Biological Evaluation of a New Sodium-Potassium Silico-Phosphate Glass for Bone Regeneration: In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Glass Production
2.2. In Vitro Studies
2.2.1. In Vitro Cytocompatibility Evaluation
2.2.2. Pro-Osteogenic Efficacy
2.2.3. Statistical Analysis
2.3. Acute Toxicity Tests: Determination of LD50 Dose
2.4. In Vivo Biocompatibility Tests
3. Results and Discussion
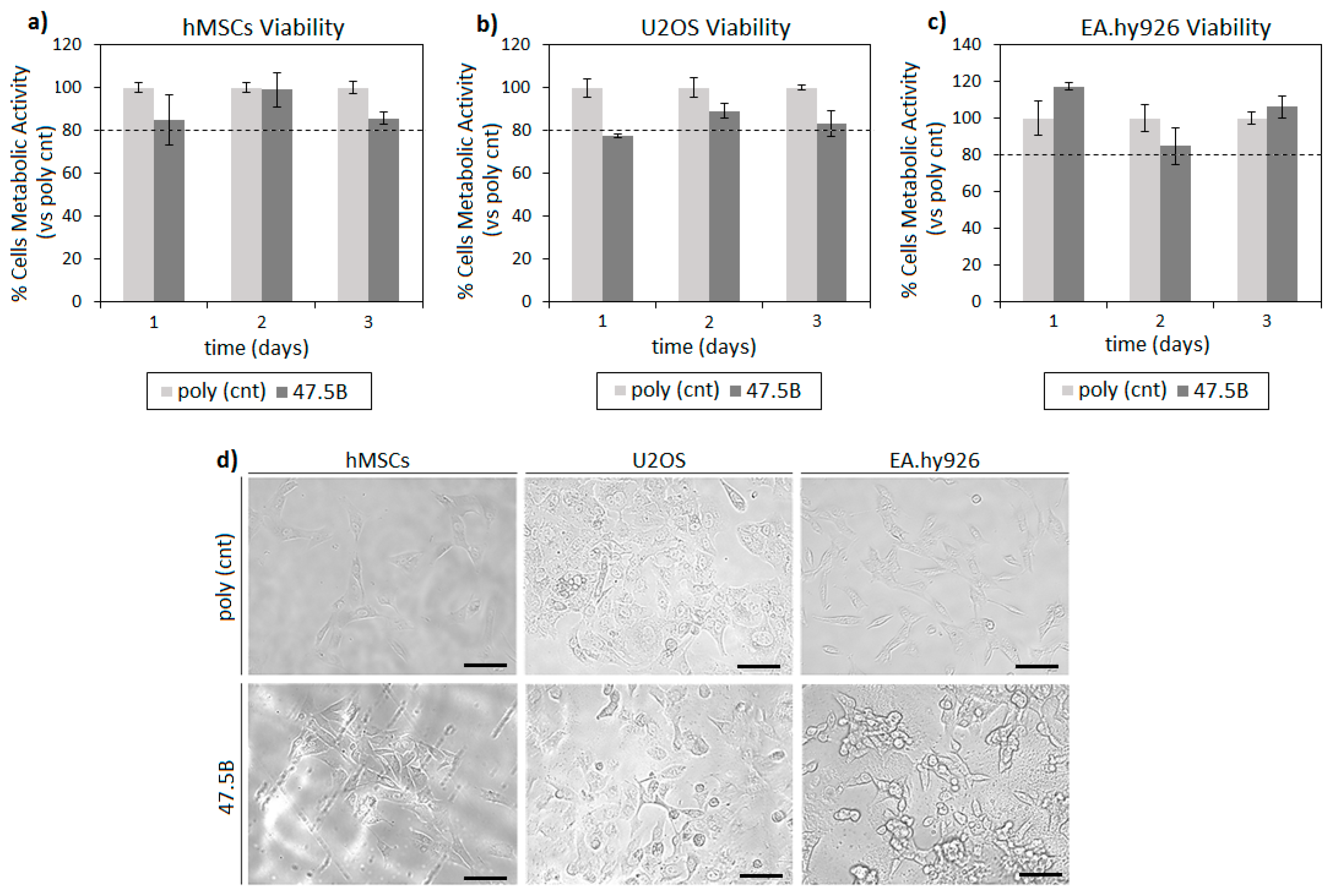
3.1. In Vitro Cytocompatibility Evaluation
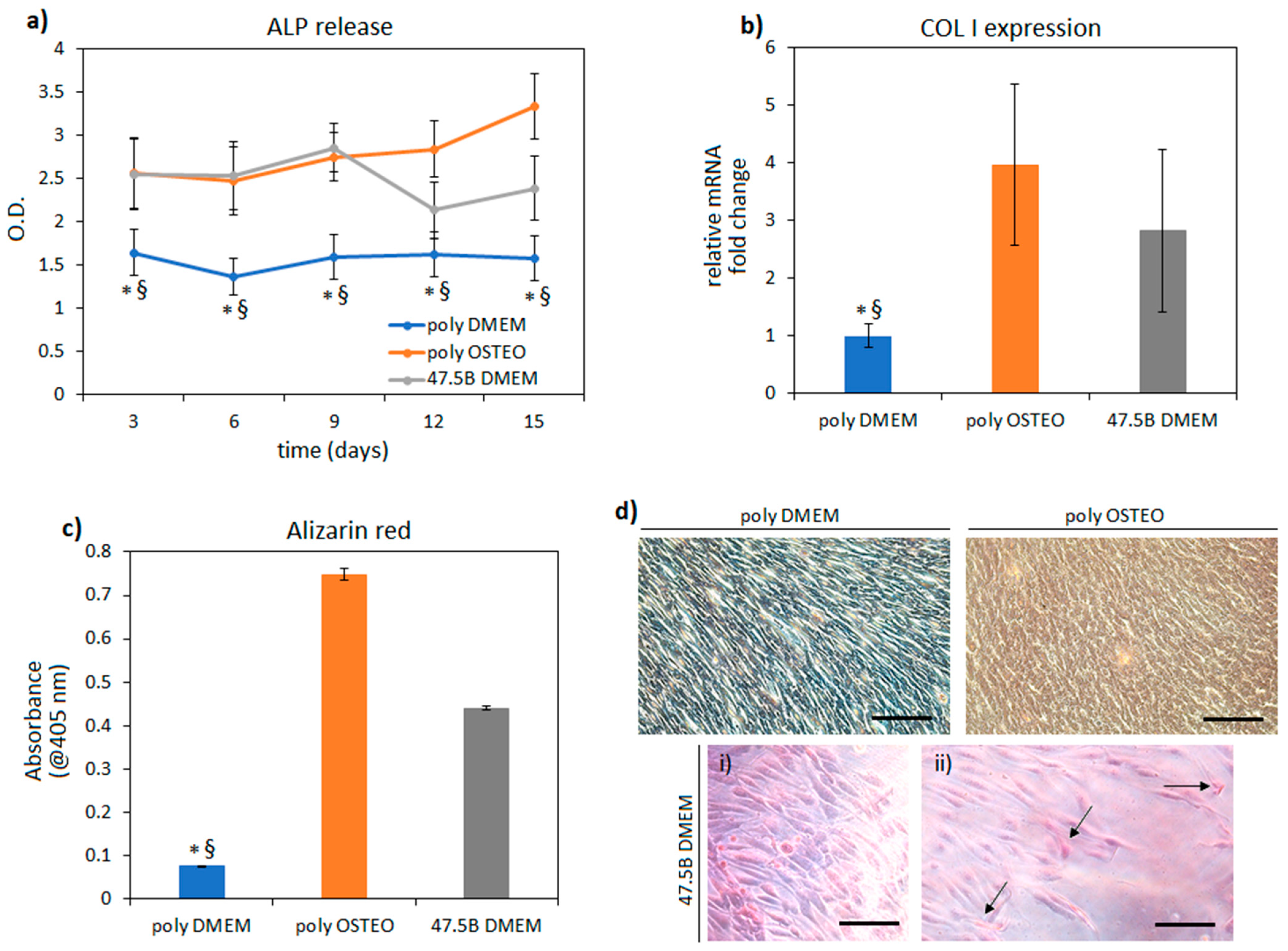
3.2. In Vitro Pro-Osteogenic Evaluation
3.3. Determination of LD50 Dose
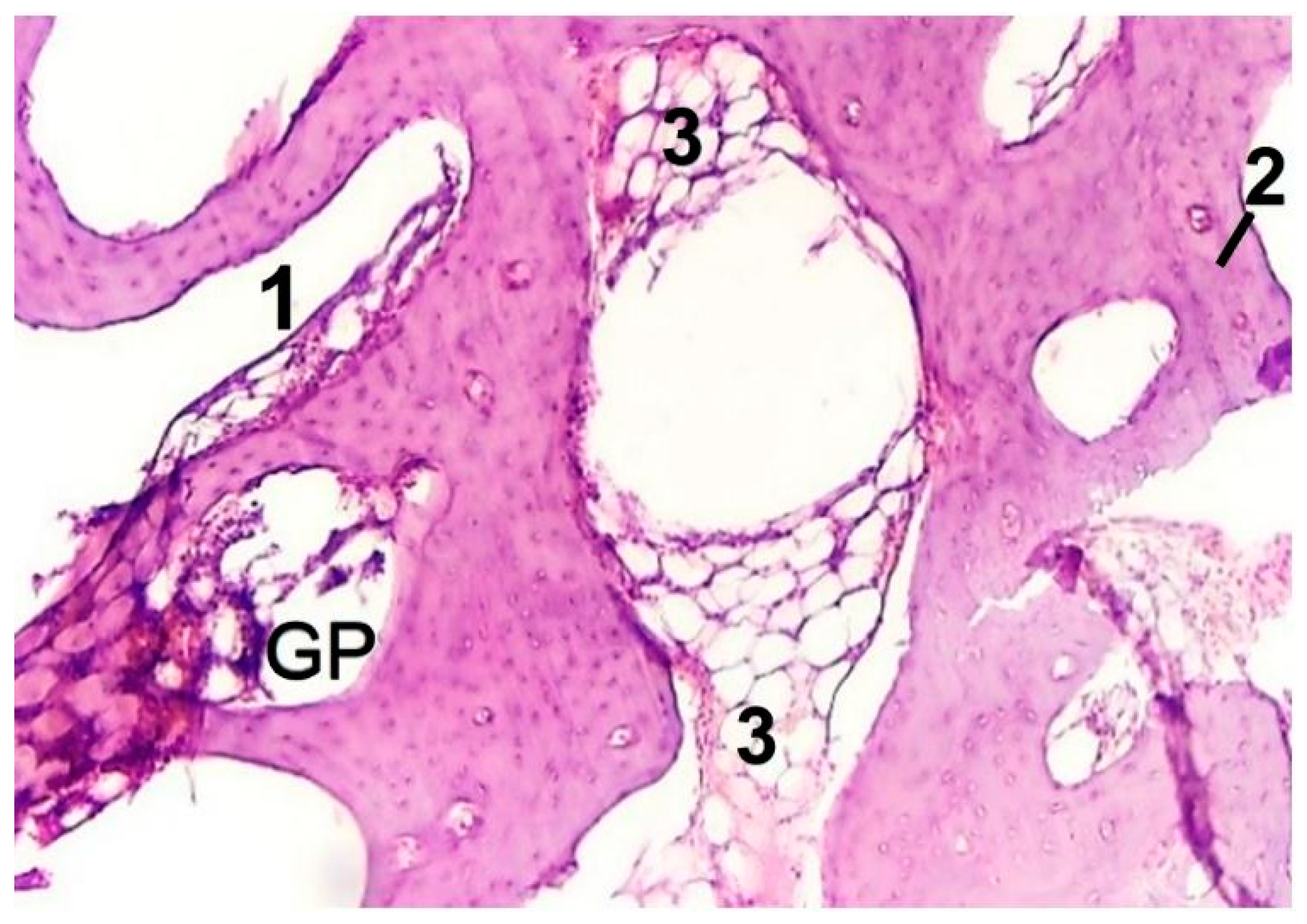
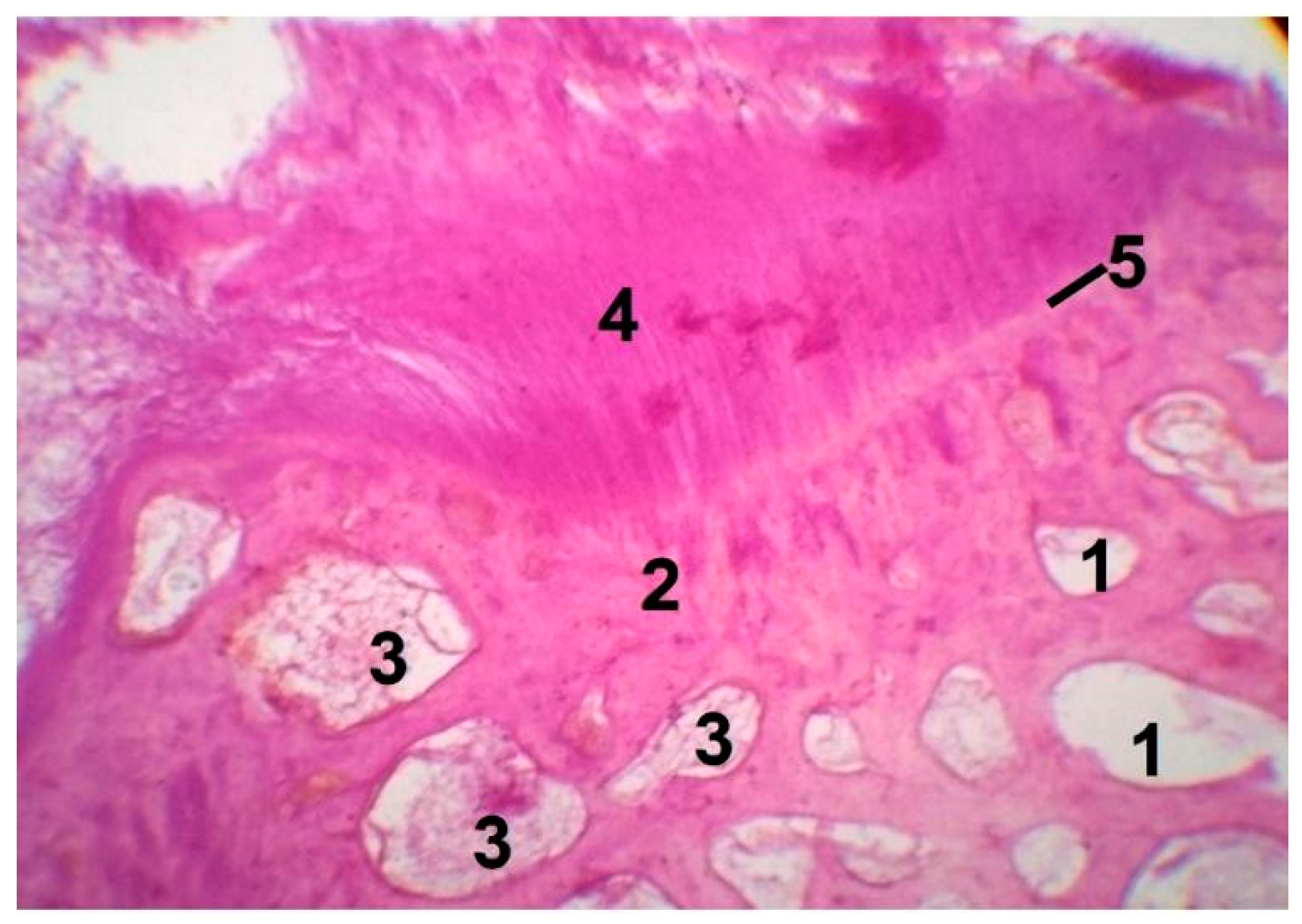
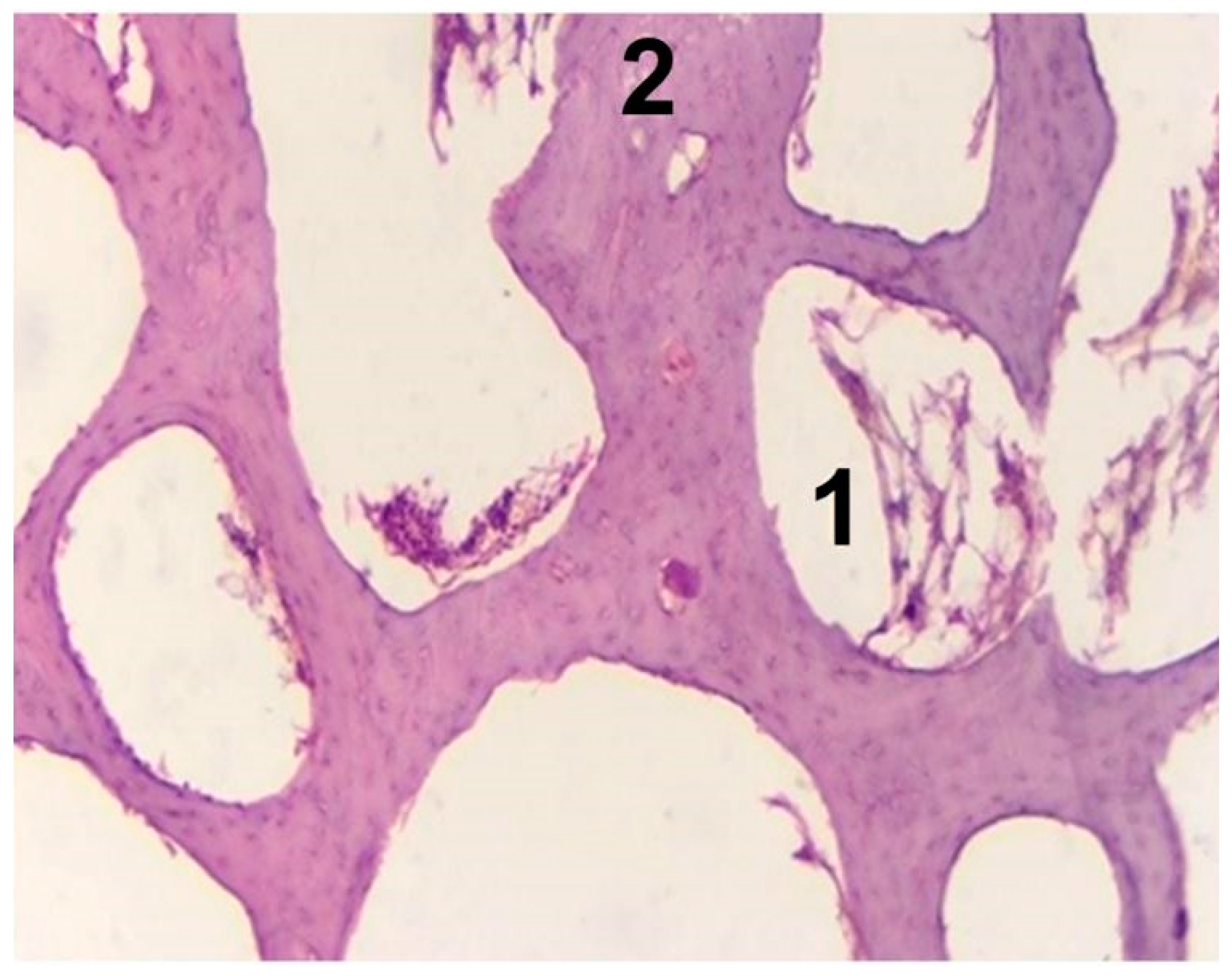
3.4. In Vivo Biocompatibility Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 452, 15–30. [Google Scholar] [CrossRef]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and bioactive glasses and their impact on healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K., Jr. Bonding Mechanisms at the Interface of Ceramic Prosthetic Materials. J. Biomed. Mater. Res. 1971, 2, 117–141. [Google Scholar] [CrossRef]
- Beckham, C.A.; Greenlee, T.K., Jr.; Crebo, A.R., Jr. Bone Formation at a Ceramic Implant Interface. Calcif. Tissue Res. 1971, 8, 165–171. [Google Scholar] [CrossRef]
- Greenlee, T.K., Jr.; Beckham, C.A.; Crebo, A.R.; Malmborg, J.C. Glass Ceramic Bone Implants. J. Biomed. Mater. Res. 1972, 6, 235–244. [Google Scholar] [CrossRef]
- Hench, L.L. Opening paper 2015 -Some comments on Bioglass: Four Eras of Discovery and Development. Biomed. Glasses 2015, 1, 1235–1244. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In Genotoxicity: A Predictable Risk to Our Actual World; Larramendy, M.L., Soloneski, S., Eds.; BoD—Books on Demand: Norderstedt, Germany, 2018; pp. 1–17. [Google Scholar]
- Jablonská, E.; Horkavcová, D.; Rohanová, D.; Brauer, D.S. Review of in vitro cell culture testing methods for bioactive glasses and other biomaterials for hard tissue regeneration. J. Mater. Chem. B 2020, 8, 10941–10953. [Google Scholar] [CrossRef]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses entering the mainstream. Drug Discov. Today 2018, 23, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Erhirhie, E.O.; Ihekwereme, C.P.; Ilodigwe, E.E. Advances in acute toxicity testing: Strengths, weaknesses and regulatory acceptance. Interdiscip. Toxicol. 2018, 11, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Chinedu, E.; Arome, D.; Ameh, F.S. A new method for determining acute toxicity in animal models. Toxicol. Int. 2013, 20, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Walum, E. Acute oral toxicity. Environ. Health Perspect. 1998, 106, 497–503. [Google Scholar]
- Akhila, J.S.; Deepa, S.; Alwar, M.C. Acute toxicity studies and determination of median lethal dose. Curr. Sci. 2007, 93, 917–920. [Google Scholar]
- Vandenberg, L.N.; Welshons, W.V.; Vom Saal, F.S.; Toutain, P.-L.; Peterson Myers, J. Should oral gavage be abandoned in toxicity testing of endocrine disruptors? Environ. Health 2014, 13, 46. [Google Scholar] [CrossRef]
- Verné, E.; Bretcanu, O.; Balagna, C.; Bianchi, C.L.; Cannas, M.; Gatti, S.; Vitale-Brovarone, C. Early stage reactivity and in vitro behavior of silica-based bioactive glasses and glass-ceramics. J. Mater. Sci. Mater. Med. 2009, 20, 75–87. [Google Scholar] [CrossRef]
- Fiume, E.; Migneco, C.; Verné, E.; Baino, F. Comparison Between Bioactive Sol-Gel and Melt-Derived Glasses/Glass-Ceramics Based on the Multicomponent SiO2-P2O5-CaO-MgO-Na2O-K2O System. Materials 2020, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Verné, E.; Baino, F. Crystallization behavior of SiO2–P2O5–CaO–MgO–Na2O–K2O bioactive glass powder. Biomed. Glasses 2019, 5, 46–52. [Google Scholar] [CrossRef]
- Fiume, E.; Serino, G.; Bignardi, C.; Verné, E.; Baino, F. Bread-Derived Bioactive Porous Scaffolds: An Innovative and Sustainable Approach to Bone Tissue Engineering. Molecules 2019, 24, 2954. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Schiavi, A.; Orlygsson, G.; Bignardi, C.; Verné, E.; Baino, F. Comprehensive assessment of bioactive glass and glass-ceramic scaffold permeability: Experimental measurements by pressure wave drop, modelling and computed tomography-based analysis. Acta Biomater. 2021, 119, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Tulyaganov, D.; Ubertalli, G.; Vernè, E.; Baino, F. Dolomite-Foamed Bioactive Silicate Scaffolds for Bone Tissue Repair. Materials 2020, 13, 628. [Google Scholar] [CrossRef]
- Baino, F.; Barberi, J.; Fiume, E.; Orlygsson, G.; Massera, J.; Verné, E. Robocasting of Bioactive SiO2-P2O5-CaO-MgO-Na2O-K2O Glass Scaffolds. J. Healthc. Eng. 2019, 2019, 5153136. [Google Scholar] [CrossRef]
- Barberi, J.; Baino, F.; Fiume, E.; Orlygsson, G.; Nommeots-Nomm, A.; Massera, J.; Verné, E. Robocasting of SiO2-Based Bioactive Glass Scaffolds with Porosity Gradient for Bone Regeneration and Potential Load-Bearing Applications. Materials 2019, 12, 2691. [Google Scholar] [CrossRef]
- Barberi, J.; Nommeots-Nomm, A.; Fiume, E.; Verné, E.; Massera, J.; Baino, F. Mechanical characterization of pore-graded bioactive glass scaffolds produced by robocasting. Biomed. Glasses 2019, 5, 140–147. [Google Scholar] [CrossRef]
- Fiume, E.; Serino, G.; Bignardi, C.; Verné, E.; Baino, F. Sintering Behavior of a Six-Oxide Silicate Bioactive Glass for Scaffold Manufacturing. Appl. Sci. 2020, 10, 8279. [Google Scholar] [CrossRef]
- Berlitz, D.L.; Giovenardi, M.; Charles, J.-F.; Fiúza, L.M. Toxicity intraperitoneal and intragastric route of Bacillus thuringiensis and Melia azedarach in mice. Arq. Inst. Biol. 2012, 79, 511–517. [Google Scholar] [CrossRef][Green Version]
- Vásquez-Padrón, R.I.; Moreno-Fierros, L.; Neri-Bazan, L.; Martínez-Gill, A.F.; De La Riva, G.A.; Lopéz-Revilla, R. Characterization of the mucosal and systemic immune response induced by Cry 1Ac protein from Bacillus thruringiensis HD 73 in mice. Braz. J. Med. Biol. Res. 2000, 33, 147–155. [Google Scholar] [CrossRef]
- Shevchuk, O.O. Study of some acute toxicity indicators of melphalan in rats. Med. Clin. Chem. 2020, 4, 113–118. [Google Scholar] [CrossRef]
- Upadhyay, A.; Pandya, P.; Parikh, P. Acute exposure of Pyrazosulfuron Ethyl induced Haematological and Blood Biochemical changes in the Freshwater Teleost fish Oreochromis mossambicus. Int. J. Adv. Res. Biol. Sci. 2014, 1, 79–86. [Google Scholar]
- Camargo, A.F.F.; Baptista, A.M.; Natalino, R.; Camargo, O.P. Bioactive glass in cavitary bone defects: A comparative experimental study in rabbits. Acta Ortop. Bras. 2015, 23, 202–207. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Anesi, A.; Salvatori, R.; Chiarini, L.; Manfredini, T.; Zaffe, D. Bone Regeneration by Novel Bioactive Glasses Containing Strontium and/or Magnesium: A Preliminary In-Vivo Study. Materials 2018, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, A.; Grjibovski, A.M. Analysis of two independent samples using stata software: Non parametric criteria. Ekologiya Cheloveka 2014, 4, 60–63. (In Russian) [Google Scholar]
- Vernè, E.; Ferraris, S.; Vitale-Brovarone, C.; Cochis, A.; Rimondini, L. Bioactive glass functionalized with alkaline phosphatase stimulates bone extracellular matrix deposition and calcification in vitro. Appl. Surf. Sci. 2014, 313, 372–381. [Google Scholar] [CrossRef]
- Cazzola, M.; Vernè, E.; Cochis, A.; Sorrentino, R.; Azzimonti, B.; Prenesti, E.; Rimondini, L.; Ferraris, S. Bioactive glasses functionalized with polyphenols: In vitro interactions with healthy and cancerous osteoblast cells. J. Mater. Sci. 2017, 52, 9211–9223. [Google Scholar] [CrossRef]
- Ahmed, M. Acute Toxicity (Lethal Dose 50 Calculation) of Herbal Drug Somina in Rats and Mice. J. Pharm. Pharmacol. 2015, 6, 185–189. [Google Scholar] [CrossRef]
- Heikkilä, J.T.; Aho, H.J.; Yli-Urpo, A.; Happonen, R.P.; Aho, A.J. Bone formation in rabbit cancellous bone defects filled with bioactive glass granules. Acta Orthop. Scand. 1995, 66, 463. [Google Scholar] [CrossRef] [PubMed]
- Tulyaganov, D.U.; Akbarov, A.; Ziyadullaeva, N.; Khabilov, B.; Baino, F. Injectable bioactive glass-based pastes for potential use in bone tissue repair. Biomed. Glasses 2020, 6, 23–33. [Google Scholar] [CrossRef]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicater—A multipurpose, highly bioactive glass-ceramic. In vitro, In vivo and clinical trials. J. Non-Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Kopp, H.-G.; Avecilla, S.T.; Hooper, A.T.; Rafii, S. The Bone Marrow Vascular Niche: Home of HSC Differentiation and Mobilization. Physiology 2005, 20, 349–356. [Google Scholar] [CrossRef]
- Bahney, C.S.; Hu, D.P.; Miclau, T., III; Marcucio, R.S. The multifaceted role of the vasculature in endochondral fracture repair. Front. Endocrinol. 2015, 6, 4. [Google Scholar] [CrossRef]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.P.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue engineering and cell-based therapies for fractures and bone defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef]
- Shapiro, F.; Wu, J.Y. Woven bone overview: Structural classification based on its integral role in development, repair and pathological bone formation throughout vertebrate groups. Eur. Cells Mater. 2019, 38, 137–167. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: A review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burns Trauma 2019, 7. [Google Scholar] [CrossRef] [PubMed]

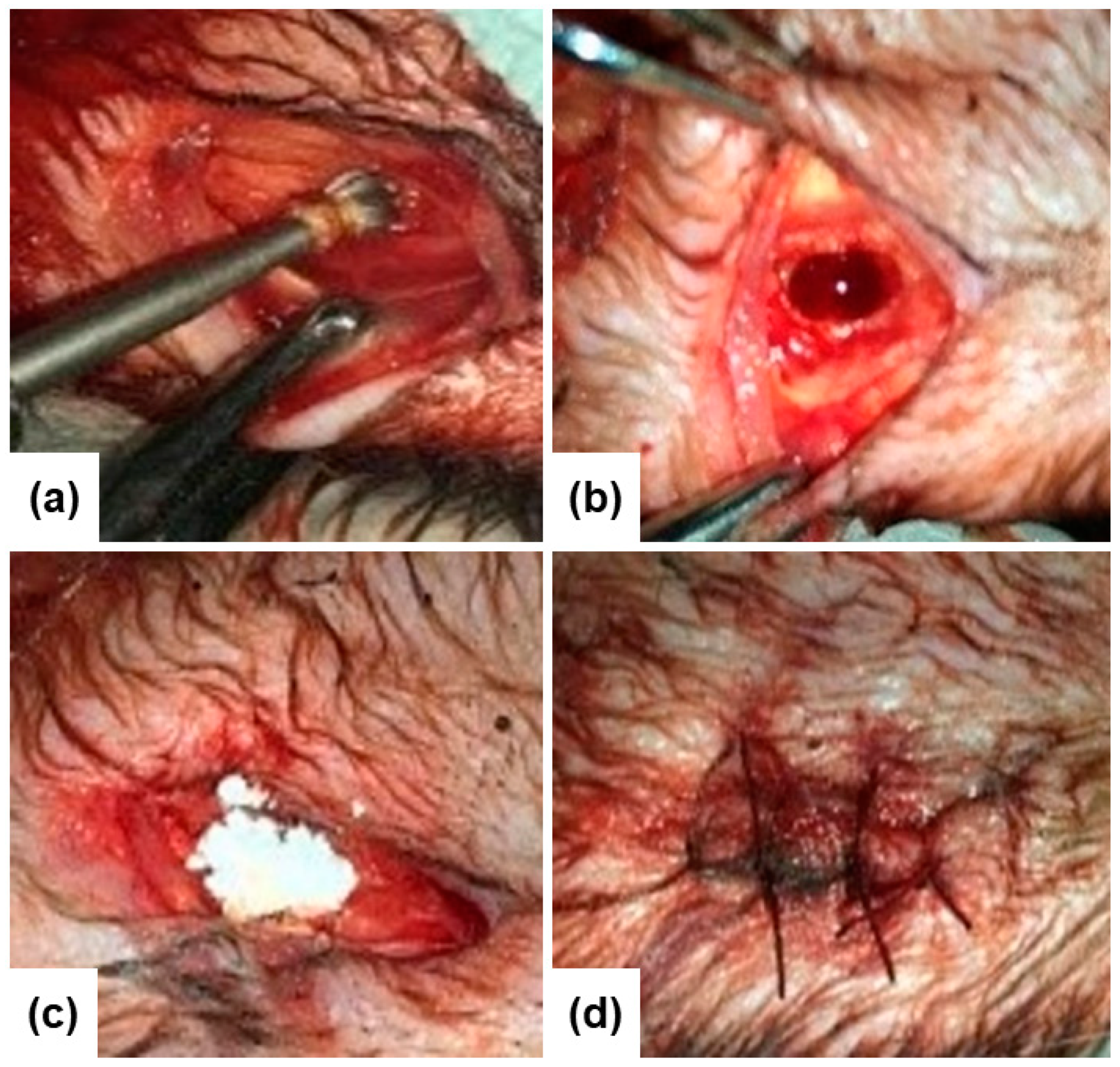
| Observation Stage | Glass Powder (Experimental) | Empty Hole (Control) |
|---|---|---|
| 1 month | 3 animals per group with numbering in the range No.1–3 | 3 animals per group with numbering in the range No.4–6 |
| 2 months | 4 animals per group with numbering in the range No.7–10 | 4 animals per group with numbering in the range No.11–14 |
| 3 months | 4 animals per group with numbering in the range No.15–18 | 4 animals per group with numbering in the range No.19–22 |
| Dose (mg/kg) | Number of Dead Rats/Total Number of Rats |
|---|---|
| 4000 | 0/6 |
| 4100 | 1/6 |
| 4250 | 2/6 |
| 4500 | 4/6 |
| 4750 | 4/6 |
| 5000 | 6/6 |
| Observation Stage | Glass Powder (Experimental Group) | Empty Hall (Control) |
|---|---|---|
| 2 months | No.7/ score 4 No.8/ score 4 No.9/ score 4 No.10/ score 5 | No.11/ score 2 No.12/ score 3 No.13/ score 3 No.14/ score 3 |
| 3 months | No.15/ score 5 No.16/ score 5 No.17/ score 6 No.18/ score 7 | No.19/ score 3 No.20/ score 4 No.21/ score 4 No.22/ score 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiume, E.; Tulyaganov, D.U.; Akbarov, A.; Ziyadullaeva, N.; Cochis, A.; Scalia, A.C.; Rimondini, L.; Verné, E.; Baino, F. Biological Evaluation of a New Sodium-Potassium Silico-Phosphate Glass for Bone Regeneration: In Vitro and In Vivo Studies. Materials 2021, 14, 4546. https://doi.org/10.3390/ma14164546
Fiume E, Tulyaganov DU, Akbarov A, Ziyadullaeva N, Cochis A, Scalia AC, Rimondini L, Verné E, Baino F. Biological Evaluation of a New Sodium-Potassium Silico-Phosphate Glass for Bone Regeneration: In Vitro and In Vivo Studies. Materials. 2021; 14(16):4546. https://doi.org/10.3390/ma14164546
Chicago/Turabian StyleFiume, Elisa, Dilshat U. Tulyaganov, Avzal Akbarov, Nigora Ziyadullaeva, Andrea Cochis, Alessandro C. Scalia, Lia Rimondini, Enrica Verné, and Francesco Baino. 2021. "Biological Evaluation of a New Sodium-Potassium Silico-Phosphate Glass for Bone Regeneration: In Vitro and In Vivo Studies" Materials 14, no. 16: 4546. https://doi.org/10.3390/ma14164546
APA StyleFiume, E., Tulyaganov, D. U., Akbarov, A., Ziyadullaeva, N., Cochis, A., Scalia, A. C., Rimondini, L., Verné, E., & Baino, F. (2021). Biological Evaluation of a New Sodium-Potassium Silico-Phosphate Glass for Bone Regeneration: In Vitro and In Vivo Studies. Materials, 14(16), 4546. https://doi.org/10.3390/ma14164546










