Fabrication and Characterization of PCL/PLGA Coaxial and Bilayer Fibrous Scaffolds for Tissue Engineering
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Solution Preparation and Electrospinning Parameter
2.3. Scanning Electron Microscopy
2.4. Water Contact Angle (WCA)
2.5. Degradation Process
2.6. Tensile Testing
2.7. Statistical Analysis
3. Results and Discussion
3.1. Scaffold Morphology
3.2. Mechanical Properties
3.3. Handleability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boffito, M.; Sartori, S.; Ciardelli, G. Polymeric scaffolds for cardiac tissue engineering: Requirements and fabrication technologies. Polym. Int. 2014, 63, 2–11. [Google Scholar] [CrossRef]
- Azevedo, H.S.; Reis, R.L. Understanding the Enzymatic Degradation of Biodegradable Polymers and Strategies to Control Their Degradation Rate; CRC Press: Boca Raton, FL, USA, 2005; pp. 177–200. [Google Scholar]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Lin, C.-C.; Anseth, K.S. The biodegradation of biodegradable polymeric biomaterials. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 716–728. [Google Scholar]
- Treiser, M.; Abramson, S.; Langer, R.; Kohn, J.; Treiser, M.; Abramson, S.; Langer, R.; Kohn, J. Degradable and resorbable biomaterials. In Biomaterials Science: An Introduction to Materials in Medicine; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Inc.: Oxford, UK, 2013; pp. 179–195. [Google Scholar]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Gajjar, C.R.; King, M.W. Resorbable Fiber-Forming Polymers for Biotextile Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Engineer, C.; Parikh, J.; Raval, A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends Biomater. Artif. Organs 2011, 25, 79–85. [Google Scholar]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly (DL-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef]
- Semler, E.J.; Tjia, J.S.; Moghe, P.V. Analysis of surface microtopography of biodegradable polymer matrices using confocal reflection microscopy. Biotechnol. Prog. 1997, 13, 630–634. [Google Scholar] [CrossRef]
- Allen, G. Crystallinity in polymers: An historical view. Eur. Rev. 1993, 1, 197–206. [Google Scholar] [CrossRef]
- Kavesh, S.; Schultz, J. Meaning and measurement of crystallinity in polymers: A review. Polym. Eng. Sci. 1969, 9, 331–338. [Google Scholar] [CrossRef]
- Kim, G.-M.; Le, K.H.T.; Giannitelli, S.M.; Lee, Y.J.; Rainer, A.; Trombetta, M. Electrospinning of PCL/PVP blends for tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1425–1442. [Google Scholar] [CrossRef]
- Nair, L.S.; Bhattacharyya, S.; Laurencin, C.T. Development of novel tissue engineering scaffolds via electrospinning. Expert Opin. Biol. Ther. 2004, 4, 659–668. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.; Pandit, A.; Zeugolis, D.I. The multifaceted potential of electro-spinning in regenerative medicine. Pharm. Nanotechnol. 2014, 2, 23–34. [Google Scholar] [CrossRef]
- Bognitzki, M.; Frese, T.; Steinhart, M.; Greiner, A.; Wendorff, J.H.; Schaper, A.; Hellwig, M. Preparation of fibers with nanoscaled morphologies: Electrospinning of polymer blends. Polym. Eng. Sci. 2001, 41, 982–989. [Google Scholar] [CrossRef]
- Li, M.; Mondrinos, M.J.; Chen, X.; Lelkes, P.I. Electrospun blends of natural and synthetic polymers as scaffolds for tissue engineering. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 5858–5861. [Google Scholar]
- Zhang, J.-G.; Mo, X.-M. Current research on electrospinning of silk fibroin and its blends with natural and synthetic biodegradable polymers. Front Mater. Sci. 2013, 7, 129–142. [Google Scholar] [CrossRef]
- Nasr, S.M.; Rabiee, N.; Hajebi, S.; Ahmadi, S.; Fatahi, Y.; Hosseini, M.; Bagherzadeh, M.; Ghadiri, A.M.; Rabiee, M.; Jajarmi, V. Biodegradable nanopolymers in cardiac tissue engineering: From concept towards nanomedicine. Int. J. Nanomed. 2020, 15, 4205. [Google Scholar] [CrossRef]
- Chen, G.; Ushida, T.; Tateishi, T. Development of biodegradable porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2001, 17, 63–69. [Google Scholar] [CrossRef]
- Jawad, H.; Ali, N.; Lyon, A.; Chen, Q.; Harding, S.; Boccaccini, A. Myocardial tissue engineering: A review. J. Tissue Eng. Regen. Med. 2007, 1, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Ganan-Calvo, A. Micro/nano encapsulation via electrified coaxial liquid jets. Science 2002, 295, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound core–shell polymer nanofibers by co-electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Romano, L.; Camposeo, A.; Manco, R.; Moffa, M.; Pisignano, D. Core–shell electrospun fibers encapsulating chromophores or luminescent proteins for microscopically controlled molecular release. Mol. Pharm. 2016, 13, 729–736. [Google Scholar] [CrossRef]
- Wang, X.; Yu, D.-G.; Li, X.-Y.; Bligh, S.A.; Williams, G.R. Electrospun medicated shellac nanofibers for colon-targeted drug delivery. Int. J. Pharm. 2015, 490, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, Z.-M.; Xu, X.; Lim, C.T.; Ramakrishna, S. Preparation of core− shell structured PCL-r-gelatin bi-component nanofibers by coaxial electrospinning. Chem. Mater. 2004, 16, 3406–3409. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-Y.; Liu, J.-J.; Fan, C.-Y.; Mo, X.-M.; Ruan, H.-J.; Li, F.-F. The effect of aligned core–shell nanofibres delivering NGF on the promotion of sciatic nerve regeneration. J. Biomater. Sci. Polym. Ed. 2012, 23, 167–184. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.-G.; Yu, J.-H.; Chen, L.; Williams, G.R.; Wang, X. Modified coaxial electrospinning for the preparation of high-quality ketoprofen-loaded cellulose acetate nanofibers. Carbohydr. Polym. 2012, 90, 1016–1023. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Luqman, M.; Fouad, H.; Khalil, K.A.; Alharthi, N.H. Viscoelastic behavior of core-shell structured nanofibers of PLA and PVA produced by coaxial electrospinning. Polym. Test 2018, 67, 136–143. [Google Scholar] [CrossRef]
- Duan, N.; Geng, X.; Ye, L.; Zhang, A.; Feng, Z.; Guo, L.; Gu, Y. A vascular tissue engineering scaffold with core–shell structured nano-fibers formed by coaxial electrospinning and its biocompatibility evaluation. Biomed. Mater. 2016, 11, 035007. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.; Ramakrishna, S. Double-layered composite nanofibers and their mechanical performance. J. Polym. Sci. B Polym. Phys. 2005, 43, 2852–2861. [Google Scholar] [CrossRef]
- Figueira, D.R.; Miguel, S.P.; de Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone-hyaluronic acid/chitosan-zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef]
- Pu, J.; Yuan, F.; Li, S.; Komvopoulos, K. Electrospun bilayer fibrous scaffolds for enhanced cell infiltration and vascularization in vivo. Acta Biomater. 2015, 13, 131–141. [Google Scholar] [CrossRef]
- Arasteh, S.; Kazemnejad, S.; Khanjani, S.; Heidari-Vala, H.; Akhondi, M.M.; Mobini, S. Fabrication and characterization of nano-fibrous bilayer composite for skin regeneration application. Methods 2016, 99, 3–12. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Zhang, G.R.; Wang, L.L.; Jiang, Y.Z.; Ouyang, H.W.; Zou, X.H. Novel biodegradable three-dimensional macroporous scaffold using aligned electrospun nanofibrous yarns for bone tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 1187–1194. [Google Scholar] [CrossRef]
- Elsayed, Y.; Lekakou, C.; Labeed, F.; Tomlins, P. Fabrication and characterisation of biomimetic, electrospun gelatin fibre scaffolds for tunica media-equivalent, tissue engineered vascular grafts. Mater. Sci. Eng. C 2016, 61, 473–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etemadi, N.; Mehdikhani, M.; Poorazizi, E.; Rafienia, M. Novel bilayer electrospun poly (caprolactone)/silk fibroin/strontium carbonate fibrous nanocomposite membrane for guided bone regeneration. J. Appl. Polym. Sci. 2021, 138, 50264. [Google Scholar] [CrossRef]
- Priya, S.G.; Jungvid, H.; Kumar, A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng. Part B Rev. 2008, 14, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Radakovic, D.; Reboredo, J.; Helm, M.; Weigel, T.; Schürlein, S.; Kupczyk, E.; Leyh, R.; Walles, H.; Hansmann, J. A multilayered electrospun graft as vascular access for hemodialysis. PLoS ONE 2017, 12, e0185916. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Tominaga, K.; Kidoaki, S. Time-programmed dual release formulation by multilayered drug-loaded nanofiber meshes. J. Control. Release 2010, 143, 258–264. [Google Scholar] [CrossRef]
- Srouji, S.; Kizhner, T.; Suss-Tobi, E.; Livne, E.; Zussman, E. 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. J. Mater. Sci. Mater. Med. 2008, 19, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wu, L.; Li, X.; Yuan, X.; Li, X.; Zhang, Y.; Yao, K. Degradation of electrospun PLGA-chitosan/PVA membranes and their cytocompatibility in vitro. J. Biomater. Sci. Polym. Ed. 2007, 18, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.A.; Min, Y.-K.; Yang, H.-M.; Lee, B.-T. Fabrication and biocompatibility of novel bilayer scaffold for skin tissue engineering applications. J. Biomater. Appl. 2013, 27, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, X.; Liang, Q.; Xu, X.; Jing, X. Enzymatic degradation of poly (L-lactide) and poly (ε-caprolactone) electrospun fibers. Macromol. Biosci. 2004, 4, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.; Jin, K.; Liu, W.; Qiu, X.; Li, C. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater. Sci. Eng. C 2016, 59, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Khang, G.; Lee, J.W.; Lee, H.B. Interaction of different types of cells on polymer surfaces with wettability gradient. J. Colloid. Interface Sci. 1998, 205, 323–330. [Google Scholar] [CrossRef]
- Song, W.; Mano, J.F. Interactions between cells or proteins and surfaces exhibiting extreme wettabilities. Soft Matter 2013, 9, 2985–2999. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Wei, Q.; Cai, Y.; Wu, N. Surface structures and contact angles of electrospun poly (vinylidene fluoride) nanofiber membranes. Int. J. Polym. Anal. Char. 2008, 13, 292–301. [Google Scholar] [CrossRef]
- Krok-Borkowicz, M.; Filova, E.; Chlupac, J.; Klepetar, J.; Bacakova, L.; Pamuła, E. Influence of pore size and hydroxyapatite deposition in poly (L-lactide-co-glycolide) scaffolds on osteoblast-like cells cultured in static and dynamic conditions. Mater. Lett. 2019, 241, 1–5. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact angle and wetting properties. In Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Ajalloueian, F.; Tavanai, H.; Hilborn, J.; Donzel-Gargand, O.; Leifer, K.; Wickham, A.; Arpanaei, A. Emulsion electrospinning as an approach to fabricate PLGA/chitosan nanofibers for biomedical applications. BioMed Res. Int. 2014, 2014, 475280. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Shivkumar, S. Nano-sized beads and porous fiber constructs of poly (ε-caprolactone) produced by electrospinning. J. Mater. Sci. 2004, 39, 3003–3013. [Google Scholar] [CrossRef]
- Perumal, G.; Sivakumar, P.M.; Nandkumar, A.M.; Doble, M. Synthesis of magnesium phosphate nanoflakes and its PCL composite electrospun nanofiber scaffolds for bone tissue regeneration. Mater. Sci. Eng. C 2020, 109, 110527. [Google Scholar] [CrossRef]
- Vasita, R.; Mani, G.; Agrawal, C.M.; Katti, D.S. Surface hydrophilization of electrospun PLGA micro-/nano-fibers by blending with Pluronic® F-108. Polymer 2010, 51, 3706–3714. [Google Scholar] [CrossRef]
- Lee, J.; Tae, G.; Kim, Y.H.; Park, I.S.; Kim, S.-H.; Kim, S.H. The effect of gelatin incorporation into electrospun poly (l-lactide-co-ε-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials 2008, 29, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Pauly, H.M.; Kelly, D.J.; Popat, K.C.; Trujillo, N.A.; Dunne, N.J.; McCarthy, H.O.; Donahue, T.L.H. Mechanical properties and cellular response of novel electrospun nanofibers for ligament tissue engineering: Effects of orientation and geometry. J. Mech. Behav. Biomed. Mater. 2016, 61, 258–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]

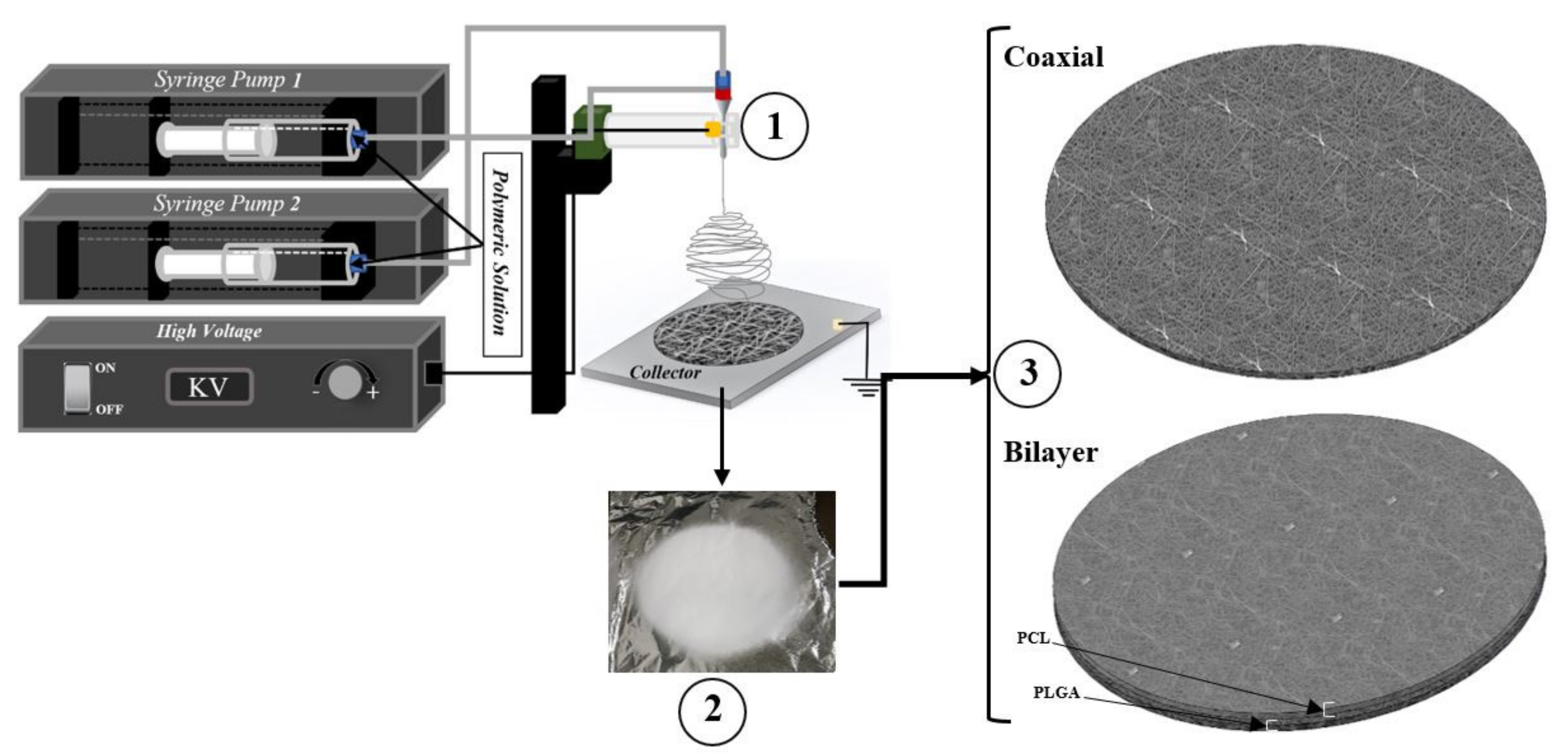
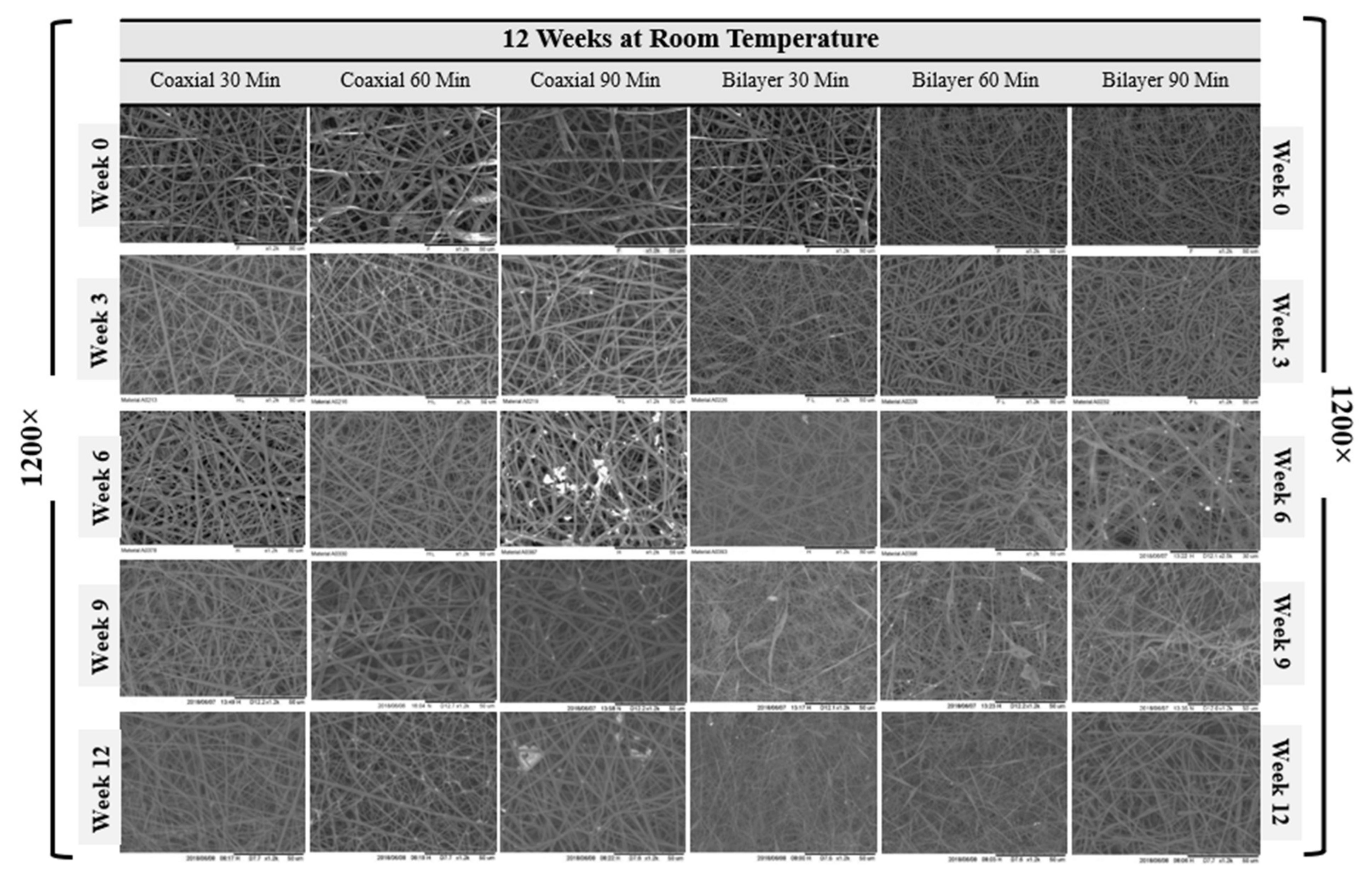
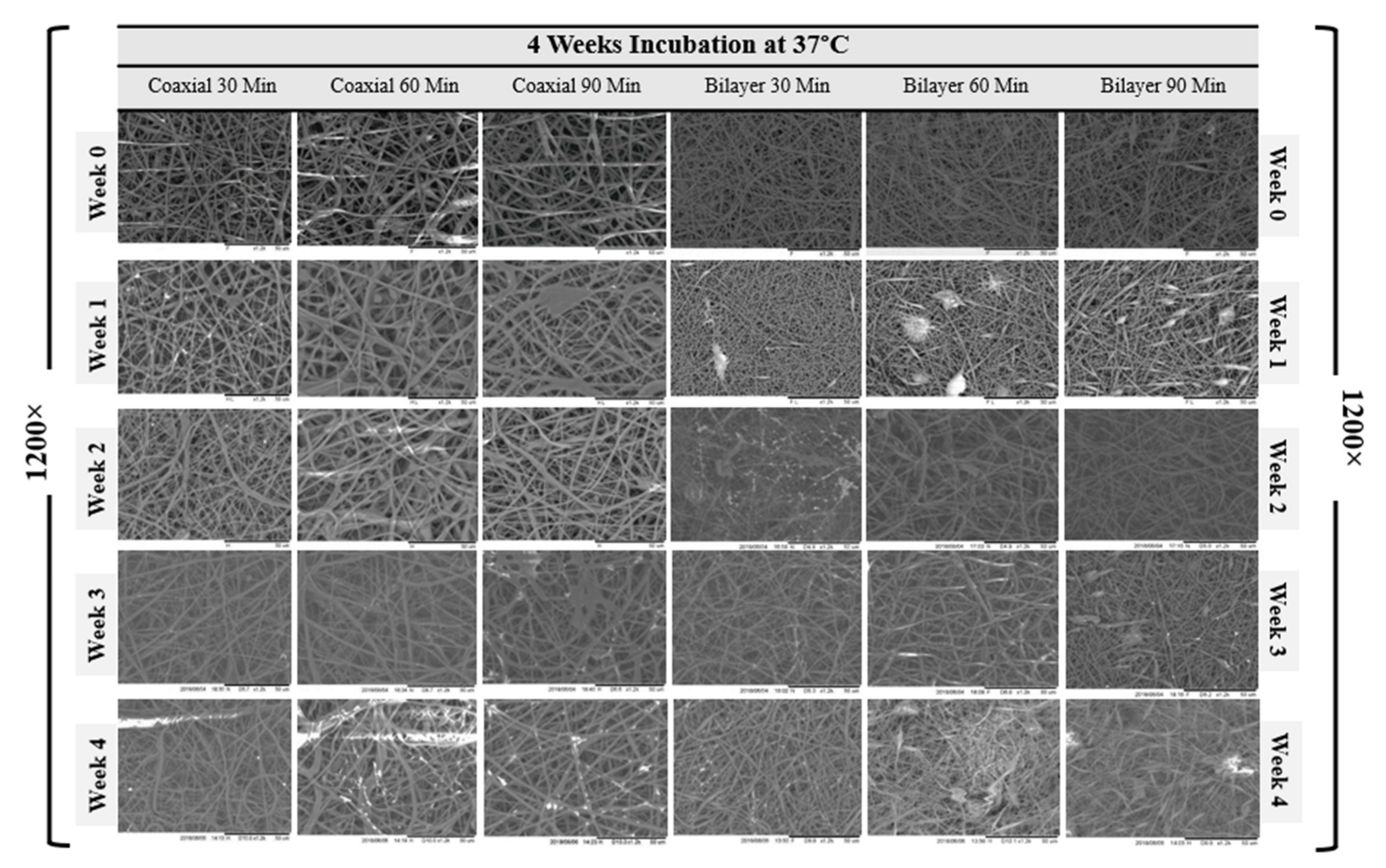
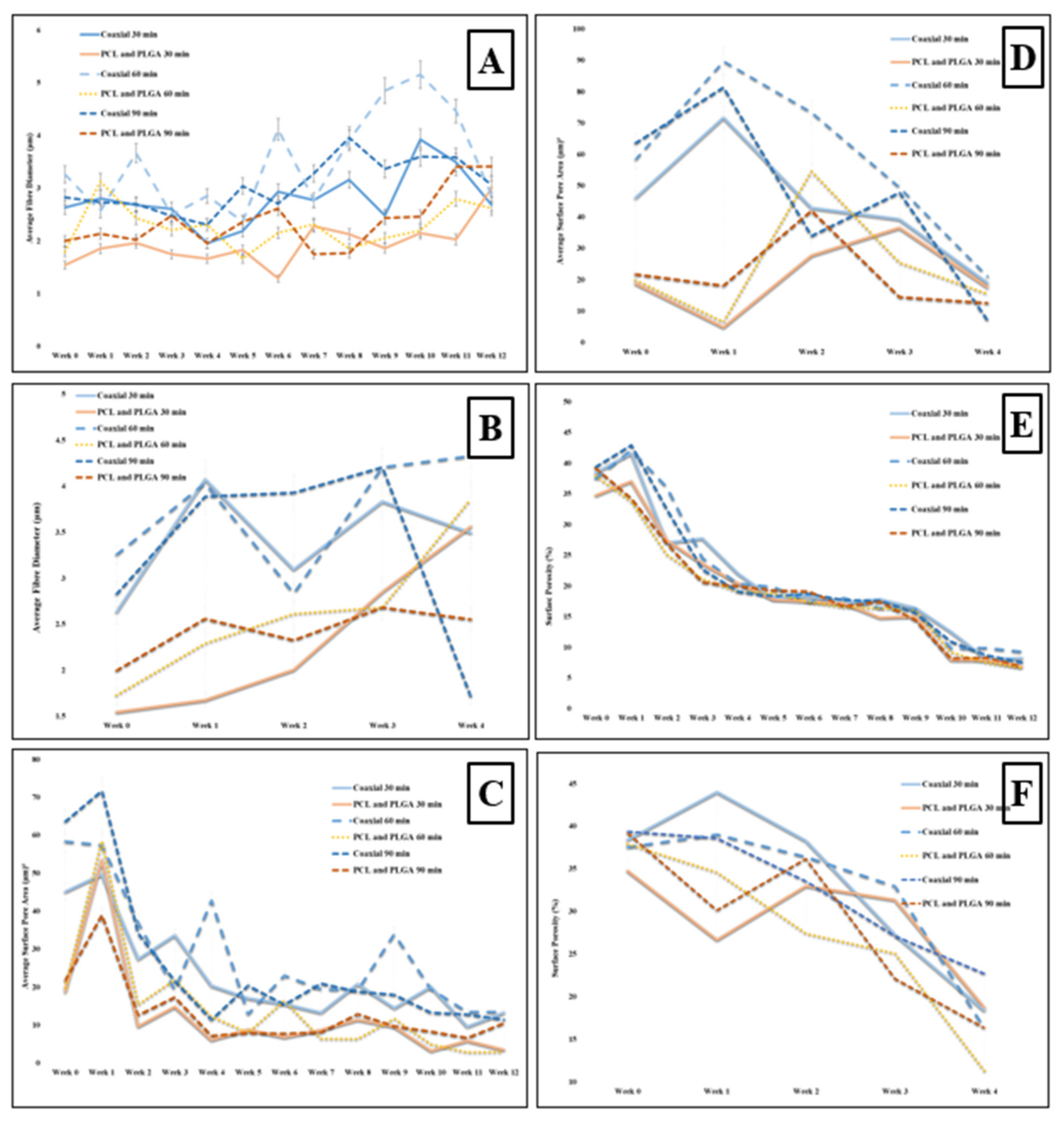

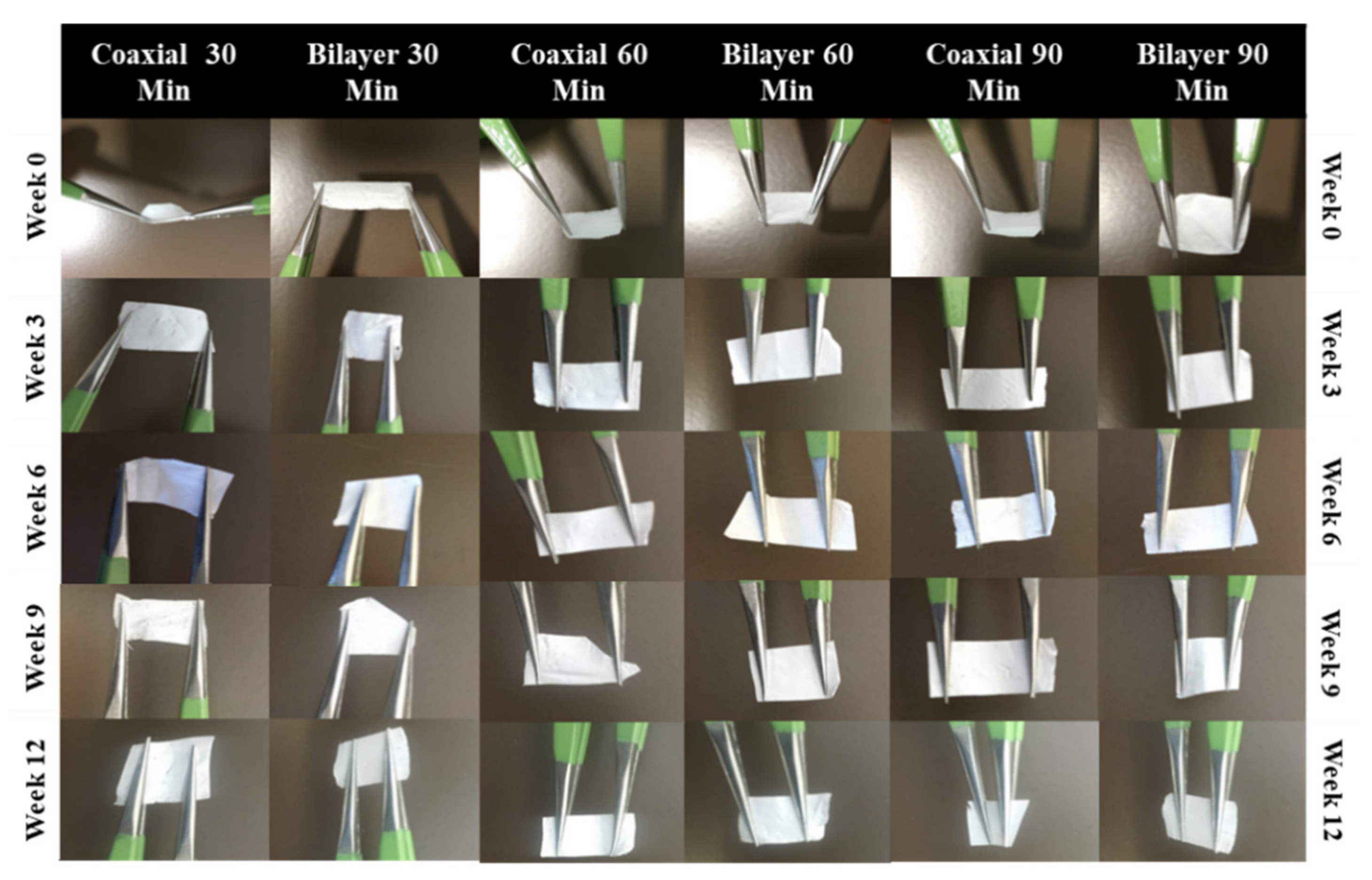
| Sample Name | Voltage (kv) | Needle Type | Distance from Tip of the Needle to the Collector (mm) | Type of Collector | Flow Rate (mL/h) | T (°C) | Humidity (%) | Time (min) | Electrospinning | |
|---|---|---|---|---|---|---|---|---|---|---|
| Coaxial | C1 | 13.84 | Coaxial | 95 | Flat | Pump1: 0.5 Pump2: 0.5 | 21.6 | 43 | 30 | 0.251 each pump |
| C2 | 14.84 | Coaxial | 95 | Flat | Pump1: 0.5 Pump2: 0.5 | 22 | 44 | 60 | 0.532 for each pump | |
| C3 | 16.53 | Coaxial | 95 | Flat | Pump1: 0.5 Pump2: 0.5 | 22.2 | 26 | 90 | 0.751 for each pump | |
| PCL and PLGA | D1 | Voltage for PCL: 7.55 Voltage for PLGA: 7.05 | 20G | 95 | Flat | 1 | 21.2 | 28 | 15 min for PCL 15 min for PGA | PCL: 0.278 mL PLGA: 0.273 mL |
| D2 | Voltage for PCL: 7.52 Voltage for PLGA: 7.00 | 20G | 95 | Flat | 1 | 21.2 | 28 | 30 min for PCL and 30 min for PLGA | PCL: 0.543 mL PLGA: 0.545 mL | |
| D3 | Voltage for PCL: 7.52 Voltage for PLGA: 7.11 | 20G | 95 | Flat | 1 | 21.2 | 28 | 45 min for PCL and 45 min for PLGA | PCL: 0.756 mL PLGA: 0.752 mL |
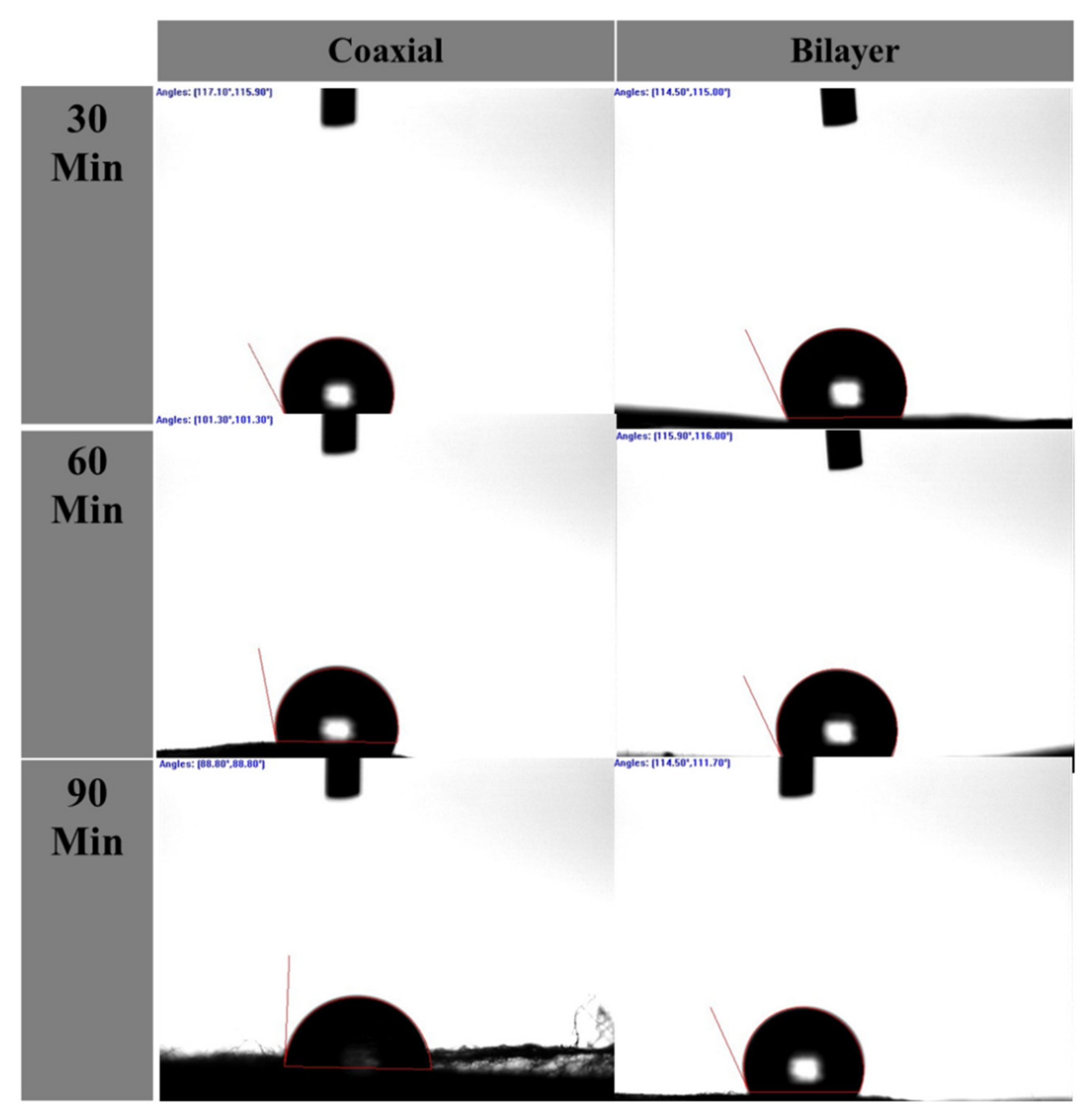
| Scaffold with Spinning Period (min) | Mean ± SD | (DH2O) | |
|---|---|---|---|
| Left Angle | Right Angle | ||
| Coaxial | 30 min (C1) | 122.8° ± 12.3 | 122.7° ± 13.8 |
| 60 min (C2) | 110.42° ± 21.5 | 107.42° ± 19.3 | |
| 90 min (C3) | 106.42° ± 35.9 | 106.2° ± 33.4 | |
| PCL and PLGA | 30 min (D1) | 120.37° ± 22.5 | 120.65° ± 23.3 |
| 60 min (D2) | 120.45° ± 13 | 120.8° ± 13.3 | |
| 90 min (D3) | 116.72° ± 17.5 | 115.45° ± 16.5 | |
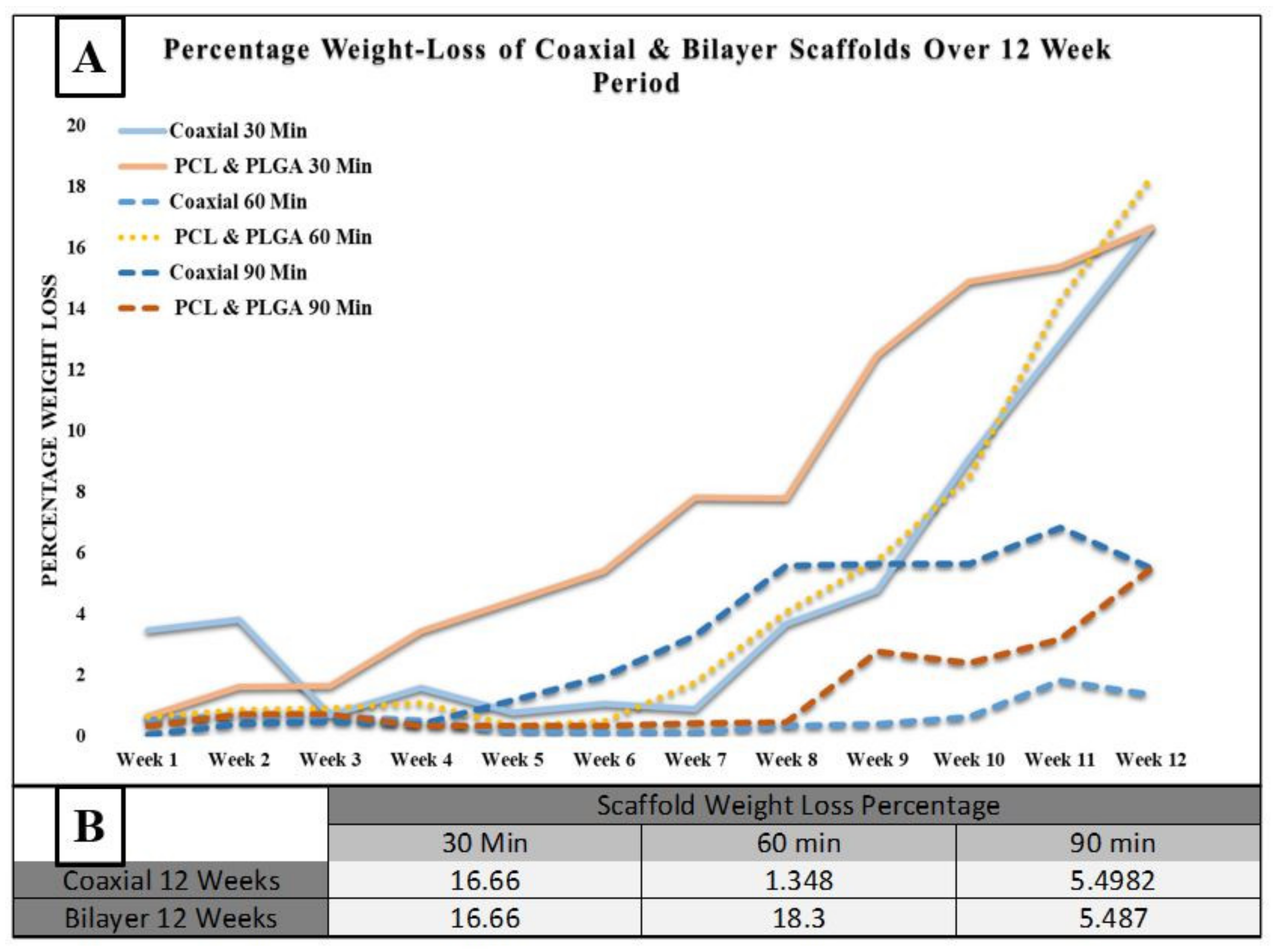
| A | Percentage Change in Fibre Diameter | ||
| 30 min | 60 min | 90 min | |
| Coaxial 12 Weeks | 2.18  | 11.97  | 8.35  |
| Bilayer 12 Weeks | 93.61  | 46.01  | 70.68  |
| Coaxial 4 Weeks at 37 °C | 32.69  | 32.82  | 39.541  |
| Bilayer 4 Weeks at 37 °C | 130.61  | 123.23  | 27.61  |
| B | Percentage Change in Pore Size | ||
| 30 min | 60 min | 90 min | |
| Coaxial 12 Weeks | 70.15  | 77.00  | 82.04  |
| Bilayer 12 Weeks | 81.60  | 85.43  | 51.53  |
| Coaxial 4 Weeks at 37 °C | 58.69  | 64.03  | 88.87  |
| Bilayer 4 Weeks at 37 °C | 5.75  | 22.27  | 42.47  |
| C | Percentage Change in Surface Porosity (%) | ||
| 30 min | 60 min | 90 min | |
| Coaxial 12 Weeks | 78.57  | 75.35  | 80.75  |
| Bilayer 12 Weeks | 80.33  | 82.53  | 82.4  |
| Coaxial 4 Weeks at 37 °C | 52.10  | 56.49  | 42.25  |
| Bilayer 4 Weeks at 37 °C | 46.08  | 70.03  | 58.17  |
| Sample Name | Time | Length (mm) | Thickness (mm) | Width (mm) | Area (mm)2 | Tensile Strength (MPa ± SD) | Elongation at Break (% ± SD) | Young Modulus (MPa ± SD) |
|---|---|---|---|---|---|---|---|---|
| Coaxial | 30 | 35.6 | 0.04 | 5 | 0.2 | 3.29 ± 1.65 | 29.16 ± 4.02 | 21.74 ± 3.45 |
| 60 | 34 | 0.06 | 5.5 | 0.33 | 3.87 ± 1.23 | 41.96 ± 2.48 | 29.44 ± 7.14 | |
| 90 | 35.3 | 0.075 | 6.2 | 0.465 | 5.39 ± 1.64 | 31.23 ± 6.74 | 33.84 ± 4.21 | |
| Bilayer | 30 | 26.8 | 0.17 | 5.3 | 0.901 | 0.68 ± 0.1 | 17.86 ± 3.11 | 3.77 ± 1.53 |
| 60 | 26 | 0.18 | 5.5 | 0.99 | 0.86 ± 0.12 | 21.44 ± 7.14 | 4.31 ± 1.22 | |
| 90 | 27.1 | 0.21 | 4.52 | 0.949 | 2.94 ± 0.52 | 22.35 ± 3.64 | 7.40 ± 2.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Fabrication and Characterization of PCL/PLGA Coaxial and Bilayer Fibrous Scaffolds for Tissue Engineering. Materials 2021, 14, 6295. https://doi.org/10.3390/ma14216295
Bazgir M, Zhang W, Zhang X, Elies J, Saeinasab M, Coates P, Youseffi M, Sefat F. Fabrication and Characterization of PCL/PLGA Coaxial and Bilayer Fibrous Scaffolds for Tissue Engineering. Materials. 2021; 14(21):6295. https://doi.org/10.3390/ma14216295
Chicago/Turabian StyleBazgir, Morteza, Wei Zhang, Ximu Zhang, Jacobo Elies, Morvarid Saeinasab, Phil Coates, Mansour Youseffi, and Farshid Sefat. 2021. "Fabrication and Characterization of PCL/PLGA Coaxial and Bilayer Fibrous Scaffolds for Tissue Engineering" Materials 14, no. 21: 6295. https://doi.org/10.3390/ma14216295
APA StyleBazgir, M., Zhang, W., Zhang, X., Elies, J., Saeinasab, M., Coates, P., Youseffi, M., & Sefat, F. (2021). Fabrication and Characterization of PCL/PLGA Coaxial and Bilayer Fibrous Scaffolds for Tissue Engineering. Materials, 14(21), 6295. https://doi.org/10.3390/ma14216295










