Structure and Heat Transfer in Zircaloy-4 Treated at High Temperatures
Abstract
:1. Introduction
2. Materials and Methods
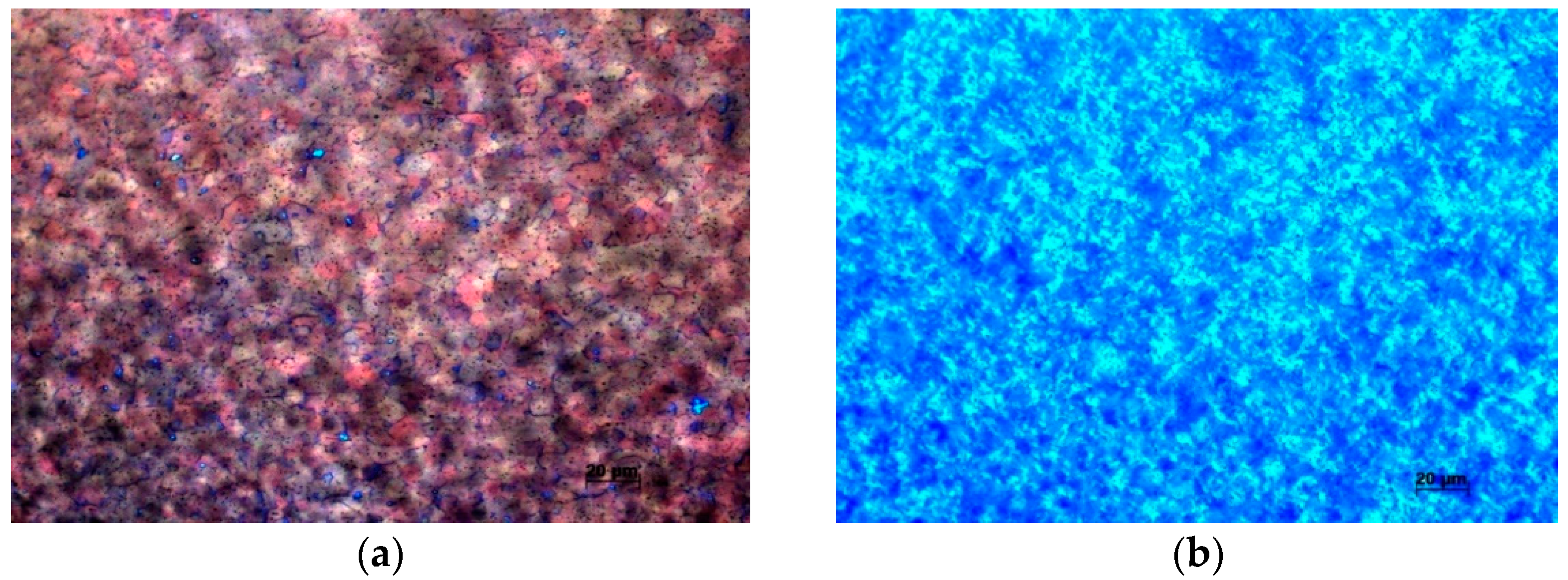
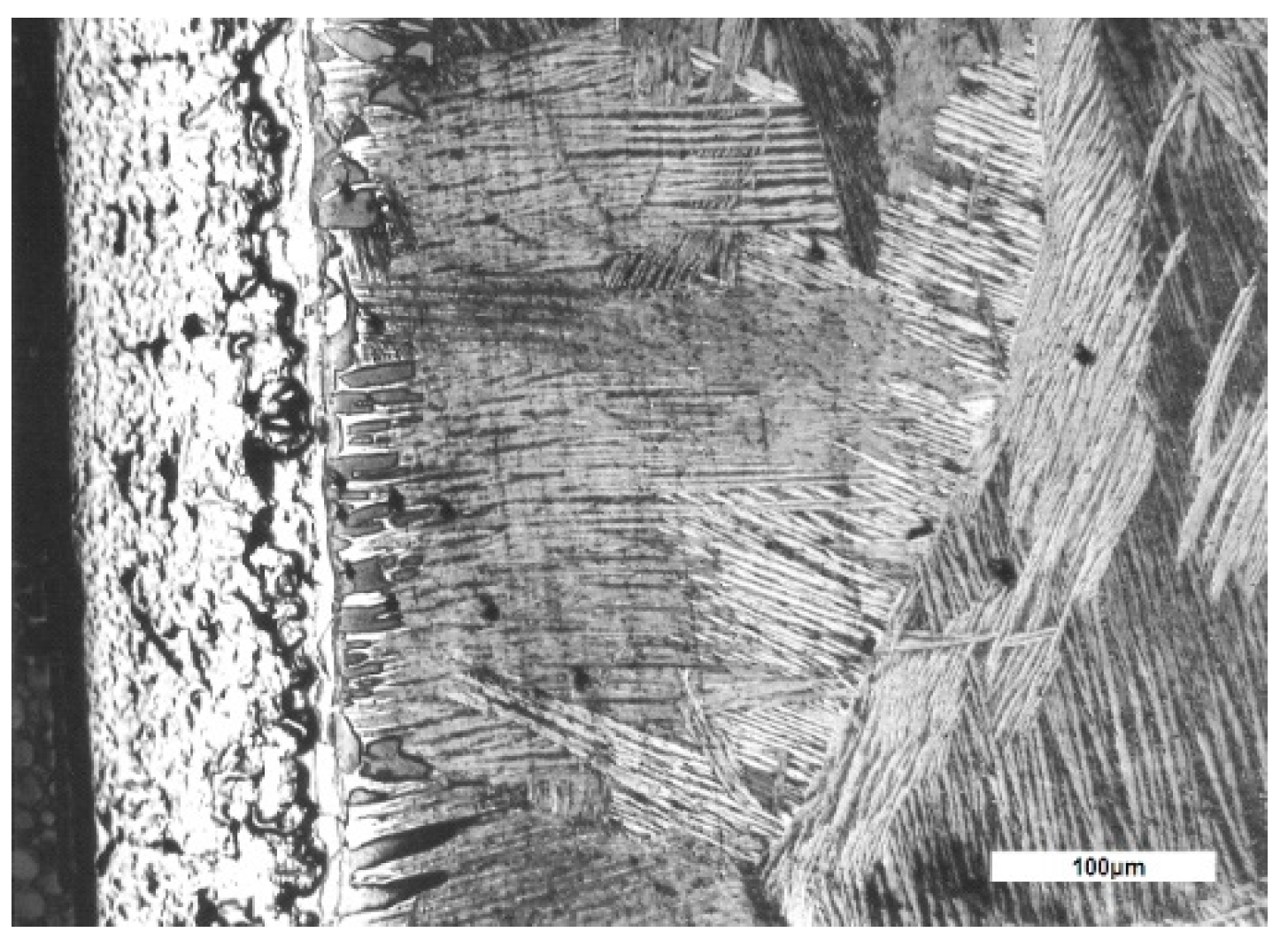
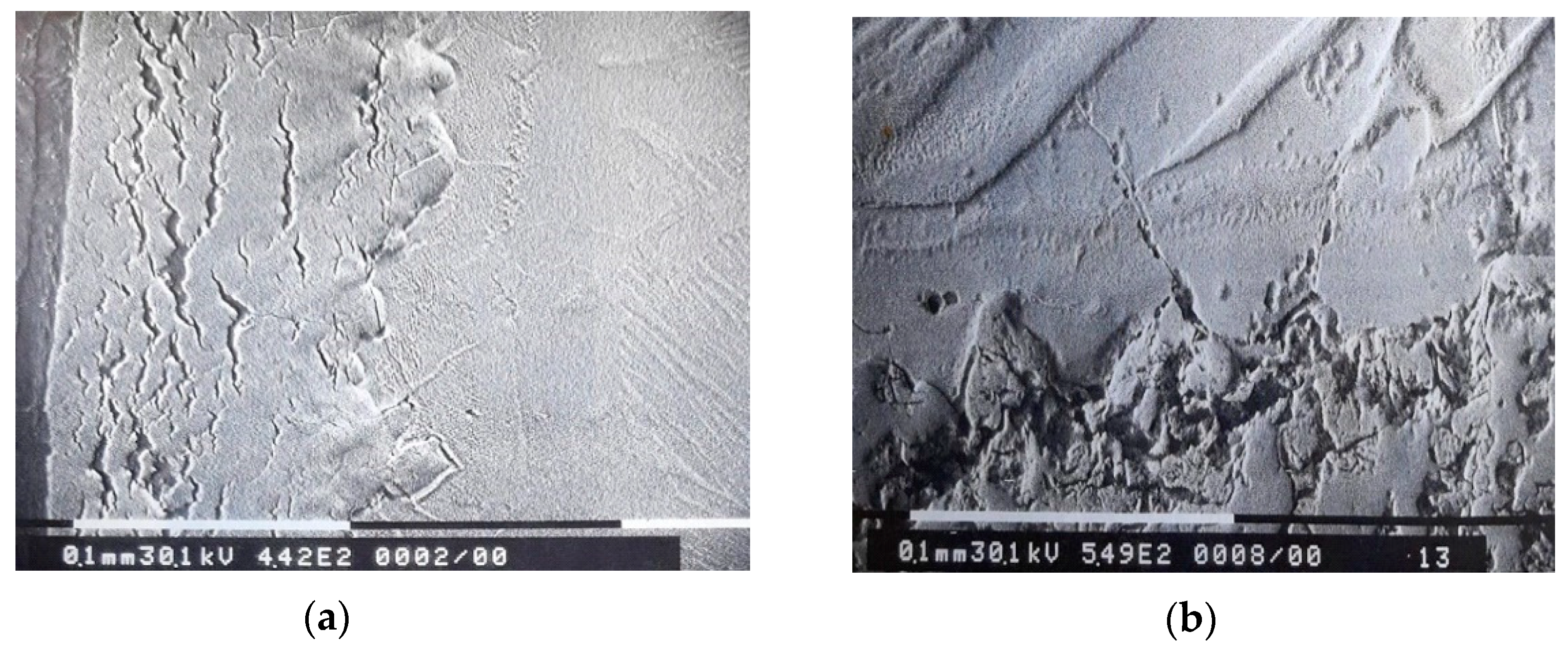
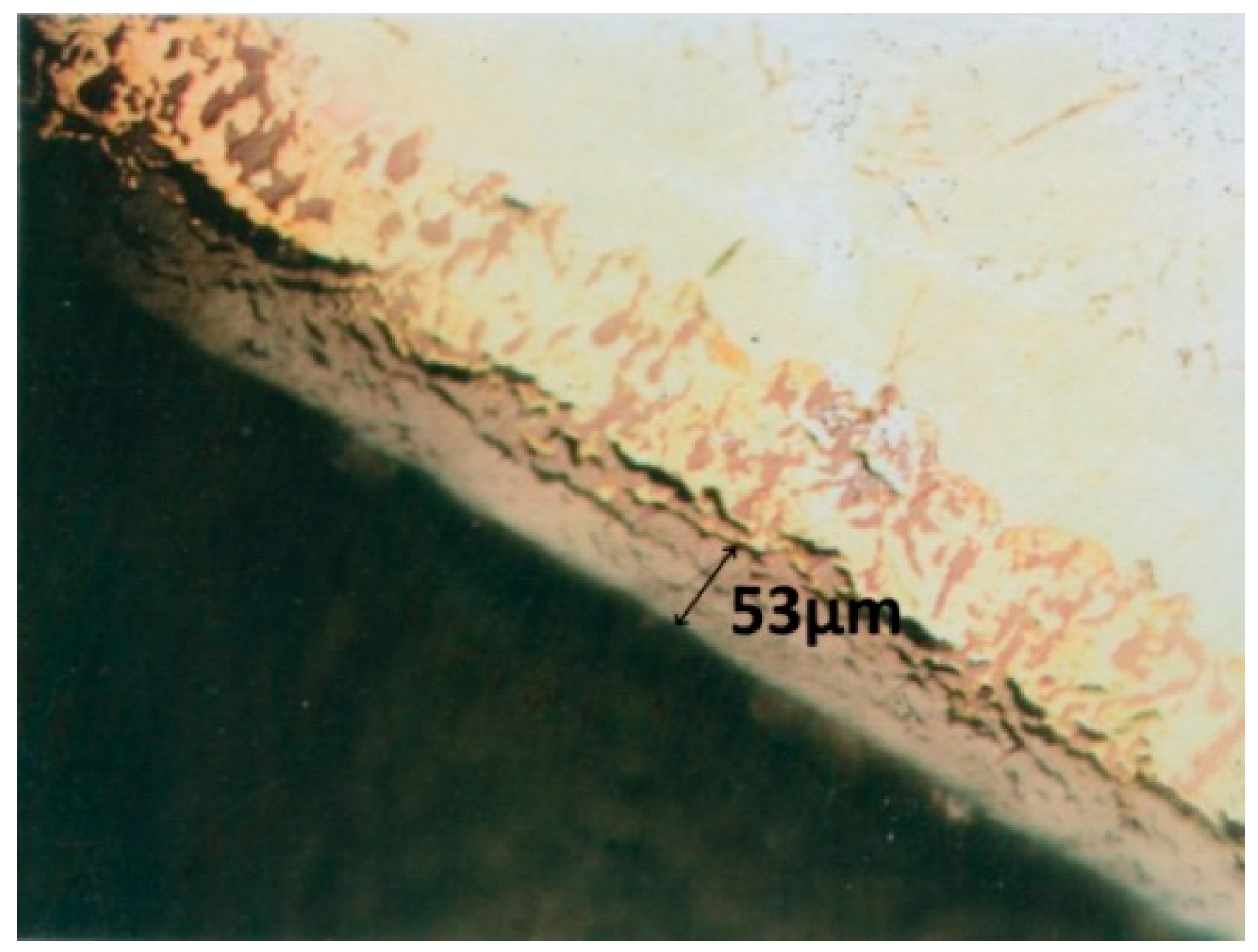
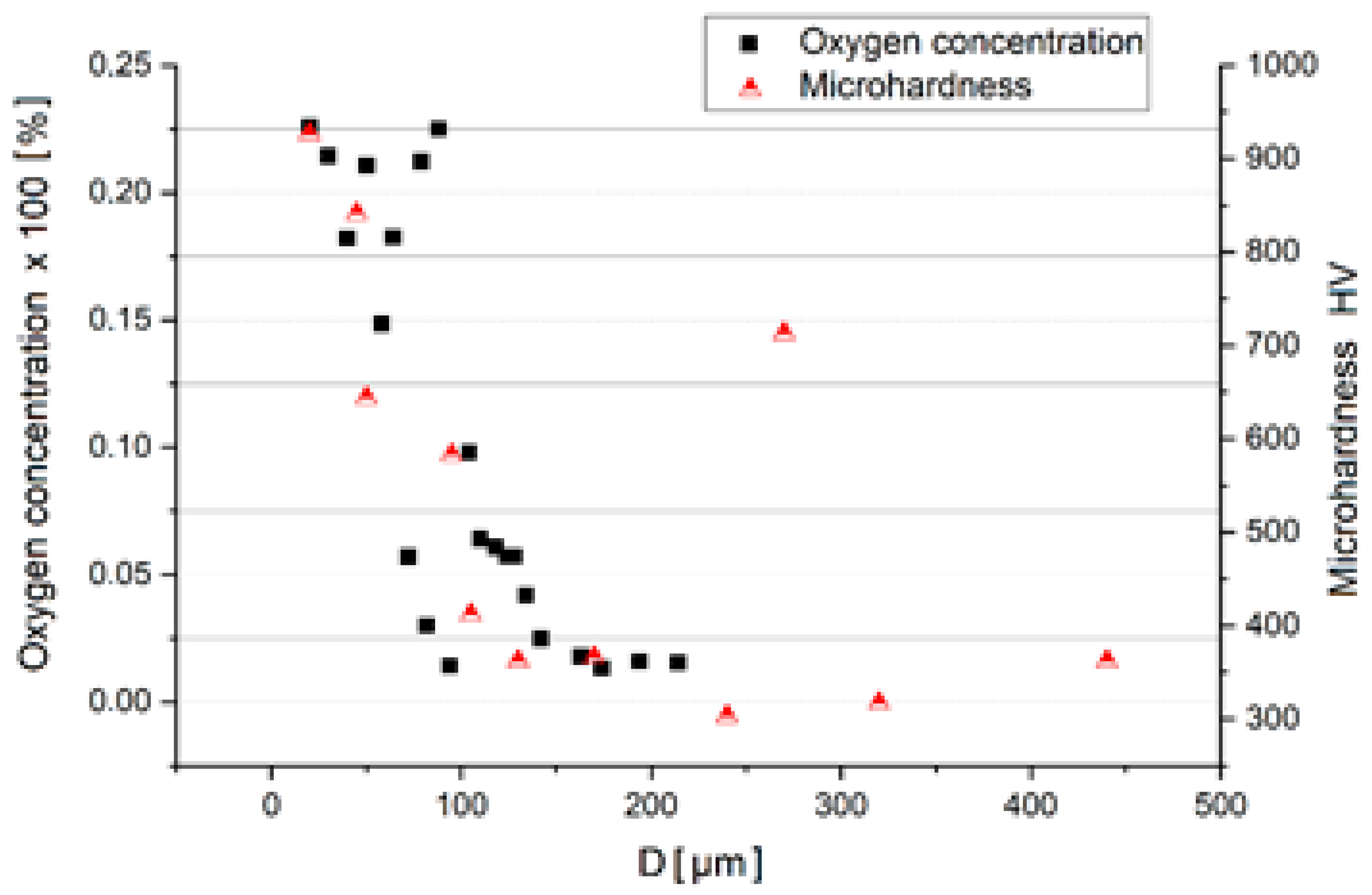
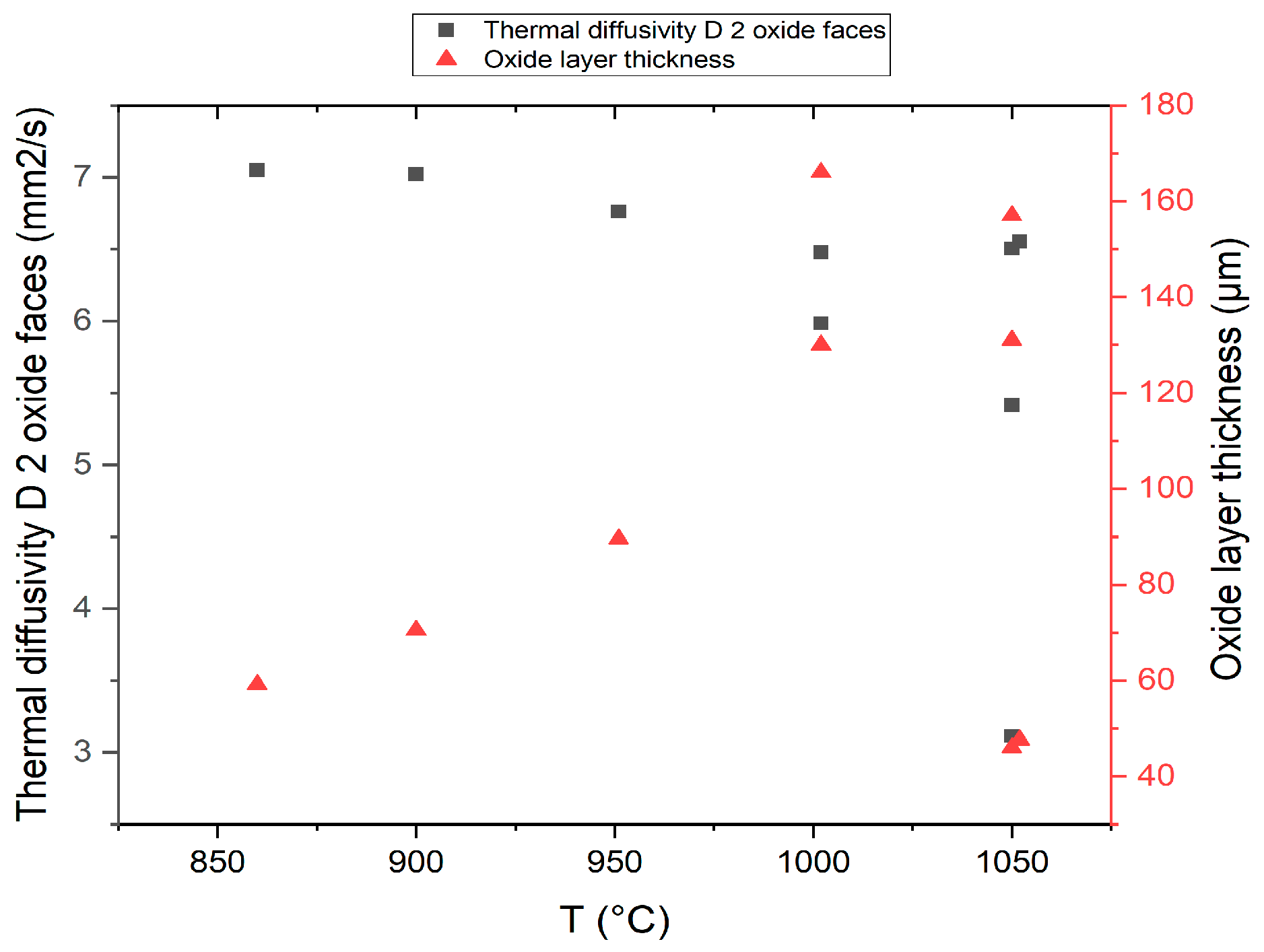
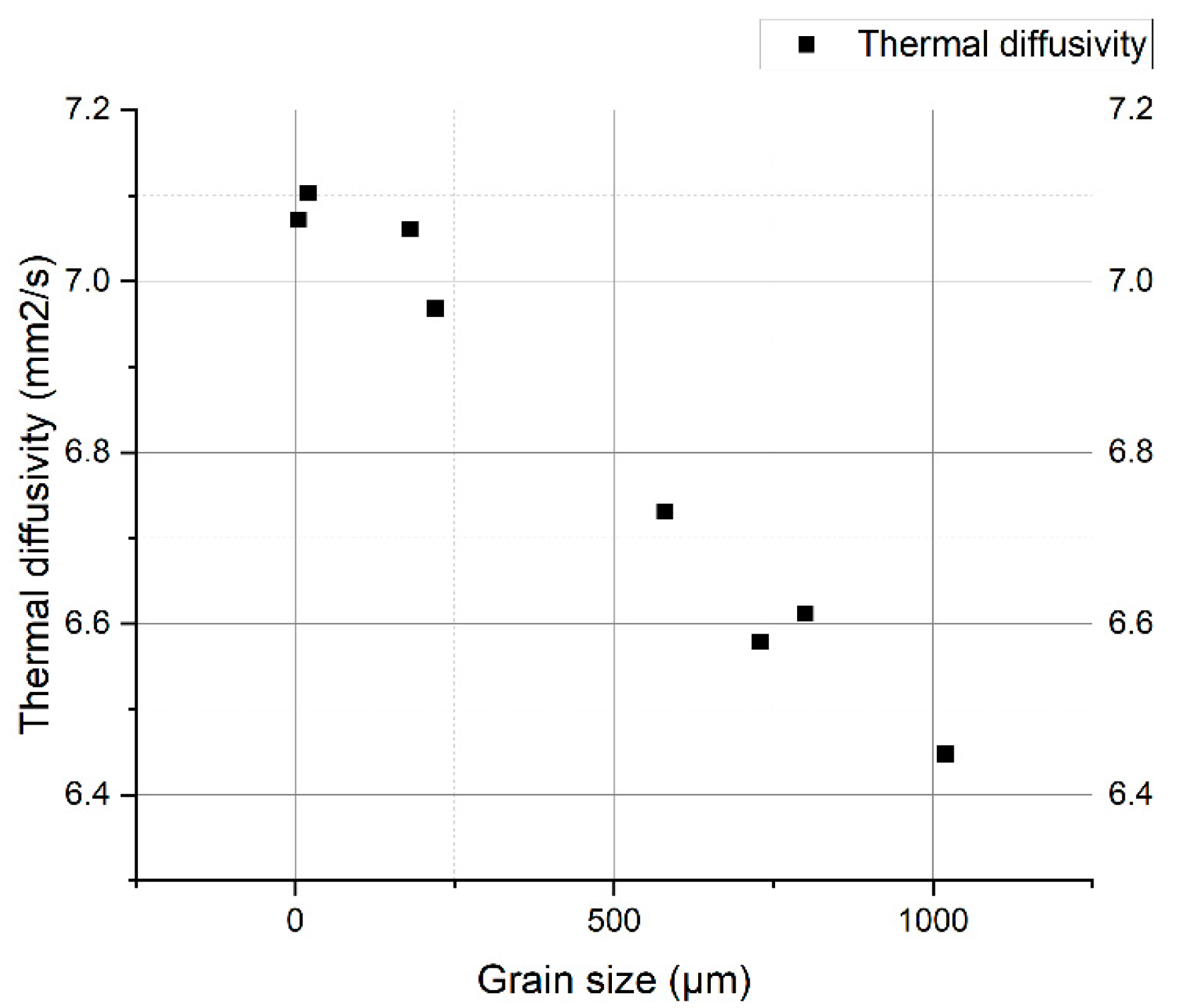
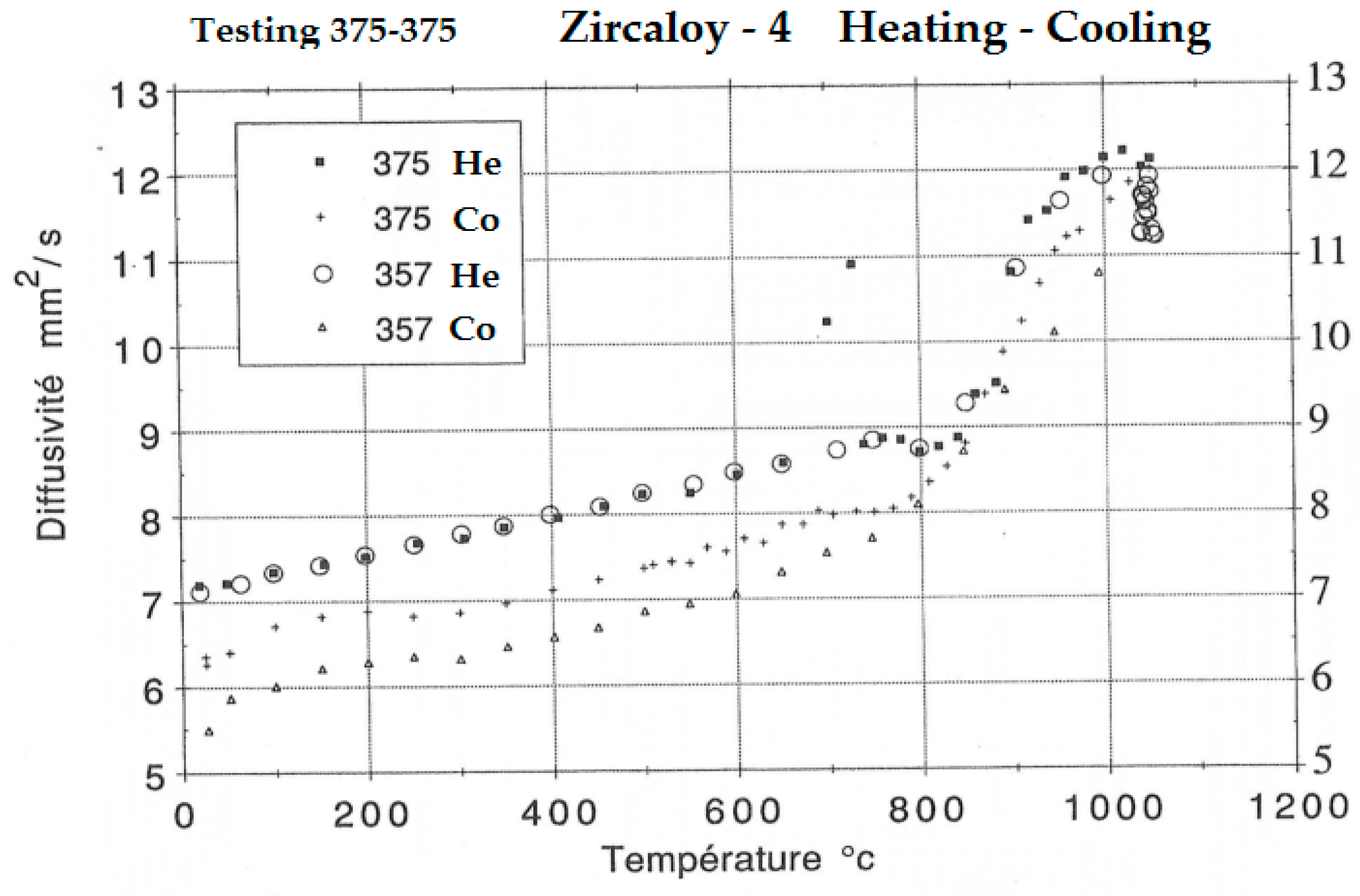
3. Experimental Results
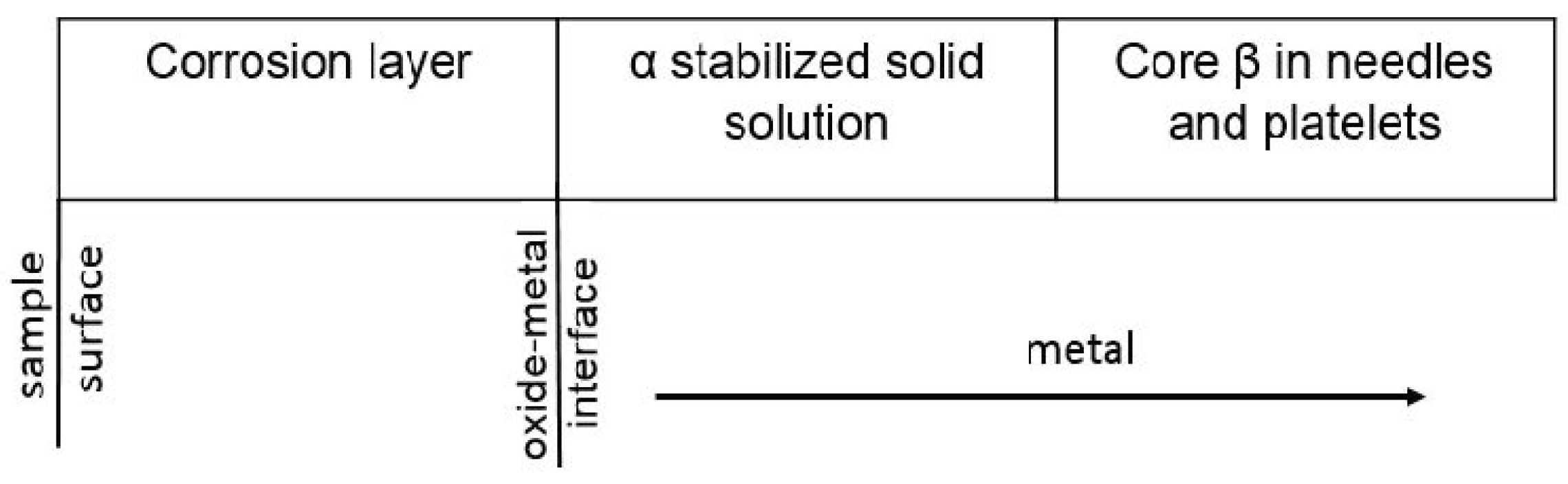
4. Interpretation of Results
- a corrosion layer with an oxide zone in the upper part, which presents degradation defects in the form of pores and cracks and with nitrogen-rich precipitates located near the oxide–metal interface
- a layer of solid solution α stabilized by dissolving oxygen in the metal, under the oxide layer, with a columnar development towards the core and
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Atomic Energy Agency. Nuclear Power Reactors in the World. In Reference Data Series, 2nd ed.; IAEA: Vienna, Austria, 2009. [Google Scholar]
- Nuclear Fuel Behaviour in Loss-of-Coolant Accident (LOCA) Conditions, State-of-the-Art Report No. 6846; The OECD Nuclear Energy Agency (NEA): Paris, France, 2009; Volume 48, p. 369, NEA-CSNI-R-2009-15; ISBN 978-92-64-99091-3.
- Health and Safety Executive, Report of the System Design and Security Review of the AP1000 Nuclear Reactor (Step 3 of the Generic Design Assessment Process), November 2009. Available online: http://www.hse.gov.uk (accessed on 30 July 2021).
- Lee, S.-S.; Kim, S.-H.; Suh, K.-Y. The design features of the Advanced Power Reactor 1400. Nucl. Eng. Technol. 2009, 41, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Chu, I.C.; Song, C.H.; Cho, B.H.; Park, J.K. Development of passive flow controlling safety injection tank for APR1400. Nucl. Eng. Des. 2008, 238, 200–206. [Google Scholar] [CrossRef]
- Douglas, D.L. Atomic Energy Review, Supplement 1971: The Metallurgy of Zirconium; IAEA: Vienna, Austria, 1971; p. 466. [Google Scholar]
- Maxim, I. Materiale Nucleare; Academiei Republicii Socialiste Romania: Bucharest, Romania, 1969. [Google Scholar]
- Waterside Corrosion of Zirconium Alloys in Nuclear Power Plants. IAEA—TECDOC—996; IAEA: Vienna, Austria, 1998.
- Ursu, I. Fizica si Tehnologia Materialelor Nucleare; Academiei Republicii Socialiste Romania: Bucharest, Romania, 1982. [Google Scholar]
- Prodea, I. Contribuţii la Proiectarea Neutronică şi Evaluarea Performanţelor Sistemului de Oprire nr 1 la un Reactor Avansat de tip CANDU. Ph.D. Thesis, Universitatea Politehnica Bucureşti, Bucharest, Romania, 2010. [Google Scholar]
- Rouben, B. CANDU Fuel-Management Course, Manager, Reactor Core Physics Branch. At. Energy Can. Ltd. 1999. Available online: https://canteach.candu.org/Content%20Library/Forms/DispForm.aspx?ID=205&RootFolder=%2A (accessed on 9 August 2021).
- Ben Ammar, Y.; Aoufi, A.; Darrieulat, M. Influence of the cooling rate on the texture and the microstructure of Zircaloy-4 studied by means of a Jominy end-quench test. Mater. Sci. Eng. 2012, 556, 184–193. [Google Scholar] [CrossRef]
- Pârvan, I. Coroziunea și Chimia Apei în Circuitul Primar al Reactorului CANDU; Institutul de Cercetări Nucleare: Pitesti, Romania, 2009; ISBN 978-973-47-0811-6. [Google Scholar]
- Radulescu, M. Studiul Cineticii Proceselor de Coroziune la Unele Aliaje de Zirconiu Folosite in Energetica Nucleara. Ph.D. Thesis, Universitatea Politehnica, Bucharest, Romania, 1997. [Google Scholar]
- Abrudeanu, M.; Archambault, P. L’influence de l’oxydation à haute température sur la diffusivité thermique de l’alliage Zy-4. In Proceedings of the 9e Congrés International du Traitement Thermique et de l’Ingénierie des Surfaces, Nice, France, 26–28 September 1994; pp. 87–93. [Google Scholar]
- Abrudeanu, M.; Archambault, P.; Petot-Ervas, G.; Petrescu, N.; Petrescu, M. Microstructure and electron microprobe study of oxide layers obtained on zircaloy-4 by oxidation at high temperature. In Proceeding of the Second International Conference, elwyn College, University of Cambridge, SCambridge, UK, 29–31 March 1993; pp. 387–395. [Google Scholar]
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Mihalache, M.; Meleg, T.; Pavelescu, M. Studiul modificarii microstructurii aliajului Zr-2,5% Nb in decursul ciclurilor termice de tip LOCA. Rev. Romana Mater. 2010, 40, 349–358. [Google Scholar]
- Anghel, D.C.; Rosu, A.E.; Neacsu, G.; Popa, I.A.; Branzei, M.; Rizea, V.; Ducu, C.M.; Dicu, M.M.; Rizea, A.D.; Ungureanu, E.; et al. The influence of thermal shocks on the thermophisics properties of the zircaloy-4. Rev. Chim. 2019, 70, 575–577. [Google Scholar] [CrossRef]
- Popa, I.; Rosu, A.E.; Neacsu, G.; Anghel, D.C.; Rizea, V.; Branzei, M.; Ducu, C.M.; Dicu, M.M.; Abrudeanu, M. The influence of the high temperatures thermal shocks on the microstructure and harness of zircaloy-4 alloy. Rev. Chim. 2018, 69, 1655–1660. [Google Scholar] [CrossRef]
- Archambault, P.; Abrudeanu, M. Influence de l’oxydation et de l’état structural sur les propriétés thermo-physiques d’un alliage a base de zirconium. J. Nucl. Mater. 1993, 200, 162–168. [Google Scholar] [CrossRef]
- Abrudeanu, M.; Archambault, P. Corrélation microstructures—propriétés pour l’alliage zircaloy 4. In Proceedings of the Conference Zr-95, Saclay, Paris, France, 1995; pp. 303–312. [Google Scholar]
- Grain Size Determination in Zirconium Alloys. Final Report of a Co-Ordinated Research Programe 1989–1992. IAEA-TECDOC-794; IAEA: Vienna, Austria, 1995.
- ASTM E 112, Standard Test Methods for Determining Average Grain Size; ASTM International: West Conshohocken, PA, USA, 1988.
- Haldik, J. Metrologie des Propriétés Thermophysiques des Matériaux; Masson: Paris, France, 1990. [Google Scholar]
- Donaldson, A.T.; Evans, H.E. Oxidation induced Creep in zircalloy-2: III. The average stress in the oxide layer. J. Nucl. Mater. 1981, 99, 57–65. [Google Scholar] [CrossRef]
- Parker, W.J.; Jenkins, R.J.; Butler, C.P.; Abbott, G.L. Flash Method of Determining Thermal Diffusivity Heat Capacity and Thermal Conductivity. J. Appl. Phys. 1961, 32, 1679. [Google Scholar] [CrossRef]
- Watt, D.A. Theory of Thermal Diffusivity of Pulse Technique. Br. J. Appl. Phys. 1966, 231, 17. [Google Scholar] [CrossRef]
- Larson, K.B.; Koyama, K. Correction for Finite Pulse-Time Effects in Very Thin Samples Using the Flash Method of Measuring Thermal Diffusivity. J. Appl. Phys. 1967, 465, 38. [Google Scholar] [CrossRef]
- Clark, L.M., III; Taylor, R.E. Radiation Loss in the Flash Method for Thermal Diffusivity. J. Appl. Phys. 1975, 714, 46. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrudeanu, M.; Dicu, M.M.; Pasăre, M.M. Structure and Heat Transfer in Zircaloy-4 Treated at High Temperatures. Materials 2021, 14, 4494. https://doi.org/10.3390/ma14164494
Abrudeanu M, Dicu MM, Pasăre MM. Structure and Heat Transfer in Zircaloy-4 Treated at High Temperatures. Materials. 2021; 14(16):4494. https://doi.org/10.3390/ma14164494
Chicago/Turabian StyleAbrudeanu, Mărioara, Maria Magdalena Dicu, and Maria Minodora Pasăre. 2021. "Structure and Heat Transfer in Zircaloy-4 Treated at High Temperatures" Materials 14, no. 16: 4494. https://doi.org/10.3390/ma14164494
APA StyleAbrudeanu, M., Dicu, M. M., & Pasăre, M. M. (2021). Structure and Heat Transfer in Zircaloy-4 Treated at High Temperatures. Materials, 14(16), 4494. https://doi.org/10.3390/ma14164494




