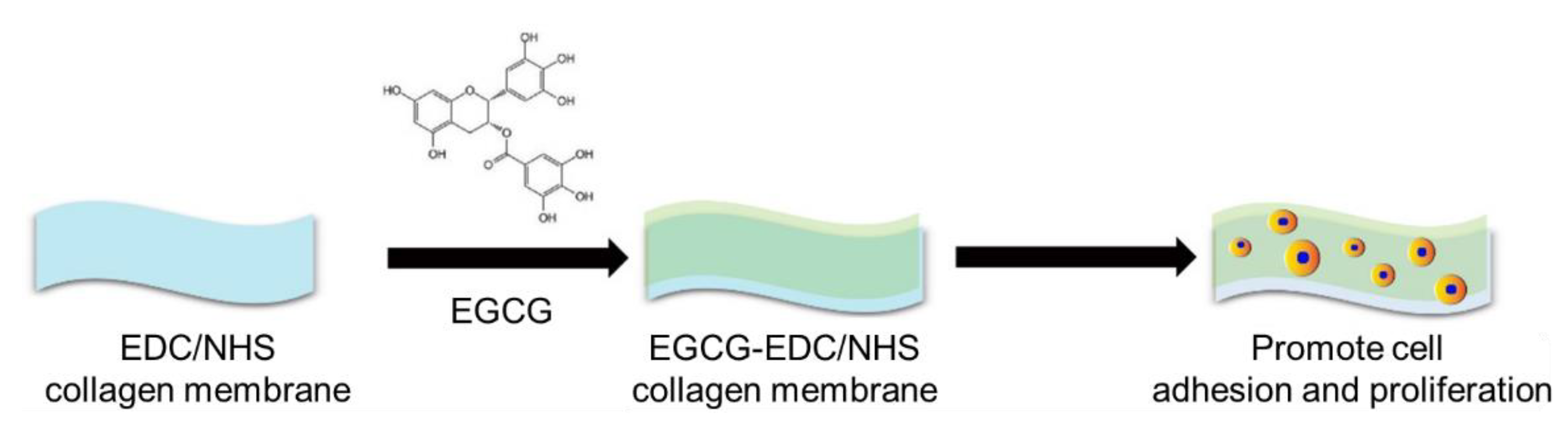
Application of Epigallocatechin-3-gallate (EGCG) Modified 1-Ethyl-3-(3-dimethylaminopropylcarbodiimide hydrochloride/N-hydroxy-succinimide (EDC/NHS) Cross-Linked Collagen Membrane to Promote Macrophage Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
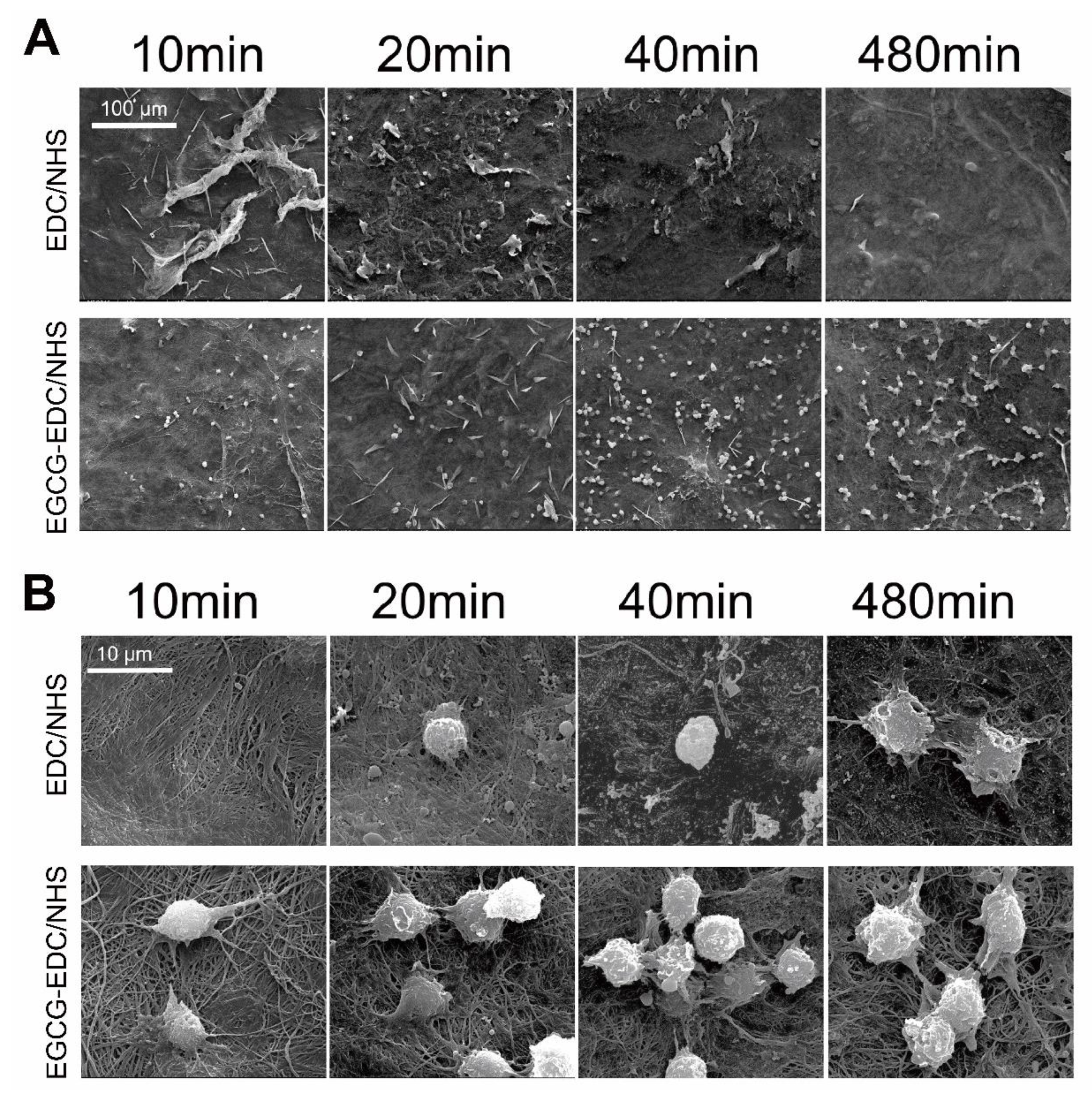
2.2. Surface Morphology
2.3. Mechanical and Chemical Properties
- a.
- Mechanical properties measurement
- b.
- Chemical properties measurement
- c.
- Release profile of EGCG-EDC/NHS-Col in vitro
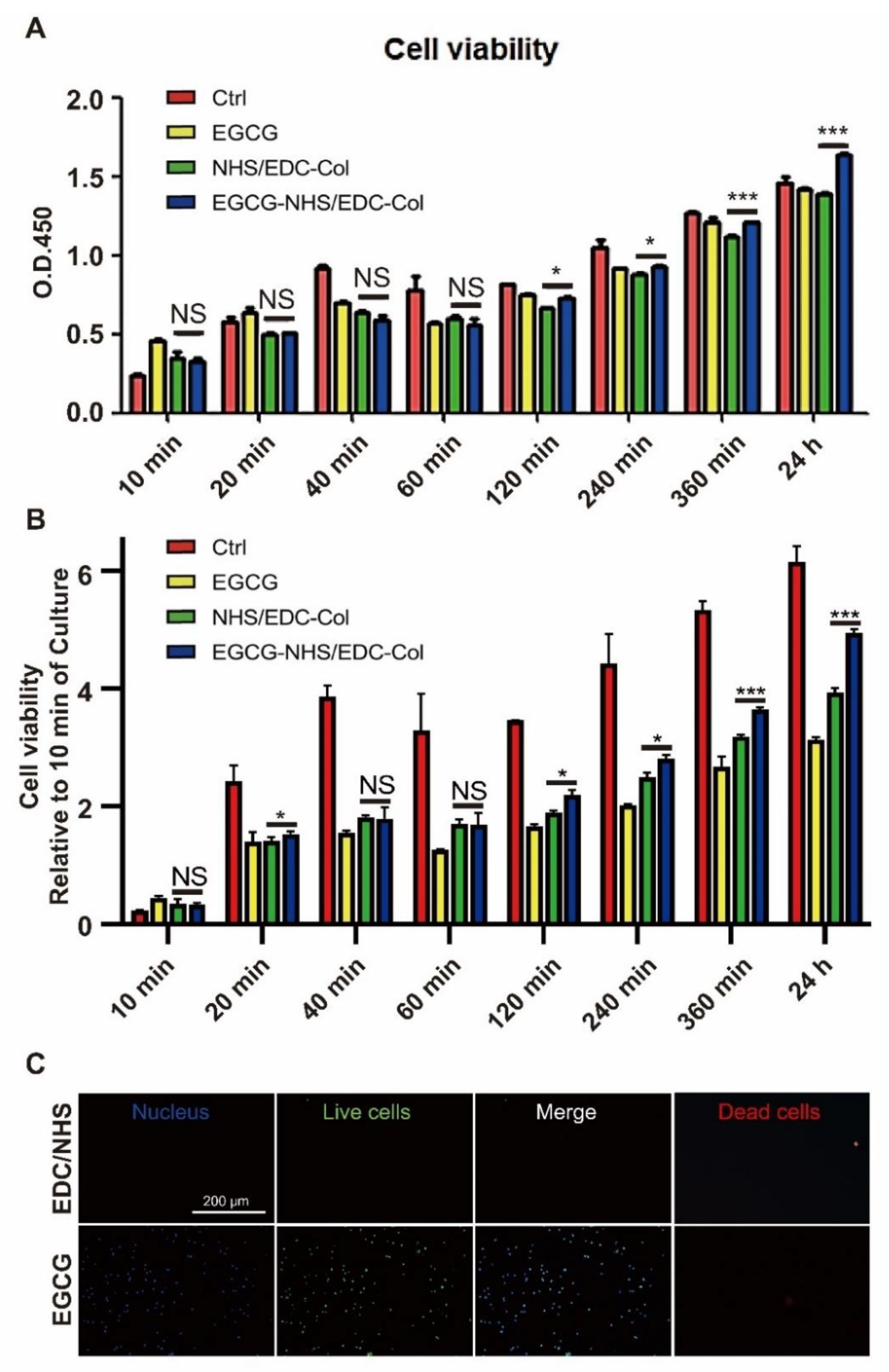
2.4. Cell Viability
2.5. Cell Adhesion
2.6. Surgical Procedures
2.7. HE Staining
- (a)
- Dehydration with ethanol;
- (b)
- Stain for 5 min using hematoxylin and differentiate for 2 s in 1% hydrochloric acid alcohol;
- (c)
- Incubate for 2 min in 0.2% ammonia water and stain for 1 min using eosin;
- (d)
- Gradually dehydrate using 95% ethanol, then clear and mount the sections with neutral resin. These sections were observed with light microscopy (Olympus, Tokyo, Japan) and scanned by the Digital Slide Scanning System (PRECICE, Beijing, China).
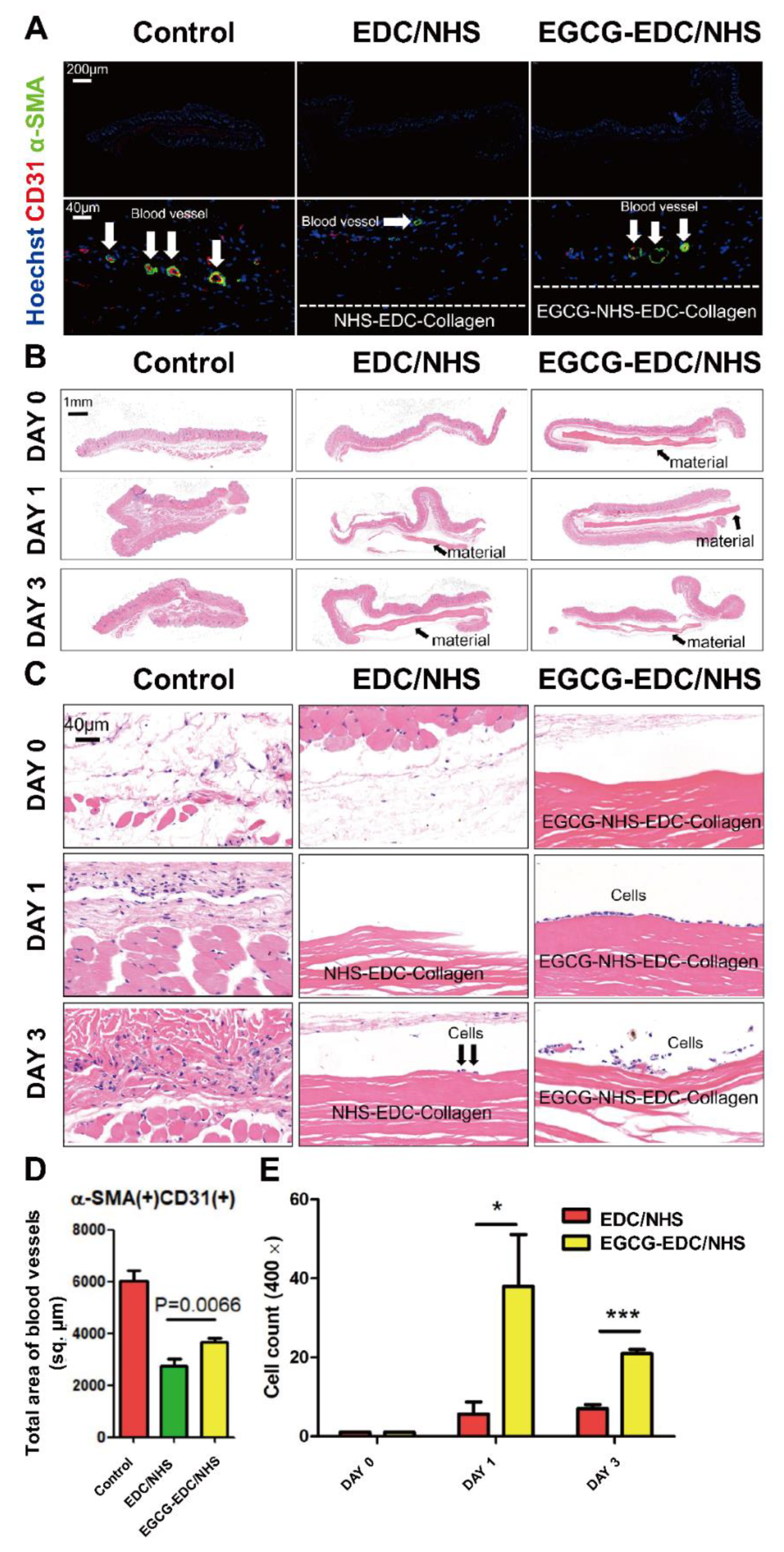
2.8. Promotion of Revascularization
2.9. Statistical Analysis
3. Results
3.1. Surface Morphology
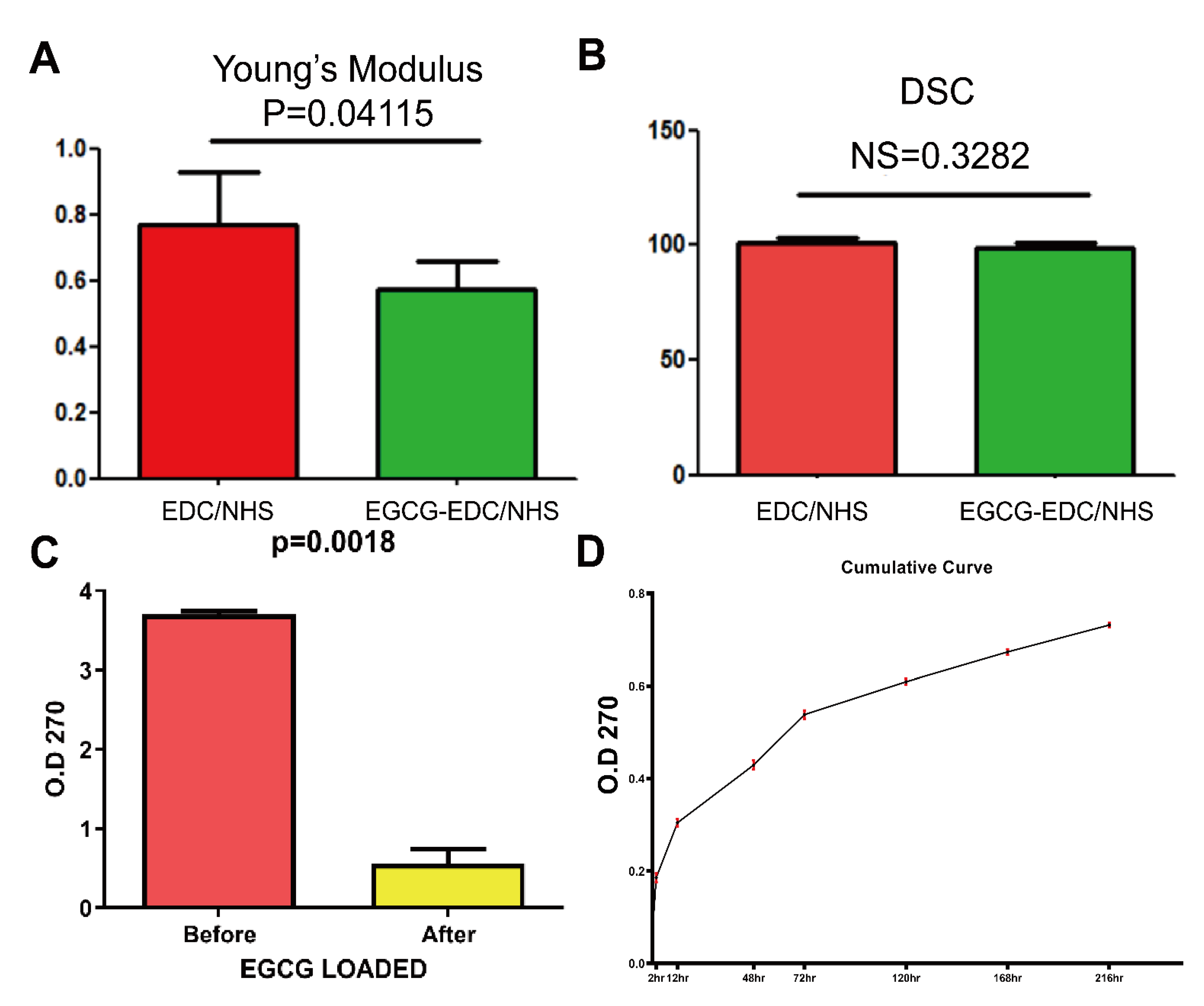
3.2. Mechanical and Chemical Properties
3.3. Cell Viability
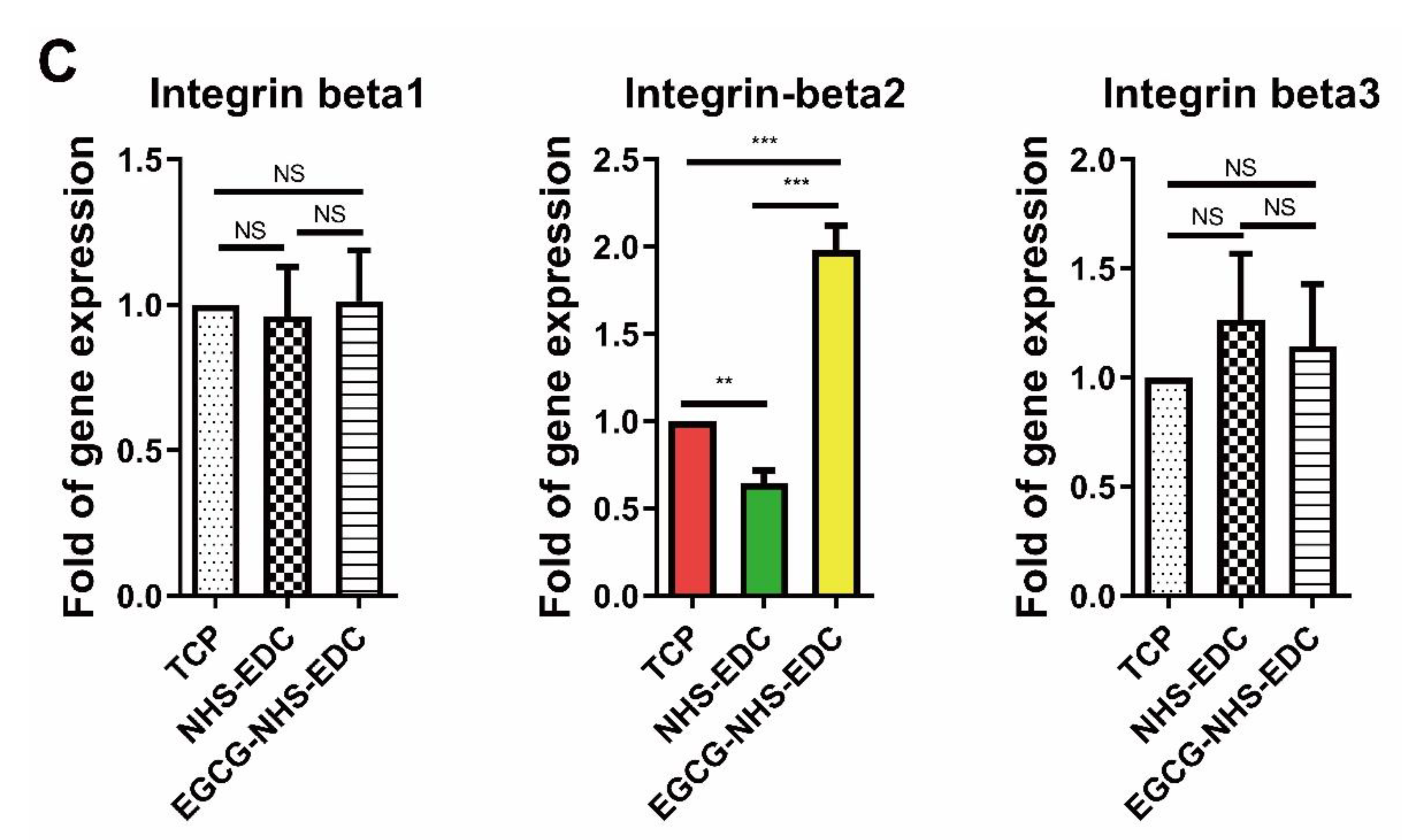
3.4. Cell Adhesion
3.5. Promotion of Revascularization
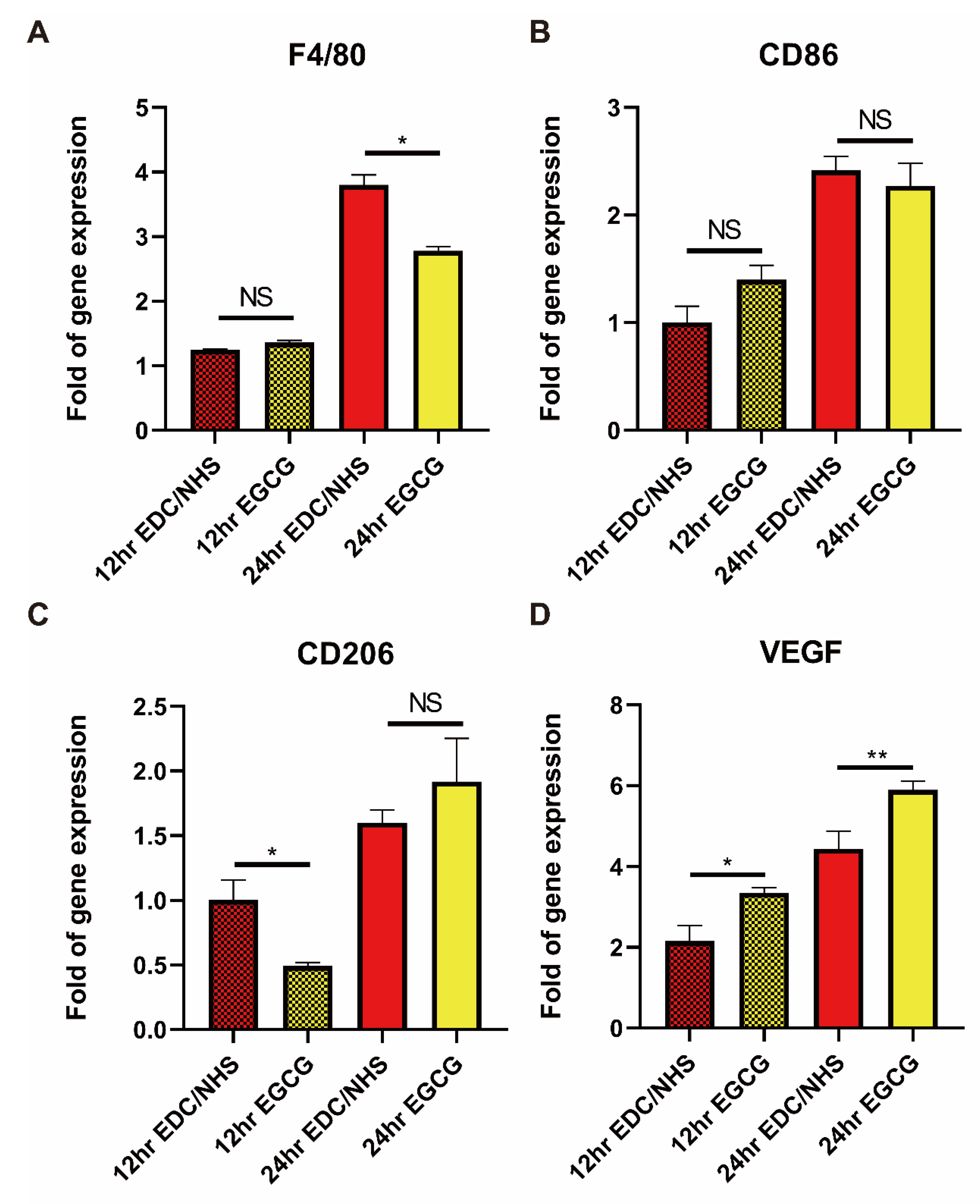
3.6. Recruitment of Monocyte/Macrophage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melcher, A.H. On the Repair Potential of Periodontal Tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef] [Green Version]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Chu, C.; Zhao, X.; Rung, S.; Xiao, W.; Liu, L.; Qu, Y.; Man, Y. Application of biomaterials in periodontal tissue repair and reconstruction in the presence of inflammation under periodontitis through the foreign body response: Recent progress and perspectives. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021. [Google Scholar] [CrossRef] [PubMed]
- El-Jawhari, J.; Jones, E.; Giannoudis, P.V. The roles of immune cells in bone healing; what we know, do not know and future perspectives. Injury 2016, 47, 2399–2406. [Google Scholar] [CrossRef]
- Fang, J.; Liu, R.; Chen, S.; Liu, Q.; Cai, H.; Lin, Y.; Chen, Z.; Chen, Z. Tuning the immune reaction to manipulate the cell-mediated degradation of a collagen barrier membrane. Acta Biomater. 2020, 109, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.; Santiago-Rodriguez, T.M.; Boehm, T.K.; Pride, D.T. Bacteriophage and their potential roles in the human oral cavity. J. Oral Microbiol. 2015, 7, 27423. [Google Scholar] [CrossRef] [PubMed]
- Salmond, G.P.C.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Hu, C.; Chu, C.; Liu, L.; Wang, C.; Jin, S.; Yang, R.; Rung, S.; Li, J.; Qu, Y.; Man, Y. Dissecting the microenvironment around biosynthetic scaffolds in murine skin wound healing. Sci. Adv. 2021, 7, eabf0787. [Google Scholar] [CrossRef]
- Chu, C.; Wang, Y.; Wang, Y.; Yang, R.; Liu, L.; Rung, S.; Xiang, L.; Wu, Y.; Du, S.; Man, Y.; et al. Evaluation of epigallocatechin-3-gallate (EGCG) modified collagen in guided bone regeneration (GBR) surgery and modulation of macrophage phenotype. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 73–82. [Google Scholar] [CrossRef]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Tang, Y.; Chu, C.; Lu, W.; Baligen, B.; Man, Y.; Qu, Y. Application of green tea extracts epigallocatechin-3-gallate in dental materials: Recent progress and perspectives. J. Biomed. Mater. Res. Part A 2020, 108, 2395–2408. [Google Scholar] [CrossRef]
- Wu, Y.R.; Choi, H.J.; Kang, Y.G.; Kim, J.K.; Shin, J.-W. In vitro study on anti-inflammatory effects of epigallocatechin-3-gallate-loaded nano- and microscale particles. Int. J. Nanomed. 2017, 12, 7007–7013. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, G.; Yuan, L.; Yang, Y.; Zhao, H.; Ho, C.-T.; Li, S. Green Tea Catechins Effectively Altered Hepatic Fibrogenesis in Rats by Inhibiting ERK and Smad1/2 Phosphorylation. J. Agric. Food Chem. 2019, 67, 5437–5445. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Kang, L.; Wang, C.-Z.; Huang, H.H.; Cheng, T.-L.; Huang, H.-T.; Lee, M.-J.; Lin, Y.-S.; Ho, M.-L.; Wang, G.-J.; et al. (−)-Epigallocatechin-3-Gallate (EGCG) Enhances Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Molecules 2018, 23, 3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.; Liu, L.; Wang, Y.; Wei, S.; Wang, Y.; Man, Y.; Qu, Y. Macrophage phenotype in the epigallocatechin-3-gallate (EGCG)-modified collagen determines foreign body reaction. J. Tissue Eng. Regen. Med. 2018, 12, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Liu, L.; Rung, S.; Wang, Y.; Ma, Y.; Hu, C.; Zhao, X.; Man, Y.; Qu, Y. Modulation of foreign body reaction and macrophage phenotypes concerning microenvironment. J. Biomed. Mater. Res. Part A 2020, 108, 127–135. [Google Scholar] [CrossRef]
- Chen, Z.; Yuen, J.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated β-tricalcium phosphate. Biomaterials 2015, 61, 126–138. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Xiang, L.; Wu, Y.; Wei, X.; Qu, Y.; Man, Y. Evaluation of epigallocatechin-3-gallate (EGCG) cross-linked collagen membranes and concerns on osteoblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 386–394. [Google Scholar] [CrossRef]
- Changede, R.; Sheetz, M. Integrin and cadherin clusters: A robust way to organize adhesions for cell mechanics. BioEssays 2016, 39, e201600123-12. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-Y.; Samarelli, A.V.; Kammerer, P.; Scholze, S.; Ziegler, T.; Immler, R.; Zent, R.; Sperandio, M.; Sanders, C.R.; Fässler, R.; et al. LCP1 preferentially binds clasped αMβ2 integrin and attenuates leukocyte adhesion under flow. J. Cell Sci. 2018, 131, jcs218214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manetti, M.; Romano, E.; Rosa, I.; Guiducci, S.; Bellando-Randone, S.; De Paulis, A.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.; Shelef, K.M.; Gonzalez, A.; Davis, C.L.; Dethlefsen, L.; Burns, A.R.; Loomer, P.M.; Armitage, G.C.; Ryder, M.I.; Millman, M.E.; et al. Microbial biogeography and ecology of the mouth and implications for periodontal diseases. Periodontology 2000 2020, 82, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Jung, I.-H.; Kim, Y.-K.; Lim, H.-C.; Lee, J.-S.; Jung, U.-W.; Choi, S.-H. Guided bone regeneration using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)-cross-linked type-I collagen membrane with biphasic calcium phosphate at rabbit calvarial defects. Biomater. Res. 2015, 19, 15. [Google Scholar] [CrossRef] [Green Version]
- Grabarek, Z.; Gergely, J. Zero-length crosslinking procedure with the use of active esters. Anal. Biochem. 1990, 185, 131–135. [Google Scholar] [CrossRef]
- Xie, X.; Shi, X.; Wang, S.; Cao, L.; Yang, C.; Ma, Z. Effect of Attapulgite-Doped Electrospun Fibrous PLGA Scaffold on Pro-Osteogenesis and Barrier Function in the Application of Guided Bone Regeneration. Int. J. Nanomed. 2020, 15, 6761–6777. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; Tan, J. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 2020, 7, 233–245. [Google Scholar] [CrossRef]
- Wissing, T.B.; Bonito, V.; Van Haaften, E.E.; Van Doeselaar, M.; Brugmans, M.M.C.P.; Janssen, H.M.; Bouten, C.; Smits, A.I.P.M. Macrophage-Driven Biomaterial Degradation Depends on Scaffold Microarchitecture. Front. Bioeng. Biotechnol. 2019, 7, 87. [Google Scholar] [CrossRef]
- Saino, E.; Focarete, M.L.; Gualandi, C.; Emanuele, E.; Cornaglia, A.I.; Imbriani, M.; Visai, L. Effect of Electrospun Fiber Diameter and Alignment on Macrophage Activation and Secretion of Proinflammatory Cytokines and Chemokines. Biomacromolecules 2011, 12, 1900–1911. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Gordana, V.-N. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, K.L.; Freytes, D.O.; Vunjak-Novakovic, G. Macrophages Modulate Engineered Human Tissues for Enhanced Vascularization and Healing. Ann. Biomed. Eng. 2015, 43, 616–627. [Google Scholar] [CrossRef] [Green Version]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Zin, A.A.M.; Ang, K.C.; Ch’Ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Genes | Oligonucleotide Sequence |
|---|---|
| GAPDH | Forward: TGAAGCAGGCATCTGAGGG Reverse: TGAAGTCGCAGGAGACAACC |
| Integrin b1 | Forward: CCAAGTGGGACACGGGTGA Reverse: CTGCTGCTGTGAGCTTGGTG |
| Integrin b2 | Forward: GCAGCAGAAGGACGGAAACG Reverse: AGGGGGTTGTCGTTGTTCCA |
| Integrin b3 | Forward: AGACAGCGCCCAGATCACTC Reverse: GCCAATCCGAAGGTTGCTGG |
| F4/80 | Forward: ATTCCCGTGTTGTTGGTGG Reverse: TGCTTTGGCTGGATGTGC |
| CD86 | Forward: TGTTTCCGTGGAGACGCAAG Reverse: TTGAGCCTTTGTAAATGGGCA |
| CD206 | Forward: CTCTGTTCAGCTATTGGACGC Reverse: CGGAATTTCTGGGATTCAGCTTC |
| VEGF | Forward: ACACGGGAGACAATGGGATG Reverse: GGCAGGCAAAAGGACTTCG |
| Groups | Ultimate Stress (MPa) | Ultimate Elongation (%) | Young’s Modulus |
|---|---|---|---|
| EDC/NHS-Col | 18.32 ± 3.12 | 22.44 ± 3.12 | 0.77 ± 0.16 |
| EGCG-EDC/NHS-Col | 18.18 ± 2.97 | 29.29 ± 2.78 | 0.57 ± 0.09 |
| Groups | 1235/1450 |
|---|---|
| NHS/EDC-Col | 1.001 ± 0.002 |
| EGCG-NHS/EDC-Col | 0.989 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rung, S.; Zhao, X.; Chu, C.; Yang, R.; Qu, Y.; Man, Y. Application of Epigallocatechin-3-gallate (EGCG) Modified 1-Ethyl-3-(3-dimethylaminopropylcarbodiimide hydrochloride/N-hydroxy-succinimide (EDC/NHS) Cross-Linked Collagen Membrane to Promote Macrophage Adhesion. Materials 2021, 14, 4660. https://doi.org/10.3390/ma14164660
Rung S, Zhao X, Chu C, Yang R, Qu Y, Man Y. Application of Epigallocatechin-3-gallate (EGCG) Modified 1-Ethyl-3-(3-dimethylaminopropylcarbodiimide hydrochloride/N-hydroxy-succinimide (EDC/NHS) Cross-Linked Collagen Membrane to Promote Macrophage Adhesion. Materials. 2021; 14(16):4660. https://doi.org/10.3390/ma14164660
Chicago/Turabian StyleRung, Shengan, Xiwen Zhao, Chenyu Chu, Renli Yang, Yili Qu, and Yi Man. 2021. "Application of Epigallocatechin-3-gallate (EGCG) Modified 1-Ethyl-3-(3-dimethylaminopropylcarbodiimide hydrochloride/N-hydroxy-succinimide (EDC/NHS) Cross-Linked Collagen Membrane to Promote Macrophage Adhesion" Materials 14, no. 16: 4660. https://doi.org/10.3390/ma14164660
APA StyleRung, S., Zhao, X., Chu, C., Yang, R., Qu, Y., & Man, Y. (2021). Application of Epigallocatechin-3-gallate (EGCG) Modified 1-Ethyl-3-(3-dimethylaminopropylcarbodiimide hydrochloride/N-hydroxy-succinimide (EDC/NHS) Cross-Linked Collagen Membrane to Promote Macrophage Adhesion. Materials, 14(16), 4660. https://doi.org/10.3390/ma14164660





