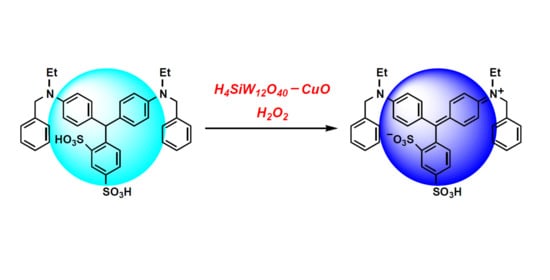
Oxidative Synthesis of Acid Blue 7 Dye Catalyzed by CuO/Silicotungstic Acid in Water-Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. General and Material
2.2. Oxidation Reaction on a Small Scale
2.3. 10 G-Scale Synthesis
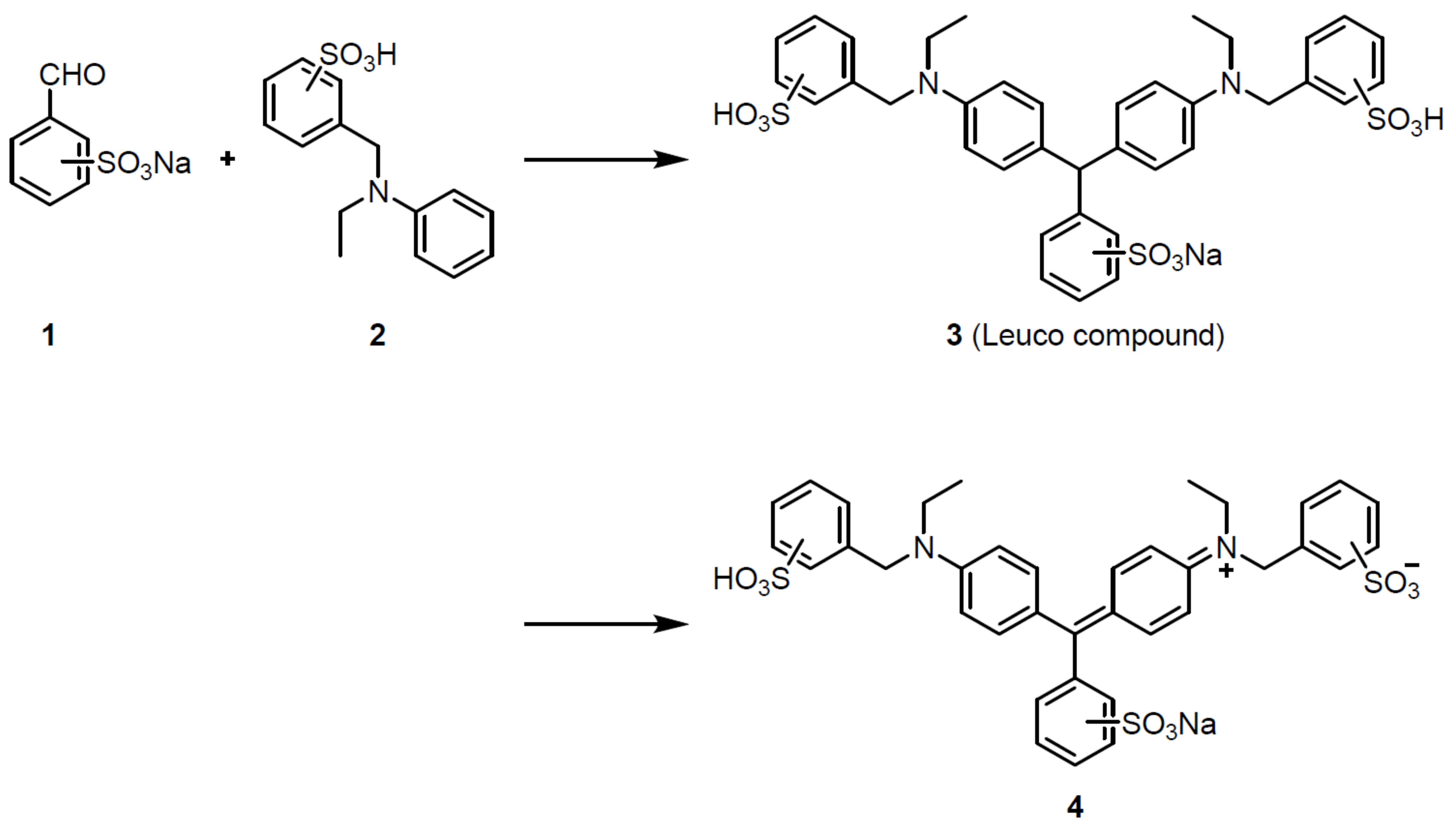
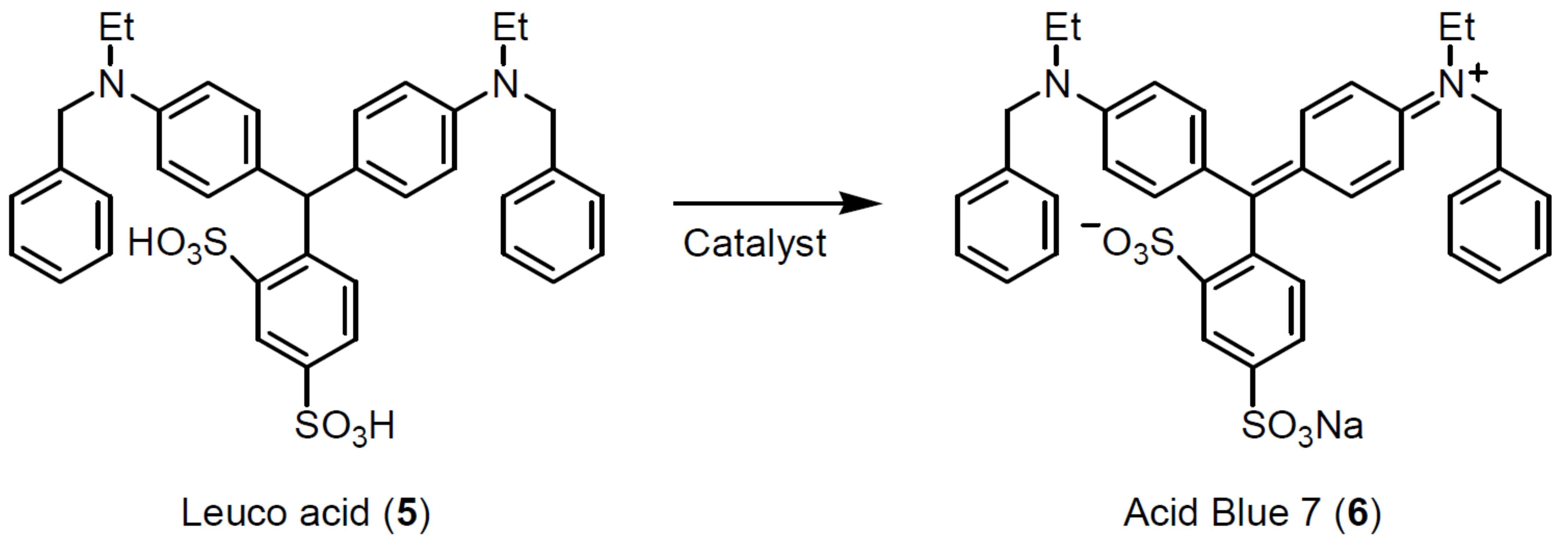
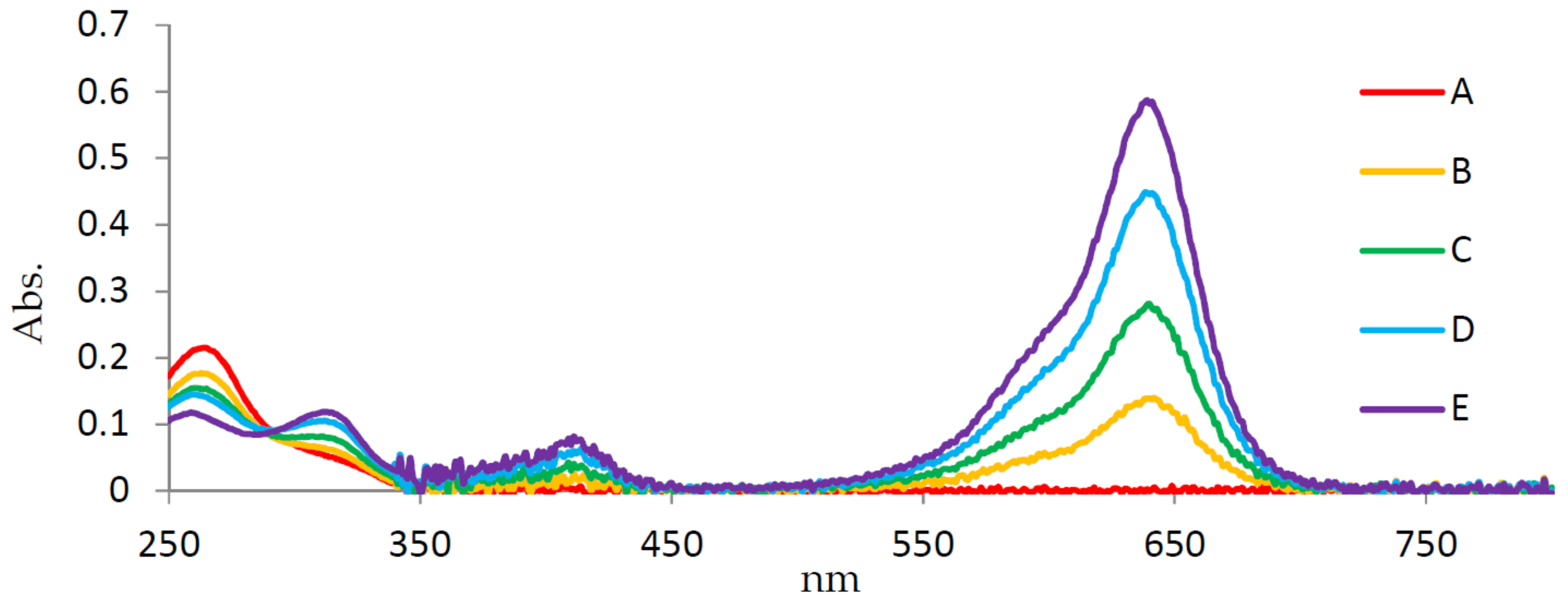
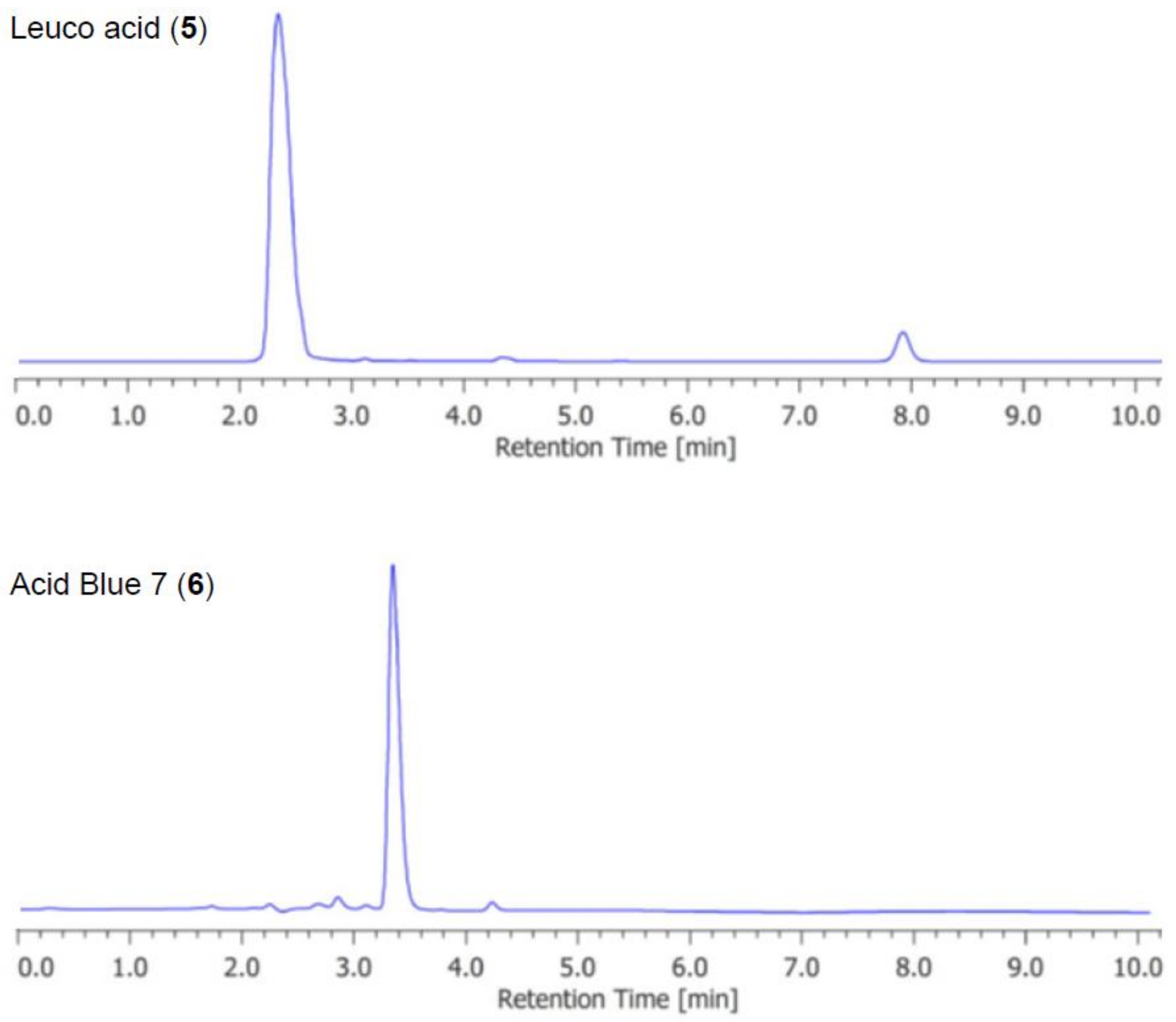
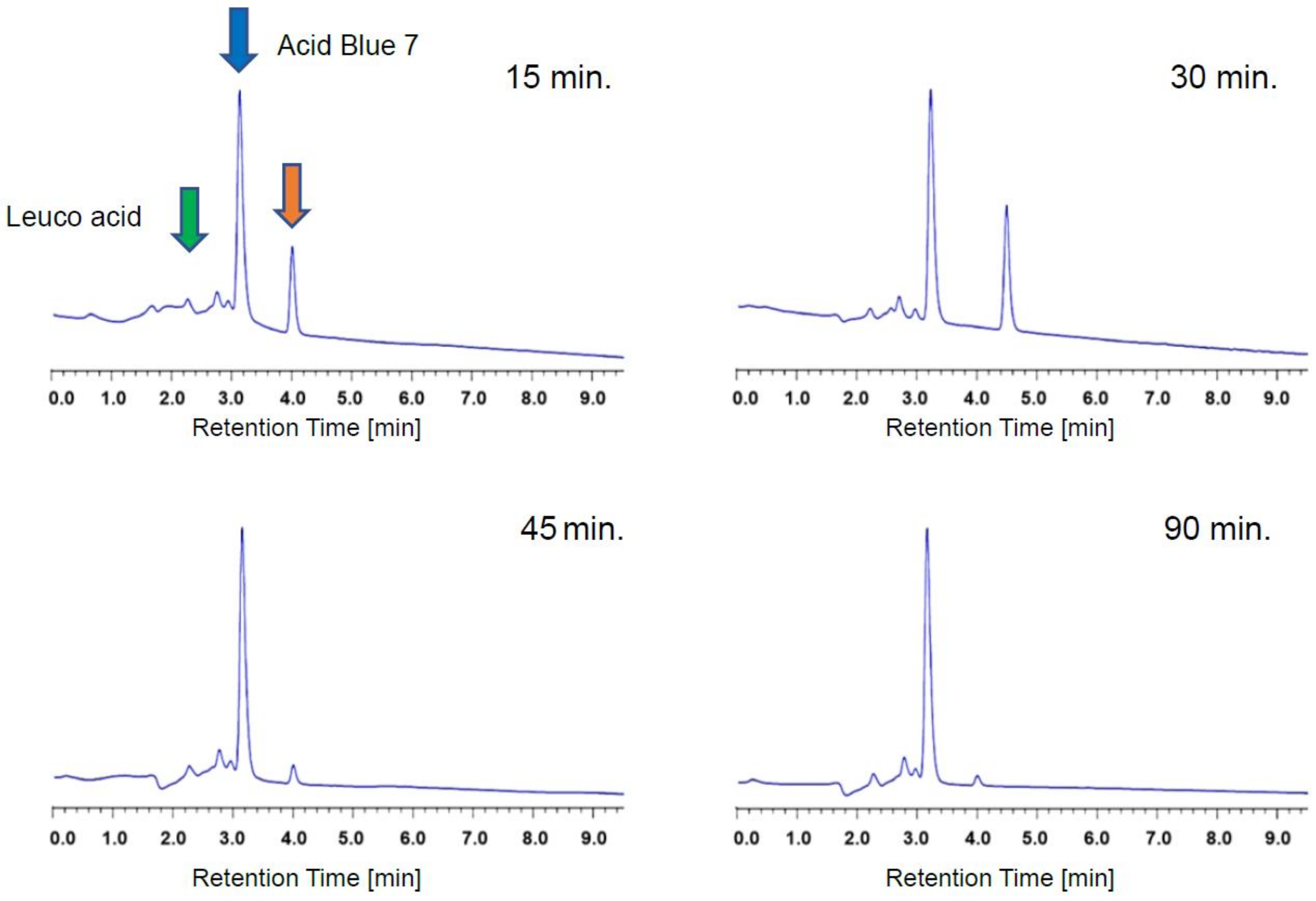
3. Results
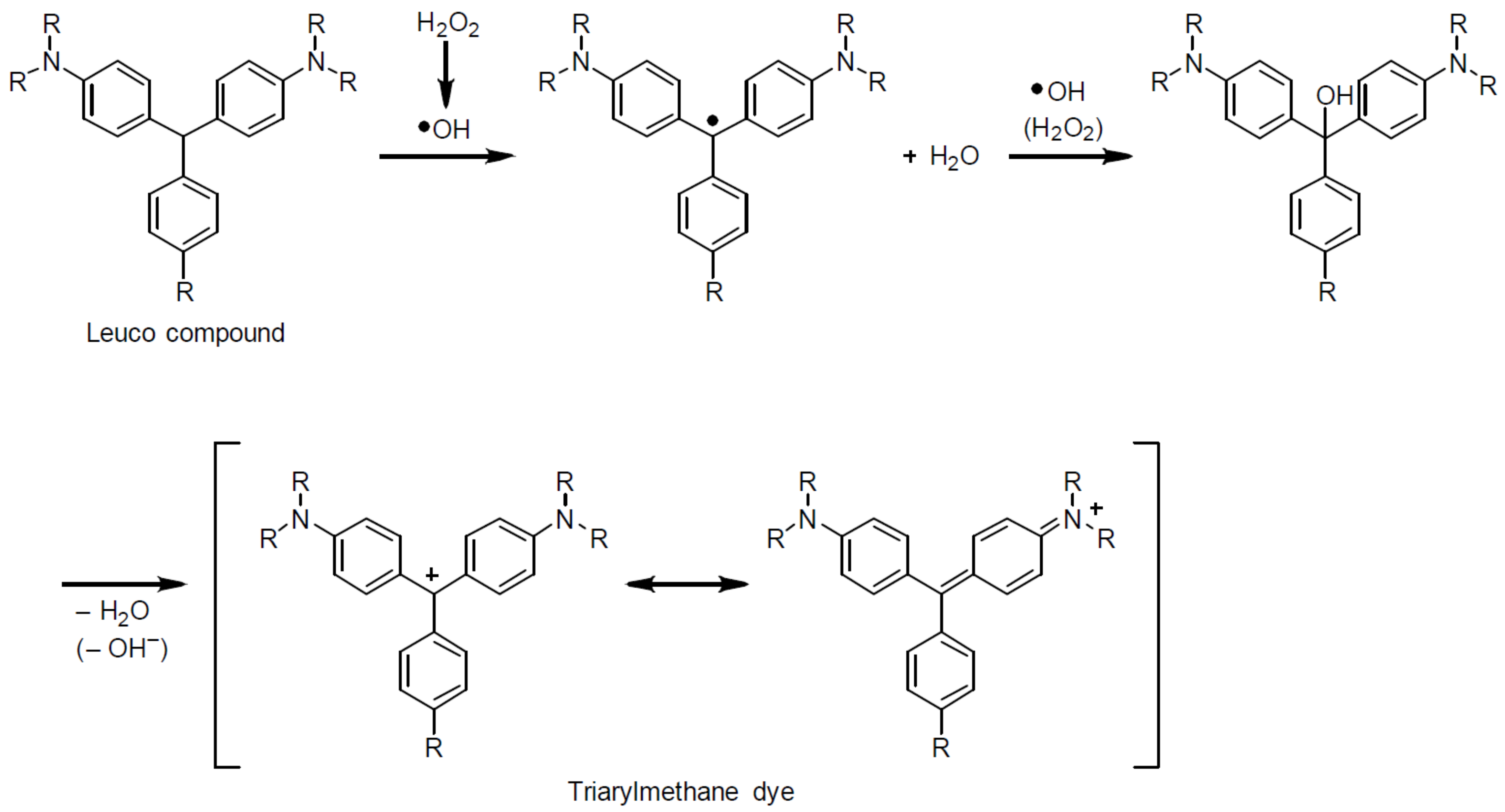
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kshatriya, R.; Jejurkar, V.P.; Saha, S. Advances in The Catalytic Synthesis of Triarylmethanes (TRAMs). Eur. J. Org. Chem. 2019, 24, 3818–3841. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, J.; Lu, Y.; Jia, R. Degradation and detoxification of the triphenylmethane dye malachite green catalyzed by crude manganese peroxidase from Irpex lacteus F17. Environ. Sci. Pollut. Res. 2016, 23, 9585–9597. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, D.F. The Photochemistry and Photophysics of Triphenylmethane Dyes in Solid and Liquid Media. Chem. Rev. 1993, 93, 381–433. [Google Scholar] [CrossRef]
- Espina, G.; Cáceres-Moreno, P.; Mejías-Navarrete, G.; Ji, M.; Sun, J.; Blamey, J.M. A novel and highly active recombinant spore-coat bacterial laccase, able to rapidly biodecolorize azo, triarylmethane and anthraquinonic dyestuffs. Int. J. Biol. Macromol. 2021, 170, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Montagut, A.M.; Gálvez, E.; Shafir, A.; Sebastián, R.M.; Vallribera, A. Triarylmethane Dyes for Artificial Repellent Cotton Fibers. Chem. Eur. J. 2017, 23, 3810–3814. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Fernandez, A.; Avlonitis, N.; Vande Velde, G.; Bradley, M.; Read, N.D.; Vendrell, M. Searching for the Optimal Fluorophore to Label Antimicrobial Peptides. ACS Comb. Sci. 2016, 18, 689–696. [Google Scholar] [CrossRef]
- Yushchenko, D.A.; Zhang, M.; Yan, Q.; Waggoner, A.S.; Bruchez, M.P. Genetically Targetable and Color-Switching Fluorescent Probe. ChemBioChem 2012, 13, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Kalluruttimmal, R.; Thekke Thattariyil, D.; Panthalattu Parambil, A.; Sen, A.K.; Chakkumkumarath, L.; Manheri, M.K. Electronically tuned triarylmethine scaffolds for fast and continuous monitoring of H2S levels in biological samples. Analyst 2019, 144, 4210–4218. [Google Scholar] [CrossRef] [PubMed]
- Cova, T.; Pais, A.; Saixas de Melo, J. Reconstructing the historical synthesis of mauveine from Perkin and Caro: Procedure and details. Sci. Rep. 2017, 7, 6806. [Google Scholar] [CrossRef]
- Plater, M.J. WH Perkin, Patent AD 1856 No 1984: A review on authentic mauveine and related compounds. J. Chem. Res. 2015, 39, 251–259. [Google Scholar] [CrossRef]
- Yi, J. An improvement on synthesis process of acid blue G. Ranliao Yu Ranse 2010, 47, 21–22, 28. [Google Scholar]
- Allen, J.L.; Meinertz, J.R. Post-column reaction for simultaneous analysis of chromatic and leuco forms of malachite green and crystal violet by high-performance liquid chromatography with photometric detection. J. Chromatogr. A 1991, 536, 217–222. [Google Scholar] [CrossRef]
- Inukai, K.; Maki, Y.; Ueda, T. Fluoro- and trifluoromethyl-substituted malachite green. Nippon Kagaku Ryoho Gakkai Zasshi 1956, 59, 515–517. [Google Scholar]
- Linnell, W.H.; Stenlake, J.B. Chlorohydroxytriphenylmethane dye. J. Pharm. Pharmacol. 1949, 1, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Perkin, W.H. The origin of the coal-tar colour industry, and the contributions of Hofmann and his pupils. J. Chem. Soc. Trans. 1896, 69, 596–637. [Google Scholar] [CrossRef]
- Kraus, G.A.; Jeon, I.; Nilsen-Hamilton, M.; Awad, A.M.; Banerjee, J.; Parvin, B. Fluorinated Analogs of Malachite Green: Synthesis and Toxicity. Molecules 2008, 13, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Agunwa, U.; Okonkwo, E. Production of malachite green by oxidation of its leuco base using potassium persulphate, potassium permanganate and manganese dioxide. Glob. J. Pure Appl. Sci. 2004, 10, 143–146. [Google Scholar] [CrossRef][Green Version]
- Sisi, A.J.; Khataee, A.; Fathinia, M.; Vahid, B. Ultrasonic-assisted degradation of a triarylmethane dye using combined peroxydisulfate and MOF-2 catalyst: Synergistic effect and role of oxidative species. J. Mol. Liq. 2020, 297, 111838. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Clean Production of Acid Blue 9 via Catalytic Oxidation in Water. Ind. Eng. Chem. Res. 2009, 48, 5548–5550. [Google Scholar] [CrossRef]
- Dong, C.-P.; Kodama, S.; Nomoto, A.; Ueshima, M.; Ogawa, A. 4,6-Dihydroxysalicylic Acid-Catalyzed Oxidative Condensation of Benzylic Amines and Aromatic Ketones for the Preparation of 2,4,6-Trisubstituted Pyridines and Its Application to Metal-Free Synthesis of G-Quadruplex Binding Ligands. ACS Omega 2019, 4, 9029–9040. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Naka, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. Transition-Metal-Free and Oxidant-Free Cross-Coupling of Arylhydrazines with Disulfides: Base-Promoted Synthesis of Unsymmetrical Aryl Sulfides. J. Org. Chem. 2017, 82, 6647–6655. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-P.; Higashiura, Y.; Marui, K.; Kumazawa, S.; Nomoto, A.; Ueshima, M.; Ogawa, A. Metal-Free Oxidative Coupling of Benzylamines to Imines under an Oxygen Atmosphere Promoted Using Salicylic Acid Derivatives as Organocatalysts. ACS Omega 2016, 1, 799–807. [Google Scholar] [CrossRef]
- Dong, C.-P.; Kodama, S.; Uematsu, A.; Nomoto, A.; Ueshima, M.; Ogawa, A. Metal-Free Blue Dye Synthesis: Oxidative Coupling of Benzylamines and N,N-Dimethylanilines to Yield 4,4′-Diaminotriarylmethanes in the Presence of Salicylic Acid as a Co-oxidant. J. Org. Chem. 2017, 82, 12530–12538. [Google Scholar] [CrossRef] [PubMed]
- Marui, K.; Nomoto, A.; Akashi, H.; Ogawa, A. Green oxidation of amines to imines based on the development of novel catalytic systems using molecular oxygen or hydrogen peroxide. Synthesis 2016, 48, 31–42. [Google Scholar]
- Kodama, S.; Yoshida, J.; Nomoto, A.; Ueta, Y.; Yano, S.; Ueshima, M.; Ogawa, A. Direct conversion of benzylamines to imines via atmospheric oxidation in the presence of VO(Hhpic)2 catalyst. Tetrahedron Lett. 2010, 51, 2450–2452. [Google Scholar] [CrossRef]
- Kodama, S.; Ueta, Y.; Yoshida, J.; Nomoto, A.; Yano, S.; Ueshima, M.; Ogawa, A. Tetranuclear vanadium complex, (VO)4(hpic)4: A recyclable catalyst for oxidation of benzyl alcohols with molecular oxygen. Dalton Trans. 2009, 44, 9708–9711. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Nomoto, A.; Yano, S.; Ueshima, M.; Ogawa, A. Novel heterotetranuclear V2Mo2 or V2W2 complexes with 4,4′-di-tert-butyl-2,2′-bipyridine: Syntheses, crystal structures, and catalytic activities. Inorg. Chem. 2011, 50, 9942–9947. [Google Scholar] [CrossRef] [PubMed]
- Vilà-Nadal, L.; Miras, H.N. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering: Coordination Chemistry in Polyoxometalates and Metal Clusters; Elsevier Inc.: New York, NY, USA, 2020; pp. 28–33. [Google Scholar]
- Ren, Y.; Wang, M.; Chen, X.; Yue, B.; He, H. Heterogeneous catalysis of polyoxometalate based organic-inorganic hybrids. Materials 2015, 8, 1545–1567. [Google Scholar] [CrossRef]
- Uehara, K.; Fukaya, K.; Mizuno, N. Reactive N-protonated isocyanate species stabilized by bis(μ-hydroxo)divanadium(IV)-substituted polyoxometalate. Angew. Chem. Int. Ed. 2012, 51, 7715–7718. [Google Scholar] [CrossRef]
- Suzuki, K.; Sugawa, M.; Kikukawa, Y.; Kamata, K.; Yamaguchi, K.; Mizuno, N. Strategic design and refinement of Lewis acid–base catalysis by rare-Earth-metal-containing polyoxometalates. Inorg. Chem. 2012, 51, 6953–6961. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Antonova, N.S.; Carbó, J.J.; Kortz, U.; Kholdeeva, O.A.; Poblet, J.M. Mechanistic insights into alkene epoxidation with H2O2 by Ti- and other TM-containing polyoxometalates: Role of the metal nature and coordination environment. J. Am. Chem. Soc. 2010, 132, 7488–7497. [Google Scholar] [CrossRef] [PubMed]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent Advances in Transition Metal Catalyzed Oxidation of Organic Substrates with Molecular Oxygen. Chem. Rev. 2005, 105, 2329–2363. [Google Scholar] [CrossRef] [PubMed]
- Adam, W.; Alsters, P.L.; Neumann, R.; Saha-Moller, C.R.; Seebach, D.; Beck, A.K.; Zhang, R. Chiral hydroperoxides as oxygen source in the catalytic stereoselective epoxidation of allylic alcohols by sandwich-type polyoxometalates: Control of enantioselectivity through a metal-coordinated template. J. Org. Chem. 2003, 68, 8222–8231. [Google Scholar] [CrossRef]
- Ohkoshi, A.; Takahashi, K.; Matsushima, A.; Abe, K.; Inada, Y. 4,4′-Tetramethyldiaminodiphenylmethanol from tetrabase with hemin in acetic acid. Tetrahedron Lett. 1989, 30, 957–958. [Google Scholar] [CrossRef]
- Swain, C.G.; Hedberg, K. The mechanism of oxidation of leuco malachite green by ceric sulfate. J. Am. Chem. Soc. 1950, 72, 3373–3375. [Google Scholar] [CrossRef]
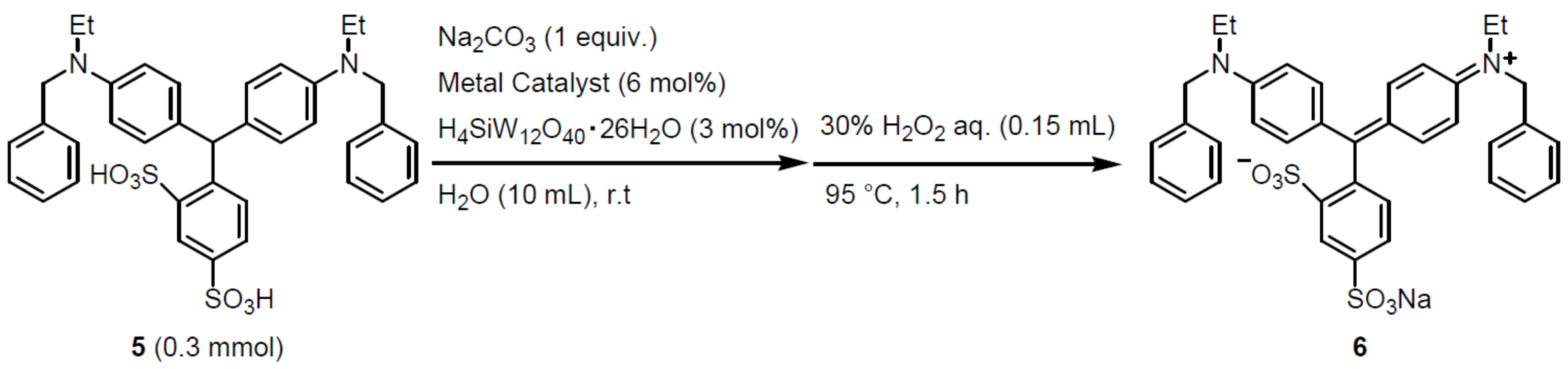


| Entry | Metal Catalyst | Yield (%) a |
|---|---|---|
| entry 1 | BaSO4 | n.d. |
| entry 2 | MgI2 | trace |
| entry 3 | MgO | n.d. |
| entry 4 | MgCl2 | 10 |
| entry 5 | MgCl2·6H2O | n.d. |
| entry 6 | (CH3COO)2·4H2O | n.d. |
| entry 7 | FeSO4·7H2O | 30 |
| entry 8 | FeCl3·6H2O | 13 |
| entry 9 | FeCl2 | 13 |
| entry 10 | FeO | 12 |
| entry 11 | CuO | 78 |
| Entry | X1/X2 | Y (mL) | Temp. (°C) c | Time (min) | Yield (%) a |
|---|---|---|---|---|---|
| entry 1 | 12/6 | 0.15 | 95 | 90 | 79 |
| entry 2 | 6/3 | 0.30 | 95 | 90 | 62 |
| entry 3 | 6/3 | 0.15 | 80 | 90 | 26 |
| entry 4 | 6/3 | 0.15 | 110 | 90 | 64 b |
| entry 5 | 6/3 | 0.15 | 95 | 45 | 74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomoto, A.; Okada, T.; Yamamoto, Y.; Kuroda, S.; Marui, K.; Yamamoto, M.; Tsujimoto, H.; Ueshima, M.; Nishigahana, T.; Itoh, K.; et al. Oxidative Synthesis of Acid Blue 7 Dye Catalyzed by CuO/Silicotungstic Acid in Water-Phase. Materials 2021, 14, 4505. https://doi.org/10.3390/ma14164505
Nomoto A, Okada T, Yamamoto Y, Kuroda S, Marui K, Yamamoto M, Tsujimoto H, Ueshima M, Nishigahana T, Itoh K, et al. Oxidative Synthesis of Acid Blue 7 Dye Catalyzed by CuO/Silicotungstic Acid in Water-Phase. Materials. 2021; 14(16):4505. https://doi.org/10.3390/ma14164505
Chicago/Turabian StyleNomoto, Akihiro, Tomoya Okada, Yuki Yamamoto, Shota Kuroda, Kuniaki Marui, Mika Yamamoto, Hidetaka Tsujimoto, Michio Ueshima, Tamotsu Nishigahana, Keiji Itoh, and et al. 2021. "Oxidative Synthesis of Acid Blue 7 Dye Catalyzed by CuO/Silicotungstic Acid in Water-Phase" Materials 14, no. 16: 4505. https://doi.org/10.3390/ma14164505
APA StyleNomoto, A., Okada, T., Yamamoto, Y., Kuroda, S., Marui, K., Yamamoto, M., Tsujimoto, H., Ueshima, M., Nishigahana, T., Itoh, K., Kobata, G., Kodama, S., & Ogawa, A. (2021). Oxidative Synthesis of Acid Blue 7 Dye Catalyzed by CuO/Silicotungstic Acid in Water-Phase. Materials, 14(16), 4505. https://doi.org/10.3390/ma14164505