Wasted Biomaterials from Crustaceans as a Compliant Natural Product Regarding Microbiological, Antibacterial Properties and Heavy Metal Content for Reuse in Blue Bioeconomy: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Samples and the Powdering Procedure
2.2. Raman Spectroscopy
2.3. Heavy Metals Analysis
2.4. Microbiological Analysis
2.4.1. Bacterial Cultures
2.4.2. Antibacterial Properties
3. Results
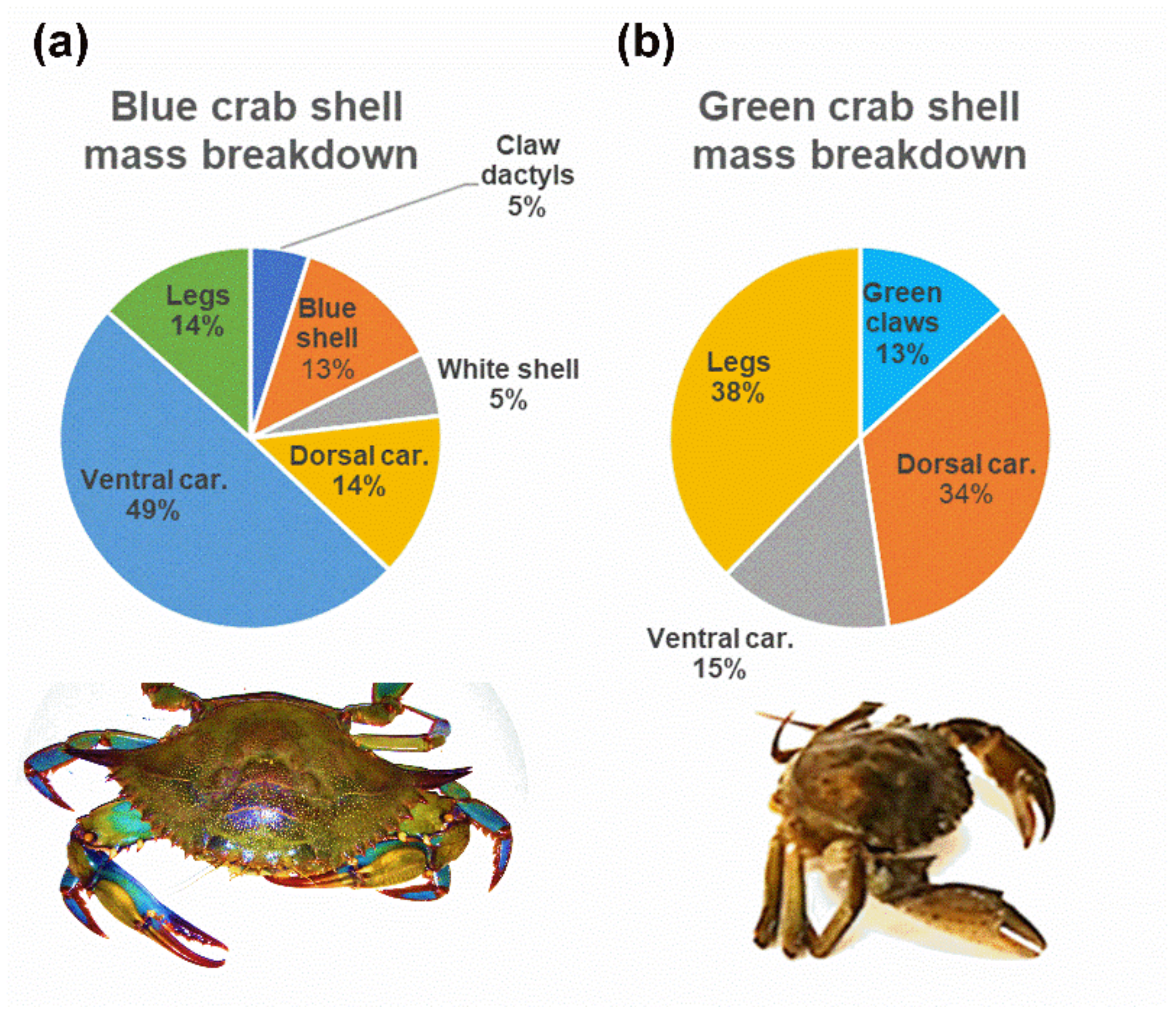
3.1. Crab Shell Powdering Technical Aspects
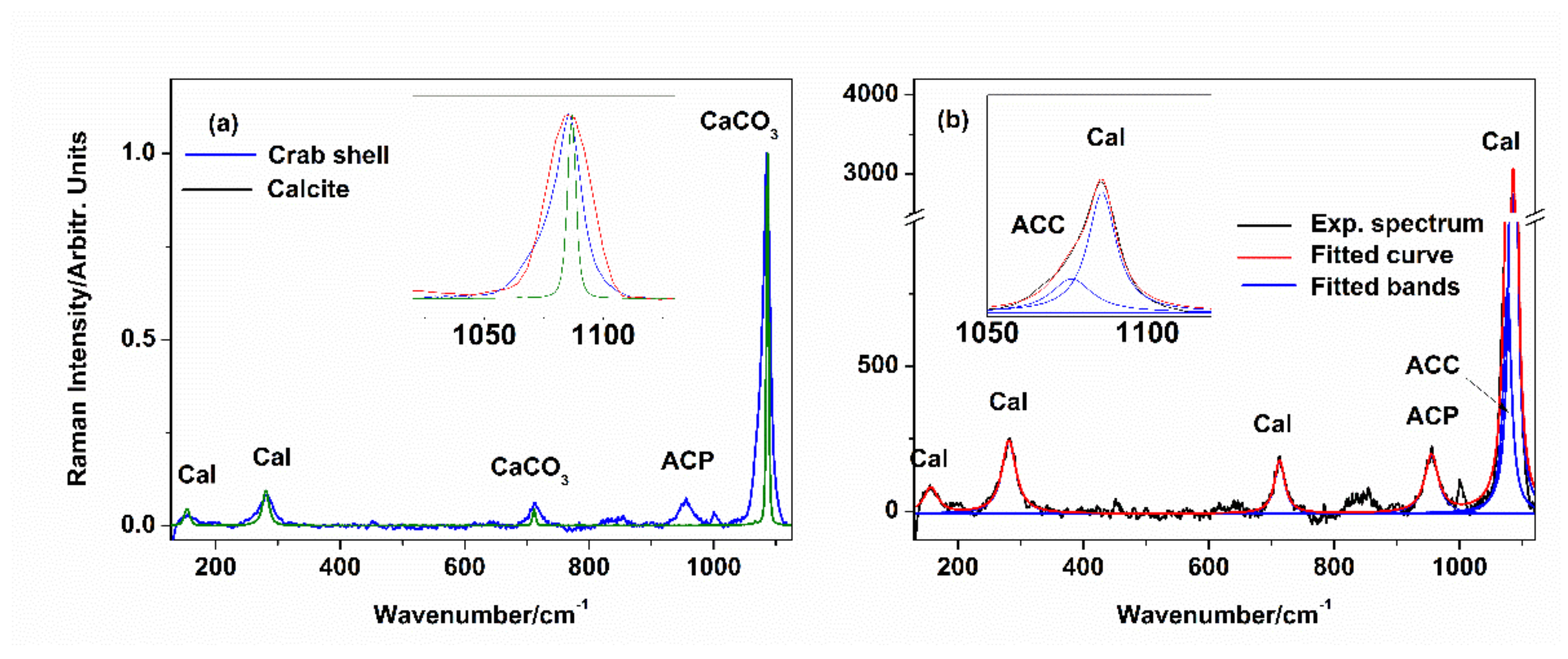
3.2. Raman Spectroscopy Tracking of Mineral Composition
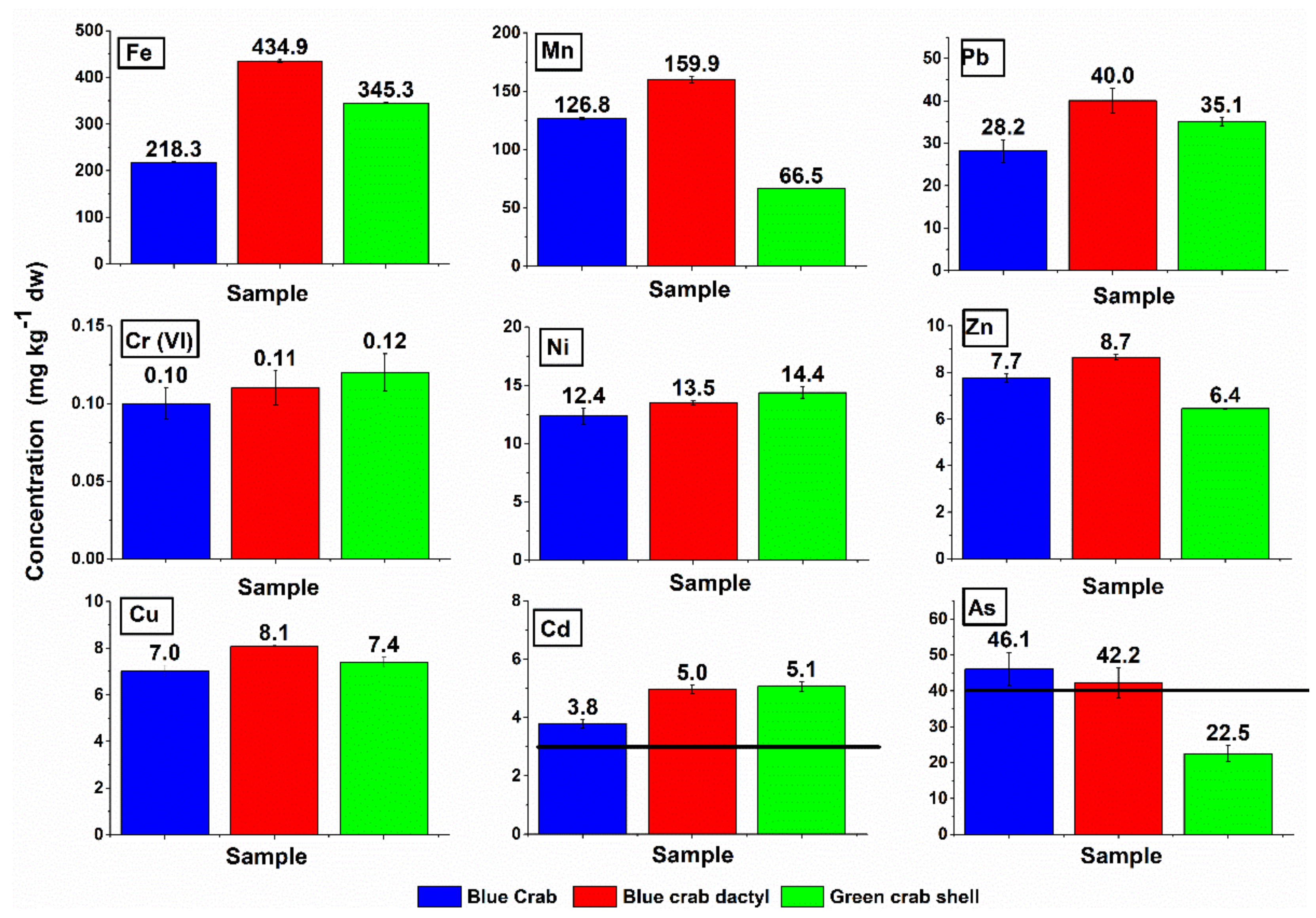
3.3. Heavy Metals in Crab Shells
3.4. Microbiology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzi, V.; Gubitosa, J.; Fini, P.; Romita, R.; Nuzzo, S.; Cosma, P. Chitosan Biopolymer from crab shell as recyclable film to remove/recover in batch ketoprofen from water: Understanding the factors affecting the adsorption process. Materials 2019, 12, 3810. [Google Scholar] [CrossRef] [Green Version]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zheng, G.; Li, W.; McDowell, M.T.; Seh, Z.; Liu, N.; Lu, Z.; Cui, Y. Crab Shells as sustainable templates from nature for nanostructured battery electrodes. Nano Lett. 2013, 13, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Nekvapil, F.; Aluas, M.; Barbu-Tudoran, L.; Suciu, M.; Bortnic, R.-A.; Glamuzina, B.; Pinzaru, S.C. From blue bioeconomy toward circular economy through high-sensitivity analytical research on waste blue crab shells. ACS Sustain. Chem. Eng. 2019, 7, 16820–16827. [Google Scholar] [CrossRef]
- Nekvapil, F.; Pinzaru, S.C.; Barbu–Tudoran, L.; Suciu, M.; Glamuzina, B.; Tamaș, T.; Chiș, V. Color-specific porosity in double pigmented natural 3d-nanoarchitectures of blue crab shell. Sci. Rep. 2020, 10, 3019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekvapil, F.; Glamuzina, B.; Barbu-Tudoran, L.; Suciu, M.; Tămaş, T.; Pinzaru, S.C. Promoting hidden natural design templates in wasted shells of the mantis shrimp into valuable biogenic composite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119223. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Naher, U.A.; Shamshuddin, J.; Ismail, M.R. Effects of biochar and ground magnesium limestone application, with or without bio-fertilizer addition, on biochemical properties of an acid sulfate soil and rice yield. Agronomy 2020, 10, 1100. [Google Scholar] [CrossRef]
- Hua, K.-H.; Wang, H.-C.; Chung, R.-S.; Hsu, J.-C. Calcium carbonate nanoparticles can enhance plant nutrition and insect pest tolerance. J. Pestic. Sci. 2015, 40, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Ah, M.; Horiuchi, T.; Miyagawa, S.; Ali, M. Effects of soil amendment with crab shell on the growth and nodulation of soybean plants (Glycine max Merr.). Plant Prod. Sci. 1998, 1, 119–125. [Google Scholar] [CrossRef]
- Adiloglu, A.; Adiloglu, S. Amendment of acid soils using crab shell powder. Asian J. Chem. 2008, 20, 2156–2162. [Google Scholar]
- Sarva, S.A.K. Effect of freshwater crab shell fog as organic fertilizer to increase plants of cucurbitaceae growth dramatically. Agrotechnology 2015, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- European Parliament and Council. Regulation (EU) 2019/1009 of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. OJ L 170, 25.6.2019, pp. 1–114. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1009 (accessed on 20 October 2020).
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. Water Quality—Determination of Chromium(VI)—Spectrometric Method Using 1,5-Diphenylcarbazide; ISO 11083:1994; ISO: Geneva, Switzerland; Available online: https://www.iso.org/standard/19070.html (accessed on 16 April 2021).
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrobial Susceptibility Testing; EUCAST: Växjö, Sweden, 2021. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). EUCAST Reading Guide for Broth Microdilution; EUCAST: Växjö, Sweden, 2021. [Google Scholar]
- Behrens, G.; Kuhn, L.T.; Ubic, R.; Heuer, A.H. Raman spectra of vateritic calcium carbonate. Spectrosc. Lett. 1995, 28, 983–995. [Google Scholar] [CrossRef]
- Katsikini, M. Detailed spectroscopic study of the role of Br and Sr in coloured parts of the Callinectes sapidus crab claw. J. Struct. Biol. 2016, 195, 1–10. [Google Scholar] [CrossRef]
- Perrin, J.; Vielzeuf, D.; Laporte, D.; Ricolleau, A.; Rossman, G.R.; Floquet, N. Raman characterization of synthetic magnesian calcites. Am. Mineral. 2016, 101, 2525–2538. [Google Scholar] [CrossRef]
- Wang, N.; Hamm, L.M.; Bodnar, R.J.; Dove, P.M. Raman spectroscopic characterization of the magnesium content in amorphous calcium carbonates. J. Raman Spectrosc. 2011, 43, 543–548. [Google Scholar] [CrossRef]
- Bukvić, V.; Dušak, V.; Kučinić, M.; Delic, A.; Dulčić, J.; Senta, I.; Glamuzina, B. Arsenic in the water, sediment and fish in the Neretva River Delta, Croatia. J. Appl. Ichthyol. 2010, 27, 908–911. [Google Scholar] [CrossRef]
- Çoğun, H.Y.; Firat, Ö.; Aytekin, T.; Firidin, G.; Varkal, H.; Temiz, Ö.; Kargin, F. Heavy metals in the blue crab (Callinectes sapidus) in Mersin Bay, Turkey. Bull. Environ. Contam. Toxicol. 2017, 98, 824–829. [Google Scholar] [CrossRef]
- Gutiérrez-Peña, L.V.; Picón, D.; Gutiérrez, I.A.; Prada, M.; Carrero, P.E.; Delgado-Cayama, Y.J.; Gutiérrez, E.O.; Morón, M.; Gon-zález, C.E.; Lara, N.D.; et al. Heavy metals in soft tissue of blue crab (Callinectes sapidus) of Puerto Concha, Colon Municipality, Zulia State. Av. Biomed. 2018, 7, 17–22. [Google Scholar]
- Abdel-Salam, H.A. Assessment of biochemical compositions and mineral contents of carapace of some important commercially crustaceans and mollusks organisms from Egyptian and Saudi Arabia coasts as a new animal feed. Am. J. BioSci. 2013, 1, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Nakal-Chidiac, A.; García, O.; García-Fernández, L.; Martín-Saavedra, F.M.; Sánchez-Casanova, S.; Escudero-Duch, C.; Román, J.S.; Vilaboa, N.; Aguilar, M.R. Chitosan-stabilized silver nanoclusters with luminescent, photothermal and antibacterial properties. Carbohydr. Polym. 2020, 250, 116973. [Google Scholar] [CrossRef]
- Rasul, R.M.; Muniandy, M.T.; Zakaria, Z.; Shah, K.; Chee, C.F.; Dabbagh, A.; Rahman, N.A.; Wong, T.W. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr. Polym. 2020, 250, 116800. [Google Scholar] [CrossRef]
- Metin, C.; Alparslan, Y.; Baygar, T.; Baygar, T. Physicochemical, microstructural and thermal characterization of chitosan from blue crab shell waste and its bioactivity characteristics. J. Polym. Environ. 2019, 27, 2552–2561. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Rinaudo, M.; Jellouli, K.; Narsi, M. Characterization and in vitro evaluation of cytotoxicity, antimicrobial and and antioxidant activities of chitosans extracted from three different marine sources. Appl. Biochem. Biotechnol. 2015, 117, 18–35. [Google Scholar] [CrossRef]
- Sharp, R.G. A Review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Sharov, A.F.; Volstad, J.H.; Davis, G.R.; Davis, B.K.; Lipcius, R.N.; Montane, M.M. Abundance and exploitation rate of the Blue crab (Callinectes sapidus) in Chesapeake Bay. Bull. Mar. Sci. 2003, 2, 543–565. [Google Scholar]
- Villegas-Hernandez, H.; Poot-Lopez, G.R.; Lopez-Rocha, J.A.; Gonzalez-Salas, C.; Gullien-Hernandez, S. Abundance and catchability estimates of the Atlantic blue crab Callinectes sapidus based on mark-recapture data from the northern Yu-catan Peninsula. J. Mar. Biol. Assoc. 2017, UK 98, 1455–1463. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Antoniadou, C. Abundance and population structure of the blue crab Callinectes sapidus (Decapoda, Portunidae) in Thermaikos Gulf (Methoni Bay), northern Aegean Sea. Crustaceana 2018, 91, 641–657. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- FAO. The State of World Fishery and Aquaculture 2020 (SOFIA); Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Eggleston, D.B.; Millstein, E.; Plaia, G. Timing and route of migration of mature female blue crabs in a tidal estuary. Biol. Lett. 2015, 11, 20140936. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. On the Atlantic blue crab (Callinectes sapidus Rathbun 1896) in southern European coastal waters: Time to turn a threat into a resource? Fish. Res. 2017, 194, 1–8. [Google Scholar] [CrossRef]

| Bacterial Strain | Sampling | Limit | ||
|---|---|---|---|---|
| n | c | CFU/mL | ||
| Salmonella spp. | 1 | 0 | 0 | Absence |
| E. coli | 1 | 1 | 3 | 100 CFU |
| Enterococcus spp. | 1 | 1 | 0 | 100 CFU |
| Powder | MIC (mg mL−1) | ||||
|---|---|---|---|---|---|
| Bacterial Strain | E. coli | P. aeruginosa | E. faecalis | S. aureus | |
| Blue crab claw shell | 1.92 | 2.92 | 1.57 | 2.56 | |
| Green crab (entire shell) | 0.41 | 0.003 | 6.96 | 6.9 | |
| Blue crab dorsal carapace | 2.3 | 7.17 | 1.41 | 0.76 | |
| Rinsed shells | - | 2.43 | 6.7 | 3.09 | |
| Waste shells | - | 2.64 | - | 3.25 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekvapil, F.; Ganea, I.-V.; Ciorîță, A.; Hirian, R.; Ogresta, L.; Glamuzina, B.; Roba, C.; Cintă Pinzaru, S. Wasted Biomaterials from Crustaceans as a Compliant Natural Product Regarding Microbiological, Antibacterial Properties and Heavy Metal Content for Reuse in Blue Bioeconomy: A Preliminary Study. Materials 2021, 14, 4558. https://doi.org/10.3390/ma14164558
Nekvapil F, Ganea I-V, Ciorîță A, Hirian R, Ogresta L, Glamuzina B, Roba C, Cintă Pinzaru S. Wasted Biomaterials from Crustaceans as a Compliant Natural Product Regarding Microbiological, Antibacterial Properties and Heavy Metal Content for Reuse in Blue Bioeconomy: A Preliminary Study. Materials. 2021; 14(16):4558. https://doi.org/10.3390/ma14164558
Chicago/Turabian StyleNekvapil, Fran, Iolanda-Veronica Ganea, Alexandra Ciorîță, Razvan Hirian, Lovro Ogresta, Branko Glamuzina, Carmen Roba, and Simona Cintă Pinzaru. 2021. "Wasted Biomaterials from Crustaceans as a Compliant Natural Product Regarding Microbiological, Antibacterial Properties and Heavy Metal Content for Reuse in Blue Bioeconomy: A Preliminary Study" Materials 14, no. 16: 4558. https://doi.org/10.3390/ma14164558
APA StyleNekvapil, F., Ganea, I.-V., Ciorîță, A., Hirian, R., Ogresta, L., Glamuzina, B., Roba, C., & Cintă Pinzaru, S. (2021). Wasted Biomaterials from Crustaceans as a Compliant Natural Product Regarding Microbiological, Antibacterial Properties and Heavy Metal Content for Reuse in Blue Bioeconomy: A Preliminary Study. Materials, 14(16), 4558. https://doi.org/10.3390/ma14164558








