Medical Plant Extract Purification from Cadmium(II) Using Modified Thermoplastic Starch and Ion Exchangers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sorbents and Ion Exchangers
2.2. Instruments
2.3. Methods; Kinetic and Adsorption Experiments
2.4. Calculations
3. Results and Discussion
3.1. Chemical Characterization of the Materials
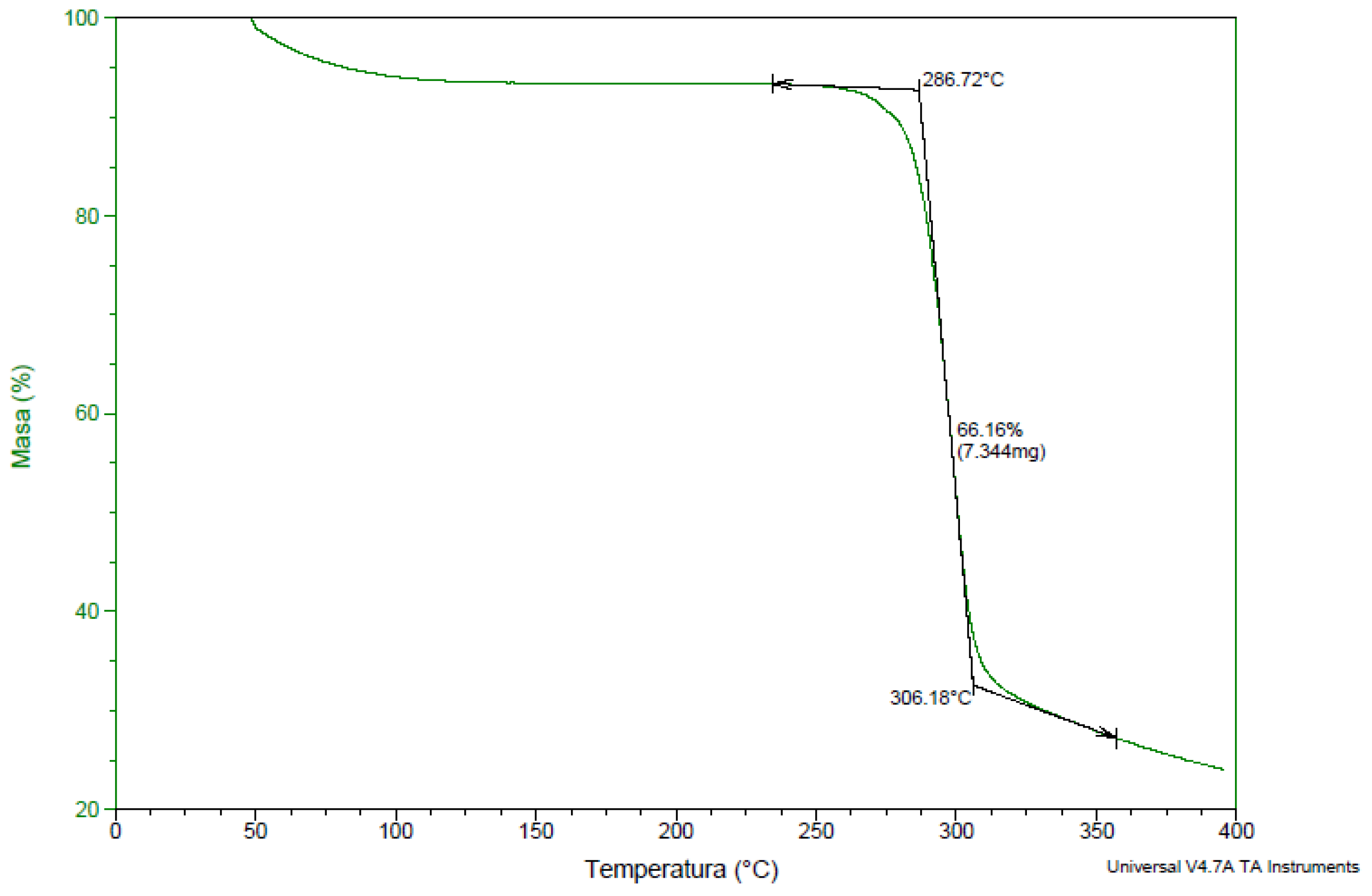
3.1.1. ATR-FTIR Analysis
3.1.2. TGA Analysis
3.1.3. XRD Analysis
3.1.4. ASAP ANALYSIS
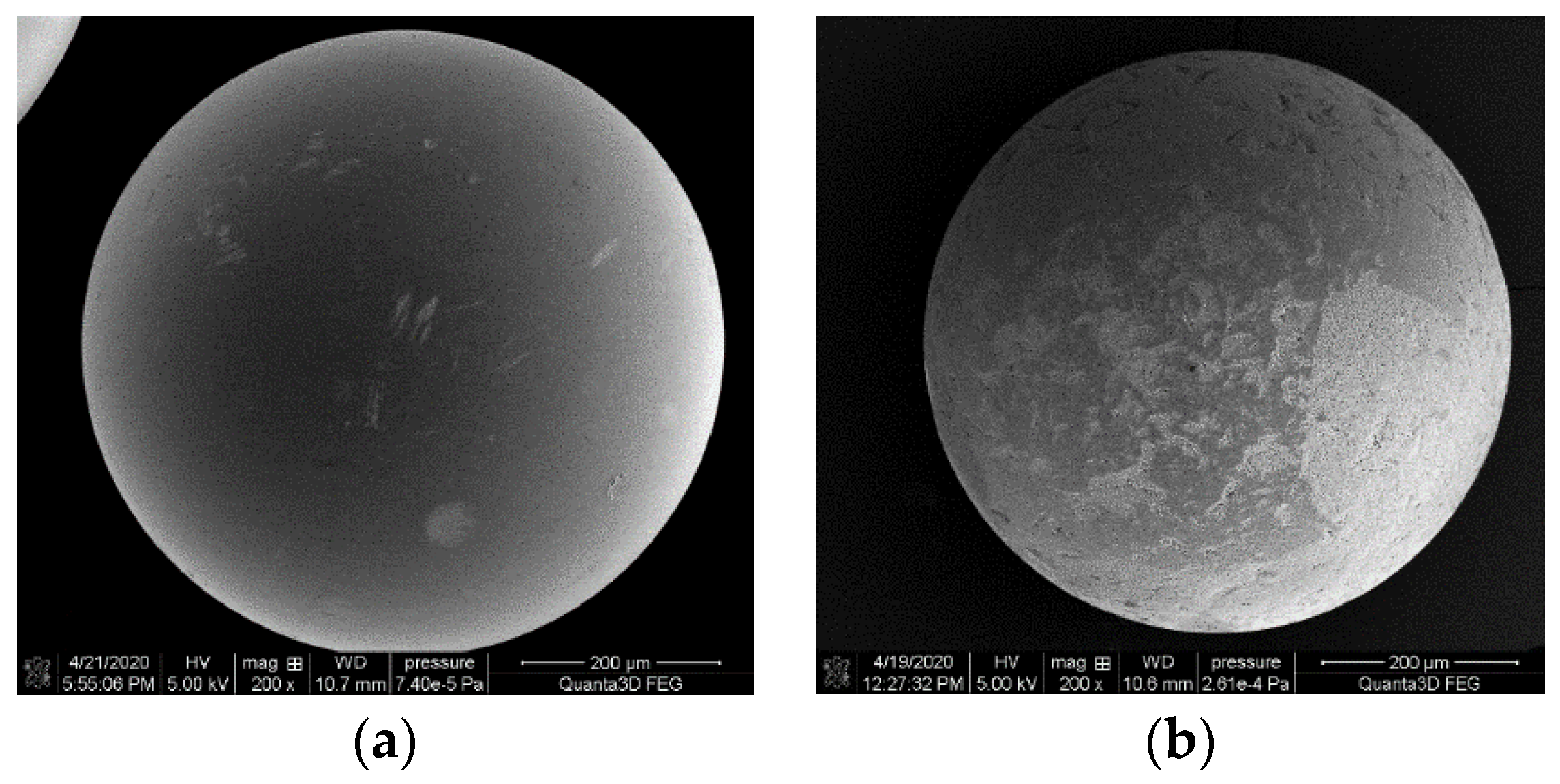
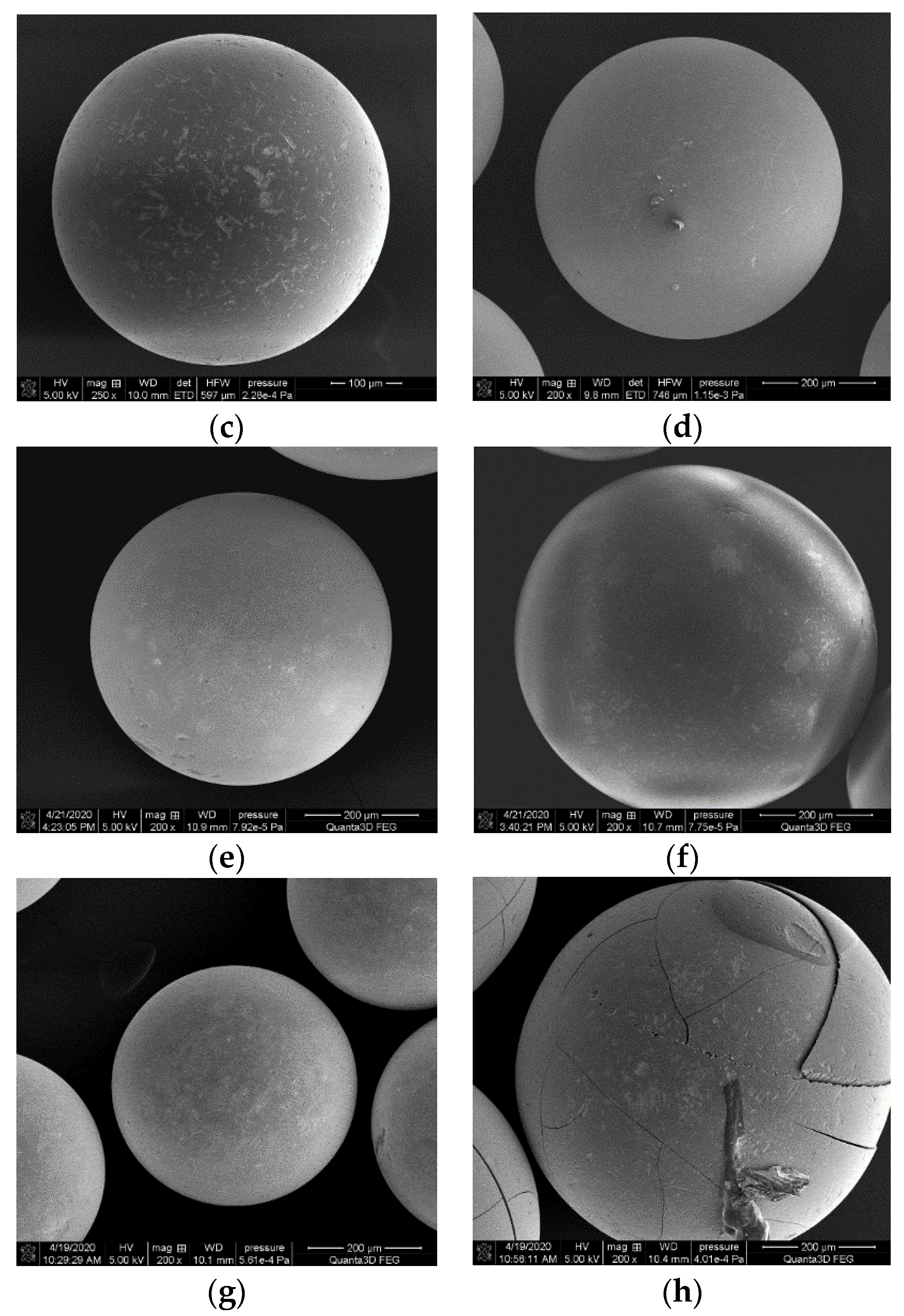
3.1.5. SEM Analysis
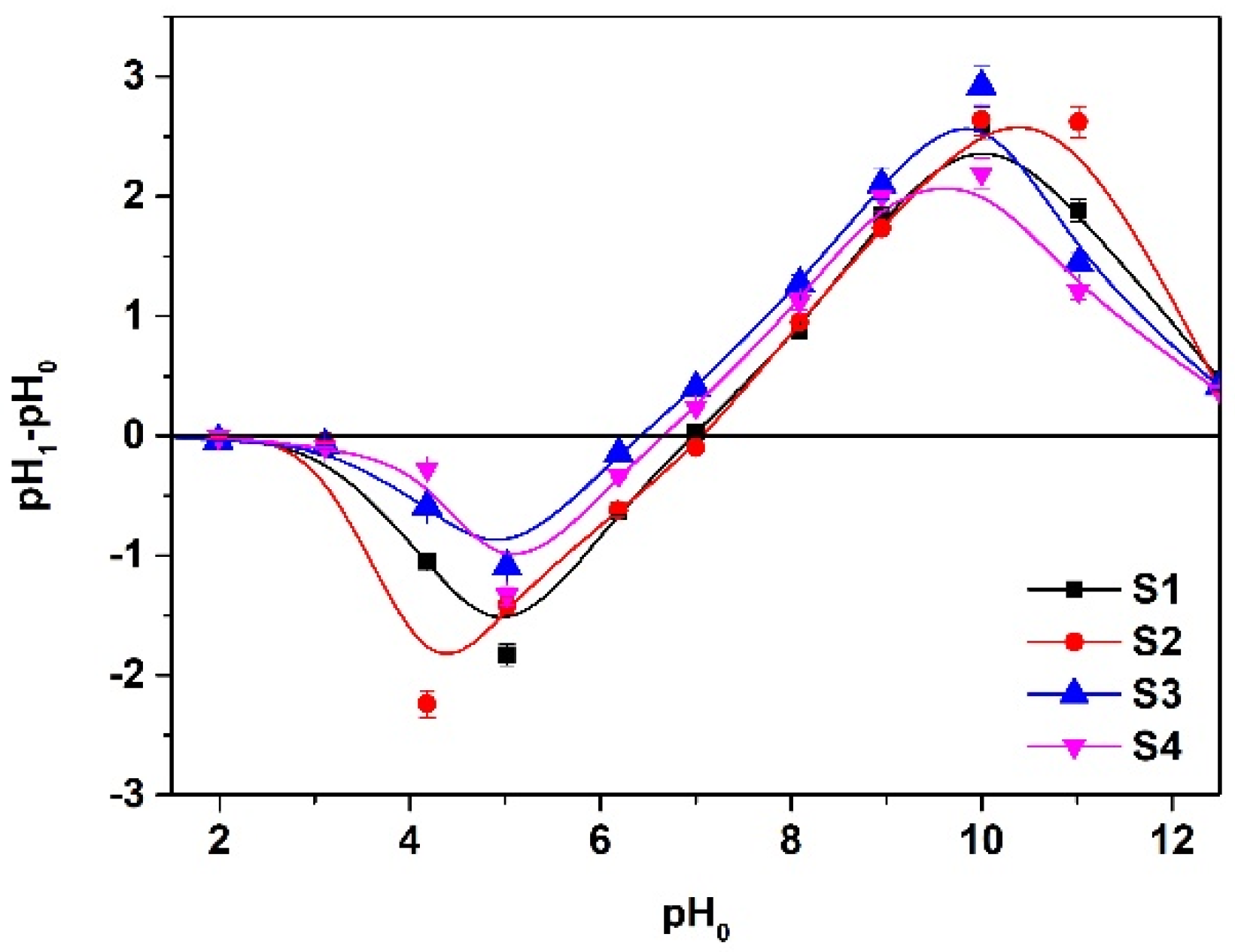
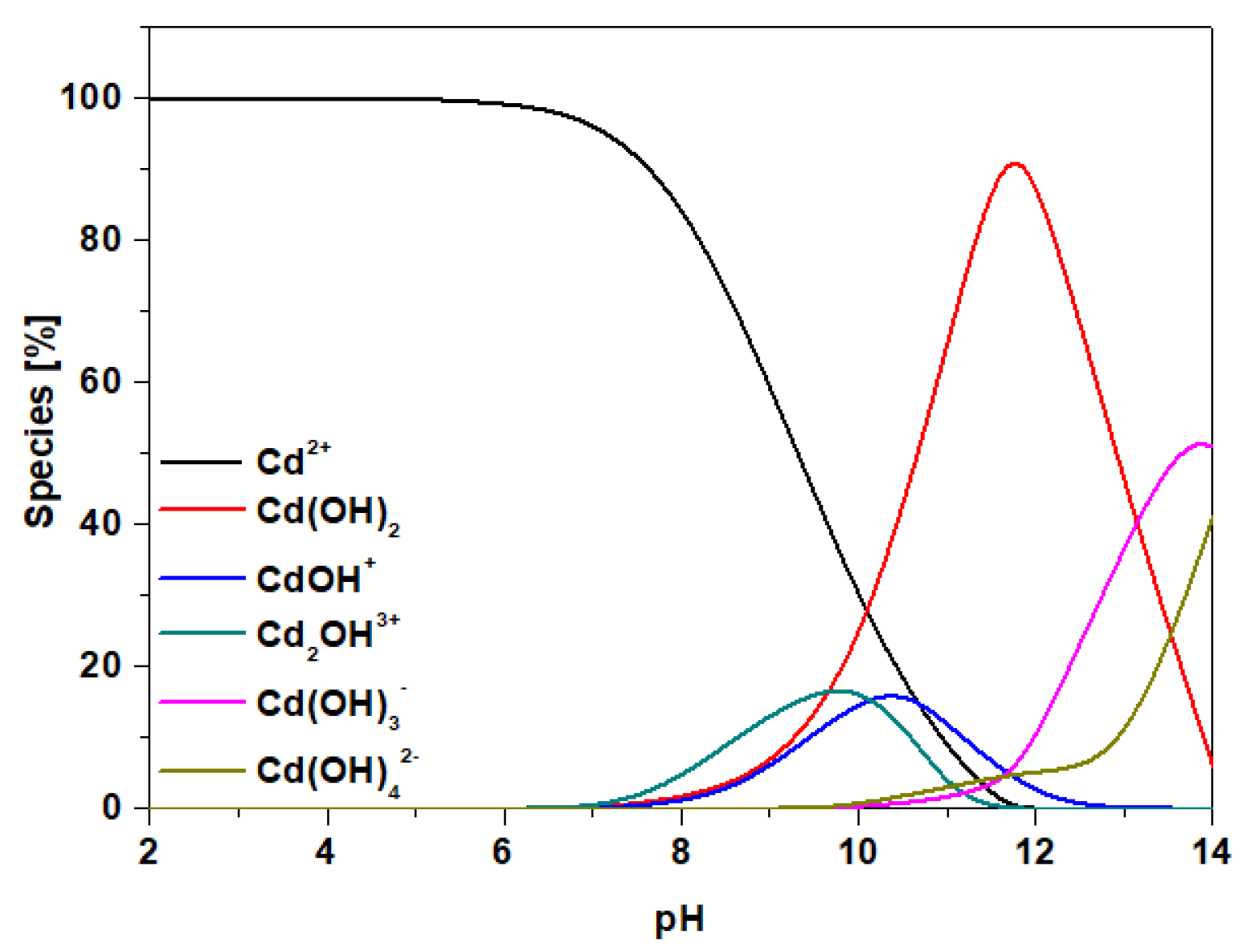
3.1.6. pHpzc Analysis
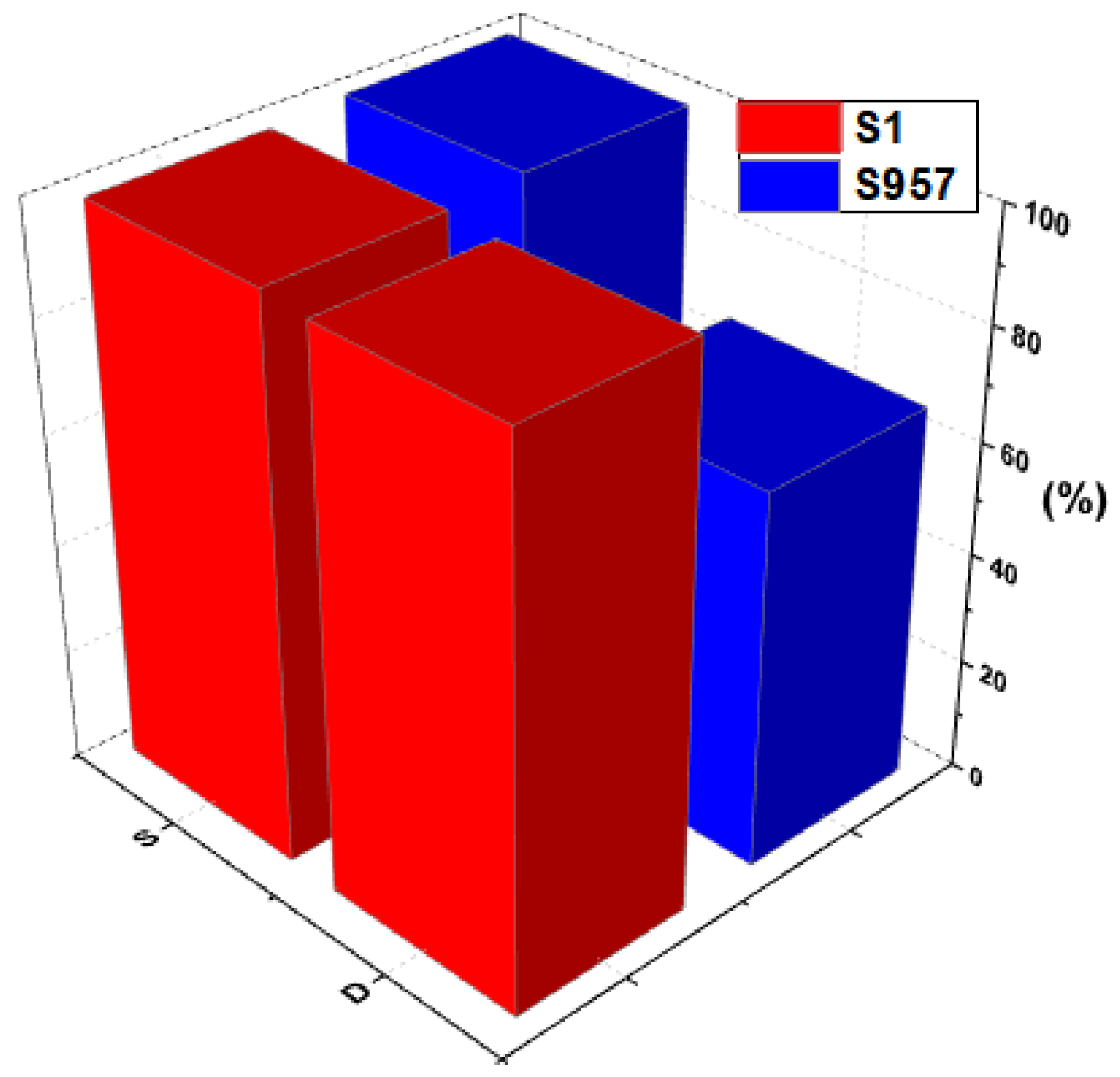
3.1.7. Degradation Analysis
3.2. Influence of Solution Ph on the Uptake of Cd(II) Ions
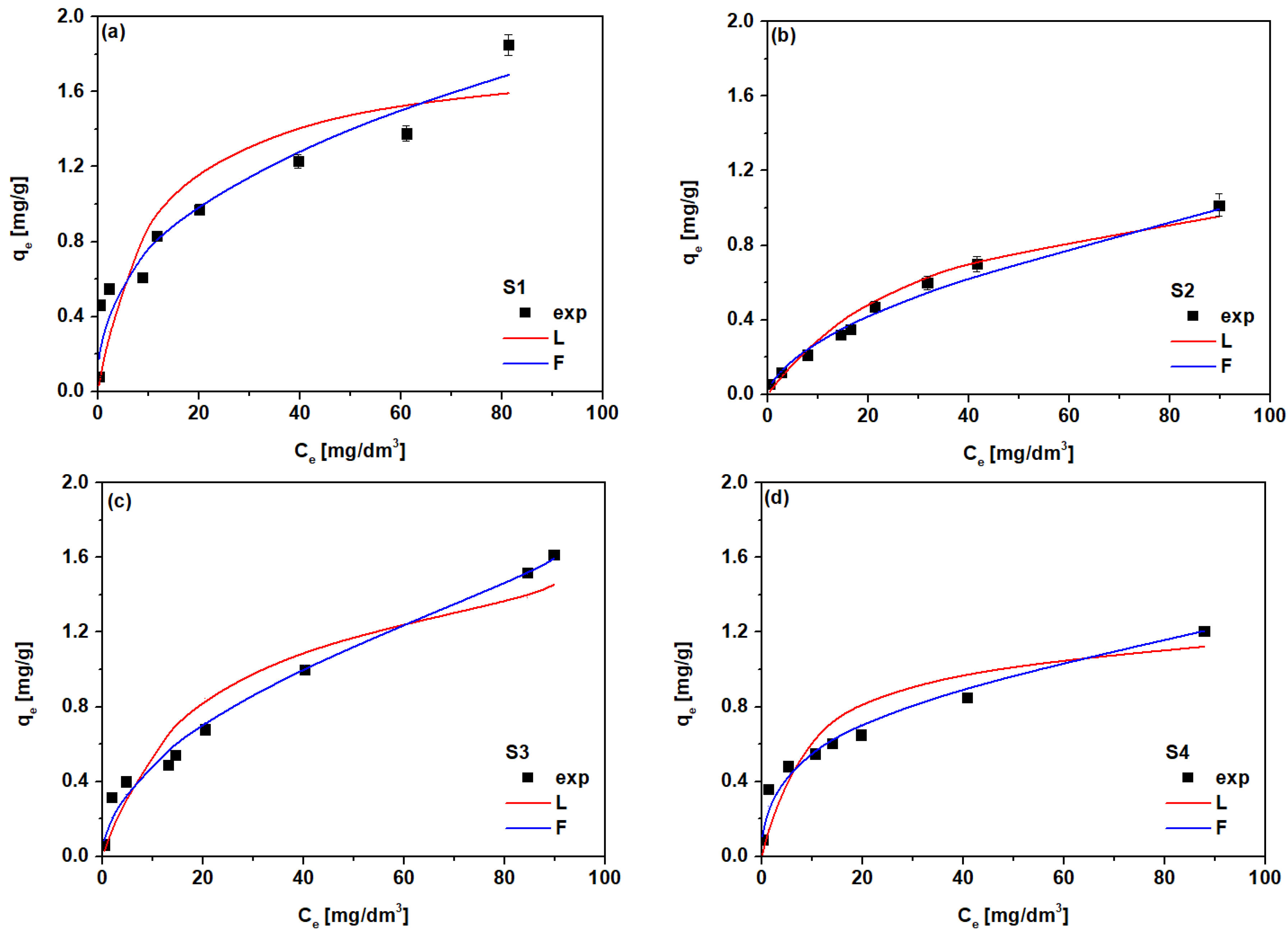
3.3. Sorption Process
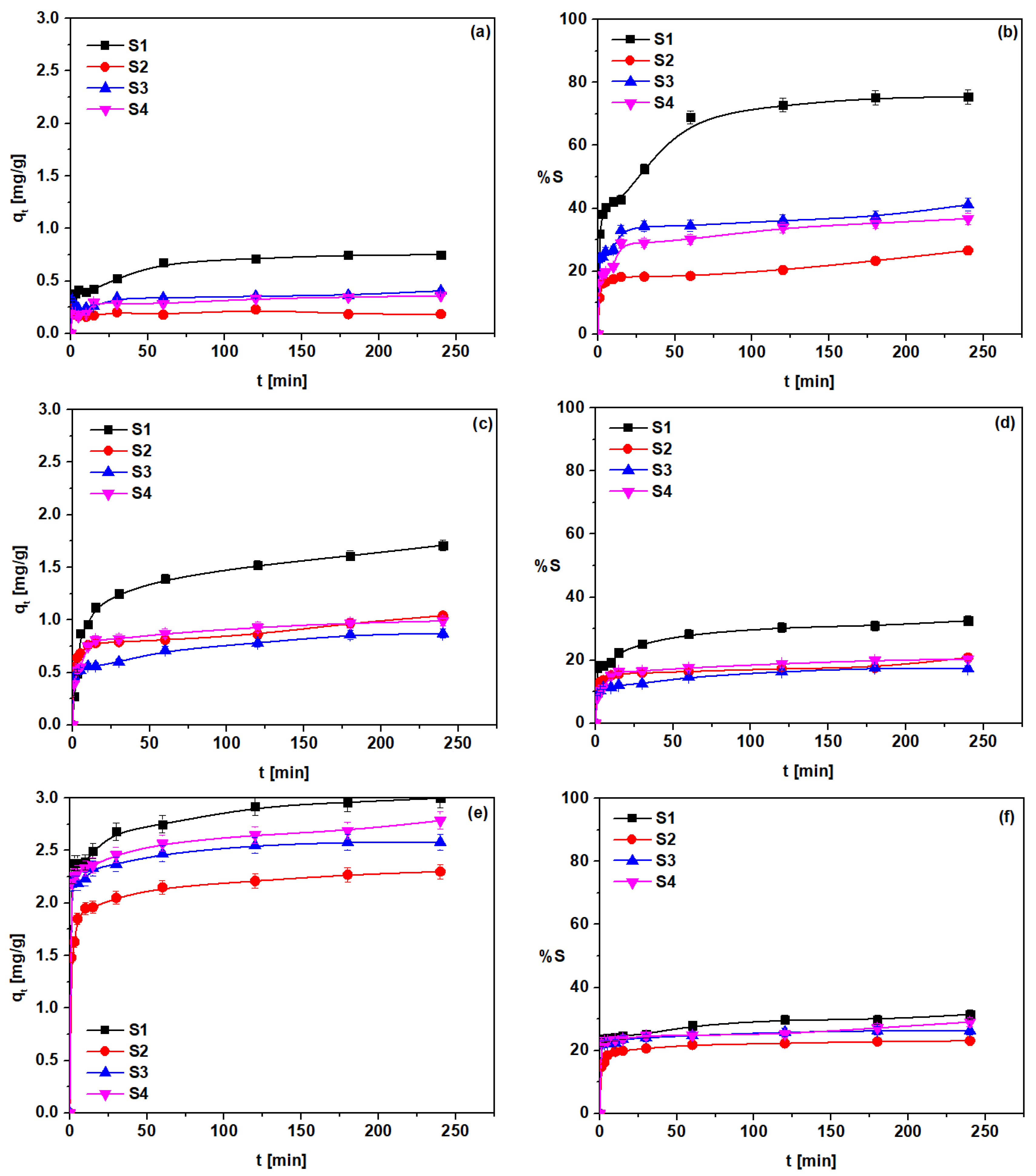
3.4. Kinetic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, L.; Jia, Y.; Ji, K.; Sanders, A.J.; Xue, K.; Ji, J.; Mason, M.D.; Jiang, W.G. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis (review). Oncol. Lett. 2015, 10, 1240–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M.; Ibrahim, F.A.; Shaban, S.A.; Youssef, N.A. Adsorption of heavy metal ion from aqueous solution by nickel oxide nano catalyst prepared by different methods. Egypt. J. Pet. 2015, 24, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer: Berlin, Germany, 2012. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, E.E.; Eide, D.J.; Guerinot, M. Lou Altered selectivity in an Arabidopsis metal transporter. Proc. Natl. Acad. Sci. USA 2000, 97, 12356–12360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditusa, S.F.; Fontenot, E.B.; Wallace, R.W.; Silvers, M.A.; Steele, T.N.; Elnagar, A.H.; Dearman, K.M.; Smith, A.P. A member of the Phosphate transporter 1 (Pht1) family from the arsenic-hyperaccumulating fern Pteris vittata is a high-affinity arsenate transporter. New Phytol. 2016, 209, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Jupa, R.; Luo, J.; Lux, A.; Ková, J.; Wen, Y.; Zhou, Y.; Jan, J.; Liang, Y.; Li, T. The apoplasmic pathway via the root apex and lateral roots contributes to Cd hyperaccumulation in the hyperaccumulator Sedum alfredii. J. Exp. Bot. 2017, 68, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, P.; Barberon, M. Polarized transport across root epithelia. Curr. Opin. Plant Biol. 2019, 52, 23–29. [Google Scholar] [CrossRef]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Shi, W.G.; Liu, W.; Yu, W.; Zhang, Y.; Ding, S.; Li, H.; Mrak, T.; Kraigher, H.; Luo, Z. Bin Abscisic acid enhances lead translocation from the roots to the leaves and alleviates its toxicity in Populus × canescens. J. Hazard. Mater. 2019, 362, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Krzesłowska, M.; Rabȩda, I.; Basińska, A.; Lewandowski, M.; Mellerowicz, E.J.; Napieralska, A.; Samardakiewicz, S.; Woźny, A. Pectinous cell wall thickenings formation—A common defense strategy of plants to cope with Pb. Environ. Pollut. 2016, 214, 354–361. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hong, X.Q.; He, H.; Luo, H.W.; Qian, T.T.; Li, R.Z.; Jiang, H.; Yu, H.Q. Biosorption of Cr (VI) by Typha angustifolia: Mechanism and responses to heavy metal stress. Bioresour. Technol. 2014, 160, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Y.; Chen, S.; Polle, A.; Rennenberg, H.; Luo, Z. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, L.; Li, X. Arabidopsis and rice showed a distinct pattern in ZIPs genes expression profile in response to Cd stress. Bot. Stud. 2018, 59, 1–10. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liu, C.; Cao, B.; Qin, M.; Long, D.; Xiang, Z.; Zhao, A. Genome-wide identification and characterization of four gene families putatively involved in cadmium uptake, translocation and sequestration in mulberry. Front. Plant Sci. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Milner, M.J.; Craft, E.; Yamaji, N.; Koyama, E.; Ma, J.F.; Kochian, L.V. Characterization of the high affinity Zn transporter from Noccaea caerulescens, NcZNT1, and dissection of its promoter for its role in Zn uptake and hyperaccumulation. New Phytol. 2012, 195, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sanjaya; Hsiao, P.Y.; Su, R.C.; Ko, S.S.; Tong, C.G.; Yang, R.Y.; Chan, M.T. Overexpression of Arabidopsis thaliana tryptophan synthase beta 1 (AtTSB1) in Arabidopsis and tomato confers tolerance to cadmium stress. Plant Cell Environ. 2008, 31, 1074–1085. [Google Scholar] [CrossRef]
- Zhang, M.; Senoura, T.; Yang, X.; Nishizawa, N.K. Functional analysis of metal tolerance proteins isolated from Zn/Cd hyperaccumulating ecotype and non-hyperaccumulating ecotype of Sedum alfredii Hance. FEBS Lett. 2011, 585, 2604–2609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migocka, M.; Kosieradzka, A.; Papierniak, A.; Maciaszczyk-dziubinska, E.; Posyniak, E.; Garbiec, A.; Filleur, S. Two metal-tolerance proteins, MTP1 and MTP4, are involved in Zn homeostasis and Cd sequestration in cucumber cells. J. Exp. Bot. 2015, 66, 1001–1015. [Google Scholar] [CrossRef] [Green Version]
- Fryxell, G.E.; Wu, H.; Lin, Y.; Shaw, W.J.; Birnbaum, J.C.; Linehan, J.C.; Nie, Z.; Kemner, K.; Kelly, S. Lanthanide selective sorbents: Self-assembled monolayers on mesoporous supports (SAMMS). J. Mater. Chem. 2004, 14, 3356–3363. [Google Scholar] [CrossRef]
- Thomine, S.; Wang, R.; Ward, J.M.; Crawford, N.M.; Schroeder, J.I. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. USA 2000, 97, 4991–4996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, M.J.; Mitani-Ueno, N.; Yamaji, N.; Yokosho, K.; Craft, E.; Fei, Z.; Ebbs, S.; Zambrano, M.C.; Ma, J.F.; Kochian, L.V. Root and shoot transcriptome analysis of two ecotypes of Noccaea caerulescens uncovers the role of NcNramp1 in Cd hyperaccumulation. Plant J. 2014, 78, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Choi, H.; Segami, S.; Cho, H.T.; Martinoia, E.; Maeshima, M.; Lee, Y. AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. Plant J. 2009, 58, 737–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorrig, W.; Abdelly, C.; Berthomieu, P. The phylogenetic tree gathering the plant Zn/Cd/Pb/Co P 1B- ATPases appears to be structured according to the botanical families. Comptes Rendus Biol. 2011, 334, 863–871. [Google Scholar] [CrossRef]
- Morel, M.; Crouzet, J.; Gravot, A.; Auroy, P.; Leonhardt, N.; Vavasseur, A.; Richaud, P. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 2009, 149, 894–904. [Google Scholar] [CrossRef] [Green Version]
- Verret, F.; Gravot, A.; Auroy, P.; Leonhardt, N.; David, P.; Nussaume, L.; Vavasseur, A.; Richaud, P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 2004, 576, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef]
- Wu, Q.; Shigaki, T.; Williams, K.A.; Han, J.S.; Kim, C.K.; Hirschi, K.D.; Park, S. Expression of an Arabidopsis Ca2+/H+ antiporter CAX1 variant in petunia enhances cadmium tolerance and accumulation. J. Plant Physiol. 2011, 168, 167–173. [Google Scholar] [CrossRef]
- Mei, H.; Cheng, N.H.; Zhao, J.; Park, S.; Escareno, R.A.; Pittman, J.K.; Hirschi, K.D. Root development under metal stress in Arabidopsis thaliana requires the H+/cation antiporter CAX4. New Phytol. 2009, 183, 95–105. [Google Scholar] [CrossRef]
- Lavid, N.; Barkay, Z.; Tel-Or, E. Accumulation of heavy metals in epidermal glands of the waterlily (Nymphaeaceae). Planta 2001, 212, 313–322. [Google Scholar] [CrossRef]
- Sawidis, T.; Breuste, J.; Mitrovic, M.; Pavlovic, P.; Tsigaridas, K. Trees as bioindicator of heavy metal pollution in three European cities. Environ. Pollut. 2011, 159, 3560–3570. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Luo, Z.-B.; He, J.; Polle, A.; Rennenberg, H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: Paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef]
- Yuan, H.M.; Huang, X. Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 120–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayed, A.; Lytle, C.M.; Qian, J.H.; Terry, N. Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 1998, 206, 293–299. [Google Scholar] [CrossRef]
- Bai, Y.; Bartkiewicz, B. Removal of cdmium from wastewater usingi on exchange resin Amberjet 1200H columns. Pol. J. Environ. Stud. 2009, 18, 1191–1195. [Google Scholar]
- Maliou, E.; Malamis, M.; Sakellarides, P.O. Lead and cadmium removal by ion exchange. Water Sci. Technol. 1992, 25, 133–138. [Google Scholar] [CrossRef]
- Hubicki, Z.; Kołodyńska, D. Selective removal of heavy metal ions from waters and waste waters using ion exchange methods. In Ion Exchange Technologies; Kilislioğlu, A., Ed.; IntechOpen: London, UK, 2012; pp. 193–240. ISBN 980-953-307-139-3. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Chughtai, S.; Keane, M.A. The removal of cadmium and lead from aqueous solution by ion exchange with Na-Y zeolite. Sep. Purif. Technol. 1998, 13, 57–64. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Lin, X.; Zhou, Y. Comparative study on the blends of PBS/thermoplastic starch prepared from waxy and normal corn starches. Starch Staerke 2013, 65, 831–839. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Shiyab, S.; Chen, J.; Han, F.X.; Monts, D.L.; Matta, F.B.; Gu, M.; Su, Y. Phytotoxicity of mercury in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 2009, 72, 619–625. [Google Scholar] [CrossRef]
- Sridhar, B.B.M.; Han, F.X.; Diehl, S.V.; Monts, D.L.; Su, Y. Monitoring the effects of arsenic and chromium accumulation in Chinese brake fern (Pteris vittata). Int. J. Remote Sens. 2007, 28, 1055–1067. [Google Scholar] [CrossRef]
- Harada, E.; Choi, Y.E. Investigation of metal exudates from tobacco glandular trichomes under heavy metal stresses using a variable pressure scanning electron microscopy system. Plant Biotechnol. 2008, 25, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Fila, D.; Hubicki, Z. Static and dynamic studies of lanthanum(III) ion adsorption/desorption from acidic solutions using chelating ion exchangers with different functionalities. Environ. Res. 2020, 191, 110171. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Fila, D.; Hubicki, Z. Recovery of lanthanum(III) and nickel(II) ions from acidic solutions by the highly effective ion exchanger. Molecules 2020, 25, 3718. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Hubicki, Z.; Fila, D. Recovery of rare earth elements from acidic solutions using macroporous ion exchangers. Sep. Sci. Technol. 2019, 54, 2059–2076. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Kozioł, M.; Łodyga, A.; Hubicki, Z. New approach towards the studies of starch modification: Properties and applications. In Green Polymer Composites Technology; CRC Press: Boca Raton, FL, USA, 2016; pp. 547–578. [Google Scholar]
- Ahn, C.K.; Park, D.; Woo, S.H.; Park, J.M. Removal of cationic heavy metal from aqueous solution by activated carbon impregnated with anionic surfactants. J. Hazard. Mater. 2009, 164, 1130–1136. [Google Scholar] [CrossRef]
- Hu, H.; Jin, Q.; Kavan, P. A study of heavy metal pollution in China: Current status, pollution-control policies and countermeasures. Sustainability 2014, 6, 5820–5838. [Google Scholar] [CrossRef] [Green Version]
- Zlobin, I.E.; Kartashov, A.V.; Shpakovski, G.V. Different roles of glutathione in copper and zinc chelation in Brassica napus roots. Plant Physiol. Biochem. 2017, 118, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA 1987, 84, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Thirumoorthy, N.; Kumar, K.T.M.; Sundar, A.S.; Panayappan, L.; Chatterjee, M. Metallothionein: An overview. World J. Gastroenterol. 2007, 13, 993–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van, H.N.; Van, H.C.; Hoang, T.L.; Nguyen, D.K.V.; Thuc, C.N.H. The starch modified montmorillonite for the removal of Pb(II), Cd(II) and Ni(II) ions from aqueous solutions. Arab. J. Chem. 2020, 13, 7212–7223. [Google Scholar] [CrossRef]
- Haroon, M.; Wang, L.; Yu, H.; Abbasi, N.M.; Zain-ul-Abdin; Saleem, M.; Khan, R.U.; Ullah, R.S.; Chen, Q.; Wu, J. Chemical modification of starch and its application as an adsorbent material. RSC Adv. 2016, 6, 78264–78285. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, X.; Luo, X.; Su, T.; Zhang, Y.; Qin, Z.; Ji, H. Mechanically activated starch magnetic microspheres for Cd(II) adsorption from aqueous solution. Chin. J. Chem. Eng. 2020, 33, 40–49. [Google Scholar] [CrossRef]
- Xie, G.; Shang, X.; Liu, R.; Hu, J.; Liao, S. Synthesis and characterization of a novel amino modified starch and its adsorption properties for Cd(II) ions from aqueous solution. Carbohydr. Polym. 2011, 84, 430–438. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Demirbas, A.; Pehlivan, E.; Gode, F.; Altun, T.; Arslan, G. Adsorption of Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) from aqueous solution on Amberlite IR-120 synthetic resin. J. Colloid Interface Sci. 2005, 282, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, E.; Altun, T. The study of various parameters affecting the ion exchange of Cu2+, Zn2+, Ni2+, Cd2+, and Pb2+ from aqueous solution on Dowex 50W synthetic resin. J. Hazard. Mater. 2006, 134, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Singh, P.; Rahman, N. Adsorption behavior of Hg(II), Pb(II), and Cd(II) from aqueous solution on Duolite C-433: A synthetic resin. J. Colloid Interface Sci. 2004, 275, 398–402. [Google Scholar] [CrossRef] [PubMed]
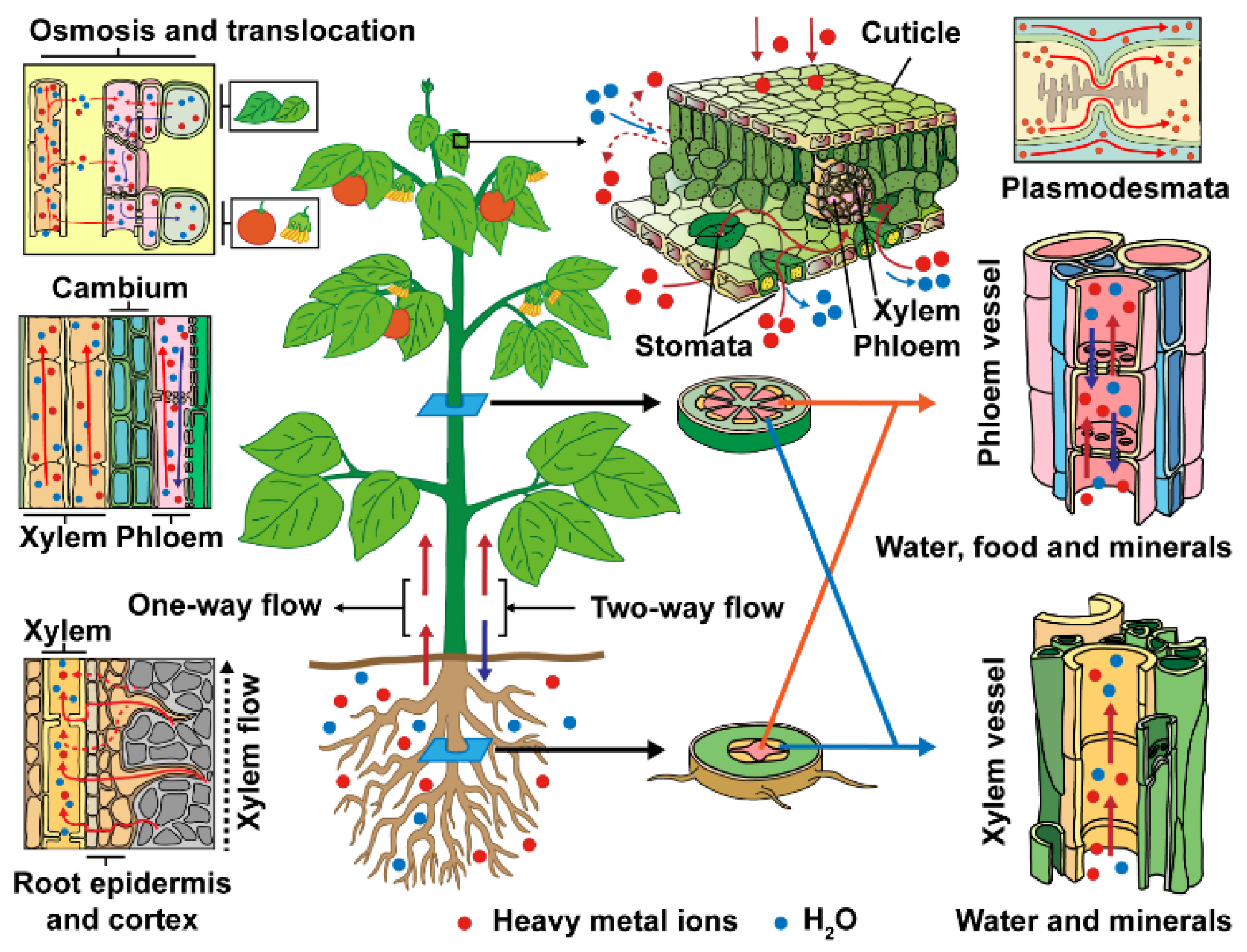
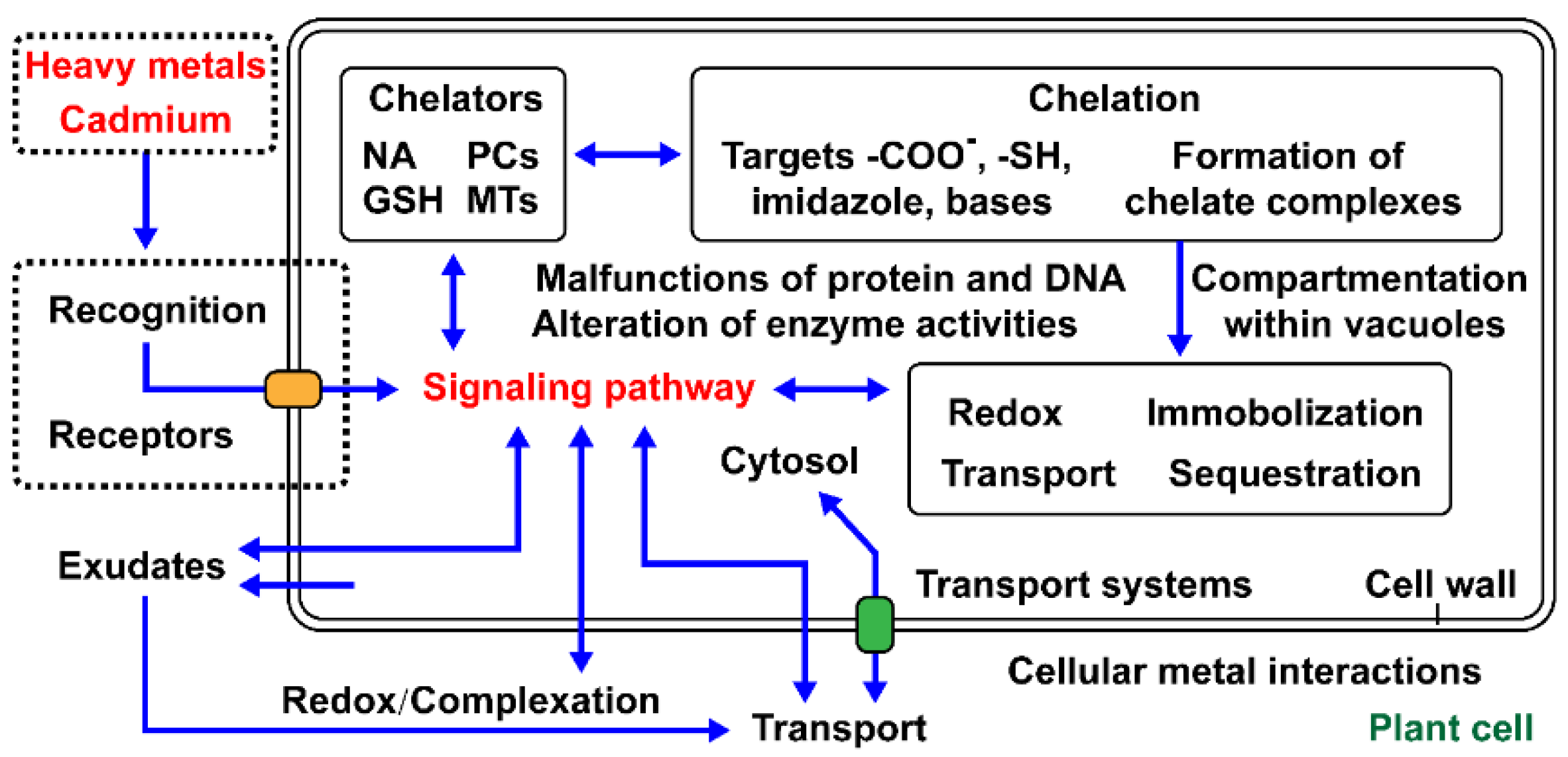
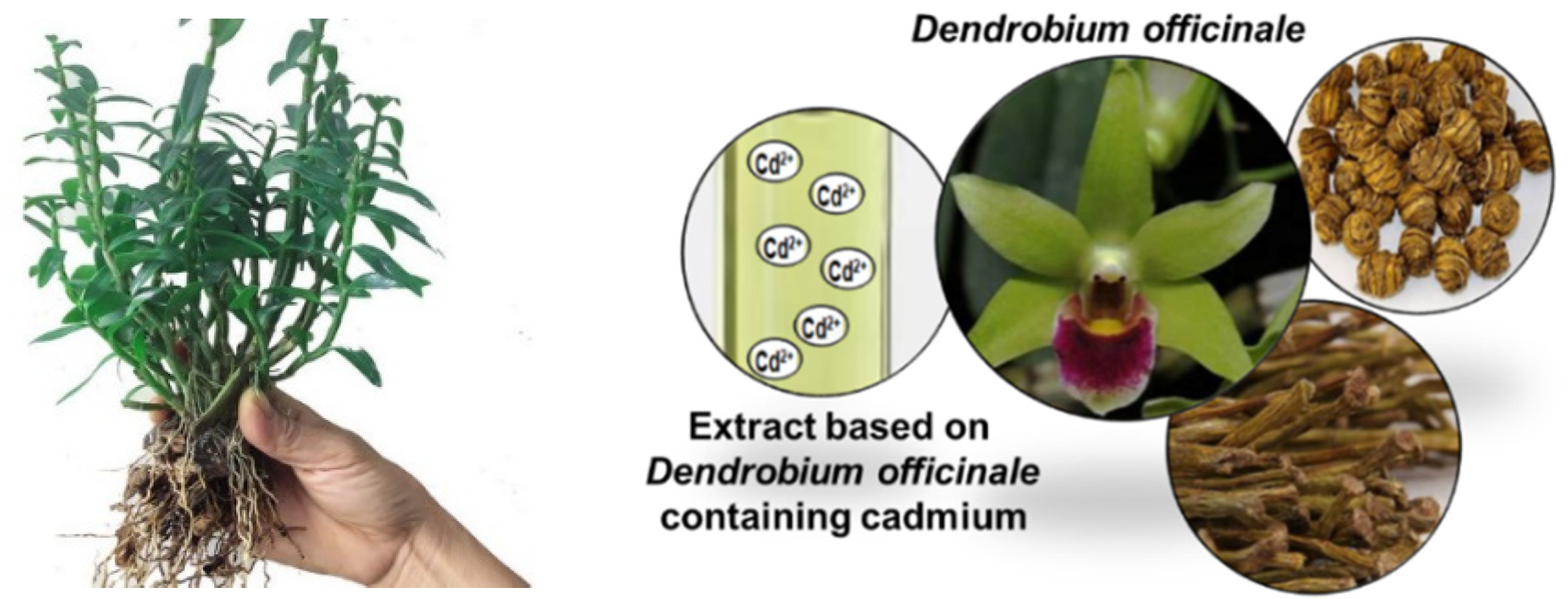




| Names | Functions Related to Heavy Metal Accumulation | Transporter Types | Reference |
|---|---|---|---|
| ZIPs family transporters (Zn, Fe, Mn, Cd, Ni, Cu, etc.) | |||
| ZIP1 | Transporting Cd to the cytosol | Divalent cations exporter transporter | [16] |
| ZIP2 | Transporting Cd(II)/Zn(II) to the root vascular system | Plasma membrane -localized transporter | [17] |
| NcZNT1 | Zn(II)/Cd(II) long-distance transport of vascular system | Plasma membrane -localized transporter | [18] |
| AtZIP9 | Marker of Zn(II) deficiency or Cd(II) excess in uptake system | Metal transporter | [19] |
| CDFs family transporters (Mn, Fe, Zn, Co, Cd, Ni, etc.) | |||
| MTP1 | Transporting cytosolic Zn/Cd into vacuoles | Vacuolar transporter | [20] |
| MTP4 | Participating in vacuolar Zn and Cd sequestration | Vacuolar transporter | [21] |
| NRAMPs family transporters (Mn, Fe, Zn, Cd, Co, Ni, Pb, etc.) | |||
| AtNRAMP1 | High-affinity transporting Mn(II) into root cells; Implicating in Cd(II) uptake in endodermal cells | Plasma membrane-localized transporter | [22] |
| AtNRAMP3/4 | Exporting vacuolar Fe(II)/Mn(II)/Cd(II) into the cytosol | Metal transporters | [23] |
| NcNRAMP1 | Transporting Cd(II) into endodermal cells; Involving Cd(II) flux movement towards the stele and root-to-shoot Cd transport | Cd hyperaccumulation transporter | [24] |
| HMAs family transporters (Cu, Ag, Zn, Cd, Pb, etc.) | |||
| AtHMA1 | Exporting excessive Cd(II), Cu(II), Zn(II) from the chloroplast to the cytosol | Chloroplast-localized transporter | [25] |
| AtHMA2 | Exporting of cytosolic Zn(II) and Cd(II) into the vascular cylinder | Plasma-membrane-localized transporters | [26] |
| AtHMA3 | Transporting cytosolic Co(II), Zn(II), Cd(II) and Pb(II) into vacuoles | Metal transporter | [27] |
| AtHMA4 | Transporting cytoplasmic Zn(II), Cd(II), Co(II) to the xylem vessels | Plasma-membrane-localized transporters | [28] |
| ABCC sub-family transporters (As, Cd, Hg, etc.) | |||
| DK/2 | Transporting Cd-PCs and Hg-PCs into vacuoles | Vacuolar phytochelatin transporters | [29] |
| CAXs (Cation/H+ antiporters) family (Mn, Zn, Cd, Ni, Cu, etc.) | |||
| CAX2 | Transporting Mn(II), Zn(II) and Cd(II) into the vacuole | Vacuolar transporter | [30] |
| AtCAX4 | Transporting Mn(II), Ni(II) and Cd(II) into the vacuole | Vacuolar transporter | [31] |
| Name | Appearance | Composition | Name | Appearance | Composition |
|---|---|---|---|---|---|
| S1 |  | TPS (containing starch + 30% glycerol + 8% H2O) | S3 |  | 50% of TPS + 50% of PLA |
| S2 |  | 25% of TPS + 75% of PBS | S4 |  | 50% of TPS + 50% of PLA (5% hemp fibers) |
| Name | Appearance | Composition | Name | Appearance | Composition |
|---|---|---|---|---|---|
| SP112 |  |  | IRC718 |  | 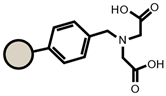 |
| S940 |  | 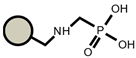 | TP207 |  | 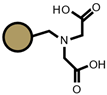 |
| IRC747 |  | 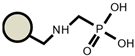 | TP208 |  | 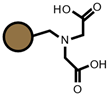 |
| IRC748 |  |  | S930 |  |  |
| Sorbent | Hydrogen Bonds | C-O in C-O-H | C-O in C-O-C |
|---|---|---|---|
| S1 | 3287 cm−1 | 1158 cm−1 and 1059 cm−1 | 1014 cm−1 and 996 cm−1 |
| S2 | 3329 cm−1 | 1207 cm−1 and 1053 cm−1 | 1020 cm−1 and 994 cm−1 |
| S3 | 3319 cm−1 | 1183 cm−1 and 1082 cm−1 | 1021 cm−1 and 998 cm−1 |
| S4 | 3319 cm−1 | 1206 cm−1 and 1083 cm−1 | 1018 cm−1 and 990 cm−1 |
| SBET [m2/g] | 14.98 |
| Vtot [cm3/g] | 0.144 |
| Sorbent | Freundlich Isotherm Parameters | Langmuir Isotherm Parameters | |||||
|---|---|---|---|---|---|---|---|
| kF (mg/g) | 1/n | R2 | Q0 (mg/g) | b (dm3/mg) | RL | R2 | |
| S1 | 0.323 | 0.377 | 0.909 | 1.79 | 0.099 | 0.092 | 0.927 |
| S2 | 0.075 | 0.573 | 0.986 | 1.32 | 0.029 | 0.257 | 0.857 |
| S3 | 0.148 | 0.524 | 0.944 | 1.74 | 0.046 | 0.178 | 0.865 |
| S4 | 0.243 | 0.358 | 0.967 | 1.26 | 0.097 | 0.094 | 0.944 |
| Adsorbent | qe (mg/g) | pH | Ref. |
|---|---|---|---|
| S1 | 1.79 | 6 | This paper |
| S2 | 1.32 | 6 | |
| S3 | 1.74 | 6 | |
| S4 | 1.26 | 6 | |
| Montmorillonite modified starch | 4.2 | 5 | [58] |
| Starch esters | 7.54 | 4–9 | [59] |
| Succinylated starch | 12.36 | 4–7 | |
| Magnetic starch microspheres | 39.98 | - | [60] |
| Amino (5.67–13.01 N%) modified starch | 69.7–139.4 | 6–7 | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-G.; Wang, Q.; Wołowicz, A.; Gładysz-Płaska, A.; Wawrzkiewicz, M.; Sofińska-Chmiel, W.; Lv, G.-Y.; Kołodyńska, D.; Chen, S.-H. Medical Plant Extract Purification from Cadmium(II) Using Modified Thermoplastic Starch and Ion Exchangers. Materials 2021, 14, 4734. https://doi.org/10.3390/ma14164734
Chen Y-G, Wang Q, Wołowicz A, Gładysz-Płaska A, Wawrzkiewicz M, Sofińska-Chmiel W, Lv G-Y, Kołodyńska D, Chen S-H. Medical Plant Extract Purification from Cadmium(II) Using Modified Thermoplastic Starch and Ion Exchangers. Materials. 2021; 14(16):4734. https://doi.org/10.3390/ma14164734
Chicago/Turabian StyleChen, Yi-Gong, Qian Wang, Anna Wołowicz, Agnieszka Gładysz-Płaska, Monika Wawrzkiewicz, Weronika Sofińska-Chmiel, Gui-Yuan Lv, Dorota Kołodyńska, and Su-Hong Chen. 2021. "Medical Plant Extract Purification from Cadmium(II) Using Modified Thermoplastic Starch and Ion Exchangers" Materials 14, no. 16: 4734. https://doi.org/10.3390/ma14164734
APA StyleChen, Y.-G., Wang, Q., Wołowicz, A., Gładysz-Płaska, A., Wawrzkiewicz, M., Sofińska-Chmiel, W., Lv, G.-Y., Kołodyńska, D., & Chen, S.-H. (2021). Medical Plant Extract Purification from Cadmium(II) Using Modified Thermoplastic Starch and Ion Exchangers. Materials, 14(16), 4734. https://doi.org/10.3390/ma14164734










