Efficiency Boost in Dye-Sensitized Solar Cells by Post- Annealing UV-Ozone Treatment of TiO2 Mesoporous Layer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Dye-Sensitized Solar Cells Preparation
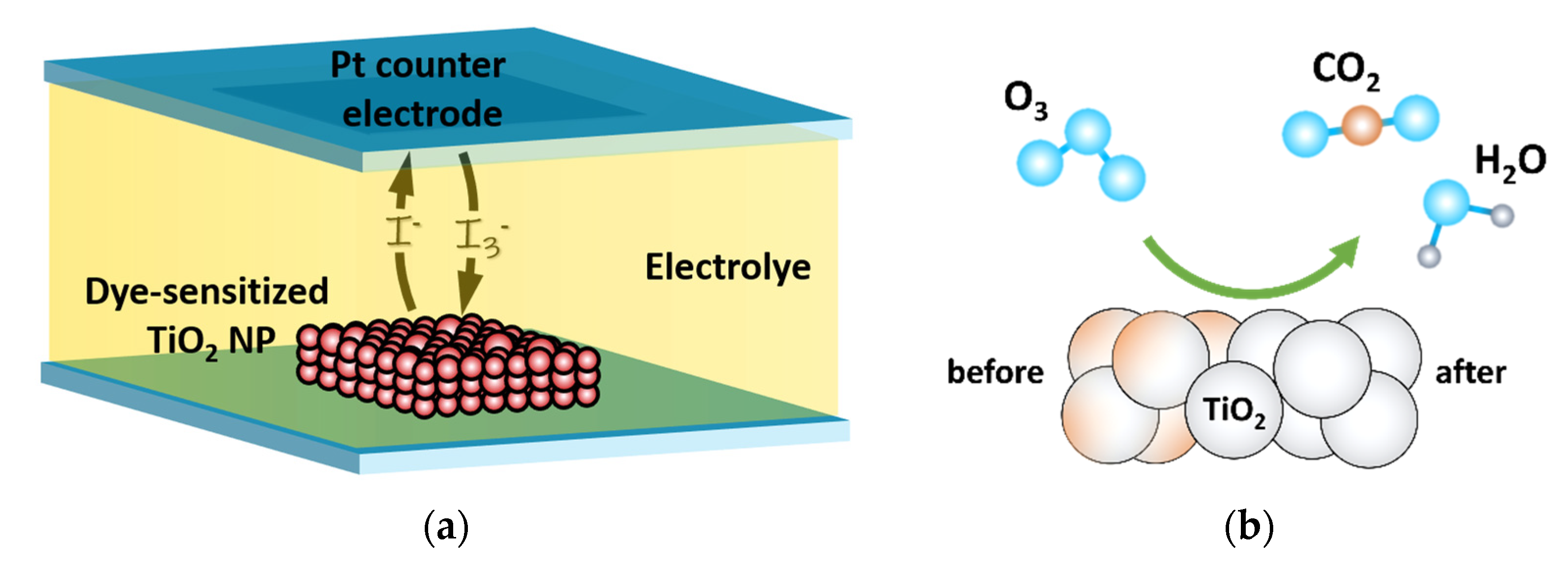
3. Results and Discussion
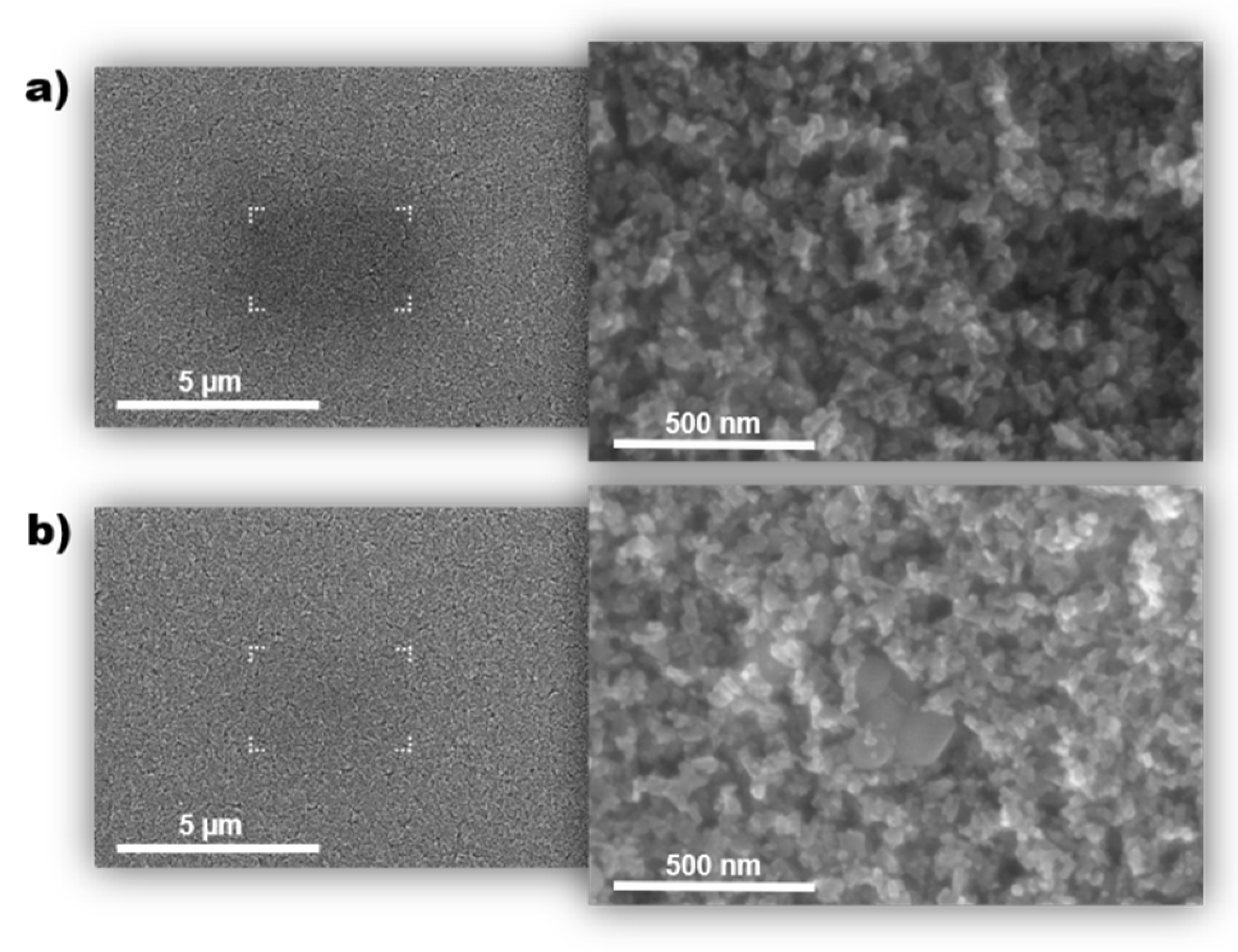
3.1. Contamination Effect in Uncleaned TiO2 Layer
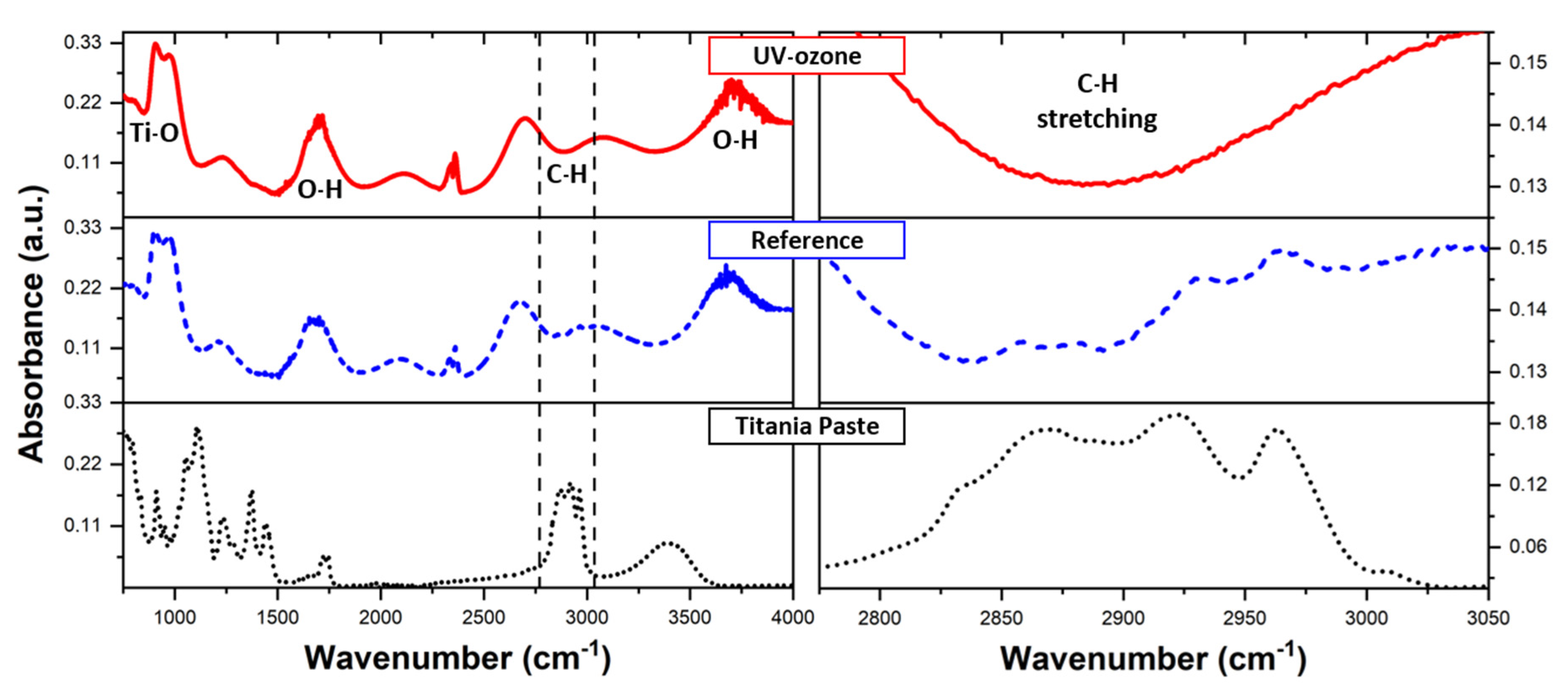
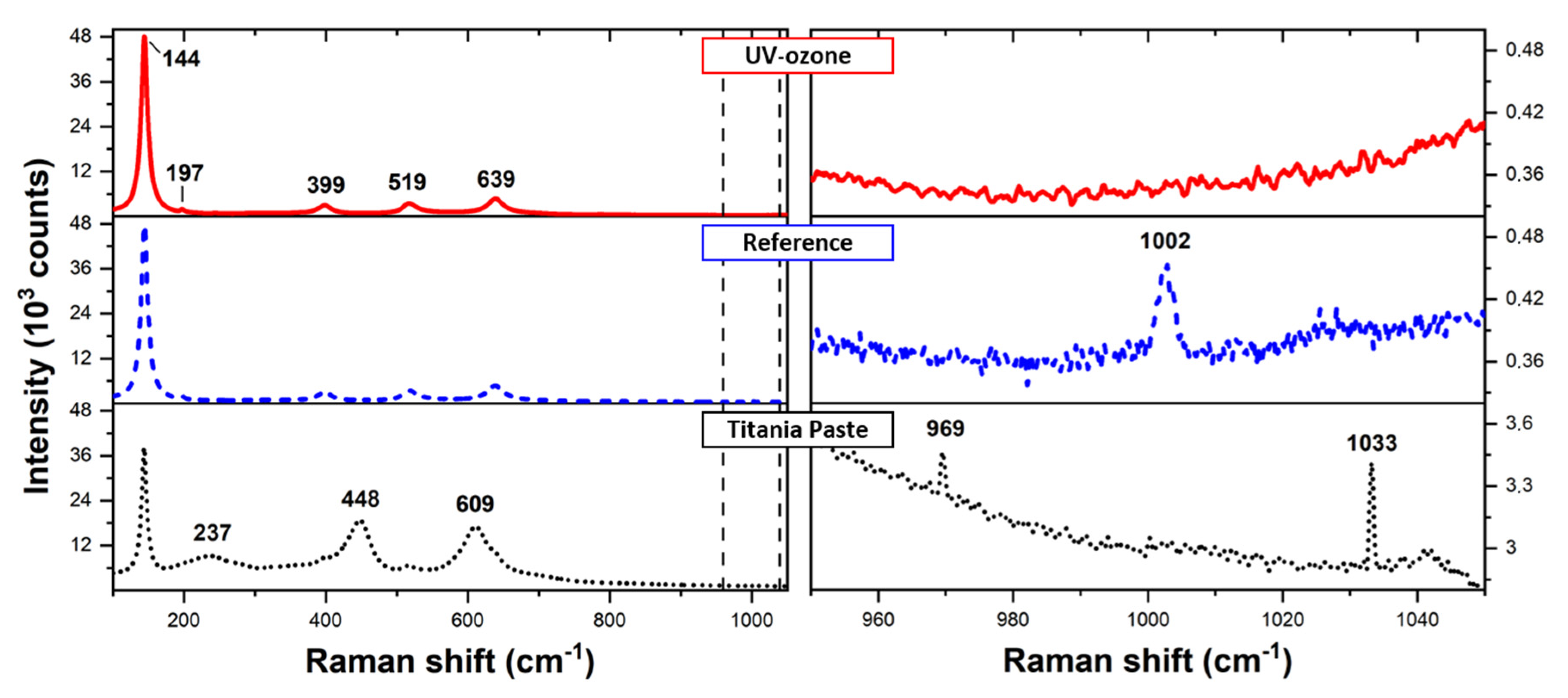
3.2. Determination of Organic Residuals Presence on TiO2
3.3. The Influence of Ozone Treatment on TiO2
3.4. Improving the Dye-Sensitizing Efficiency
3.5. Performance of DSSC Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M.; Gr, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Peter, L.M. The Grätzel Cell: Where Next? J. Phys. Chem. Lett. 2011, 2, 1861–1867. [Google Scholar] [CrossRef]
- O’Regan, B.C.; Durrant, J.R.; Sommeling, P.M.; Bakker, N.J. Influence of the TiCl4 Treatment on Nanocrystalline TiO2 Films in Dye-Sensitized Solar Cells. 2. Charge Density, Band Edge Shifts, and Quantification of Recombination Losses at Short Circuit. J. Phys. Chem. C 2007, 111, 14001–14010. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xu, Q.; Xu, J.; Wu, B.; Gong, M.; Chu, J.; Xiong, S. A Low-Cost Quasi-Solid DSSC Assembled with PVDF-Based Gel Electrolyte Plasticized by PC-EC & Electrodeposited Pt Counter Electrode. J. Photochem. Photobiol. A Chem. 2015, 311, 112–117. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Augustowski, D.; Dziedzic, J.; Kwaśnicki, P.; Malej, W.; Iwan, A. Preparation and Characterization of Novel Polymer-Based Gel Electrolyte for Dye-Sensitized Solar Cells Based on Poly(Vinylidene Fluoride-Cohexafluoropropylene) and Poly(Acrylonitrile-Cobutadiene) or Poly(Dimethylsiloxane) Bis(3-Aminopropyl) Copolymers. Materials 2020, 13, 2721. [Google Scholar] [CrossRef] [PubMed]
- Tinguely, J.C.; Solarska, R.; Braun, A.; Graule, T. Low-Temperature Roll-to-Roll Coating Procedure of Dye-Sensitized Solar Cell Photoelectrodes on Flexible Polymer-Based Substrates. Semicond. Sci. Technol. 2011, 26, 045007. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Pathan, H.M.; Salunke-Gawali, S.; Butcher, R.J. Aminonaphthoquinones as Photosensitizers for Mesoporous ZnO Based Dye-Sensitized Solar Cells. J. Alloys Compd. 2020, 845. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Tao, X.; Wang, L.-X.; Xu, H.; Hou, Q.; Zhou, W.-L.; Chen, J.-F. Novel ZnO-Based Film with Double Light-Scattering Layers as Photoelectrodes for Enhanced Efficiency in Dye-Sensitized Solar Cells. Chem. Mater. 2010, 22, 928–934. [Google Scholar] [CrossRef]
- Bonomo, M.; Di Girolamo, D.; Piccinni, M.; Dowling, D.P.; Dini, D. Electrochemically Deposited NiO Films as a Blocking Layer in P-Type Dye-Sensitized Solar Cells with an Impressive 45% Fill Factor. Nanomaterials 2020, 10, 167. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, F.; Huang, Q.-L.; Zhou, G.; Wang, Z.-S. Dye-Sensitized W-Doped TiO2 Solar Cells with a Tunable Conduction Band and Suppressed Charge Recombination. J. Phys. Chem. C 2011, 115, 18. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Akhtar, M.S.; Mohamed, I.M.A.; Hamdan, R.; Dakka, Y.A.; Barakat, N.A.M. Demonstrated Photons to Electron Activity of S-Doped TiO2 Nanofibers as Photoanode in the DSSC. Mater. Lett. 2018, 225, 77–81. [Google Scholar] [CrossRef]
- Dahlan, D.; Md Saad, S.K.; Berli, A.U.; Bajili, A.; Umar, A.A. Synthesis of Two-Dimensional Nanowall of Cu-Doped TiO2 and Its Application as Photoanode in DSSCs. Phys. E Low-Dimens. Syst. Nanostructures 2017, 91, 185–189. [Google Scholar] [CrossRef]
- Yun, S.; Hagfeldt, A.; Ma, T. Pt-Free Counter Electrode for Dye-Sensitized Solar Cells with High Efficiency. Adv. Mater. 2014, 26, 6210–6237. [Google Scholar] [CrossRef]
- Murakami, T.N.; Ito, S.; Wang, Q.; Nazeeruddin, M.K.; Bessho, T.; Cesar, I.; Liska, P.; Humphry-Baker, R.; Comte, P.; Péchy, P.; et al. Highly Efficient Dye-Sensitized Solar Cells Based on Carbon Black Counter Electrodes. J. Electrochem. Soc. 2006, 153, 2255–2261. [Google Scholar] [CrossRef]
- Hussain, S.; Shaikh, S.F.; Vikraman, D.; Mane, R.S.; Joo, O.S.; Naushad, M.; Jung, J. Sputtering and Sulfurization-Combined Synthesis of a Transparent WS2 Counter Electrode and Its Application to Dye-Sensitized Solar Cells. RSC Adv. 2015, 5, 103567–103572. [Google Scholar] [CrossRef]
- Li, L.; Sui, H.; Zhao, K.; Zhang, W.; Li, X.; Liu, S.; Yang, K.; Wu, M.; Zhang, Y. Preparation of Carbon Nanofibers Supported MoO2 Composites Electrode Materials for Application in Dye-Sensitized Solar Cells. Electrochim. Acta 2018, 259, 188–195. [Google Scholar] [CrossRef]
- Law, C.; Pathirana, S.C.; Li, X.; Anderson, A.Y.; Barnes, P.R.F.; Listorti, A.; Ghaddar, T.H.; O′Regan, B.C. Water-Based Electrolytes for Dye-Sensitized Solar Cells. Adv. Mater. 2010, 22, 4505–4509. [Google Scholar] [CrossRef] [PubMed]
- Su’ait, M.S.; Rahman, M.Y.A.; Ahmad, A. Review on Polymer Electrolyte in Dye-Sensitized Solar Cells (DSSCs). Sol. Energy 2015, 115, 452–470. [Google Scholar] [CrossRef]
- Manafi, P.; Nazockdast, H.; Karimi, M.; Sadighi, M.; Magagnin, L. Microstructural Development and Rheological Study of a Nanocomposite Gel Polymer Electrolyte Based on Functionalized Graphene for Dye-Sensitized Solar Cells. Polymers 2020, 12, 1443. [Google Scholar] [CrossRef]
- Chou, C.S.; Guo, M.G.; Liu, K.H.; Chen, Y.S. Preparation of TiO2 Particles and Their Applications in the Light Scattering Layer of a Dye-Sensitized Solar Cell. Appl. Energy 2012, 92, 224–233. [Google Scholar] [CrossRef]
- Zhu, K.; Schiff, E.A.; Park, N.G.; Van De Lagemaat, J.; Frank, A.J. Determining the Locus for Photocarrier Recombination in Dye-Sensitized Solar Cells. Appl. Phys. Lett. 2002, 80, 685–687. [Google Scholar] [CrossRef][Green Version]
- Ramasamy, P.; Kang, M.S.; Cha, H.J.; Kim, J. Highly Efficient Dye-Sensitized Solar Cells Based on HfO2 Modified TiO2 Electrodes. Mater. Res. Bull. 2013, 48, 79–83. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, X.; Chen, S.; Lin, L.; Zhang, B.; Feng, Y. Performance Improvement of Dye-Sensitized Solar Cells by Introducing a Hierarchical Compact Layer Involving ZnO and TiO2 Blocking Films. Ceram. Int. 2014, 40, 15199–15206. [Google Scholar] [CrossRef]
- Shaban, Z.; Ara, M.H.M.; Falahatdoost, S.; Ghazyani, N. Optimization of ZnO Thin Film through Spray Pyrolysis Technique and Its Application as a Blocking Layer to Improving Dye Sensitized Solar Cell Efficiency. Curr. Appl. Phys. 2016, 16, 131–134. [Google Scholar] [CrossRef]
- Augustowski, D.; Kwaśnicki, P.; Dziedzic, J.; Rysz, J. Magnetron Sputtered Electron Blocking Layer as an Efficient Method to Improve Dye-Sensitized Solar Cell Performance. Energies 2020, 13, 2690. [Google Scholar] [CrossRef]
- Taguchi, T.; Zhang, X.-T.; Sutanto, I.; Tokuhiro, K.-I.; Rao, T.N.; Watanabe, H.; Nakamori, T.; Uragami, M.; Fujishima, A. Improving the Performance of Solid-State Dye-Sensitized Solar Cell Using MgO-Coated TiO2 Nanoporous Film. Chem. Commun. 2003, 2480–2481. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Baroughi, M.F.; Galipeau, D. Effect of Atomic Layer Deposited Ultra Thin HfO2 and Al2O3 Interfacial Layers on the Performance of Dye Sensitized Solar Cells. Thin Solid Film. 2010, 518, 2678–2682. [Google Scholar] [CrossRef]
- Deepa, K.G.; Lekha, P.; Sindhu, S. Efficiency enhancement in DSSC using metal nanoparticles: A size dependent study. Sol. Energy 2012, 86, 326–330. [Google Scholar] [CrossRef]
- Shah, S.; Noor, I.M.; Pitawala, J.; Albinson, I.; Bandara, T.M.W.J.; Mellander, B.-E.; Arof, A.K. Plasmonic Effects of Quantum Size Metal Nanoparticles on Dye-Sensitized Solar Cell. Opt. Mater. Express 2017, 7, 2069. [Google Scholar] [CrossRef]
- Lim, S.P.; Pandikumar, A.; Lim, H.N.; Ramaraj, R.; Huang, N.M. Boosting Photovoltaic Performance of Dye-Sensitized Solar Cells Using Silver Nanoparticle-Decorated N,S-Co-Doped-TiO2 Photoanode OPEN. Nat. Publ. Gr. 2015, 5, 11922. [Google Scholar] [CrossRef]
- Saeed, M.A.; Kang, H.C.; Yoo, K.; Asiam, F.K.; Lee, J.J.; Shim, J.W. Cosensitization of Metal-Based Dyes for High-Performance Dye-Sensitized Photovoltaics under Ambient Lighting Conditions. Dye. Pigment. 2021, 194, 109624. [Google Scholar] [CrossRef]
- Senadeera, G.K.R.; Balasundaram, D.; Dissanayake, M.A.K.L.; Karunaratne, B.A.; Weerasinghe, J.S.; Thotawatthage, C.A.; Jaseetharan, T.; Kumari, K.W.; Jayathilaka, D.L.N. Efficiency Enhancement in Dye-Sensitized Solar Cells with Co-Sensitized, Triple Layered Photoanode by Enhanced Light Scattering and Spectral Responses. Bull. Mater. Sci. 2021, 44, 68. [Google Scholar] [CrossRef]
- Buhbut, S.; Clifford, J.N.; Kosa, M.; Anderson, A.Y.; Shalom, M.; Major, D.T.; Palomares, E.; Zaban, A. Controlling Dye Aggregation, Injection Energetics and Catalytic Recombination in Organic Sensitizer Based Dye Cells Using a Single Electrolyte Additive. Energy Environ. Sci. 2013, 6, 3046–3053. [Google Scholar] [CrossRef]
- Zama, I.; Martelli, C.; Gorni, G. Preparation of TiO2 Paste Starting from Organic Colloidal Suspension for Semi-Transparent DSSC Photo-Anode Application. Mater. Sci. Semicond. Process. 2017, 61, 137–144. [Google Scholar] [CrossRef]
- Park, K.-H.; Dhayal, M. High efficiency solar cell based on dye sensitized plasma treated nano-structured TiO2 films. Electrochem. Commun. 2009, 11, 75–79. [Google Scholar] [CrossRef]
- Dawo, C.; Afroz, M.A.; Iyer, P.K.; Chaturvedi, H. Effect of UV-Ozone Exposure on the Dye-Sensitized Solar Cells Performance. Sol. Energy 2020, 208, 212–219. [Google Scholar] [CrossRef]
- Saekow, S.; Maiakgree, W.; Jarernboon, W.; Pimanpang, S.; Amornkitbamrung, V. High Intensity UV Radiation Ozone Treatment of Nanocrystalline TiO2 Layers for High Efficiency of Dye-Sensitized Solar Cells. J. Non-Cryst. Solids 2012, 358, 2496–2500. [Google Scholar] [CrossRef]
- Lin, Y.H.; Weng, C.H.; Srivastav, A.L.; Lin, Y.T.; Tzeng, J.H. Facile Synthesis and Characterization of N-Doped TiO2 Photocatalyst and Its Visible-Light Activity for Photo-Oxidation of Ethylene. J. Nanomater. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Hitachi High-Tech Corporation. ZONE II for SEM: Tabletop Specimen Cleaner; Hitachi High-Tech Corporation: Tokyo, Japan, 2017. [Google Scholar]
- Chaichi, A.; Prasad, A.; Gartia, M.R. Raman Spectroscopy and Microscopy Applications in Cardiovascular Diseases: From Molecules to Organs. Biosensors 2018, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.; Dey, S.C.; Rashid, T.U.; Ashaduzzaman, M.; Shamsuddin, S.M. Solar Assisted Photocatalytic Degradation of Reactive Azo Dyes in Presence of Anatase Titanium Dioxide. Int. J. Latest Res. Eng. Technol. (IJLRET) 2016, 2, 14–21. [Google Scholar]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Kuś, P.M.; Jerković, I. Mediterranean Propolis from the Adriatic Sea Islands as a Source of Natural Antioxidants: Comprehensive Chemical Biodiversity Determined by GC-MS, Ftiratr, UHPLC-DAD-QQTOF-MS, DPPH and FRAP Assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Sahu, B.B.; Shin, K.S.; Han, J.G. Integrated Approach for Low-Temperature Synthesis of High-Quality Silicon Nitride Films in PECVD Using RF–UHF Hybrid Plasmas. Plasma Sources Sci. Technol. 2016, 25, 015017. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Borodin, A.; Reichling, M. Characterizing TiO2 Surface States by Their Work Function. Phys. Chem. Chem. Phys. 2011, 13, 15442–15447. [Google Scholar] [CrossRef]
- Kashiwaya, S.; Morasch, J.; Streibel, V.; Toupance, T.; Jaegermann, W.; Klein, A. The Work Function of TiO2. Surfaces 2018, 1, 7. [Google Scholar] [CrossRef]
- Nam, J.; Kim, J.H.; Kim, C.S.; Kwon, J.-D.; Jo, S. Surface Engineering of Low-Temperature Processed Mesoporous TiO2 via Oxygen Plasma for Flexible Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 12648–12655. [Google Scholar] [CrossRef]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced Charge-Collection Efficiencies and Light Scattering in Dye-Sensitized Solar Cells Using Oriented TiO2 Nanotubes Arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef]
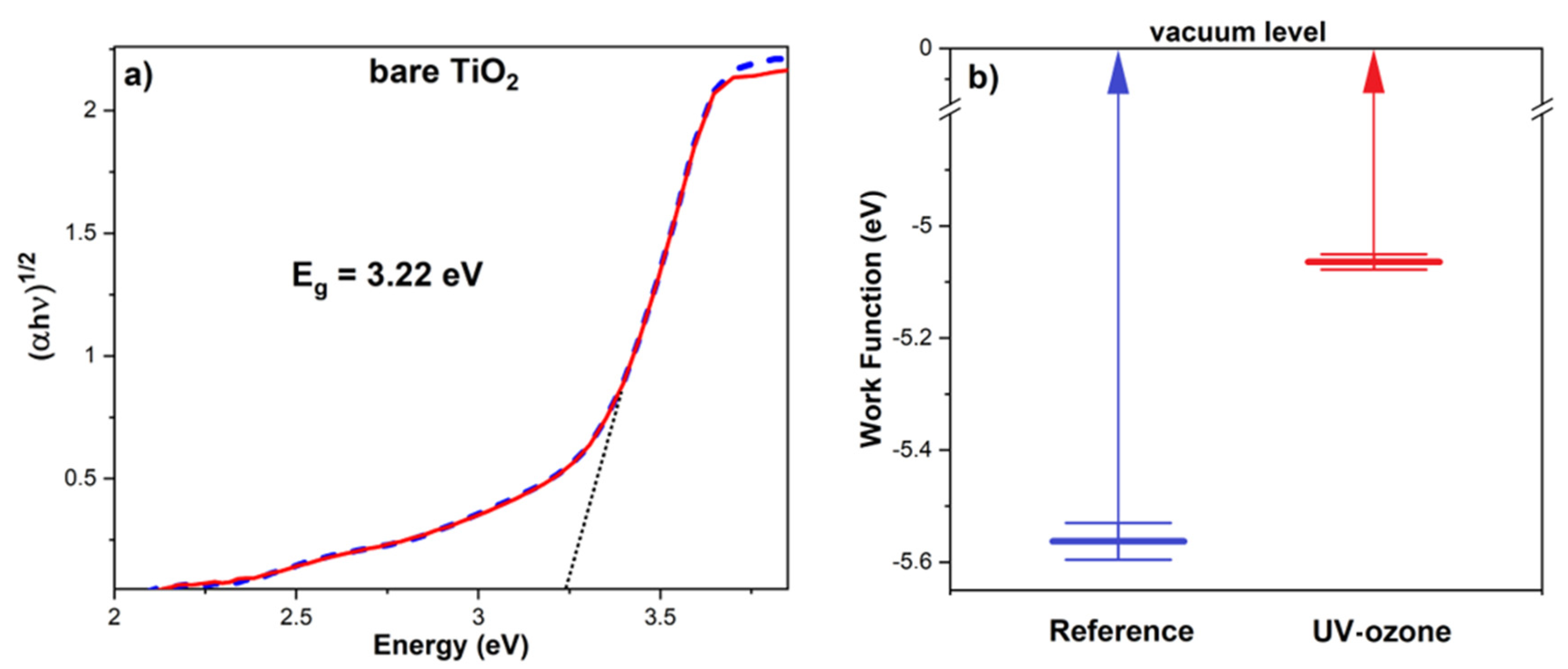
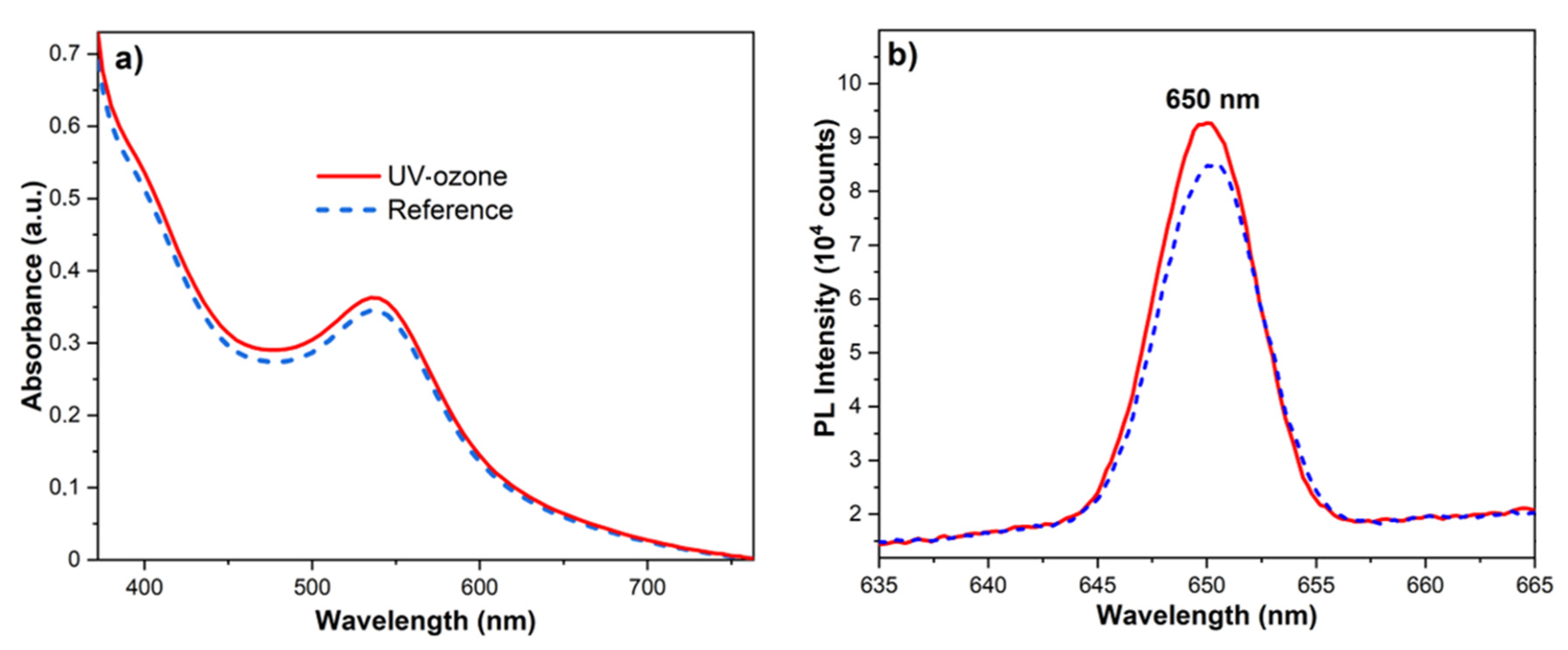
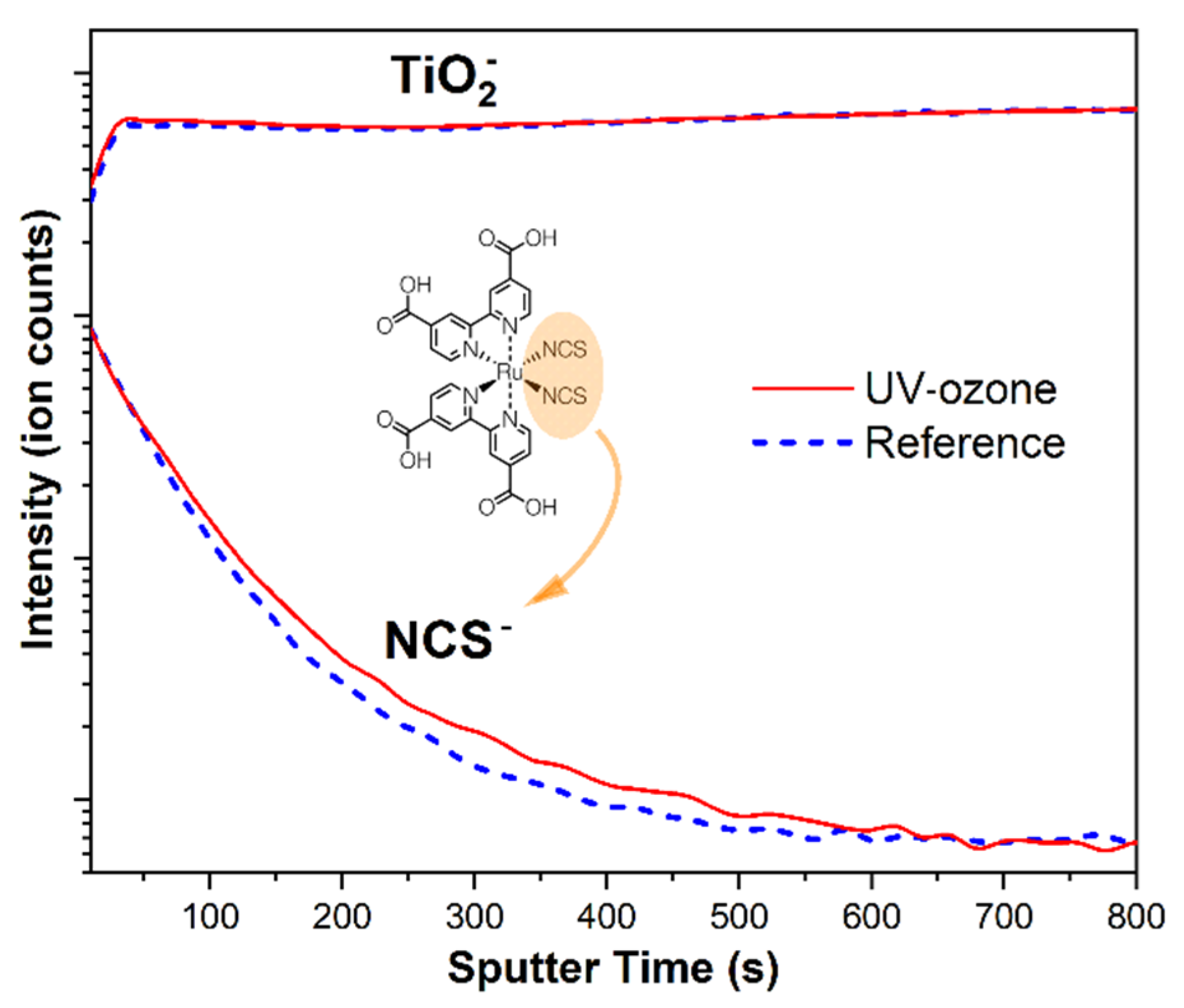
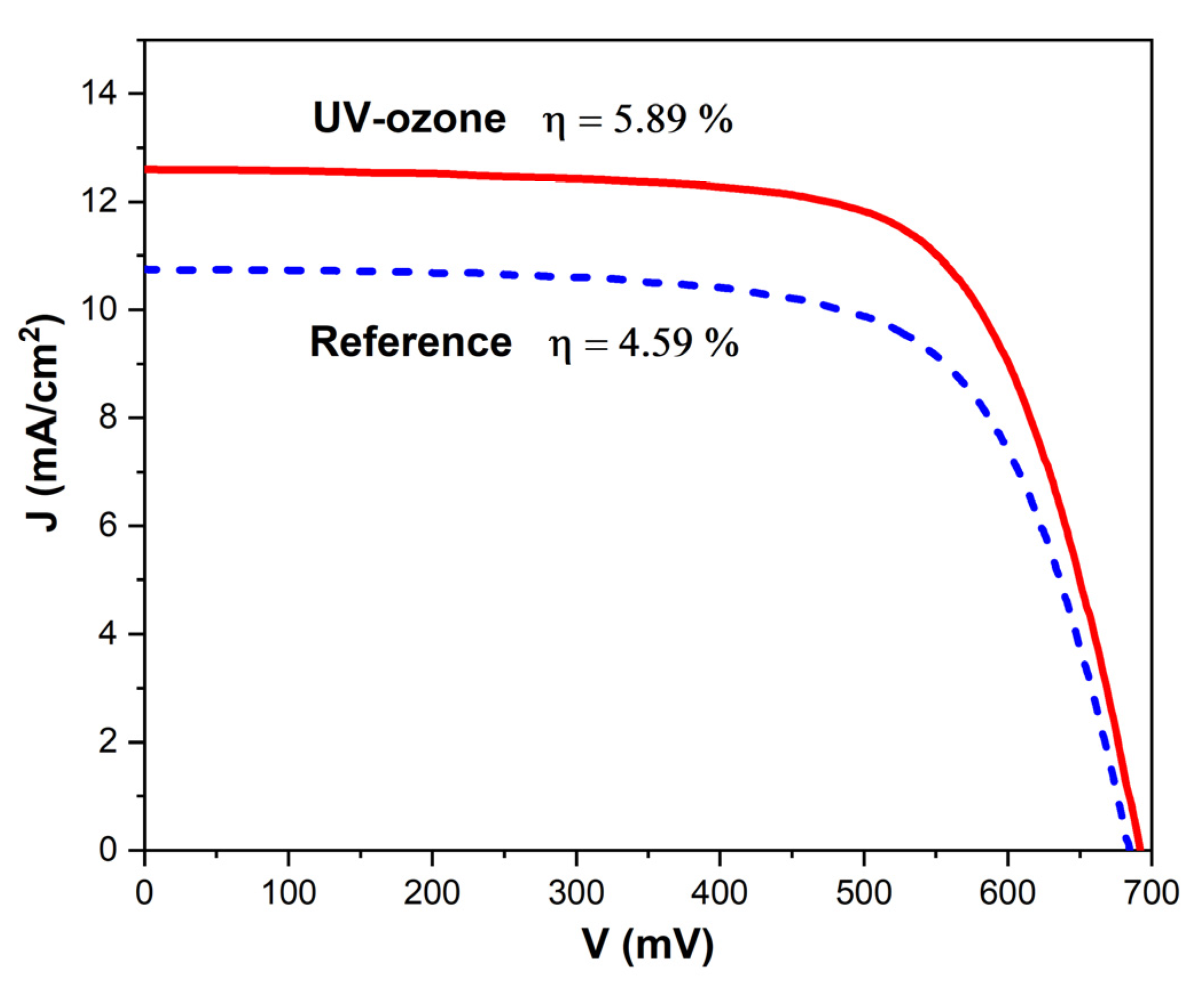

| Sample | Voc (mV) | Jsc (mA/cm2) | FF | PCE (%) | Rs (Ω) | Rsh (kΩ) |
|---|---|---|---|---|---|---|
| Reference | 667 ± 10 | 10.3 ± 1.0 | 0.67 ± 0.01 | 4.59 ± 0.45 | 58.1 ± 4.3 | 22.0 ± 5.3 |
| UV-ozone | 679 ± 10 | 12.3 ± 0.7 | 0.70 ± 0.01 | 5.89 ± 0.38 | 49.1 ± 3.9 | 39.0 ± 4.8 |
| Sample | fIMVS (Hz) | τrec (ms) | fIMPS (Hz) | τtr (ms) |
|---|---|---|---|---|
| Reference | 9.6 ± 0.5 | 16.6 ± 0.8 | 13.3 ± 0.2 | 12.0 ± 0.9 |
| UV-ozone | 21.9 ± 0.3 | 7.3 ± 0.3 | 18.5 ± 0.7 | 8.6 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augustowski, D.; Gala, M.; Kwaśnicki, P.; Rysz, J. Efficiency Boost in Dye-Sensitized Solar Cells by Post- Annealing UV-Ozone Treatment of TiO2 Mesoporous Layer. Materials 2021, 14, 4698. https://doi.org/10.3390/ma14164698
Augustowski D, Gala M, Kwaśnicki P, Rysz J. Efficiency Boost in Dye-Sensitized Solar Cells by Post- Annealing UV-Ozone Treatment of TiO2 Mesoporous Layer. Materials. 2021; 14(16):4698. https://doi.org/10.3390/ma14164698
Chicago/Turabian StyleAugustowski, Dariusz, Maciej Gala, Paweł Kwaśnicki, and Jakub Rysz. 2021. "Efficiency Boost in Dye-Sensitized Solar Cells by Post- Annealing UV-Ozone Treatment of TiO2 Mesoporous Layer" Materials 14, no. 16: 4698. https://doi.org/10.3390/ma14164698
APA StyleAugustowski, D., Gala, M., Kwaśnicki, P., & Rysz, J. (2021). Efficiency Boost in Dye-Sensitized Solar Cells by Post- Annealing UV-Ozone Treatment of TiO2 Mesoporous Layer. Materials, 14(16), 4698. https://doi.org/10.3390/ma14164698







