3.2. Microstructure of Brazed Joints
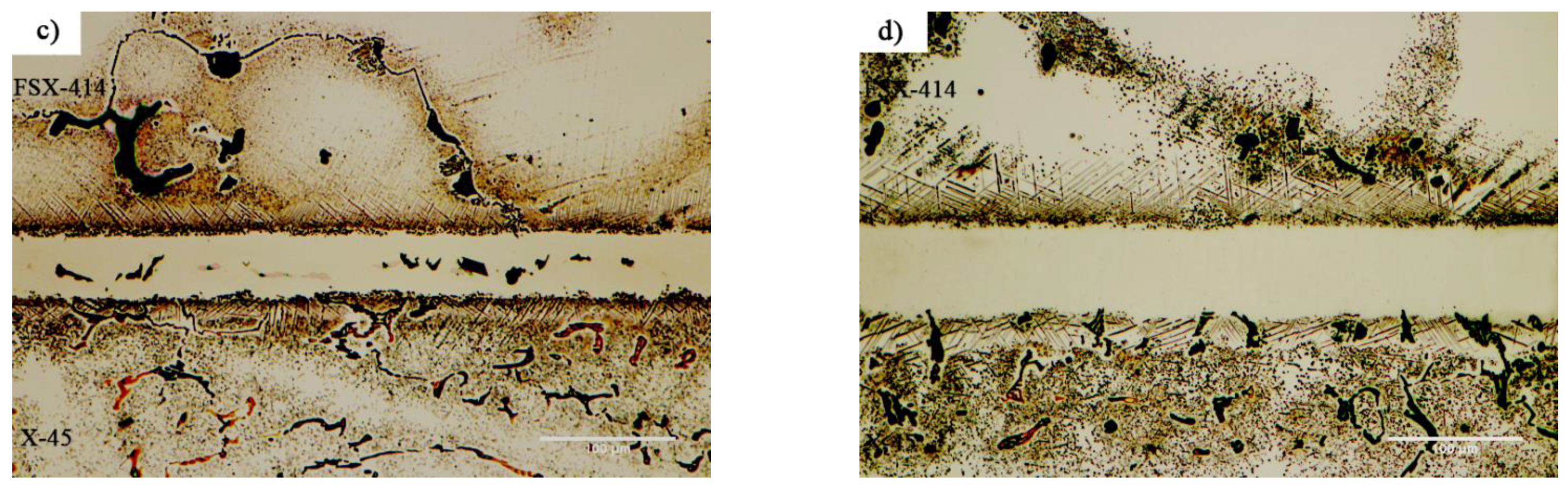
Figure 7 and
Figure 8 show images from obtained with optical and scanning electron microscopy of the microstructure of the brazed joints at temperatures of 1010, 1050, 1100, and 1150 °C with a constant holding time of 10 min. In fact, this image explains the effect of temperature on the bonding of the transient liquid phase of the X-45 and FSX-414 cobalt-based superalloys with the BNi-3 interlayer. As
Figure 7a shows, although bonding took place at a temperature lower than the liquidus temperature of the interlayer, the two base metals were bonded together and there was a misbonded zone.
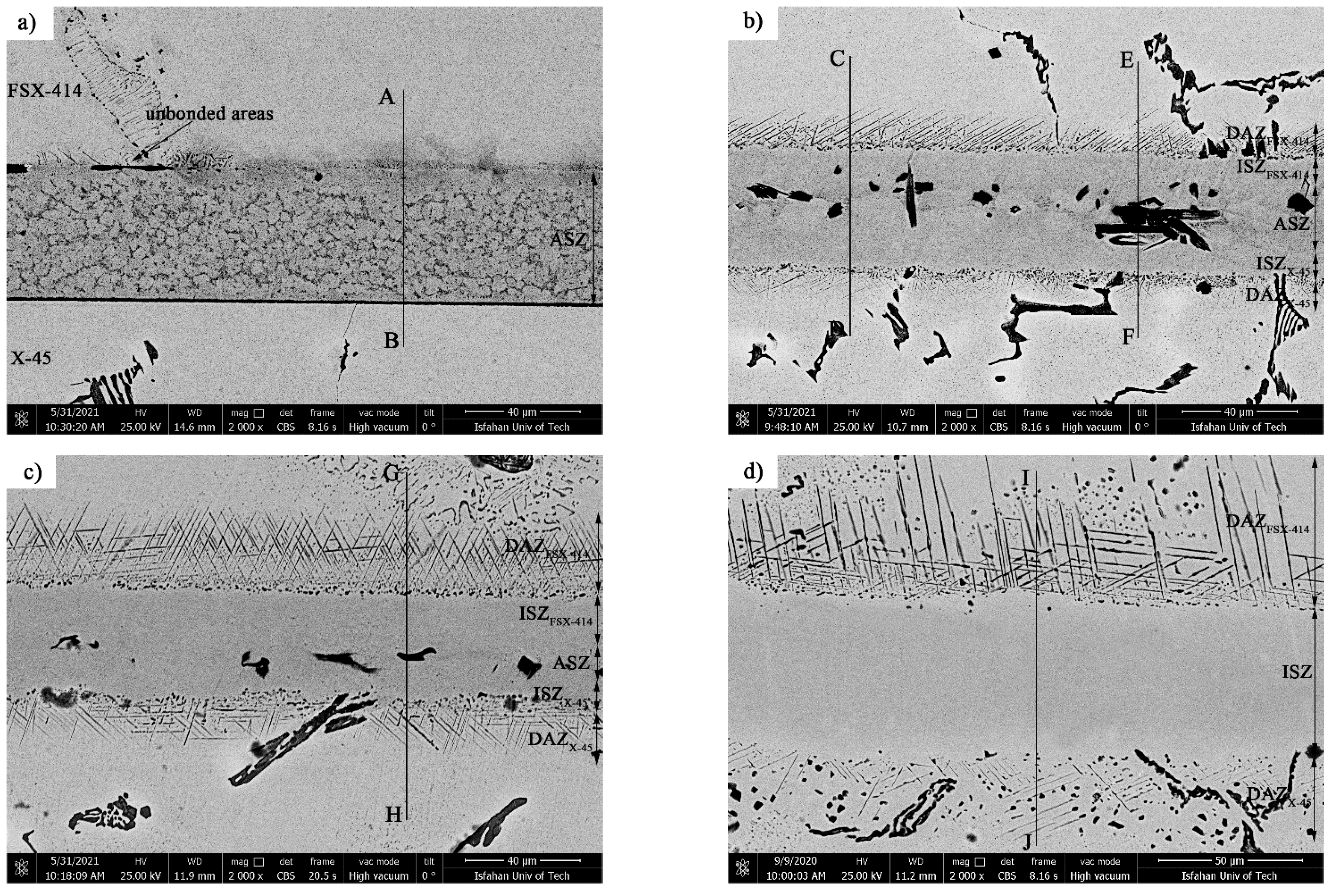
Figure 8a presents an SEM micrograph for the microstructure of the bonded area at 1010 °C/10 min. It is obvious that 93% of the bonding zone included globular-like intermetallic compounds. There were dense regions in the proximity of the bonding zone in the base metals, indicating solid-state diffusion before the liquidus temperature of the interlayer was reached.
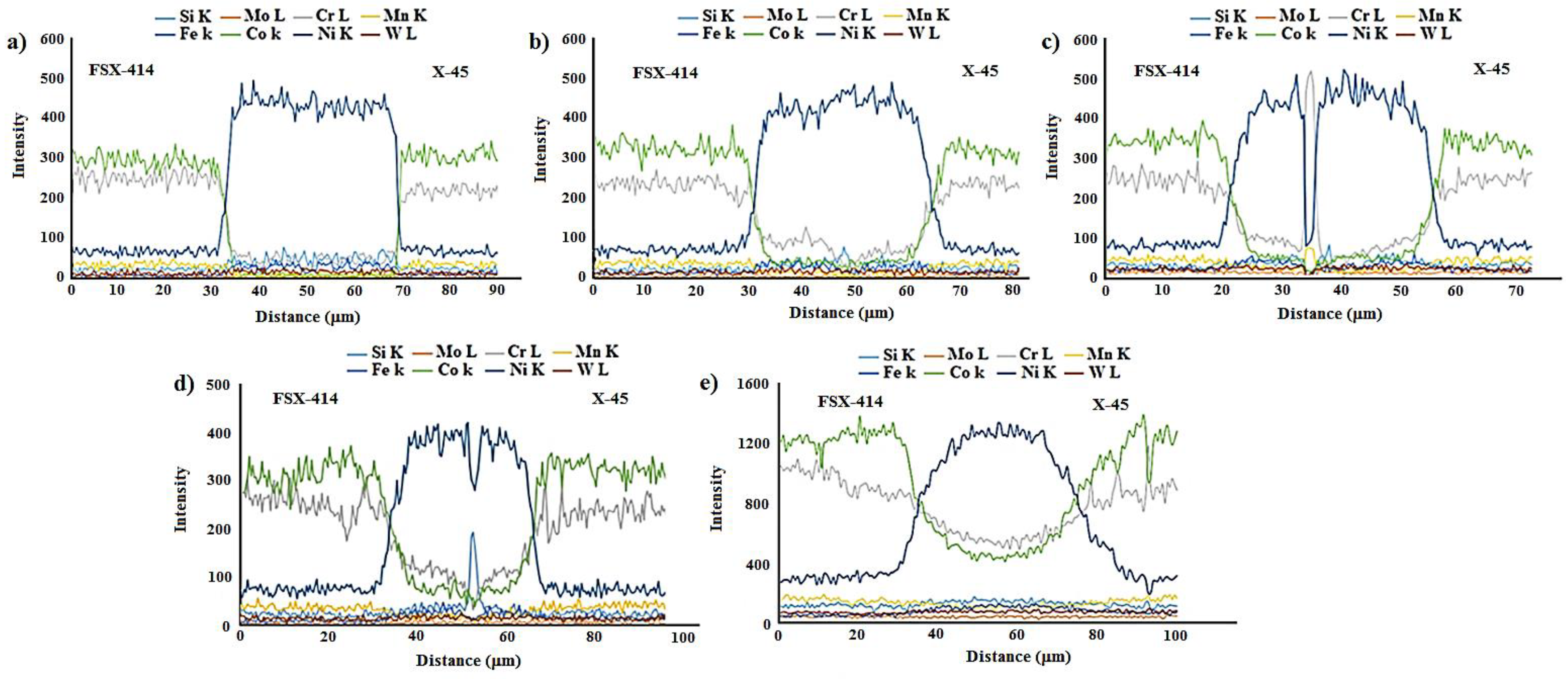
Figure 9a presents the linear analysis for the bonded zone of the sample at 1010 °C/10 min (A–B). There was a constant concentration of nickel in the base metals X-45 and FSX-414 which would increase suddenly when the interface of the base metals/bond region was reached due to the ineffectual vacillations in the bond zone. There was an essentially constant concentration of cobalt in the base metals with very few changes. The maximum concentration of cobalt was in the interface of the base metals with the bonding zone and the lowest concentration was in the bounding zone. There was a highly interesting point in the linear analysis of the sample at 1010 °C/10 min regarding chromium. The BNi-3 interlayer included only boron and silicon as melting point depressant elements. The bonding temperature was lower than the liquidus temperature of the interlayer at this condition, and there was not enough time for the solid-state diffusion and dissolution of the base metals in the small melt of the interlayer. Hence, the lowest chromium concentration was in the bonding zone, and alloying elements aggregated as precipitation increased their local concentration.
Figure 7b shows images obtained with optical microscopy of the microstructures of the bonded samples treated at 1050 °C for 10 min. The microstructures were different from the samples treated at 1010 °C/10 min. An increase 40 °C caused the microstructure of the central bonding line to be changed from a globular-like state to a continuous and long shape.
Figure 8b shows an SEM micrograph of the bonded zone of the sample at 1050 °C/10 min. Precipitations, including constant and distinct phase contrast, formed new phases. Linear analysis was performed for the bonding seam, including two phases (C–D and E–F), as shown in
Figure 9b,c.
Figure 9b shows that the nickel concentration reached its maximum at a short distance from the interface of the base metal/bonded zone in the bonding centerline. This issue was also noted in the chromium element (
Figure 9c). There was an interesting issue regarding the entry of chromium from base metals into the interlayer melt and its collection in the form of a chromium-rich phase in the bonding central line.
Figure 7c shows a light microscopy image of the microstructure of the bonded sample treated at 1100 °C/10 min. It was obvious that there were some intermetallic compounds from the remaining melt of the interlayer, but the precipitations came out of the constant state and could be observed discontinuously in the centerline of the bounding as narrow bands. Linear analysis for the joint showed that the distance between the concentrations of nickel and chromium, from the interface to the central line of the joint, increased with slight but constant fluctuations. Also, the concentration of cobalt increased significantly, which indicated the diffusion and dissolution of base metals in the melt of the interlayer (G–H,
Figure 9d).
Figure 7d presents images obtained with optical microscopy of the microstructure of the bonded sample treated at 1150 °C/10 min. The bonding zone had no eutectic-like compounds and indicated that a single-phase zone had been achieved. Linear analysis for the joint (I–J,
Figure 9e) indicated that the concentrations of cobalt, chromium, tungsten, and molybdenum in the bonding zone had increased and these elements had been replaced as substitutes in the nickel crystal lattice. On the other hand, alloy elements were more uniformly distributed. The formation of a single-phase zone by keeping the interlayer melt at a constant temperature explains the isothermal solidification of the interlayer melt (
Figure 8d).
It is possible to state, based on microstructural observations and linear analysis, that the concentration gradient along the joint seam caused a microstructural gradient. Consequently, according to
Figure 8, it is possible to divide the bonded zone into seven microstructural zones, which we list from top to bottom: (1) FSX-414 cobalt-based superalloy, (2) diffusion-affected zone on the FSX-414 side (DAZ
FSX-414), (3) isothermal solidification zone on the side of FSX-414 (ISZ
FSX-414), (4) athermal solidification zone (ASZ), (5) isothermal solidification zone on the side of the superalloy X-45 (ISZ
X-45), (6) diffusion-affected zone on the X-45 side (DAZ
X-45), and (7) X-45 cobalt-based superalloy.
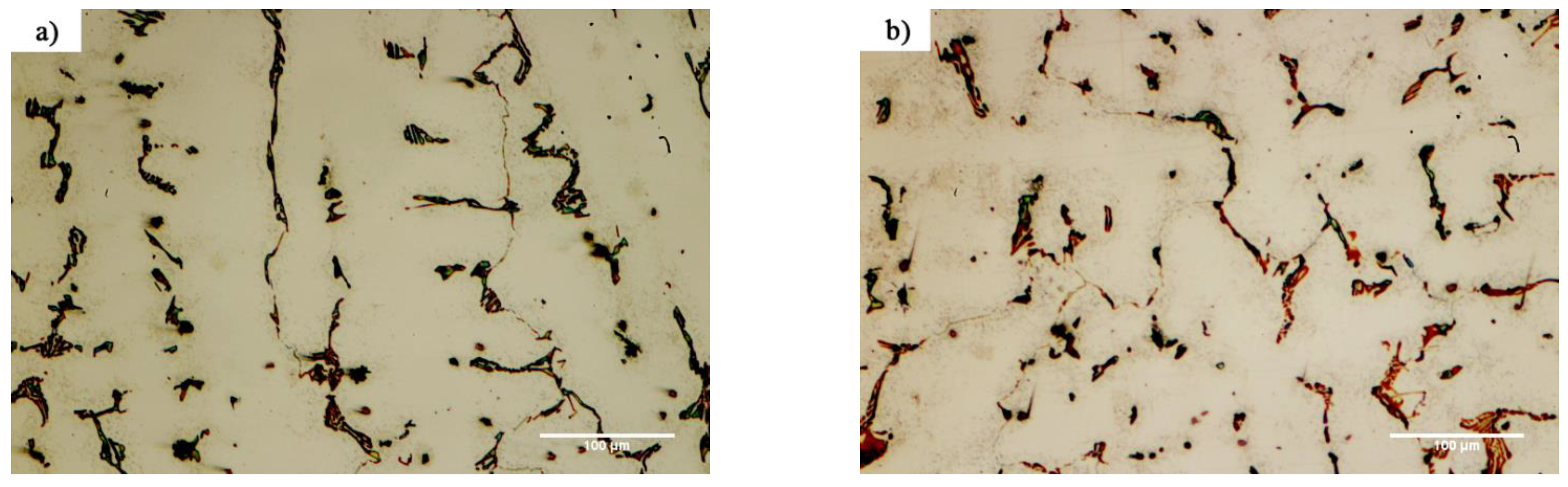
Figure 10 shows the microstructural evolution in the FSX-414 superalloy at a fixed time of 10 min and temperatures of 1010, 1050, 1100, and 1150 °C. Carbides with eutectic morphology can maintain their stability, and there were no traces of secondary phase particles in the rich field of cobalt (α-Co), indicating that increasing the amount of chromium by 4 wt.% could ensure the temperature stability of the FSX-414 superalloy was maintained. Wei et al. [
29] examined the microstructural changes of a 6509 K superalloy at different temperatures with various holding times. They argued that cobalt-based cast alloys are thermodynamically unstable and that their stability completely depends on the temperature. At high temperatures, M
23C
6 carbides comprise the most stable carbide state in the conditions of service. Accordingly, if carbides are deposited as eutectic islands, their temperature stability will be increased [
30]. There were completely different microstructural conditions in the X-45 cobalt-based superalloy.
Figure 11 shows the microstructural changes in the X-45 superalloy at a fixed time of 10 min and temperatures of 1010, 1050, 1100, and 1150 °C. It is obvious that increasing temperature caused the dark particles to be deposited in the matrix. These precipitations were deposited as the kind of clouds that have a maximum volume fraction around the grain boundaries and interdendritic zones, and their amount decreased in the center of cobalt-rich grains (α-Co).
Figure 12 exhibited SEM micrographs and EDX spectra of X-45 superalloy.
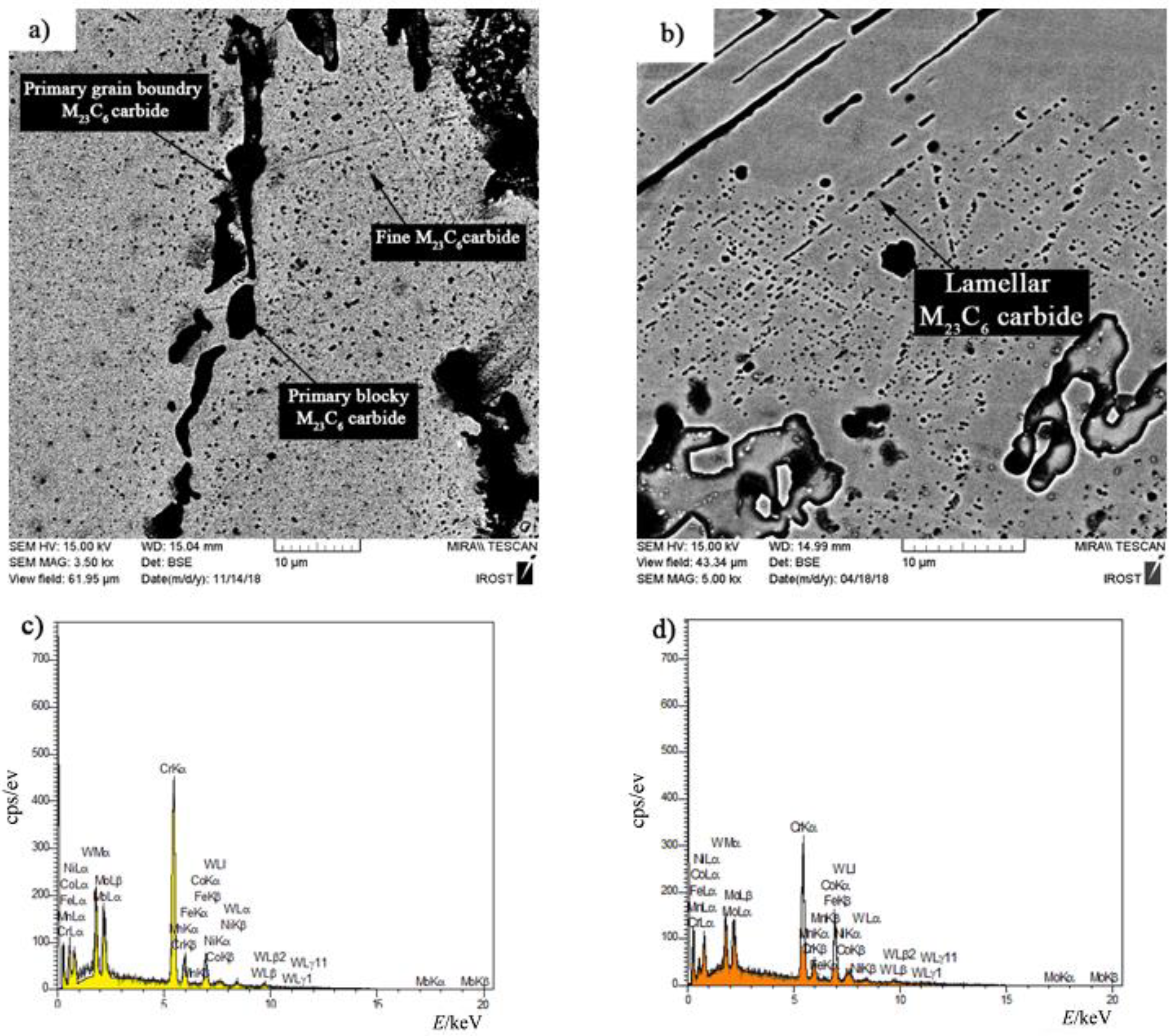
Figure 12a shows an SEM micrograph of fine particle deposition around the initial X-45 superalloy carbides at 1150 °C/10 min. EDX analysis of the deposits (
Figure 12c) indicated that they were rich in chromium. Examining the chemical analysis and previously published papers [
31,
32], we judged that these particles were M
23C
6 carbides. In fact, these were secondary M
23C
6 (M = Co, Cr, W, Mo) carbide particles that had been deposited during the cooling of the room temperature due to solid-state diffusion. This type of carbide particle is precipitated in aging conditions (deposition in the standard heat treatment conditions of 980 °C/240 min) or during high-temperature service in cobalt-based superalloys [
30], nickel-based superalloys [
33], and even steels [
34]. These precipitations are called secondary carbides and are formed in situ and have a particle, cube, or disc morphology. Also, it was possible to observe a series of deposits with a dotted morphology at 1150 °C/10 min in the X-45 superalloy at a distance of 50 μm from the interface of the bonding zone, as shown in
Figure 12b. EDX analysis showed a chemical composition similar to deposits (
Figure 12d). This morphology of M
23C
6 is named lamellar carbide and it has been observed in the DZ40M alloy [
35] and in a Co-Cr-Mo-C base alloy [
36]. In their study, Gui et al. [
35] stated that the released atoms were absorbed during heating by stacking faults in the cobalt matrix and local segregation zones were created. M
23C
6 carbides nucleate and grow a lamellar character due to the in situ reaction between chromium and carbon in local segregation situations.
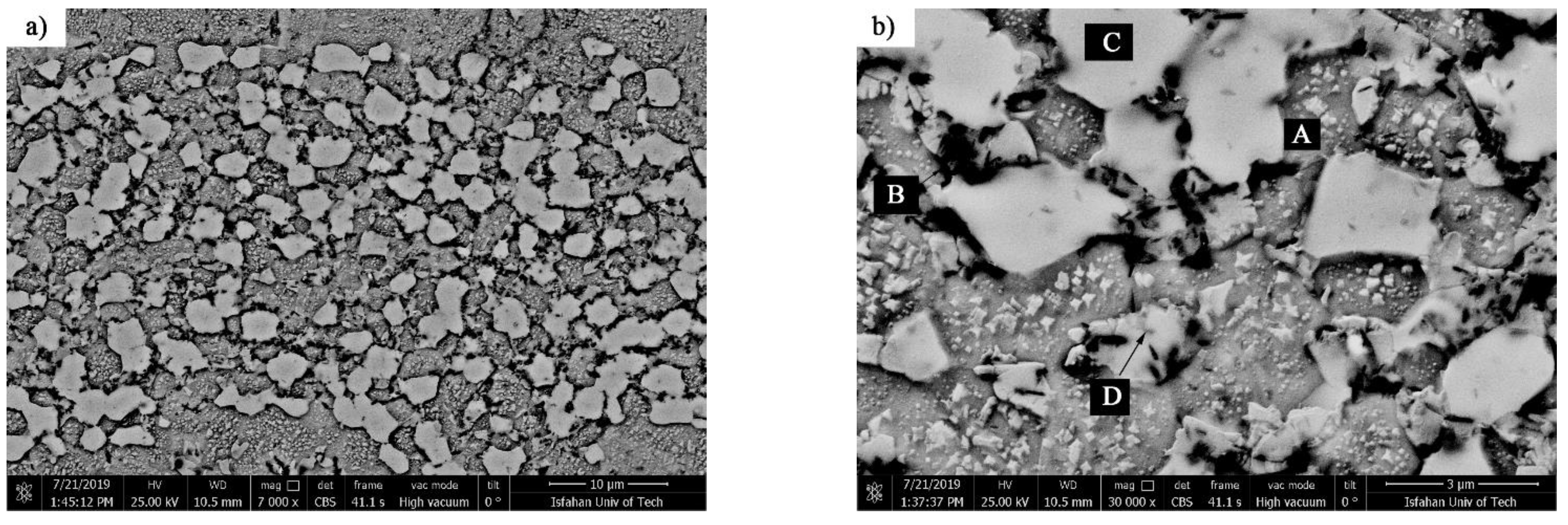
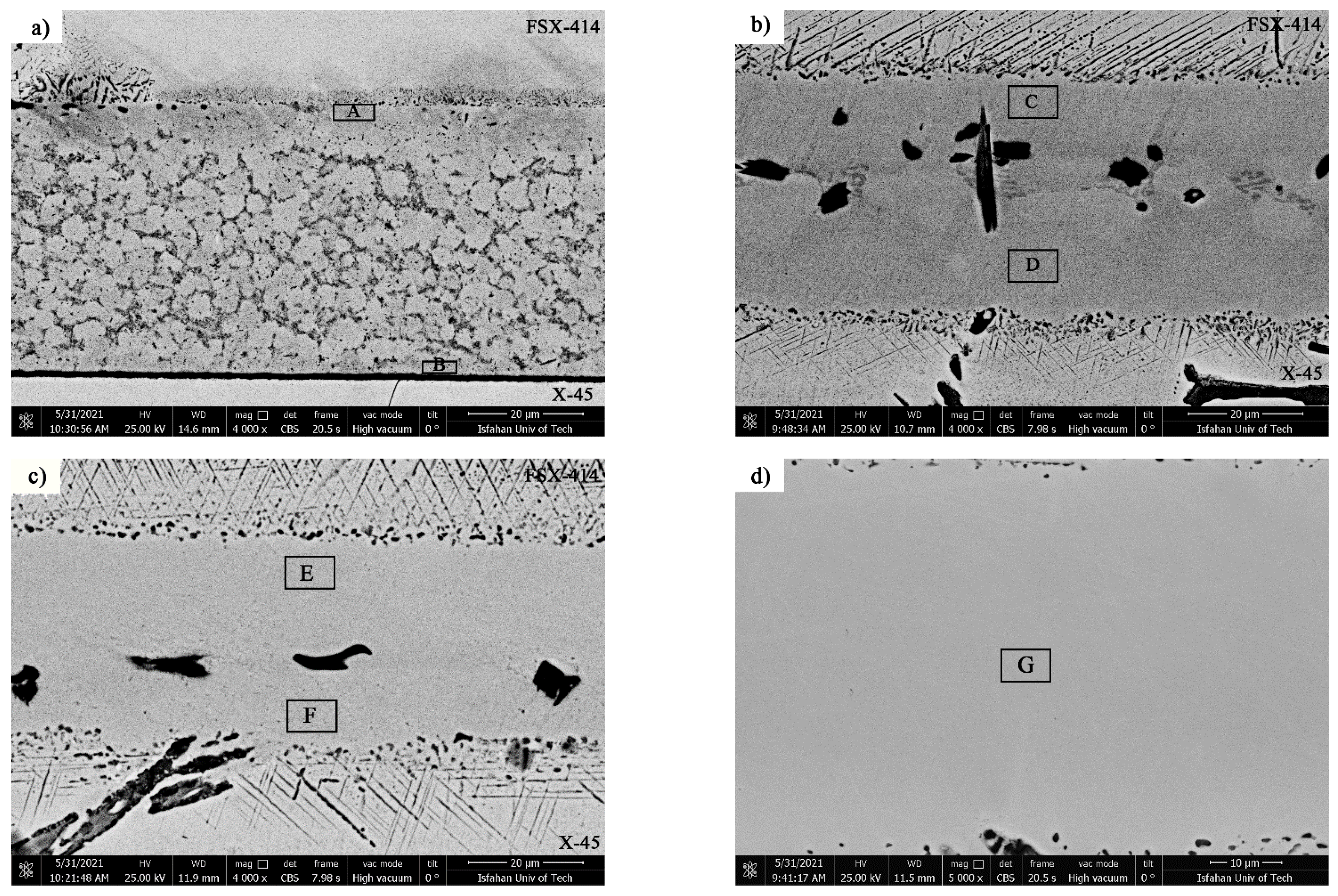
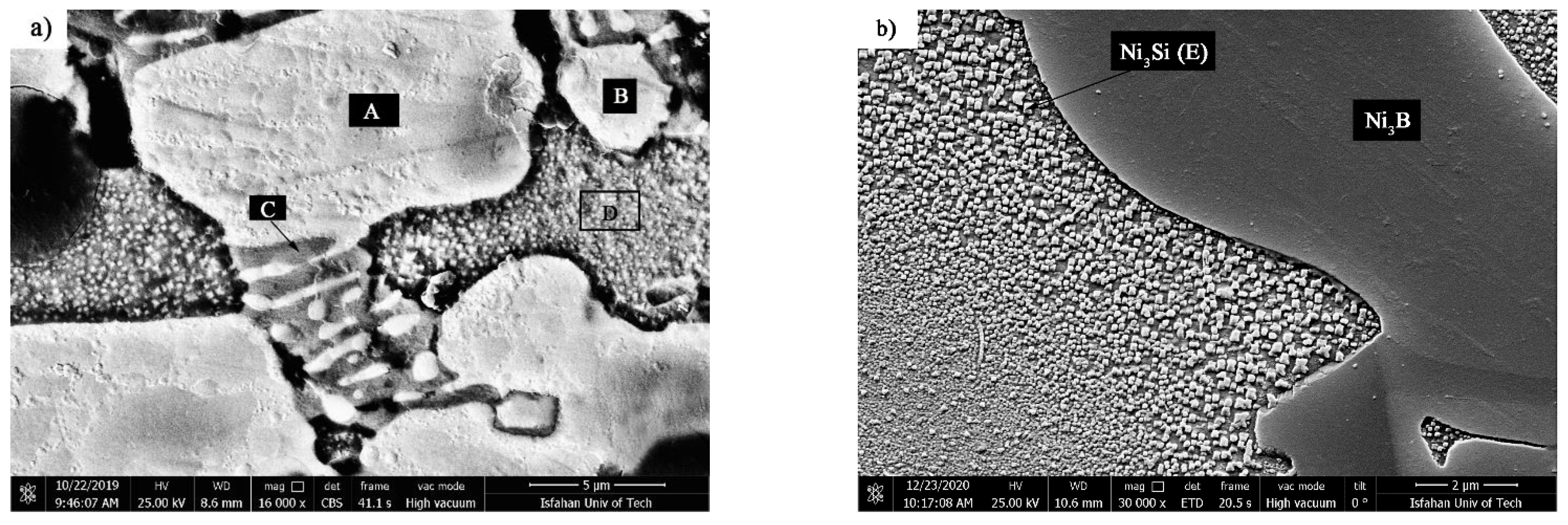
Figure 13 shows SEM micrographs of TLP-bonded samples with greater magnification at temperatures of 1010, 1050, 1100, and 1150 °C and a constant holding time of 10 min. The dense microstructure with a globular-shaped appearance for the 1010 °C/10 min treatment (
Figure 13a) indicated the simultaneous existence of melt and solid together, and the conditions for the complete melting of the crystallized components in the interlayer were not present, as the liquidus temperature of the interlayer was 1012.2 °C. The amount of the formed molt that, among the particles’ crystallized phases, reached the middle layer/base metal interfaces through capillary force was very small, and, through only a little diffusion of the melting point depressant elements into the base metals, very narrow regions of the isothermal solidification zone were created in close proximity to the interfaces, and the rest of the molten components and crystallized components were athermally solidified. Regarding the nickel–silicon–boron phase diagram, the first phase, composed of a solid/liquid joint interface as epitaxial growth on the base metal grains, was a nickel-rich γ proeutectic solid solution formed as a narrow band on both sides of the interface of the base metals inside the bonding zone. Chemical analysis of the EDX data for ISZ
FSX-414 (A in
Table 4) and ISZ
X-45 (B in
Table 4) indicated the formation of a nickel-rich solid solution. The existence of very small amounts of the alloying elements chromium, tungsten, and molybdenum indicated the absence of sufficient molt for the dissolution of base metals. In the second step, a binary eutectic of Ni-rich boride and Ni-rich c solid solution was formed. The growth of nickel-rich dendrites from the interface of the molten interlayer/base metals enriched the residual molt with elements (boron and silicon) with a distribution coefficient smaller than the unit. The low solubility of boron in nickel (0.3 at. % based on a binary nickel–boron phase diagram [
37]) and the low boron distribution coefficient in nickel (0.008 based on a binary nickel–boron phase diagram [
37]) caused the rejection of the boron element to adjacent areas of nickel-rich dendrites.
This unequal solute rejection caused the residual molten chemical composition to move towards the eutectic chemical composition of nickel–boron and then followed the eutectic line of γ and Ni
3B.
Figure 14 shows the SEM micrograph of the athermal solidification zone of the 1010 °C/10 min sample with a higher magnification. Point EDX analysis from the island phase with an irregular shape (A in
Table 5) and the distributed fine phase (B in
Table 5) revealed that these phases were rich in nickel and the presence of the boron element was confirmed. Thus, accumulation of the nickel and boron elements together indicated a nickel-rich phase, which was likely to be nickel-rich boride (Ni
3B). In the third step, ternary eutectic nickel–boron–silicon was formed. Due to the lack of solubility of silicon in nickel-rich boride, the formation of the boride phase was associated with the rejection of the silicon element in the residual molten. A distribution coefficient smaller than the unit in nickel enriched the residual molt with a silicon element. Although boron was consumed in the previous steps, there was still some of it in the final molt. Hence, simultaneous enrichment of the chemical compound with boron and silicon caused the chemical composition to move towards the ternary eutectic and the formation of γ + Ni
3B + Ni
6Si
2B. Based on
Figure 14a, this phase was formed of the residual molten phase in the last step; hence, it precipitated among the Ni
3B phases. Point analysis of this phase showed that it included significant amounts of nickel and silicon and, in addition, a boron element was detected (C in
Table 5). Thus, it can be claimed that it was probably Ni
6Si
2B. EDX area analysis (D in
Table 5) indicated that it included a considerable amount of silicon (4.24 wt.%).
Figure 14b shows an SEM micrograph of the microstructure of the nickel-rich matrix at a greater magnification. In the nickel-rich matrix, particles that had cubic shapes and a size of 200 nm were regularly distributed. Chemical analysis of the EDX data showed that they were rich in nickel and silicon (E in
Table 5). The precipitation method and particle size indicated that these precipitations were deposited during cooling due to solid-state diffusion. The silicon value of these particles was about 12.2 at. %. Considering the nickel–silicon binary phase diagram [
37], the solubility of silicon in nickel at 1050 °C is about 15 at. %, while its solubility at room temperature is 8 at. %; therefore, while cooling from bonding temperature to room temperature, this difference in solubility caused the precipitation of fine particles, which were probably Ni
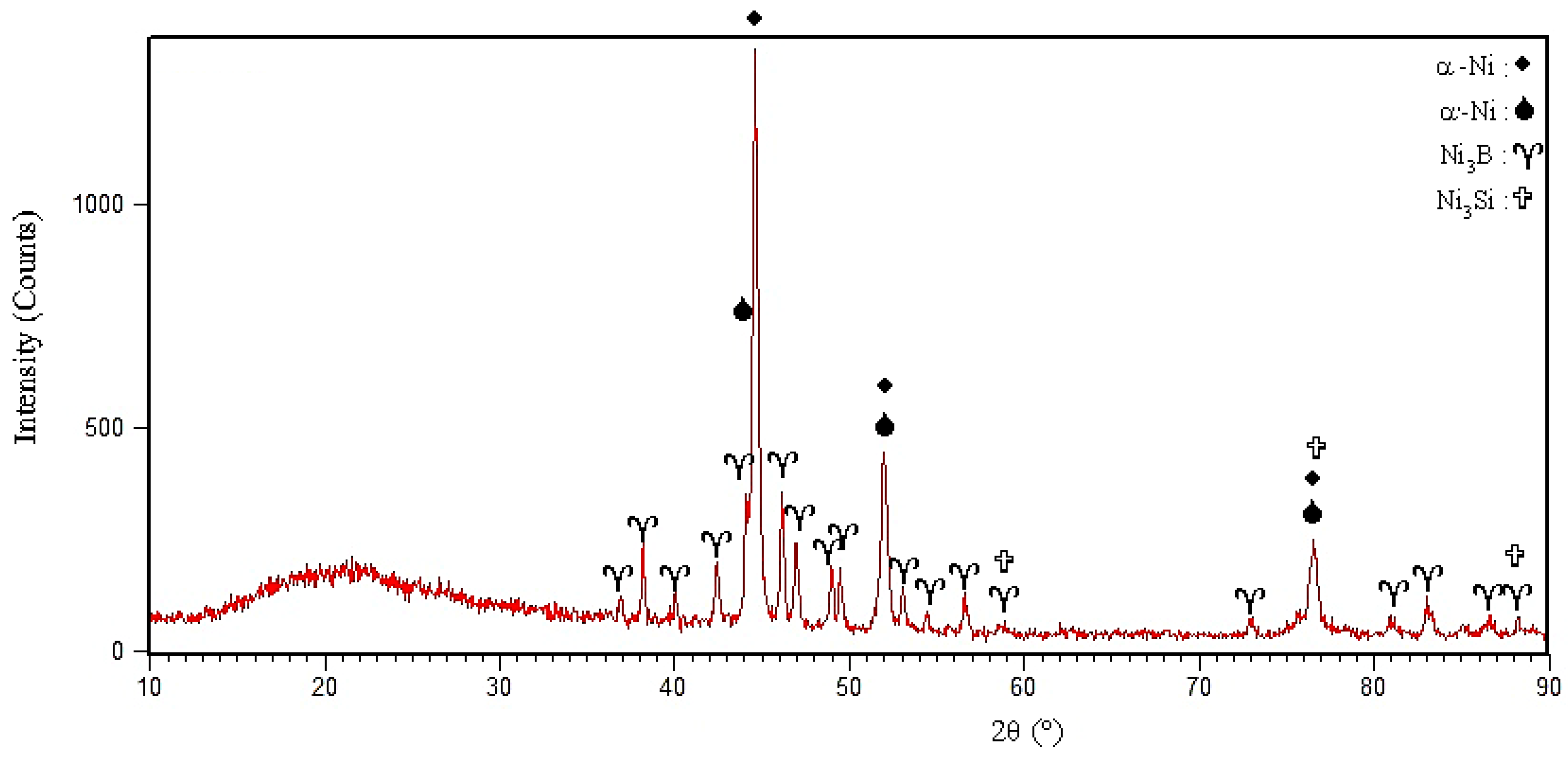
3Si. The X-ray diffraction pattern from the fracture surface of the 1010 °C/10 min and 1050 °C/10 min sample is shown in
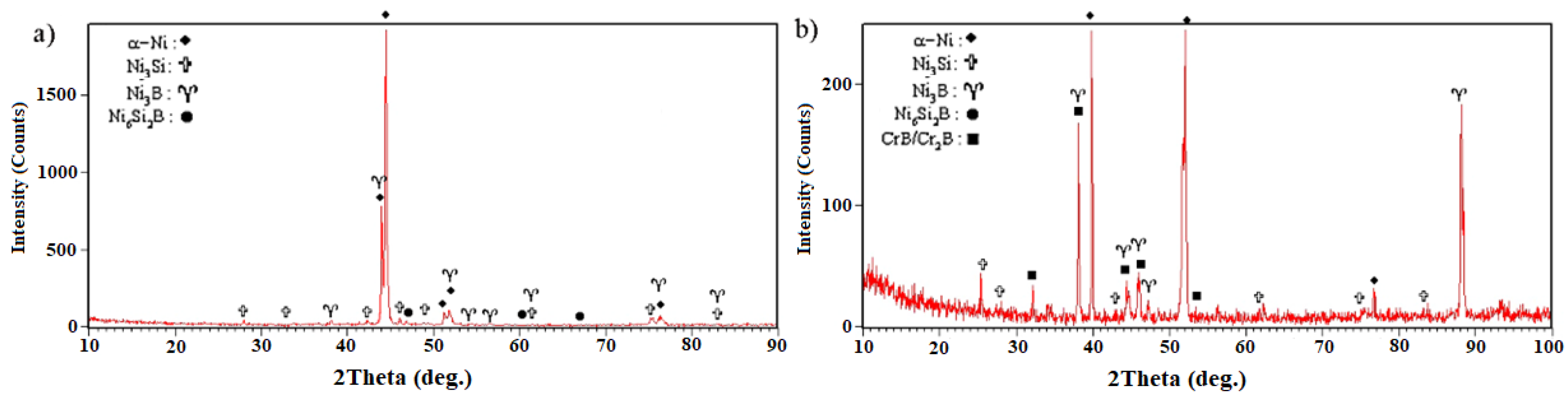
Figure 15. It is strongly evident that the γ, Ni
3B, Ni
6Si
2B, and Ni
3Si phases were formed in the molten and solidified interlayer (
Figure 15a). It should be noted that, in the X-ray diffraction pattern, the chromium-rich boride phase was not detected, which might have been related to there being a very low amount of it or it not forming at all.
Figure 13b shows the SEM micrograph of the TLP-bonded sample at 1050 °C/10 min. The extent of the isothermal solidification zones on both sides of the interface considerably increased. EDX analysis of ISZ
FSX-414 and ISZ
X-45 (C and D, respectively) showed the entry of chromium, cobalt, tungsten, and molybdenum elements from the base metals to the bonding area (
Table 4). Increasing the extent of the nickel-rich isothermal solidification zone through increasing temperature revealed an increase in the diffusion rate of the melting depressant elements (boron and silicon) to base metals. Although the extent of the athermal solidification zone was reduced, its microstructure was generally altered. New precipitations were formed with discontinuous morphologies and continuous morphologies.
Figure 16 shows the SEM micrograph of the athermal solidification zone of the 1050 °C/10 min sample with greater magnification. EDX analysis of phase A (
Table 6) showed that it was rich in nickel. EDX analysis of phase B (
Table 6) showed that it was rich in chromium. Thus, the formation of chromium-rich boride was confirmed. Regarding microstructural observations, the solidification mechanism in the 1050 °C/10 min sample was completely different from that of the 1050 °C/10 min sample. The nickel, chromium, silicon, and boron changed the solidification mode. As the ternary phase diagrams of nickel–chromium–boron [
38] and nickel–silicon–boron [
28] show, the first phase that grew dendritically from the solid/molten interlayer interface was the nickel-rich solid-solution proeutectic phase. The maximum solubilities of boron, chromium, and silicon in nickel are 0.3, 15, and 47 at. % (at eutectic Ni-Cr temperature), respectively; consequently, the unsteady-state rejection of these elements enriched the remaining melt of chromium, boron, and silicon. As the binary nickel–boron and chromium–boron phase diagrams [
37] show, the binary eutectic temperatures of the formation of the nickel-rich and chromium-rich borides were 1089 °C and 1260–1630 °C, respectively. Accordingly, since the formation temperature of chromium borides through the binary eutectic reaction was much higher than that of the binary nickel–boron eutectic reaction, there was a greater possibility of forming nickel-rich boride due to boron rejection. Consequently, according to the bonded sample at 1010 °C/10 min, the boron-rich residual melt was transformed into L→α(Ni) + Ni
3B + L(rem). The formation of nickel-rich boride enriched the remaining melt with chromium and silicon. The low solubility of chromium in nickel boride (10.11 at. %) and solid solution γ (18 at. %) enriched the remaining melted materials of chromium [
39]. Therefore, the conditions were present to perform a ternary eutectic reaction: L→α(Ni) + Ni
3B + CrB + L(rem). The formation of the boride phases resulted from the rejection of silicon into the remaining melt because of the insolubility of silicon in nickel- and chromium-rich borides. The less-than-unity distribution coefficient of silicon in nickel, along with a very small amount of boron in the remaining melt, caused the interlayer to be solidified due to the ternary eutectic reaction: L→α(Ni) + Ni
3B + Ni
6Si
2B. The X-ray diffraction pattern from the fracture surface of the 1050 °C/10 min sample is presented in
Figure 15b and it confirmed the formation of chromium-rich boride.
Figure 13c shows the SEM micrograph of the TLP-bonded sample at 1100 °C/10 min. The extent of the isothermal solidification zones on both sides of interface considerably increased. EDX analysis of ISZ
FSX-414 and ISZ
X-45 (E and F, respectively) showed that the enrichment of the isothermal solidification resulted more from chromium, cobalt, tungsten, and molybdenum elements transferred from the base metals to the bonding area (
Table 4). The solidification mode of the TLP-bonded sample at 1100 °C/10 min was similar to the 1050 °C/10 min one, but the intermetallic compounds were discontinuous at the bonding centerline.
Figure 13d shows the SEM micrograph of the sample bonding zone for 1150 °C/10 min. The most significant phenomenon in the sample was the absence of nickel- and chromium-rich intermetallic boride compounds, which were thoroughly eliminated, and the creation of a single-phase zone. EDX analysis of the solidification zone revealed the alloying of the interlayer melt with the base metals. Each alloying element solved with lower solubility in the interlayer melt and formed a single-phase solid solution. It is very important to precisely choose the bonding temperature and holding time in reaching the single-phase isothermal solidification zone because the isothermal solidification phase is controlled by the solid-state diffusion of melting point depressant elements into the base metals. Nickel-rich proeutectic γ dendrites moved to the bonding centerline as an epitaxial growth from the base metal/molten interlayer interface in the optimal temperature and time conditions. The melt solidification was completed by diffusing the solid state of the melting point depressant elements (boron and silicon) into the base metals through nickel-rich dendrites and the conditions were close to equilibrium; thus, the absence of solute rejection at the solid/liquid interface during the solidification stopped the formation of destructive phases. If there is a concentration gradient for the melting point depressant elements between the base metal and the molten interlayer, the isothermal solidification process will continue so that the concentration of melting point depressant elements in the bonding joint is equal to their solubility in the nickel-rich solid solution matrix at the bonding temperature. Diffusion of melting point depressant elements into the base metals resulted in increasing the melting point of the interlayer. Once the boron concentration reached the solubility of boron in nickel (0.3 at. %) and the chemical compound of the interlayer was solid Ni-17.42 Cr-16Co-3.5Fe-2.31Si-2.32W- 1.26Mn-0.16Mo, it became possible to apply the software Image J to identify the melting range of the nickel-rich solid solution in the bonding joint: the solidus temperature was 1162 °C and the liquidus temperature was 1372.39 °C.
As
Figure 8 indicates, increasing temperature caused microstructural changes in the bonding zone and removed the brittle intermetallic compounds, which were caused by the change in the solidification mode. At low temperatures, there was a low diffusion coefficient for the melting point depressant elements to diffuse to zones away from the base metals. These elements reacted with the strong boride-forming elements of the base metals at the adjacent surfaces and precipitated as boride. These borides acted like strong barriers and inhibited the long-range diffusion of the melting point depressant elements. Increasing the bonding temperature made it possible to obtain the conditions for breaking, decomposition, and partial dissolution of the boride deposition. Moreover, the diffusion coefficient of the melting point depressant elements was increased and, finally, the isothermal solidification rate was also increased [
40].
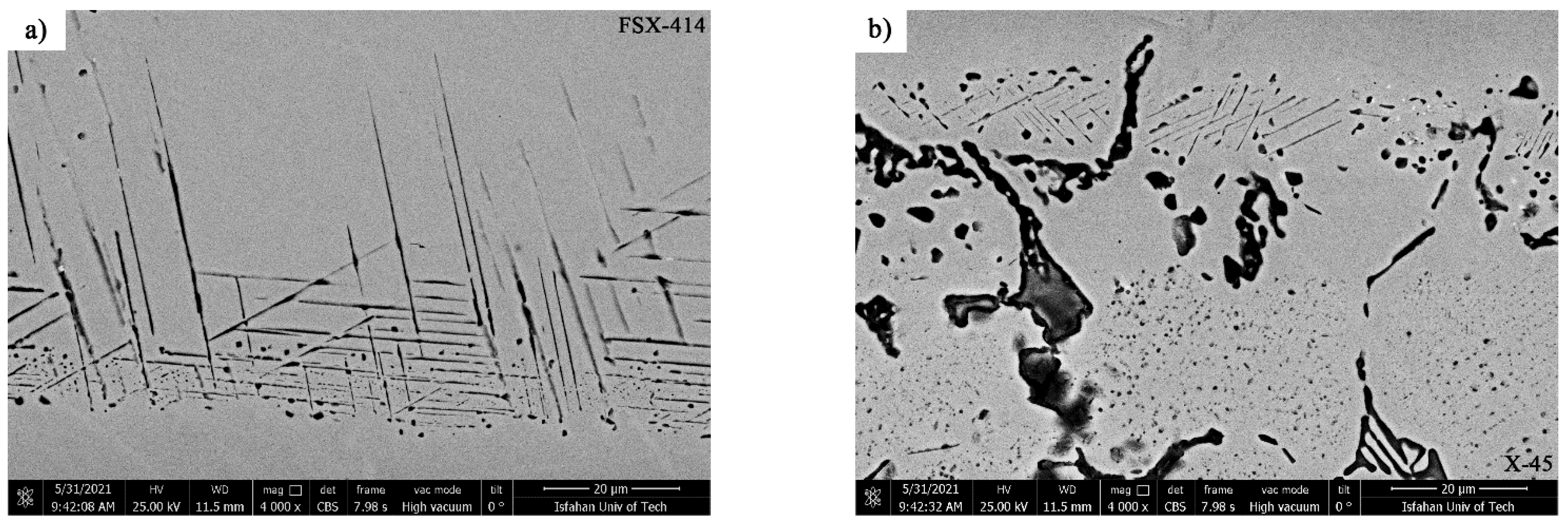
The effect of the chemical composition of the base metal directly influenced the microstructure of the diffusion-affected zone. The difference of 4% in weight between the chromium superalloys X-45 and FSX-414 made the microstructural slopes different in the side diffusion-affected zones of each base metal.
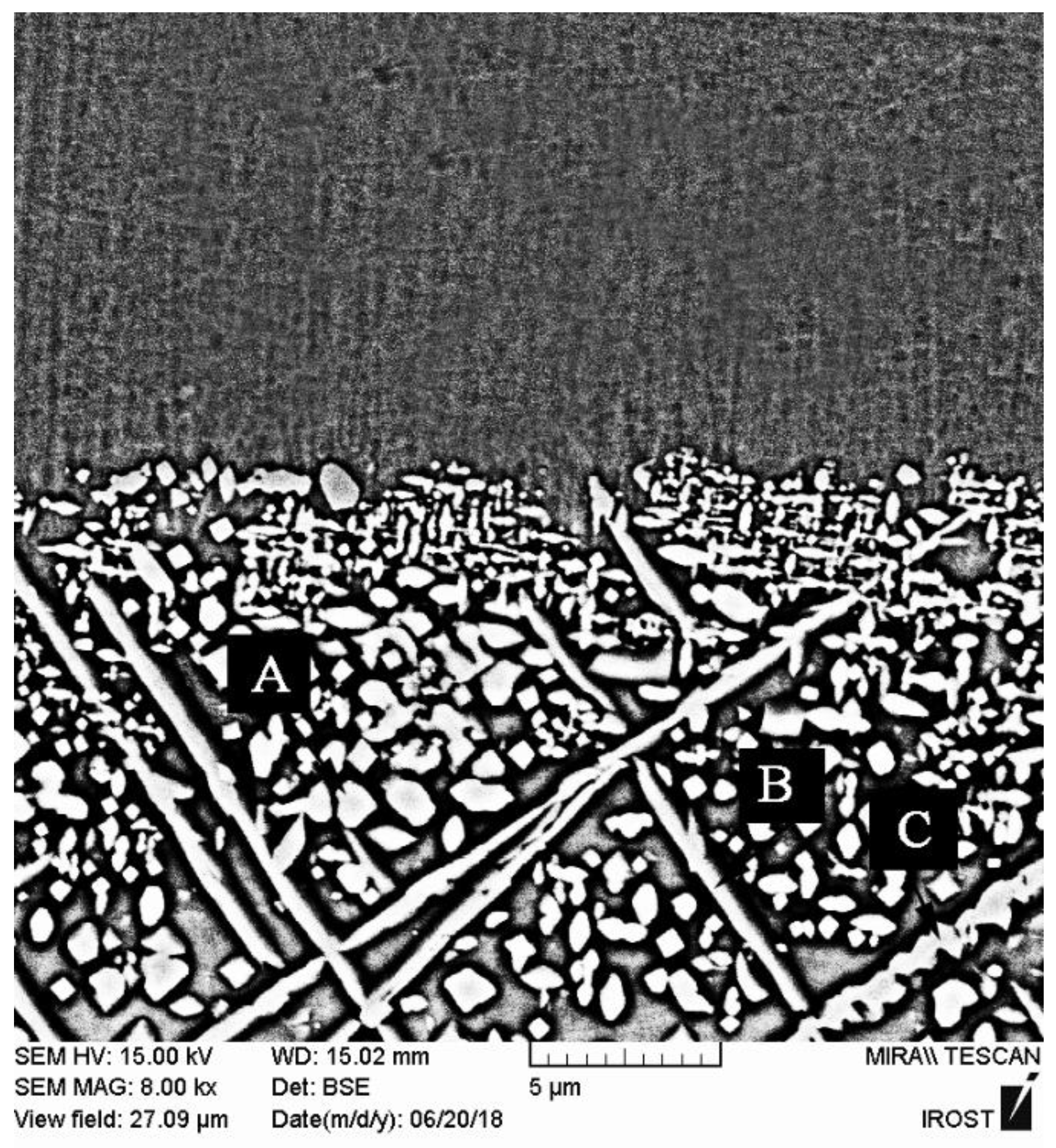
Figure 17 shows the SEM micrographs of the microstructures of the diffusion-affected zones of the X-45 superalloy side and the FSX-414 superalloy side in the TLP-bonded sample with the 1150 °C/10 min condition. As the figure clearly shows, the DAZ
FSX-414 was more extensive than the DAZ
X-45 (56.24 vs. 13.05 μm), which was possibly due to the higher diffusion coefficient of boron in X-45 than in FSX-414 because of the increasing chromium concentration. These precipitations may have possibly precipitated and been solved in the matrix. This area had a considerably high phase density; however, close examination reveals that the diffusion-affected zone also had a microstructural slope and could be classified into three sub-zones:
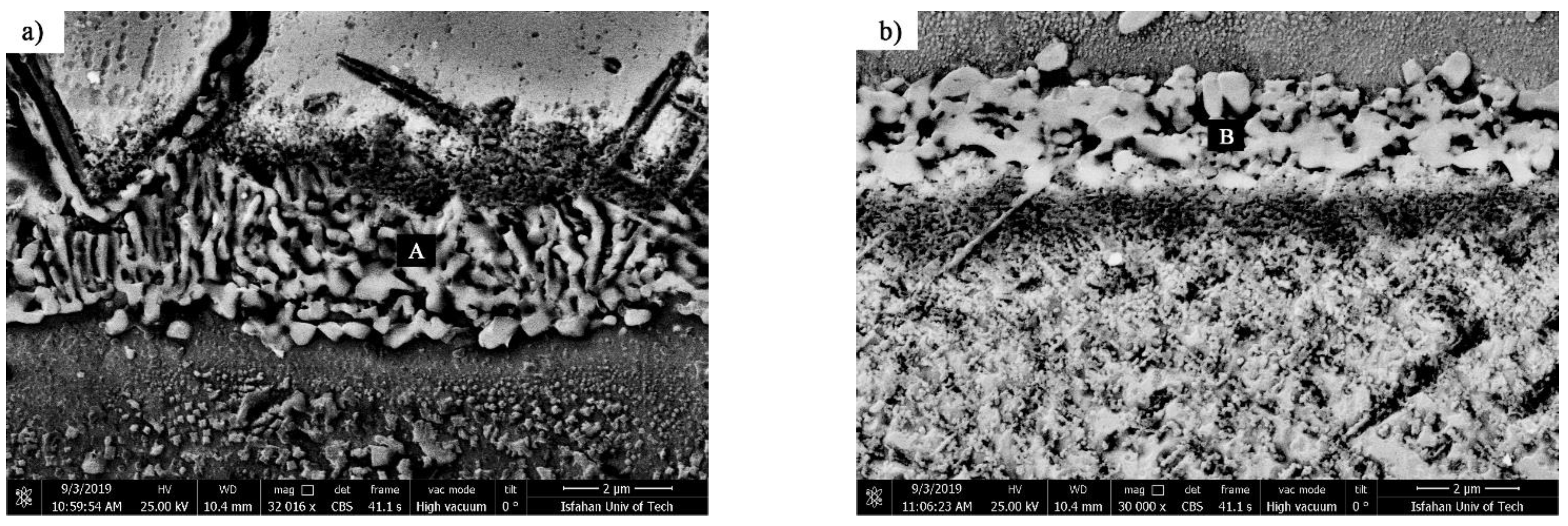
(1) Coarse block precipitation zone: These precipitations were provided in the interface of the isothermally solidified zone with the base metals with a coarse block morphology. These precipitations had a more complex nature compared to other zones in the diffusion-affected zone because they were formed adjacent to the isothermally solidified zone and made it difficult to investigate. The point element analysis of these precipitations revealed that they were rich in nickel, chromium, and cobalt. Also, they carried small amounts of heavy elements, such as tungsten and molybdenum, which, due to the diffusion of boron into the base metals, makes it possible to claim that they were nickel-chromium-cobalt-rich borides. This coarse block structure had flat edges in some zones of the interface and sharp edges in other zones, which indicate their dissolution in the melt of the interlayer.
Figure 18a,b show that the diffusion-affected zone was formed before the liquidus temperature of interlayer, which indicates the diffusion of the solid state during heating to the bonding temperature. These images show that the zone of coarse block precipitations was formed continuously and completely densely and allocated a significant part of the diffusion-affected zone to itself. This continuous and dense zone prevented the diffusion of boron and silicon to more inaccessible zones and stopped the spread of other zones. Increasing the bonding temperature at a constant time reduced the adherence of the coarse blocky precipitations in the zone so that it became discontinuous in the sample at 1150 °C/10 min (
Figure 17), which indicated their dissolution in the melt of the interlayer. Ruiz-Vargas et al. [
41] examined borides in the interface at temperatures lower and higher than the liquidus temperature of the amorphous BNi-2 interlayer. They stated that there was a low amount of boron available at low temperatures to diffuse the base metals and more inaccessible zones through the melt of the interlayer; hence, the first-generation interface borides were formed, which were caused by the accumulation of boron in the base metal/interlayer of the interface. Increasing the temperature completely melts the brazing alloy and more boron becomes available. It was necessary for the first-generation boride to be solved in order to establish the balance of the chemical composition at the molten interlayer and the base metal interfaces. The authors determined the interface borides as Ni
3B. The borides identified in this study contained nickel, chromium, and cobalt (
Table 7), and thus it is possible to claim that the interface borides were of the type (Ni, Cr, Co)
3B.
(2) Fine and needle-like mixed-precipitation zone: This zone of precipitation was directly located inside the base metals and adjacent to the surfaces of the joint, and it involved a mixture of fine precipitations among the needle-like ones with different lengths.
Figure 19 presents the SEM micrograph of the fine and needle-like mixed-precipitation zone in a sample bonded with the 1100 °C/10 min condition. Fine precipitations were scattered between the blades of the needle-like ones. EDX analysis of the fine particle showed that they were rich in cobalt, chromium, tungsten, and molybdenum (A in
Table 8). Additionally, needle-like precipitations were formed with two, continuous and discontinuous, morphologies and their EDX analysis revealed that they had the same chemical compositions as the fine particles (B and C in
Table 8). Consequently, these borides were rich in cobalt–chromium–tungsten–molybdenum. There were many sites inclined to concentration gradients for boron diffusion, including grain boundaries, interdendritic sites, crystalline defects (dislocations and stacking faults), and bulk material [
42]. After passing the boron element from the diffusion barrier of the coarse borides of the interface, it was absorbed by the stress field of the crystalline defects in order to reduce the free energy of the system. If boron is adsorbed by the stress fields of the dislocations, it will be partitioned in these linear defects, deform the crystal lattice of the base material, and ultimately, cause the precipitation of fine borides. If boron is adsorbed by the stress fields of stacking fault defects, it will precipitate as continuous needle-like borides with a specific crystallographic direction relative to the interface of the bonding zone/metal base. Wang et al. [
10] examined the formation of borides in a cobalt-based single-crystal superalloy reinforced with the γ′phase during the bonding of the transient liquid phase. They stated that, in the transient liquid phase bonding, boron diffused from the interlayer melt to the alloy matrix and was absorbed by the stacking fault defects. When the stacking fault defects were completely filled with boron, it reacted with the alloying elements cobalt, chromium, tungsten, and molybdenum to reduce the system’s free energy. Therefore, needle-like borides were formed. Unbalanced boron segregation in grain boundaries leads to the formation of discontinuous needle-like borides with the same chemical composition as continuous needle-like borides.
Figure 8 shows that increasing the bonding temperature increased the area of the fine and needle-like mixed zone, which was due to the division of the interface boride barrier and the possibility of boron diffusion into more inaccessible zones.
(3) Needle-like precipitation zone: The large needle-like precipitations comprise the last zone where the boron element diffused and formed secondary phase precipitations. The formation of these precipitations showed that the boron element had diffused from the molten zone of the interlayer to zones farther from the interface through the defects of the crystal lattice and formed needle-like precipitations by reacting with strong boride-forming elements. Some of these precipitations started continuously from the fine and needle-like mixed zone and became discontinuous by progressing to the base metal at the end, which indicated the absence of sufficient boron.
Lerner et al. [
43] performed X-ray diffraction and electrical resistance measurement and noted that the maximum solubility of boron in cobalt at 1000 °C is about 0.2 at. %, which is distinctly reduced when the temperature is increased such that the solubility of boron in cobalt reaches 0.16 at. % at 1095 °C. According to the cobalt–boron binary phase diagram, there is a possibility of forming borides of the cobalt system including CoB, Co
2B, and Co
3B. Bakhtiari at al. [
17] applied an X-ray spectroscopy method based on the wavelength to quantify the boron element in the borides of the diffusion-affected zone. They recognized the amount of boron detected in the diffusion-affected zone as about 1 wt.%, which, referring to the cobalt–boron binary phase diagram, enables to state that the formation of Co
2B and Co
3B borides is more likely in this situation than the formation of complex (Co, Cr, W, Mo)
2B and (Co, Cr, W, Mo)
3B borides is in the presence of tungsten and molybdenum. The presence of these secondary phase compounds in the adjacent surfaces of the joint influenced the mechanical properties of the joint. Bakhtiari et al. [
44] stated with regard to the bonding of the cobalt-based FSX-414 superalloy with a BNi-9 interlayer at 1150 °C that the fracture happened through nucleation and propagation of the crack in the diffusion-affected zone. Also, depletion of chromium in adjacent zones through the consumption of secondary phase precipitations reduces corrosion resistance, particularly at high temperatures. Consuming alloying elements of base metals in area adjacent to the bonding zone reduces the alloying elements, leading to reinforcement with a solid solution and carbide deposition (such as tungsten, molybdenum, and chromium) [
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58]. Consequently, it is essential to apply post-bond heat treatment to remove secondary phase compounds in the diffusion-affected zones.