The Effect of Heat Treatment on the Structure of Zeolite A
Abstract
:1. Introduction
2. Materials and Methods
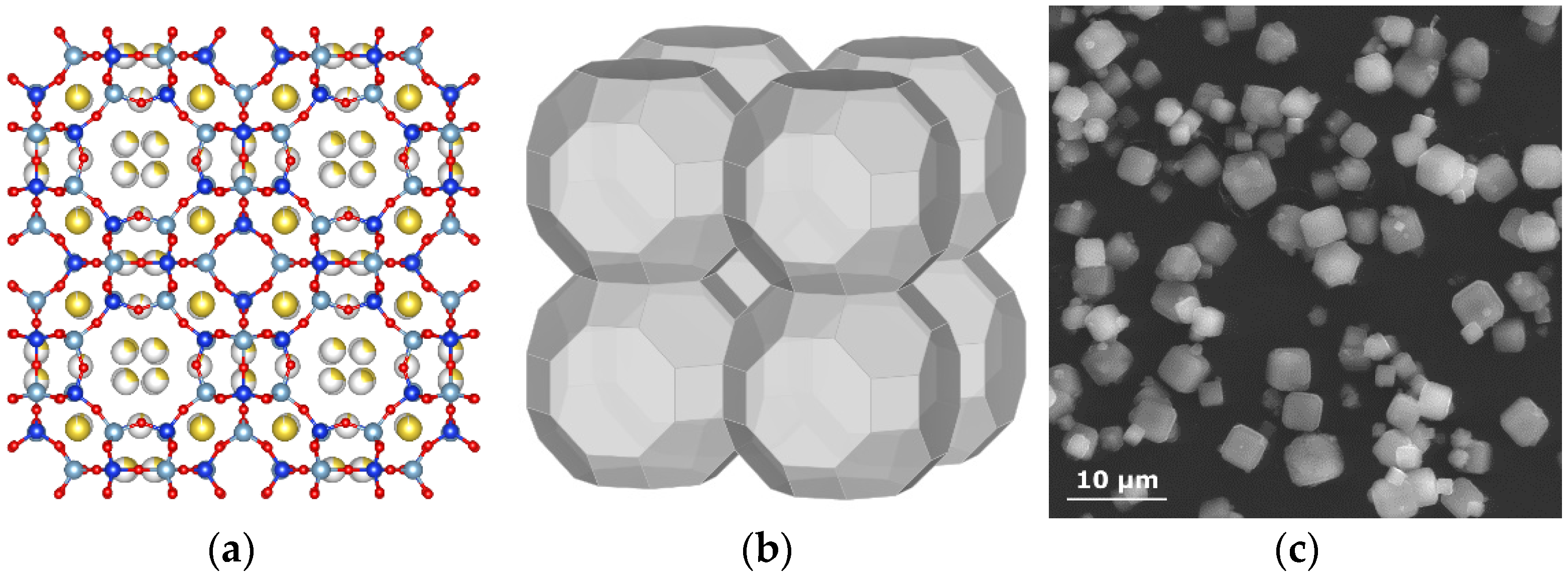
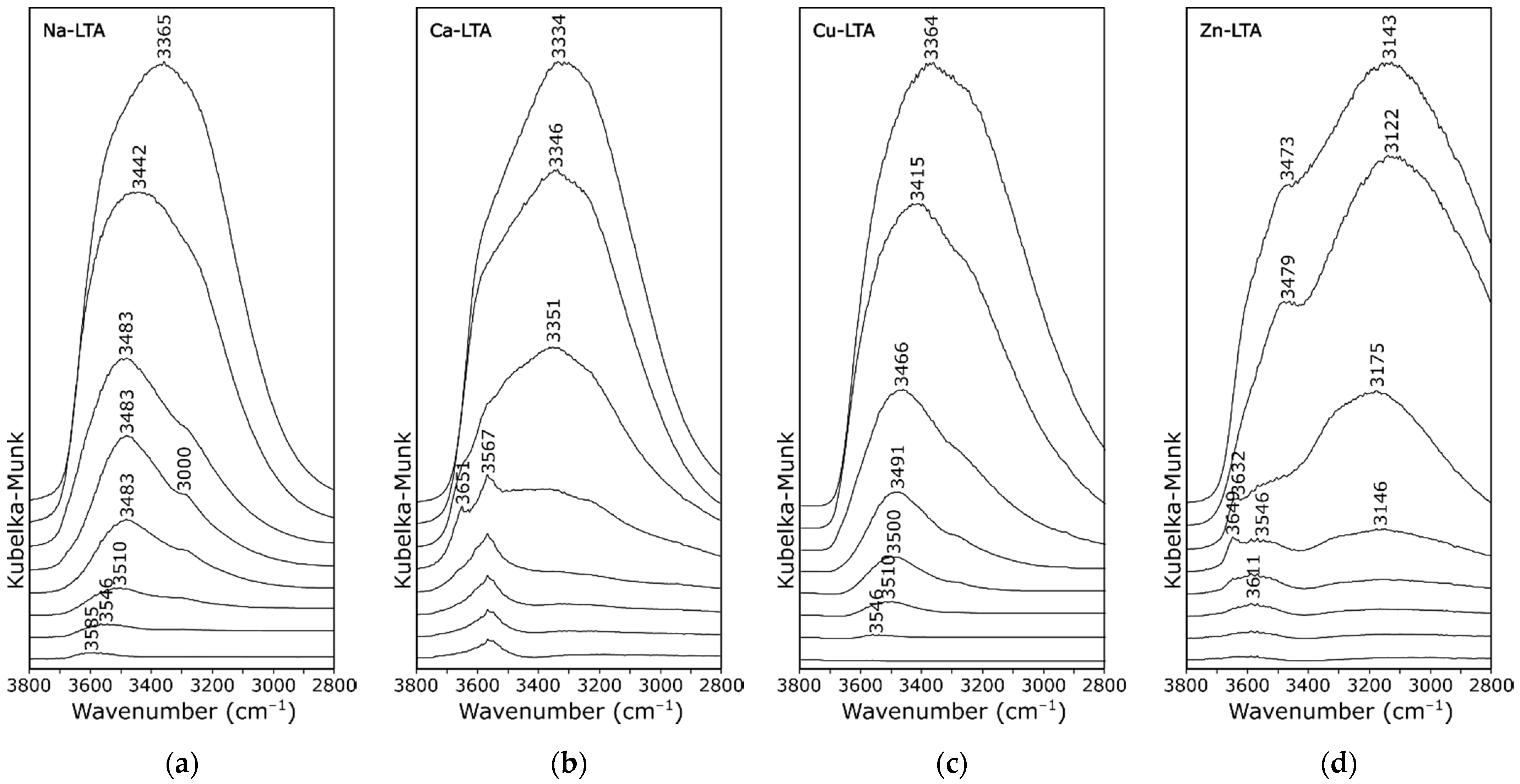
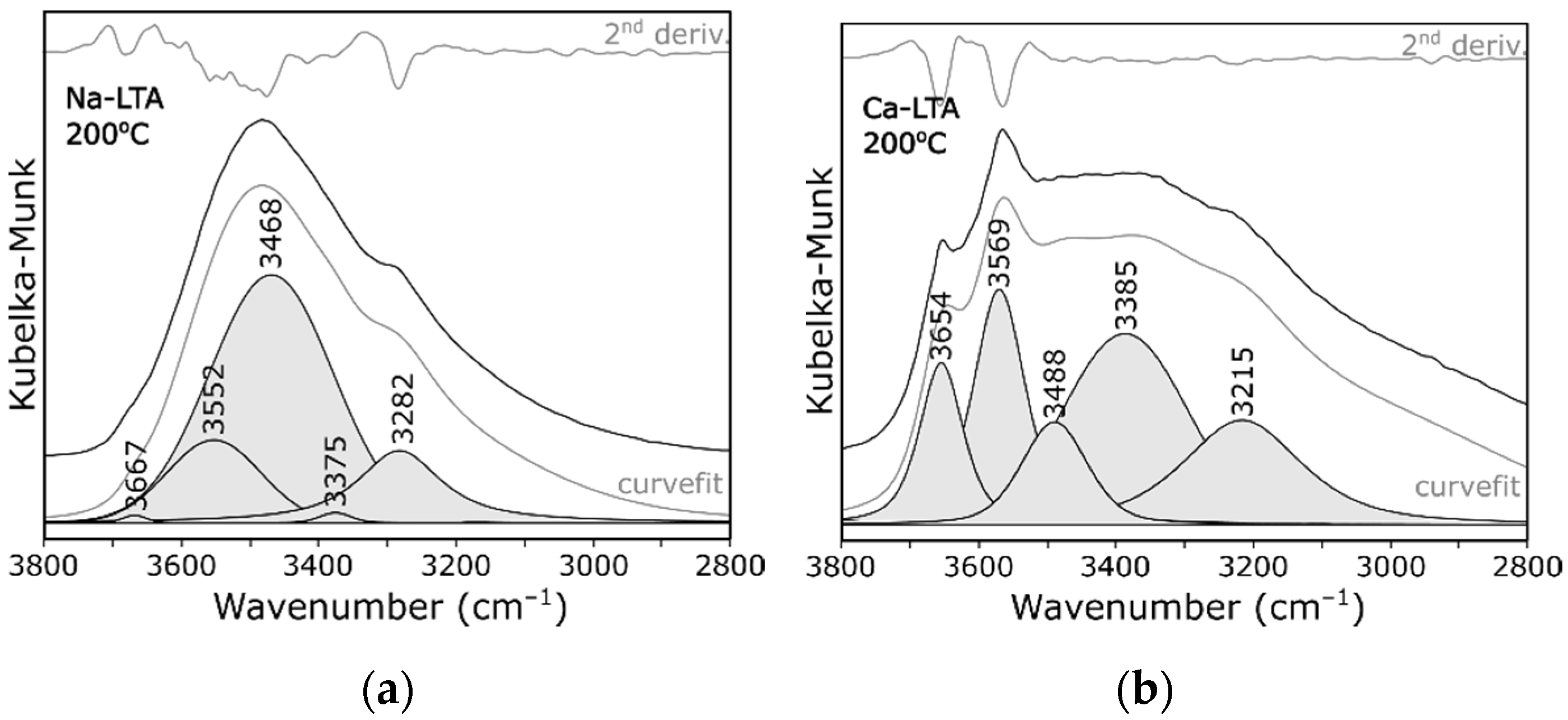
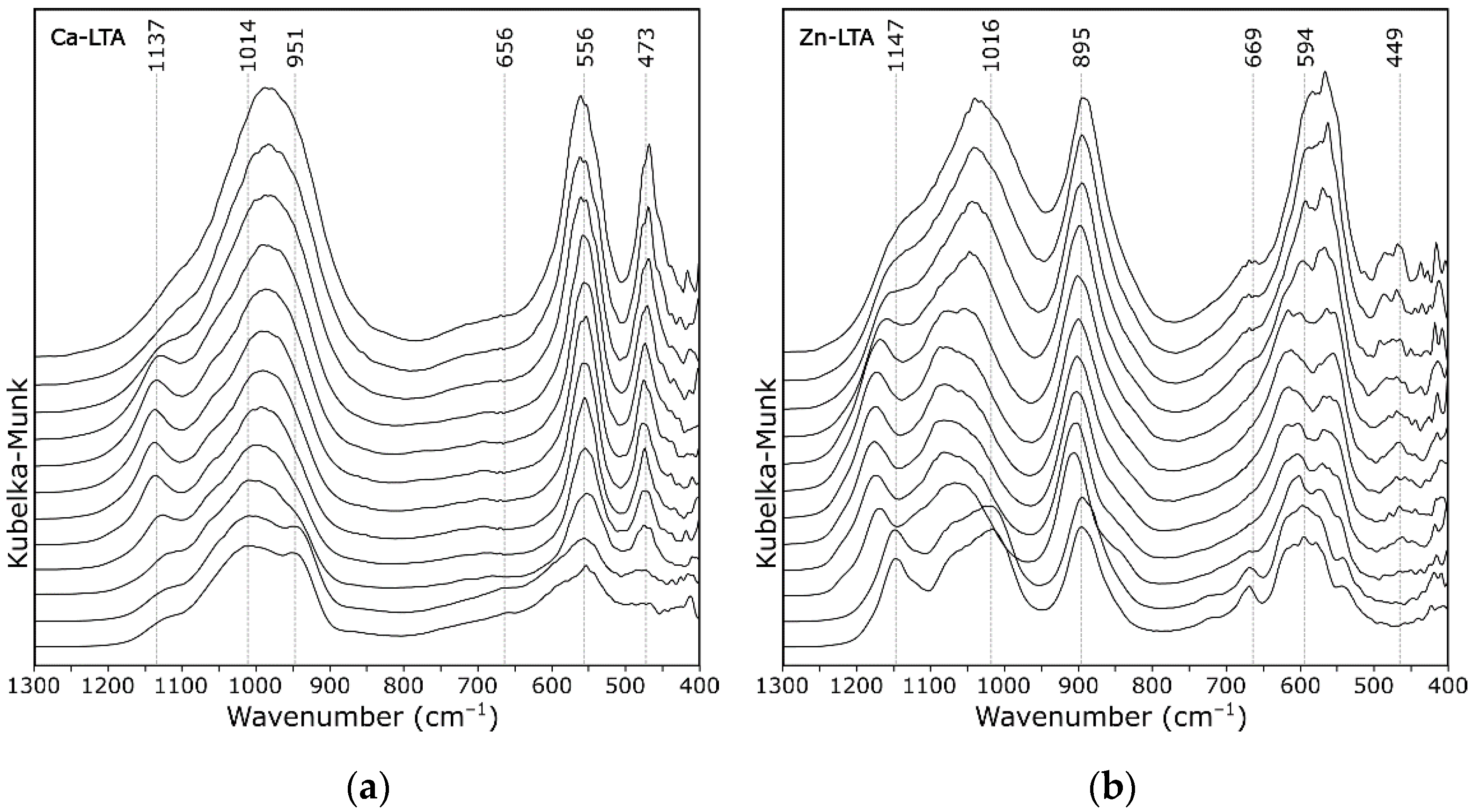
3. Results and Discussion
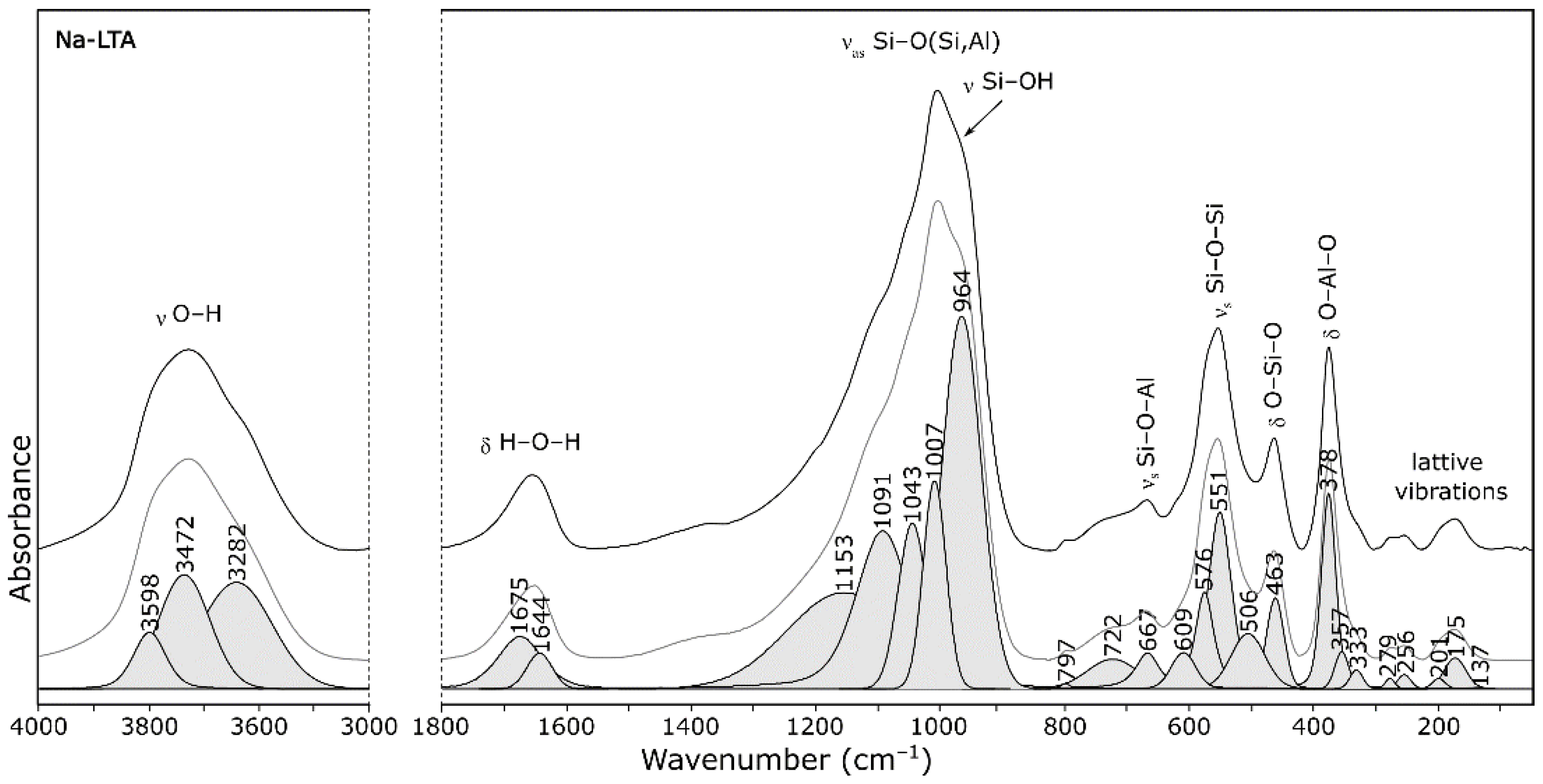
- 1005 cm−1—asymmetric stretching vibrations of bridge bonds—νas Si–O(Al)
- 726 cm−1—symmetric stretching vibrations of bridge bonds—νs Si–O–Si
- 666 cm−1—symmetric stretching vibrations of bridge bonds—νs Si–O–Al
- 554 cm−1—(complex band) symmetric stretching vibrations of bridge bonds—νs Si–O–Si and bending vibrations—δ O–Si–O
- 465 cm−1—bending vibrations—δ O–Si–O, occurring in “antiphase”
- 379 cm−1—bending vibrations—δ O–Si–O and δ O–Al–O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breck, D.W. Zeolite Molecular Sieves, Chemistry and Use; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Framework Type LTA (Material: Linde Type A, Zeolite A). 2020. Available online: http://www.iza-structure.org/databases/ (accessed on 19 July 2021).
- Cejka, J.; van Bekkum, H. Zeolites and Ordered Mesoporous Materials: Progress and Prospects. Studies in Surface Science and Catalysis; Elsevier: Prague, Czech Republic, 2005. [Google Scholar]
- Kühl, G.H. Preparation of Shape Selective Zeolite Alpha as Catalyst. U.S. Patent No. 4,191,663, 1979. [Google Scholar]
- Reed, T.B.; Breck, D.W. Crystalline zeolites. II. Crystal structures of synthetic zeolite, type A. J. Am. Chem. Soc. 1956, 78, 5972–5977. [Google Scholar] [CrossRef]
- Gramlich, V.; Meier, W.M. The crystal structure of hydrated NaA: A detailed refinement of a pseudosymmetric zeolite structure. Z. Kristallogr. 1971, 133, 134–149. [Google Scholar] [CrossRef]
- Mortier, W.J. Compilation of Extra Framework Sites in Zeolites; Butterworth & Co.: Leuven, Belgium, 1992. [Google Scholar]
- Mikuła, A.; Król, M.; Koleżyński, A. Periodic model of an LTA framework. J. Mol. Model. 2015, 21, 275. [Google Scholar] [CrossRef]
- Vanagida, R.Y.; Amaro, A.A.; Seff, K. A redetermination of the crystal structure of dehydrated zeolite 4A. J. Phys. Chem. 1973, 77, 805–809. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Peuker, C. In-Situ DRIFT measurements of OH vibrations in MFI-type zeolites. J. Mol. Struct. 1995, 349, 317–320. [Google Scholar] [CrossRef]
- Beta, I.A.; Böhlig, H.; Hunger, B. Investigation of the non-isothermal water desorption on alkali-metal cation-exchanged X-type zeolites: A temperature-programmed diffuse reaction infrared Fourier transform spectroscopic (TP-DRIFTS) study. Thermochim. Acta 2000, 361, 61–68. [Google Scholar] [CrossRef]
- Jentys, A.; Warecka, G.; Derewinski, M.; Lercher, J.A. Adsorption of water on ZSM 5 zeolites. J. Phys. Chem. 1989, 93, 4837–4843. [Google Scholar] [CrossRef]
- Ozorio, L.P.; Pianzolli, R.; da Cruz Machado, L.; Miranda, J.L.; Turci, C.C.; Guerra, A.C.O.; Falabella Souza-Aguiara, E.; Mota, C.J.A. Metal-impregnated zeolite Y as efficient catalyst for the direct carbonation of glycerol with CO2. Appl. Catal. A Gen. 2015, 504, 187–191. [Google Scholar] [CrossRef]
- Conte, M.; Lopez-Sanchez, J.A.; He, Q.; Morgan, D.J.; Ryabenkova, Y.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Kiely, C.J.; Khalid, K.; et al. Modified zeolite ZSM-5 for the methanol to aromatics reaction. Catal. Sci. Technol. 2012, 2, 105–112. [Google Scholar] [CrossRef]
- Smith, L.H., Jr. Inorganic Synthesis; Wiley: New York, NY, USA, 1983; p. 33. [Google Scholar]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Sun, H.; Wu, D.; Liu, K.; Guo, X.; Navrotsky, A. Energetics of alkali and alkaline earth ion-exchanged zeolite A. J. Phys. Chem. C 2016, 120, 15251–15256. [Google Scholar] [CrossRef]
- Singh, J.; White, R.L. A variable temperature infrared spectroscopy study of CaA zeolite dehydration and carbonate formation. Spectrochim. Acta A 2019, 207, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Bish, D.L.; Carey, B. Thermal behavior of natural zeolites. Rev. Mineral. Geochem. 2001, 45, 403–452. [Google Scholar] [CrossRef]
- Kosanovic, C.; Subotic, B.; Smit, I. Thermally induced phase transformations in cationexchanged zeolites 4A, 13X and synthetic mordenite and their amorphous derivatives obtained by mechanochemical treatment. Thermochim. Acta 1998, 317, 25–37. [Google Scholar] [CrossRef]
- Afzal, M.; Yasmeen, M.; Saleem, P.K.; Butt, A.K.; Afzal, J. TG and DTA study of the thermal dehydration of metal-exchanged zeolite-4A samples. J. Therm. Anal. Calorim. 2000, 62, 721–727. [Google Scholar] [CrossRef]
- Mozgawa, W.; Jastrzębski, W.; Handke, M. Vibrational spectra of D4R and D6R structural units. J. Mol. Struct. 2005, 744–747, 663–670. [Google Scholar] [CrossRef]
- Koleżyński, A.; Mikuła, A.; Król, M. Periodic model of LTA framework containing various non-tetrahedral cations. Spectrochim. Acta A 2016, 157, 17–25. [Google Scholar] [CrossRef]
- Katoh, M.; Koide, R.; Yamada, K.; Yoshida, T.; Horikawa, T. IR spectroscopic analysis of thermal behavior of adsorbed water on Y-type zeolite. Int. J. Mod. Phys. Conf. Ser. 2012, 6, 437–442. [Google Scholar] [CrossRef]
- Crupi, V.; Majolino, D.; Venuti, V. Diffusional and vibrational dynamics of water in NaA zeolites by neutron and fourier transform infrared spectroscopy. J. Phys. Condens. Matter. 2004, 16, S5297–S5316. [Google Scholar] [CrossRef]
- Singh, J.; White, R.L. A variable temperature infrared spectroscopy study of NaA zeolite dehydration. Vib. Spectrosc. 2018, 94, 37–42. [Google Scholar] [CrossRef]
- Gil, B.; Broclawik, E.; Datka, J.; Klinowski, J. Acidic hydroxyl groups in zeolites X and Y: A correlation between infrared and solid-state NMR spectra. J. Phys. Chem. 1994, 98, 930–933. [Google Scholar] [CrossRef]
- Jiang, M.; Karge, H.G. Investigation of acid properties of dealuminated H-mordenite zeolites by low-temperature diffuse reflectance FTIR. J. Chem. Soc. Faraday Trans. 1996, 92, 2641–2649. [Google Scholar] [CrossRef]
- Montanari, T.; Busca, G. On the mechanism of adsorption and separation of CO2 on LTA zeolites: An IR investigation. Vib. Spectrosc. 2008, 46, 45–51. [Google Scholar] [CrossRef]
- Jobic, H. Observation of the fundamental bending vibrations of hydroxyl groups in HNa–Y zeolite by neutron inelastic scattering. J. Catal. 1991, 131, 289–293. [Google Scholar] [CrossRef]
- Beck, K.; Pfeifer, H.; Staudte, B. Assignment of novel bands observed in the near infrared spectra of shallow-bed treated H-Y and H-ZSM-5 zeolites. Micropor. Mater. 1993, 2, 1–6. [Google Scholar] [CrossRef]
- Abd El Maksod, I.H.; Saleh, T.S.; Hegazy, E.Z. Preparation, characterization and utilization of (Ni:Cu) bimetallic system loaded on zeolites. J. Alloys Compd. 2010, 506, 923–927. [Google Scholar] [CrossRef]
- Ohnishi, N.; Horikawa, Y.; Kajitani, T.; Morii, Y.; Hiraga, K. Neutron powder diffraction study of Ni-LTA zeolite. Phys. B Condens. Matter 1995, 213–214, 408–410. [Google Scholar] [CrossRef]
- Esquivel, D.; Cruz-Cabeza, A.J.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Transition metal exchanged β zeolites: Characterization of the metal state and catalytic application in the methanol conversion to hydrocarbons. Microporous Mesoporous Mater. 2013, 179, 30–39. [Google Scholar] [CrossRef]
- Nyankson, E.; Efavi, J.K.; Yaya, A.; Manu, G.; Asare, K.; Daafuor, J.; Abrokwah, R.Y. Synthesis and characterisation of zeolite-A and Zn-exchanged zeolite-A based on natural aluminosilicates and their potential applications. Cogent. Eng. 2018, 5, 1440480. [Google Scholar] [CrossRef]
- Majdan, M.; Pikus, S.; Kowalska-Ternes, M.; Gładysz-Płaska, A.; Staszczuk, P.; Fuks, L.; Skrzypek, H. Equilibrium study of selected divalent d-electron metals adsorption on A-type zeolite. J. Colloid Interface Sci. 2003, 262, 321–330. [Google Scholar] [CrossRef]
- Baker, M.D.; Godber, J.; Helwig, K.; Ozin, G.A. Probing extra-framework cations in alkali- and alkaline-earth-metal linde type A zeolites by Fourier Transform Far-Infrared Spectroscopy. J. Phys. Chem. 1988, 92, 6017–6024. [Google Scholar] [CrossRef]
- Blackwell, C.S. Investigation of zeolite frameworks by vibrational properties. 1. The double-four-ring in group 3 zeolites. J. Phys. Chem. 1979, 83, 3251–3257. [Google Scholar] [CrossRef]
- Takaishi, T.; Yatsurugi, Y.; Yusa, A.; Kuratomi, T. Changes in the sieving action and thermal stability of zeolite a produced by ion-exchange. J. Chem. Soc. Faraday Trans. 1976, 71, 97–105. [Google Scholar] [CrossRef]
- Radulović, A.; Dondur, V.; Dimitrijević, R.; Arandjelović, D. Thermal transformation of Na-LTA zeolite into low-carnegieite: The influence of residual sodium and aluminium species. Thermochim. Acta 2010, 511, 37–42. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, M.K.; Jeleń, P. The Effect of Heat Treatment on the Structure of Zeolite A. Materials 2021, 14, 4642. https://doi.org/10.3390/ma14164642
Król MK, Jeleń P. The Effect of Heat Treatment on the Structure of Zeolite A. Materials. 2021; 14(16):4642. https://doi.org/10.3390/ma14164642
Chicago/Turabian StyleKról, Magdalena Katarzyna, and Piotr Jeleń. 2021. "The Effect of Heat Treatment on the Structure of Zeolite A" Materials 14, no. 16: 4642. https://doi.org/10.3390/ma14164642
APA StyleKról, M. K., & Jeleń, P. (2021). The Effect of Heat Treatment on the Structure of Zeolite A. Materials, 14(16), 4642. https://doi.org/10.3390/ma14164642





