior cruciate ligament replacements. Clin. Orthop. Relat. Res. 1996, 324, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Malloy, K.M.; Hilibrand, A.S. Autograft versus allograft in degenerative cervical disease. Clin. Orthop. Relat. Res. 2002, 394, 27–38. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Xie, C.; Zhang, X.; Benoit, D.S. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials 2013, 34, 8887–8898. [Google Scholar] [CrossRef]
- Sasso, R.C.; LeHuec, J.C.; Shaffrey, C.; Group, S.I.R. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: A prospective patient satisfaction outcome assessment. Clin. Spine Surg. 2005, 18, S77–S81. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yong, T.; Teo, W.E.; Ma, Z.; Ramakrishna, S. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: Potential vascular graft for blood vessel tissue engineering. Tissue Eng. 2005, 11, 1574–1588. [Google Scholar] [CrossRef]
- Mooney, D.; Organ, G.; Vacanti, J.; Langer, R. Design and fabrication of biodegradable polymer devices to engineer tubular tissues. Cell Transplant. 1994, 3, 203–210. [Google Scholar] [CrossRef]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Vacanti, C.A.; Upton, J. Tissue-engineered morphogenesis of cartilage and bone by means of cell transplantation using synthetic biodegradable polymer matrices. Clin. Plast. Surg. 1994, 21, 445–462. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, H.; Zhang, J.; Ding, J. Fabrication of three-dimensional porous scaffolds of complicated shape for tissue engineering. I. Compression molding based on flexible–rigid combined mold. Tissue Eng. 2005, 11, 1105–1114. [Google Scholar] [CrossRef]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. Tissue engineering interventions for esophageal disorders—promises and challenges. Biotechnol. Adv. 2012, 30, 1481–1492. [Google Scholar] [CrossRef]
- Vasanthan, K.S.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Role of biomaterials, therapeutic molecules and cells for hepatic tissue engineering. Biotechnol. Adv. 2012, 30, 742–752. [Google Scholar] [CrossRef]
- Wen, X.; Tresco, P.A. Fabrication and characterization of permeable degradable Poly(DL-lactide-co-glycolide)(PLGA) hollow fiber phase inversion membranes for use as nerve tract guidance channels. Biomaterials 2006, 27, 3800–3809. [Google Scholar] [CrossRef]
- Abdul-Al, M.; Zaernia, A.; Sefat, F. Biomaterials for breast reconstruction: Promises, advances, and challenges. J. Tissue Eng. Regen. Med. 2020, 14, 1549–1569. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Sefat, F. Scaffolds for Bone Tissue Engineering. In Handbook of Tissue Engineering Scaffolds: Volume Two; Elsevier: Amsterdam, The Netherlands, 2019; pp. 449–474. [Google Scholar]
- Zhang, X.; Wang, C.; Liao, M.; Dai, L.; Tang, Y.; Zhang, H.; Coates, P.; Sefat, F.; Zheng, L.; Song, J. Aligned electrospun cellulose scaffolds coated with rhBMP-2 for both in vitro and in vivo bone tissue engineering. Carbohydr. Polym. 2019, 213, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, F.; Ebrahimi-Barough, S.; Nourani, M.R.; Mansoori, K.; Salehi, M.; Alizadeh, A.A.; Tavangar, S.M.; Sefat, F.; Sharifi, S.; Ai, J. Enhanced sciatic nerve regeneration by human endometrial stem cells in an electrospun Poly(ε-caprolactone)/collagen/NBG nerve conduit in rat. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Hancox, Z.; Yousaf, S.; Khurshid, Z.; Zafar, M.S.; Youseffi, M.; Mozafari, M.; Tuinea-Bobe, C.; Sefat, F. Scaffolds for dental cementum. In Handbook of Tissue Engineering Scaffolds: Volume One; Elsevier: Amsterdam, The Netherlands, 2019; pp. 563–594. [Google Scholar]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of electrospun nanofibers for biomedical and dental applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Bye, F.J.; Bullock, A.J.; Singh, R.; Sefat, F.; Roman, S.; MacNeil, S. Development of a basement membrane substitute incorporated into an electrospun scaffold for 3D skin tissue engineering. J. Biomater. Tissue Eng. 2014, 4, 686–692. [Google Scholar] [CrossRef]
- Mahjour, S.B.; Fu, X.; Yang, X.; Fong, J.; Sefat, F.; Wang, H. Rapid creation of skin substitutes from human skin cells and biomimetic nanofibers for acute full-thickness wound repair. Burns 2015, 41, 1764–1774. [Google Scholar] [CrossRef]
- Mahjour, S.B.; Sefat, F.; Polunin, Y.; Wang, L.; Wang, H. Improved cell infiltration of electrospun nanofiber mats for layered tissue constructs. J. Biomed. Mater. Res. Part A 2016, 104, 1479–1488. [Google Scholar] [CrossRef]
- Deshpande, P.; Ortega, Í.; Sefat, F.; Sangwan, V.S.; Green, N.; Claeyssens, F.; MacNeil, S. Rocking media over ex vivo corneas improves this model and allows the study of the effect of proinflammatory cytokines on wound healing. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1553–1561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deshpande, P.; Ramachandran, C.; Sefat, F.; Mariappan, I.; Johnson, C.; McKean, R.; Hannah, M.; Sangwan, V.S.; Claeyssens, F.; Ryan, A.J. Simplifying corneal surface regeneration using a biodegradable synthetic membrane and limbal tissue explants. Biomaterials 2013, 34, 5088–5106. [Google Scholar] [CrossRef] [PubMed]
- Hancox, Z.; Keshel, S.H.; Yousaf, S.; Saeinasab, M.; Shahbazi, M.-A.; Sefat, F. The progress in corneal translational medicine. Biomater. Sci. 2020, 8, 6469–6504. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Í.; Sefat, F.; Deshpande, P.; Paterson, T.; Ramachandran, C.; Ryan, A.J.; MacNeil, S.; Claeyssens, F. Combination of microstereolithography and electrospinning to produce membranes equipped with niches for corneal regeneration. J. Vis. Exp. 2014, 51826. [Google Scholar] [CrossRef]
- Sefat, F.; McKean, R.; Deshpande, P.; Ramachandran, C.; Hill, C.J.; Sangwan, V.S.; Ryan, A.J.; MacNeil, S. Production, sterilisation and storage of biodegradable electrospun PLGA membranes for delivery of limbal stem cells to the cornea. Procedia Eng. 2013, 59, 101–116. [Google Scholar] [CrossRef]
- Yousaf, S.; Keshel, S.H.; Farzi, G.A.; Momeni-Moghadam, M.; Ahmadi, E.D.; Asencio, I.O.; Mozafari, M.; Sefat, F. Scaffolds for corneal tissue engineering. In Handbook of Tissue Engineering Scaffolds: Volume Two; Elsevier: Amsterdam, The Netherlands, 2019; pp. 649–672. [Google Scholar]
- Yousaf, S.; Keshel, S.H.; Farzi, G.A.; Momeni-Moghadam, M.; Ahmadi, E.D.; Mozafari, M.; Sefat, F. Scaffolds for intraocular lens. In Handbook of Tissue Engineering Scaffolds: Volume Two; Elsevier: Amsterdam, The Netherlands, 2019; pp. 693–709. [Google Scholar]
- Bazgir, M.; Raja, T.; Hancox, Z.; Gentile, P.; Ferreira, A.M.; Zhang, W.; Mozafari, M.; Youseffi, M.; Coates, P.; Sefat, F. Scaffolds for Blood Vessels Tissue Engineering. In Handbook of Tissue Engineering Scaffolds: Volume One; Elsevier: Amsterdam, The Netherlands, 2019; pp. 563–594. [Google Scholar]
- Oluwadamilola, A.; Yousaf, S.; Zare, M.; Mozafari, M.; Youseffi, M.; Twigg, P.; Sefat, F. Scaffolds for ligament tissue engineering. In Handbook of Tissue Engineering Scaffolds: Volume One; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–327. [Google Scholar]
- Hancox, Z.; Yousaf, S.; Shah, T.; Fhong, S.C.; Zhang, W.; Ahmed, N.; Mozafari, M.; Nair, K.; Coates, P.; Sefat, F. Scaffolds for reconstruction of the diaphragm. In Handbook of Tissue Engineering Scaffolds: Volume Two; Elsevier: Amsterdam, The Netherlands, 2019; pp. 449–474. [Google Scholar]
- Hancox, Z.; Yousaf, S.; Farzi, G.A.; Momeni-Moghadam, M.; Shakeri, S.; Youseffi, M.; Mozafari, M.; Sefat, F. Scaffolds for Tracheal Tissue Engineering. In Handbook of Tissue Engineering Scaffolds: Volume Two; Elsevier: Amsterdam, The Netherlands, 2019; pp. 361–391. [Google Scholar]
- Raja, T.I.; Mozafari, M.; Milan, P.B.; Samadikuchaksaraei, A.; Sefat, F. Nanoengineered biomaterials for tracheal replacement. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–303. [Google Scholar]
- Moztarzadeh, S.; Mottaghy, K.; Sefat, F.; Samadikuchaksaraei, A.; Mozafari, M. Nanoengineered biomaterials for lung regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 305–323. [Google Scholar]
- Sefat, F.; Raja, T.I.; Zafar, M.S.; Khurshid, Z.; Najeeb, S.; Zohaib, S.; Ahmadi, E.D.; Rahmati, M.; Mozafari, M. Nanoengineered biomaterials for cartilage repair. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–71. [Google Scholar]
- Sefat, F.; Raja, T.I.; Moghadam, Z.S.; Milan, P.B.; Samadikuchaksaraei, A.; Mozafari, M. Nanoengineered biomaterials for bladder regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 459–474. [Google Scholar]
- Urbanska, A.M.; Sefat, F.; Yousaf, S.; Kargozar, S.; Milan, P.B.; Mozafari, M. Nanoengineered biomaterials for intestine regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 363–378. [Google Scholar]
- Amado, S.; Simoes, M.; da Silva, P.A.; Luís, A.; Shirosaki, Y.; Lopes, M.; Santos, J.; Fregnan, F.; Gambarotta, G.; Raimondo, S. Use of hybrid chitosan membranes and N1E-115 cells for promoting nerve regeneration in an axonotmesis rat model. Biomaterials. 2008, 29, 4409–4419. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Vats, A.; Tolley, N.; Polak, J.; Gough, J. Scaffolds and biomaterials for tissue engineering: A review of clinical applications. Otolaryngol. Allied Sci. 2003, 28, 165–172. [Google Scholar] [CrossRef]
- Alves da Silva, M.; Martins, A.; Costa-Pinto, A.; Costa, P.; Faria, S.; Gomes, M.; Reis, R.; Neves, N. Cartilage tissue engineering using electrospun PCL nanofiber meshes and MSCs. Biomacromolecules 2010, 11, 3228–3236. [Google Scholar] [CrossRef]
- Franco, R.A.; Nguyen, T.H.; Lee, B.-T. Preparation and characterization of electrospun PCL/PLGA membranes and chitosan/gelatin hydrogels for skin bioengineering applications. J. Mater. Sci. Mater. Med. 2011, 22, 2207–2218. [Google Scholar] [CrossRef]
- Han, J.; Lazarovici, P.; Pomerantz, C.; Chen, X.; Wei, Y.; Lelkes, P.I. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 2011, 12, 399–408. [Google Scholar] [CrossRef]
- Maurmann, N.; Pereira, D.P.; Burguez, D.; de S Pereira, F.D.; Neto, P.I.; Rezende, R.A.; Gamba, D.; da Silva, J.V.; Pranke, P. Mesenchymal stem cells cultivated on scaffolds formed by 3D printed PCL matrices, coated with PLGA electrospun nanofibers for use in tissue engineering. Biomed. Phys. Eng. Express 2017, 3, 045005. [Google Scholar] [CrossRef]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef]
- Padalhin, A.R.; Thuy Ba Linh, N.; Ki Min, Y.; Lee, B.-T. Evaluation of the cytocompatibility hemocompatibility in vivo bone tissue regenerating capability of different PCL blends. J. Biomater. Sci. Polym. Ed. 2014, 25, 487–503. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Electrospinning of manmade and biopolymer nanofibers—progress in techniques, materials, and applications. Adv. Funct. Mater. 2009, 19, 2863–2879. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kotaki, M.; Zhang, Y.; Mo, X.; Ramakrishna, S. Recent advances in polymer nanofibers. J. Nanosci. Nanotechnol. 2004, 4, 52–65. [Google Scholar]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Lowery, J.L.; Datta, N.; Rutledge, G.C. Effect of fiber diameter, pore size and seeding method on growth of human dermal fibroblasts in electrospun Poly(ɛ-caprolactone) fibrous mats. Biomaterials 2010, 31, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Cooper-White, J.J. Increasing electrospun scaffold pore size with tailored collectors for improved cell penetration. Acta Biomater. 2011, 7, 2544–2557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules 2008, 9, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Kim, G.H. Electrospun PCL nanofibers with anisotropic mechanical properties as a biomedical scaffold. Biomed. Mater. 2008, 3, 025010. [Google Scholar] [CrossRef]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. BioMed Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned biodegradable nanofibrous structure: A potential scaffold for blood vessel engineering. Biomaterials 2004, 25, 877–886. [Google Scholar] [CrossRef]
- Zamanlui, S.; Mahmoudifard, M.; Soleimani, M.; Bakhshandeh, B.; Vasei, M.; Faghihi, S. Enhanced chondrogenic differentiation of human bone marrow mesenchymal stem cells on PCL/PLGA electrospun with different alignments and compositions. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 50–60. [Google Scholar] [CrossRef]
- Chang, H.-I.; Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering-Cells and Biomaterials, Eberli, D., Ed.; InTechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Dewez, J.L.; Schneider, Y.J.; Rouxhet, P.G. Coupled influence of substratum hydrophilicity and surfactant on epithelial cell adhesion. J. Biomed. Mater. Res. 1996, 30, 373–383. [Google Scholar] [CrossRef]
- Shafrin, E.G.; Zisman, W.A. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers1. J. Phys. Chem. 1960, 64, 519–524. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Báez, J.E.; Kenny, J.M.; Marcos-Fernández, A. Effect of the molecular weight on the crystallinity of PCL-b-PLLA di-block copolymers. Polymer 2012, 53, 4561–4568. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kamada, F.; Kawazoe, N.; Tateishi, T.; Chen, G. Structural changes and biodegradation of PLLA, PCL, and PLGA sponges during in vitro incubation. Polym. Eng. Sci. 2010, 50, 1895–1903. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C. Chitosan/oligo L-lactide graft copolymers: Effect of hydrophobic side chains on the physico-chemical properties and biodegradability. Carbohydr. Polym. 2006, 64, 254–266. [Google Scholar] [CrossRef]
- Haeri, M.; Haeri, M. ImageJ plugin for analysis of porous scaffolds used in tissue engineering. J. Open Res. Softw. 2015, 3. [Google Scholar] [CrossRef]
- Heydarkhan-Hagvall, S.; Schenke-Layland, K.; Dhanasopon, A.P.; Rofail, F.; Smith, H.; Wu, B.M.; Shemin, R.; Beygui, R.E.; MacLellan, W.R. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 2008, 29, 2907–2914. [Google Scholar] [CrossRef]
- Kwon, I.K.; Kidoaki, S.; Matsuda, T. Electrospun nano-to microfiber fabrics made of biodegradable copolyesters: Structural characteristics, mechanical properties and cell adhesion potential. Biomaterials 2005, 26, 3929–3939. [Google Scholar] [CrossRef]
- Tornello, P.R.C.; Caracciolo, P.C.; Roselló, J.I.I.; Abraham, G.A. Electrospun scaffolds with enlarged pore size: Porosimetry analysis. Mater. Lett. 2018, 227, 191–193. [Google Scholar] [CrossRef]
- Kim, C.H.; Jung, Y.H.; Kim, H.Y.; Lee, D.R.; Dharmaraj, N.; Choi, K.E. Effect of collector temperature on the porous structure of electrospun fibers. Macromol. Res. 2006, 14, 59–65. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Vieira, T.; Silva, J.C.; do Rego, A.B.; Borges, J.P.; Henriques, C. Electrospun biodegradable chitosan based-poly (urethane urea) scaffolds for soft tissue engineering. Mater. Sci. Eng. C 2019, 103, 109819. [Google Scholar] [CrossRef]
- Diego, R.B.; Estellés, J.M.; Sanz, J.A.; García-Aznar, J.M.; Sánchez, M.S. Polymer scaffolds with interconnected spherical pores and controlled architecture for tissue engineering: Fabrication, mechanical properties, and finite element modeling. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81, 448–455. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Ding, S.; Liu, C.; Ai, J. Effect of porous structure and pore size on mechanical strength of 3D-printed comby scaffolds. Mater. Lett. 2018, 223, 21–24. [Google Scholar] [CrossRef]
- Takahashi, H.; Itoga, K.; Shimizu, T.; Yamato, M.; Okano, T. Human neural tissue construct fabrication based on scaffold-free tissue engineering. Adv. Healthc. Mater. 2016, 5, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef]
- Eichhorn, S.; Sampson, W. Relationships between specific surface area and pore size in electrospun polymer fibre networks. J. R. Soc. Interface 2010, 7, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Garcia, C.A.; Mikos, A.G. In vitro degradation of thin poly(DL-lactic-co-glycolic acid) films. J. Biomed. Mater. Res. 1999, 46, 236–244. [Google Scholar] [CrossRef]
- Kim, S.E.; Heo, D.N.; Lee, J.B.; Kim, J.R.; Park, S.H.; Jeon, S.H.; Kwon, I.K. Electrospun gelatin/polyurethane blended nanofibers for wound healing. Biomed. Mater. 2009, 4, 044106. [Google Scholar] [CrossRef] [PubMed]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Sienkiewicz, M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Soares, R.M.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Bellis, S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 2012, 33, 524–534. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Cipriani, E.; Gnavi, S.; Chiono, V.; Mattu, C.; Gentile, P.; Perroteau, I.; Zanetti, M.; Ciardelli, G. Crosslinked gelatin nanofibres: Preparation, characterisation and in vitro studies using glial-like cells. Mater. Sci. Eng. 2013, 33, 2723–2735. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
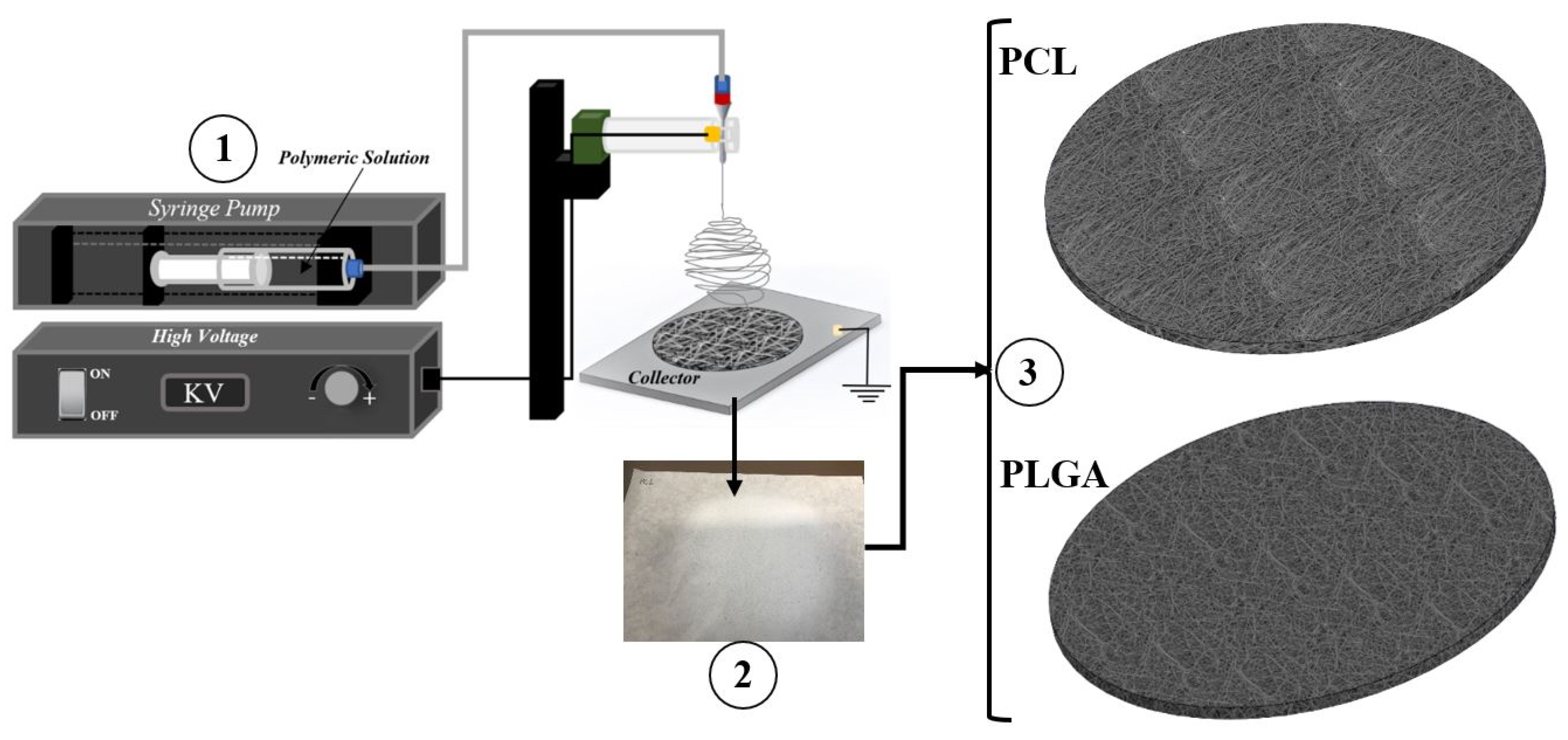
| Electrospinning | Sample Name | Voltage (kV) | Needle Type | Distance from Tip of the Needle to the Collector (mm) | Type of Collector | Flow Rate (mL/h) | T (°C) | Humidity (%) | Time (min) | Solution Dispensed (mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| PCL Only | A1 | 7.90 | 20 G | 95 | Flat | 1 | 22.1 | 46 | 30 | 0.498 |
| A2 | 7.91 | 20 G | 95 | Flat | 1 | 22.3 | 46 | 60 | 1.01 | |
| A3 | 7.36 | 20 G | 95 | Flat | 1 | 22.3 | 44 | 90 | 1.507 | |
| PLGA Only | B1 | 7.90 | 20 G | 95 | Flat | 1 | 22.2 | 46 | 30 | 0.509 |
| B2 | 8.56 | 20 G | 95 | Flat | 1 | 23.1 | 39 | 60 | 1.005 | |
| B3 | 8.74 | 20 G | 95 | Flat | 1 | 23.1 | 39 | 90 | 1.576 |
| Scheme | Mean ± SD (DH2O) | ||
|---|---|---|---|
| Left Angle | Right Angle | ||
| PCL | 30 min (A1) | 122.77° ± 3.72 | 122.40° ± 4.51 |
| 60 min (A2) | 111.40° ± 3.23 | 123.13° ± 2.70 | |
| 90 min (A3) | 125.57° ± 4.75 | 125.07° ± 4.80 | |
| PLGA | 30 min (B1) | 127.83° ± 6.16 | 129.03° ± 3.55 |
| 60 min (B2) | 131.73° ± 3.46 | 132.73° ± 3.32 | |
| 90 min (B3) | 129.83° ± 5.93 | 129.27° ± 5.60 | |
| A | Percentage Change in Fibre Diameter | ||
| 30 Min | 60 min | 90 min | |
| PCL 12 Weeks | 9.04 (−) | 34.50 (+) | 1.72 (−) |
| PLGA 12 Weeks | 35.60 (+) | 25.07 (+) | 1.38 (−) |
| PCL 4 Weeks at 37 oC | 33.60 (+) | 14.09 (+) | 28.13 (+) |
| PLGA 4 Weeks at 37 oC | 88.07 (+) | 32.95 (+) | 50.48 (+) |
| B | Percentage Change in Pore size | ||
| 30 Min | 60 min | 90 min | |
| PCL 12 Weeks | 88.03 (−) | 83.86 (−) | 82.85 (−) |
| PLGA 12 Weeks | 86.61 (−) | 81.09 (−) | 84.97 (−) |
| PCL 4 Weeks at 37 oC | 54.52 (−) | 35.39 (−) | 54.21 (−) |
| PLGA 4 Weeks at 37 oC | 39.54 (−) | 46.76 (−) | 60.18 (−) |
| C | Percentage Change in Surface Porosity (%) | ||
| 30 Min | 60 min | 90 min | |
| PCL 12 Weeks | 88.21 (−) | 87.97 (−) | 81.57 (−) |
| PLGA 12 Weeks | 86.74 (−) | 83.64 (−) | 85.79 (−) |
| PCL 4 Weeks at 37 oC | 33.25 (−) | 23.55 (−) | 36.95 (−) |
| PLGA 4 Weeks at 37 oC | 53.64 (−) | 40.41 (−) | 41.762 (−) |
| Sample Name | Time | Length (mm) | Thickness (mm) | Width (mm) | Area (mm2) | Tensile Strength (MPa ± SD) | Elongation at Break (% ± SD) | Young Modulus (MPa ± SD) |
|---|---|---|---|---|---|---|---|---|
| PCL | 30 | 35 | 0.06 | 6 | 0.36 | 0.99 ± 0.17 | 24.03 ± 2.24 | 8.07 ± 2.14 |
| 60 | 37.31 | 0.09 | 5.6 | 0.504 | 1.32 ± 0.49 | 29.83 ± 3.9 | 11.71 ± 2.96 | |
| 90 | 37.2 | 0.11 | 5.69 | 0.6259 | 1.49 ± 0.37 | 28.15 ± 2.94 | 13.69 ± 3.14 | |
| PLGA | 30 | 35 | 0.09 | 5.8 | 0.522 | 1.03 ± 0.25 | 34.36 ± 5.77 | 10.15 ± 1.64 |
| 60 | 35 | 0.12 | 6 | 0.72 | 0.92 ± 0.45 | 21.74 ± 3.28 | 9.64 ± 2.17 | |
| 90 | 35 | 0.12 | 5.9 | 0.708 | 1.76 ± 0.79 | 36.33 ± 2.96 | 15.15 ± 5.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials 2021, 14, 4773. https://doi.org/10.3390/ma14174773
Bazgir M, Zhang W, Zhang X, Elies J, Saeinasab M, Coates P, Youseffi M, Sefat F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials. 2021; 14(17):4773. https://doi.org/10.3390/ma14174773
Chicago/Turabian StyleBazgir, Morteza, Wei Zhang, Ximu Zhang, Jacobo Elies, Morvarid Saeinasab, Phil Coates, Mansour Youseffi, and Farshid Sefat. 2021. "Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering" Materials 14, no. 17: 4773. https://doi.org/10.3390/ma14174773
APA StyleBazgir, M., Zhang, W., Zhang, X., Elies, J., Saeinasab, M., Coates, P., Youseffi, M., & Sefat, F. (2021). Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials, 14(17), 4773. https://doi.org/10.3390/ma14174773










